Abstract
Pyruvate-fortified cardioplegia protects myocardium and hastens postsurgical recovery of patients undergoing cardiopulmonary bypass (CPB). Pyruvate reportedly suppresses degradation of the α-subunit of hypoxia-inducible factor-1 (HIF-1), an activator of the gene encoding the cardioprotective cytokine erythropoietin (EPO). This study tested the hypothesis that pyruvate-enriched cardioplegia evoked EPO expression and mobilized EPO signaling mechanisms in myocardium. Hearts of pigs maintained on CPB were arrested for 60 min with 4:1 blood-crystalloid cardioplegia. The crystalloid component contained 188 mM glucose ± 24 mM pyruvate. After 30-min cardiac reperfusion with cardioplegia-free blood, the pigs were weaned from CPB. Left ventricular myocardium was sampled 4 h after CPB for immunoblot assessment of HIF-1α, EPO and its receptor, the signaling kinases Akt and ERK, and endothelial nitric oxide synthase (eNOS), an effector of EPO signaling. Pyruvate-fortified cardioplegia stabilized arterial pressure post-CPB, induced myocardial EPO mRNA expression, and increased HIF-1α, EPO, and EPO-R protein contents by 60, 58, and 123%, respectively, vs. control cardioplegia (P < 0.05). Pyruvate cardioplegia also increased ERK phosphorylation by 61 and 118%, respectively, vs. control cardioplegia-treated and non-CPB sham myocardium (P < 0.01), but did not alter Akt phosphorylation. Nitric oxide synthase (NOS) activity and eNOS content fell 32% following control CPB vs. sham, but pyruvate cardioplegia prevented these declines, yielding 49 and 80% greater NOS activity and eNOS content vs. respective control values (P < 0.01). Pyruvate-fortified cardioplegia induced myocardial EPO expression and mobilized the EPO-ERK-eNOS mechanism. By stabilizing HIF-1α, pyruvate-fortified cardioplegia may evoke sustained activation of EPO's cardioprotective signaling cascade in myocardium.
Keywords: endothelial nitric oxide synthase, hypoxia-inducible factor
cardioplegia-induced cardiac arrest is a mainstay of cardiac surgery but imposes myocardial ischemia by interrupting coronary blood flow (1, 33). In a small, randomized trial in patients undergoing coronary artery revascularization on cardiopulmonary bypass (CPB), the use of pyruvate-fortified vs. conventional cardioplegia attenuated myocardial injury, hastened recovery of cardiac function, and shortened hospitalization (27). In a swine CPB model, cardioplegic arrest and reperfusion provoked oxidative stress, inactivated myocardial enzymes, and produced cardiac edema, but pyruvate cardioplegia prevented these detrimental acute effects (14, 15). However, pyruvate-enriched cardioplegia is rapidly cleared when the heart is reperfused with whole blood (35), so it seems unlikely that pyruvate's direct metabolic properties could explain its persistent, favorable cardiac effects in the clinical trial (27).
The heterodimeric transcription factor hypoxia-inducible factor 1 (HIF-1) activates an extensive gene program encoding proteins that mediate erythropoiesis, angiogenesis, ATP production, and antioxidant defense (4, 29, 34, 40). HIF-1's α- and β-subunits are constitutively expressed in cardiomyocytes, but rapid degradation of HIF-1α under normoxic conditions limits HIF-1-induction of gene expression. Fe2+-, O2-, and α-ketoglutarate-dependent hydroxylation of two proline residues, catalyzed by prolyl hydroxylase, targets HIF-1α for proteosomal degradation (4, 11, 34, 40). Pyruvate can interfere with this mechanism by competing with α-ketoglutarate for access to prolyl hydroxylase's catalytic core (19). By stabilizing HIF-1α, pyruvate may augment induction of the HIF-1 gene program.
The HIF-1-induced hematopoietic cytokine erythropoietin (EPO) has been found to protect myocardium from ischemic injury (5–7, 30, 37, 39). Interaction of EPO with its membrane receptor (EPO-R) initiates signaling cascades, mediated by Akt and/or extracellular signal-regulated kinase (ERK), which suppress mitochondrial permeability transition pore opening (22, 23), an activator of apoptotic cell death. Although myocardium expresses EPO-R (7, 41), reports of myocardial expression of the ligand EPO are sparse and equivocal.
This study tested the hypothesis that administration of pyruvate-fortified cardioplegia during CPB augmented myocardial HIF-1α content, enhanced expression and content of EPO and EPO-R, and activated the kinases that mediate EPO signaling, relative to conventional pyruvate-free cardioplegia. This potentially cardioprotective mechanism was examined in porcine myocardium 4 h after the pig was separated from CPB and the cardioplegia had cleared.
METHODS
Surgical preparation.
Animal experimentation was approved by the Animal Care and Use Committee of the University of North Texas Health Science Center and conducted in accordance with the Guide to the Care and Use of Laboratory Animals (National Institutes of Health publication 85–23, revised 1996). Twenty-four Yorkshire swine of either sex, 8–9 mo of age and weighing 50–70 kg, were assigned randomly to three groups of eight animals. The CPB groups received control or pyruvate-enriched cardioplegia. Shams were surgically prepared and studied for the same duration as the CPB experiments, but not subjected to cardiac arrest or CPB. After overnight fast, pigs were sedated with ketamine (10 mg/kg im) and xylazine (1 mg/kg im), anesthetized with propofol (2 mg/kg iv), and ventilated with 0.5–2% isoflurane supplemented with O2 to maintain a surgical plane of anesthesia. During CPB, anesthesia was maintained by continuous infusion of propofol, 0.2 mg·kg−1·min−1 iv. Body temperature was kept at 36–37°C throughout the protocol by use of circulating water heating pads.
The femoral artery and vein were cannulated for sampling blood and infusing medications, respectively. The arterial cannula was advanced into the thoracic aorta and connected to a pressure transducer (model 1290C, Hewlett-Packard) to monitor blood pressure and pulse. The heart was exposed by median sternotomy and supported in a pericardial cradle. Catheters were inserted into the coronary sinus to sample coronary venous blood and into the inferior vena cava via the right atrium to withdraw blood for the extracorporeal circuit. The descending aorta was cannulated to deliver oxygenated blood from the heart-lung machine. A cannula was inserted into the aortic root for cardioplegia infusion. Crystalloid cardioplegia (pH 7.6) contained 104 mM NaCl, 135 mM NaHCO3, 91 mM KCl, 6 mM CaCl2, 188 mM glucose, 68 U/l insulin, and 676 mg/l lidocaine (14). Pyruvate cardioplegia was prepared by equimolar substitution of 24 mM sodium pyruvate for NaCl. Crystalloid solutions were combined with four volumes of whole blood before infusion.
Experimental protocol.
The pig was connected to the heart-lung machine following heparin administration (300 U/kg iv). After aortic cross-clamp, cardiac arrest was induced by antegrade infusion of 1,200 ml hypothermic (20°C) cardioplegia. Additional cardioplegia (400 ml) was infused at 20- and 40-min arrest. Oxygenated blood from the heart-lung machine was delivered to the systemic circulation at a rate of ∼2.2 l/min, which maintained an aortic pressure of 60 mmHg throughout CPB. After 60-min arrest, the heart was reperfused for 30 min with cardioplegia-free blood, and then the pig was separated from bypass and allowed to recover off-pump for 4 h. Arterial Po2, Pco2, and pH, and HCO3− and K+ concentrations were measured in an Instrumentation Laboratory model 1730 blood-gas analyzer. Na-HCO3− and KCl were administered intravenously to correct acidemia and hypokalemia. Between 30-min and 2-h recovery, the α-adrenergic vasoconstrictor phenylephrine was infused intravenously as needed to maintain mean arterial pressure at 60–70 mmHg.
Plasma EPO.
Coronary sinus and systemic arterial plasma was obtained by centrifugation. Plasma EPO concentrations were measured by enzyme-linked immunosorbent assay (Biochain, Hayward, CA). Immune complexes detected at 450 nm were quantified by comparison with a recombinant EPO standard curve. Myocardial EPO uptake or release was assessed from coronary sinus-arterial EPO concentration differences.
Myocardial biopsies, metabolite analyses, and water content.
At 4-h recovery, myocardium was sampled for measurements of proteins, mRNA, metabolites, and water content. For metabolite and water measurements, the left ventricular anterior wall was snap-frozen in situ with liquid N2-precooled tongs (10). Next, transmural samples were quickly excised and snap-frozen for analyses of myocardial mRNA and proteins. Pyruvate, ATP, phosphocreatine (PCr), creatine (Cr), inorganic phosphate (Pi), glutathione (GSH), and glutathione disulfide (GSSG) were extracted and colorimetrically assayed, as previously described (10, 14). Myocardial energy state was assessed from ATP content and PCr phosphorylation potential, i.e., [PCr]/([Cr][Pi]), and myocardial antioxidant defenses were assessed from GSH redox state, i.e., [GSH]/[GSSG] (where brackets denote concentration) (14, 35). Myocardial water content was determined by weighing tissue samples (∼200 mg) before and after dessication to constant mass in an oven.
Analyses of myocardial proteins.
Proteins were extracted from left ventricular myocardium (15, 36) for immunoblot analyses of HIF-1α, EPO, EPO-R, total and phosphorylated ERK and Akt, and endothelial nitric oxide synthase (eNOS). Total protein concentrations in extracts were measured by the Bradford method (2). Proteins (50 μg/lane) were separated by electrophoresis (100 V for 90 min) on 10% SDS-PAGE gels, and then electrophoretically transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies for 2 h at room temperature, washed in Tween-20 Tris-buffered saline, and then exposed to horseradish peroxidase-conjugated secondary antibodies for 1 h. Immune complexes were detected by enhanced chemiluminescence (Thermo-Fisher, Rockford, IL) of H2O2. Primary antibodies were mouse monoclonal anti-HIF-1α (Abcam, Cambridge, MA), anti-EPO (R&D Systems, Minneapolis, MN), anti-(P-473S)-Akt, anti-ERK1/2, and anti-(P-202T, P-204Y)-ERK-1/2 (Cell Signaling, Danvers, MA), and rabbit polyclonal anti-EPO-R (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Akt, and anti-eNOS (Stressgen, Ann Arbor, MI). Anti-mouse and anti-rabbit secondary antibodies were from Kirkegaard and Perry (Gaithersburg, MD). Each membrane was stripped and reprobed with anti-actin antibody (Stressgen, Victoria, BC) to detect actin as loading control. Band densities were measured in an AlphaEase FC 4.0 densitometer (AlphaInnotech, San Leandro, CA), and then normalized to actin band densities. NOS activity was measured (31) with a Griess reaction-based kit (Oxis International, Portland, OR).
Real-time reverse transcriptase-polymerase chain reaction measurements of mRNA.
Real-time reverse transcriptase-polymerase chain reaction was used to assess myocardial abundances of HIF-1α, EPO, and actin mRNA. Total RNA was isolated using Trizol Regent (Invitrogen). cDNA was synthesized from total RNA with Taqman reverse transcriptase (Applied Biosystems, Foster City, CA). HIF-1α, EPO, and actin cDNA were amplified in a Smart cycler II (Cepheid, Sunnyvale, CA) by using Applied Biosystems SYBR Green Master Mix Kit with specific primers for HIF-1α (forward: 5′-GCCAGAACCTCCTGTAACCA-3′; reverse: 5′-CCTTTTCCTGCTCTGTTTGG-3′), EPO (forward: 5′-CCAAAGCAGGAGGAATTCAG-3′; reverse: 5′-GCTGTTGTGGGAGTCTCCAT-3′), and α-actin (forward: 5′-TCATCACCATCGGCAACG-3′; reverse: 5′-TTCCTGATGTCCACGTCGC-3′). Abundances of amplified genes were assessed by analysis of cycle threshold.
Statistical analysis.
Values are means ± SE. Between-group comparisons of mean values were accomplished by single-factor ANOVA. Between-group comparisons of hemodynamic variables (Fig. 1) and myocardial EPO balance (Fig. 4) at multiple time points were analyzed by repeated-measures ANOVA. When ANOVA detected statistical significance, Tukey's post hoc test was applied to identify the specific differences. Correlations between HIF-1α and EPO band densities (Fig. 5A) and EPO band density vs. myocardial water content (Fig. 5B) were analyzed by least squares linear regression. P values < 0.05 were taken to indicate statistically significant differences.
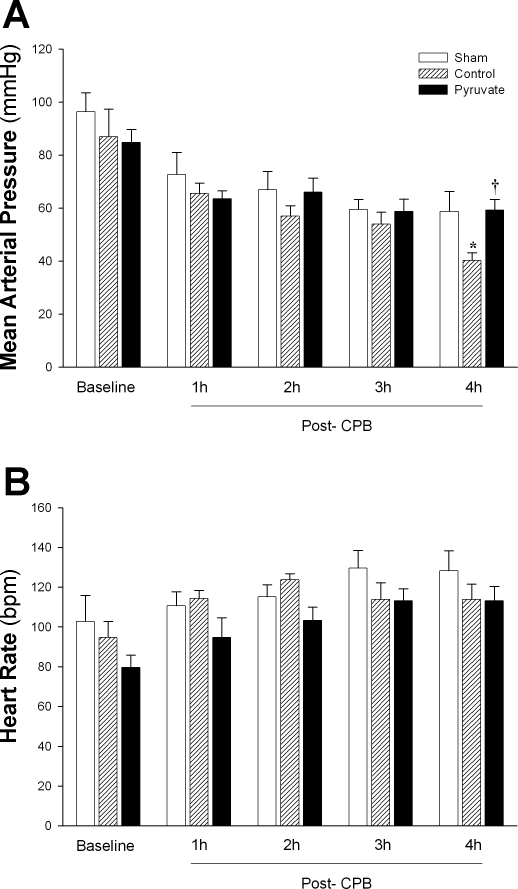
Fig. 1.
Systemic arterial blood pressure and heart rate during post-cardiopulmonary bypass (CPB) recovery. Mean arterial pressure (A) and heart rate (B) were measured at pre-CPB baseline and at 60-min intervals during post-CPB recovery in the control and pyruvate cardioplegia groups and at the corresponding time points in non-CPB sham experiments. Values are means ± SE; n = 8 experiments/group. *P < 0.05 vs. sham at the same time; †P < 0.05 vs. control CPB at the same time. No statistically significant between-group differences in heart rates were detected at any time point. bpm, Beats/min.
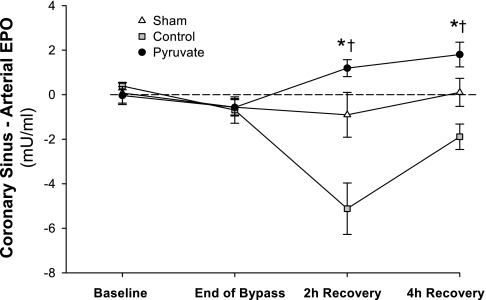
Fig. 4.
Myocardial EPO uptake/release. Coronary sinus-systemic arterial EPO concentration differences are plotted; values below the dotted line indicate net myocardial EPO uptake. Plasma EPO was measured at pre-CPB baseline, immediately before separation from CPB, and at 2 and 4 h post-CPB recovery. Values are means ± SE; n = 8 experiments/group. *P < 0.001 vs. control; †P < 0.001 vs. sham.
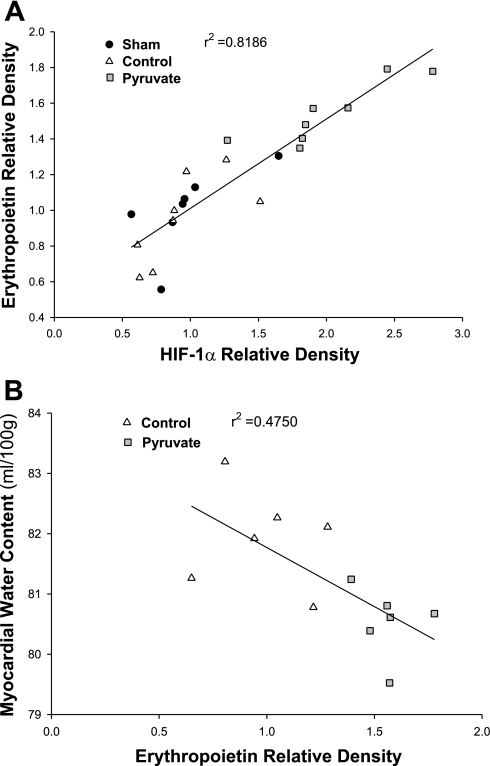
Fig. 5.
Myocardial HIF-1α vs. EPO contents (A) and EPO vs. water contents (B). Variables were measured in myocardium sampled 4 h after CPB with control (triangles) or pyruvate-fortified (squares) cardioplegia, or at the corresponding sham time point (circles). A: HIF-1α and EPO band densities were normalized to actin densities measured on the same immunoblots. Mean sham values = 1.0. The line indicates the least squares linear regression (P < 0.001). B: plotted points are from individual CPB experiments in which both EPO and water contents were measured (n = 6 per group). The line plots the least squares linear regression (P = 0.013).
RESULTS
Hemodynamic function during recovery from CPB.
In the non-CPB sham group, mean arterial pressure fell from 96 ± 8 to 72 ± 8 mmHg during the period corresponding to 60-min cardioplegic arrest + 30-min reperfusion on-pump + the first 60-min post-CPB, before stabilizing (Fig. 1A). This result indicates that surgical trauma and anesthesia contributed to a decline in blood pressure, even in the absence of cardioplegic arrest and extracorporeal circulation. In the control CPB group, arterial pressure fell by another 20 mmHg between 2 and 4 h post-CPB. Administration of pyruvate-enriched cardioplegia during CPB prevented the late hemodynamic decline, maintaining blood pressure at the sham-CPB level (Fig. 1A). Heart rate did not differ among the groups at any time (Fig. 1B).
Myocardial pyruvate content, energy metabolites, and GSH redox state.
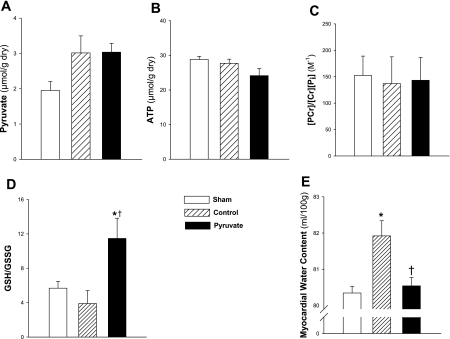
Recent studies in this model (14, 15) demonstrated that pyruvate-enriched cardioplegia acutely increased myocardial pyruvate and ATP contents, PCr phosphorylation potential, i.e., [PCr]/([Cr][Pi]), and GSH redox state, i.e., [GSH]/[GSSG], during initial reperfusion on-pump. At 4-h recovery, myocardial pyruvate content (μmol/g dry tissue) trended higher in the control (3.0 ± 0.4) and pyruvate (3.0 ± 0.2) groups vs. shams (2.0 ± 0.2), although the differences were not statistically significant (0.1 > P > 0.05). Moreover, pyruvate content was essentially identical in the two CPB groups (Fig. 2A) indicating complete clearance of the pyruvate cardioplegia. Similarly, pyruvate enhancement of myocardial ATP content (Fig. 2B) and phosphorylation potential (Fig. 2C) subsided by 4 h post-CPB. In contrast, [GSH]/[GSSG], an indicator of the redox state of the myocardium's antioxidant systems (32), was increased greater than or equal to twofold (P < 0.05) 4 h after pyruvate treatment vs. sham and control cardioplegia-treated myocardium (Fig. 2D).
Fig. 2.
Myocardial pyruvate content, energy and antioxidant redox state, and water content. Metabolites and water were measured in left ventricular myocardium sampled 4 h after CPB with control (hatched bars) or pyruvate-fortified (solid bars) cardioplegia, or in nonarrested sham time control (open bars). A and B: pyruvate and ATP contents, respectively. C: phosphocreatine (PCr) phosphorylation potential. D: glutathione (GSH)/glutathione disulfide (GSSG) redox state. E: water content. Cr, creatine; Pi, inorganic phosphate. Values are means ± SE; n = 8 experiments/group. *P < 0.05 vs. sham; †P < 0.05 vs. control.
Myocardial water content.
Myocardial edema is associated with contractile dysfunction following CPB (21). Myocardium maintained on control cardioplegia during CPB contained 82.0 ± 0.3 ml H2O/100 g at 4 h post-CPB (Fig. 2E), a marked increase from the water content of sham myocardium (80.3 ± 0.2 ml H2O/100 g), indicating the presence of tissue edema. Pyruvate-fortified cardioplegia prevented the subsequent increase in tissue water content (80.5 ± 0.2 ml H2O/100 g).
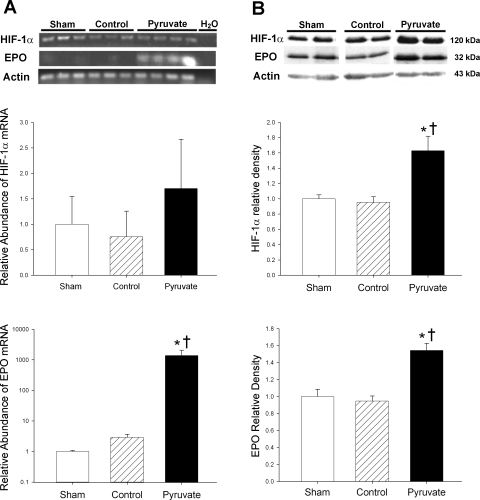
Expression and content of HIF-1α.
Myocardial HIF-1α mRNA abundances at 4-h recovery were similar in the control cardioplegia, pyruvate cardioplegia, and sham groups, although a modest upward trend (P > 0.2) in the pyruvate group was noted (Fig. 3A). In contrast, HIF-1α protein content was 60–70% greater (P < 0.05) in the pyruvate cardioplegia group vs. the other two groups (Fig. 3B). Thus 60-min exposure to pyruvate-fortified cardioplegia during CPB augmented myocardial HIF-1α content 4 h later, without a significant increase in the corresponding mRNA. These results suggest that pyruvate augments HIF-1α content by suppressing degradation, not increasing expression of HIF-1α.
Fig. 3.
Hypoxia-inducible factor (HIF)-1α and erythropoietin (EPO) mRNA expression and protein contents in left ventricular myocardium. Messenger RNA (A) and protein (B) were measured in myocardium sampled 4 h after CPB with control (hatched bars) or pyruvate-fortified (solid bars) cardioplegia, or at the corresponding sham time point (open bars). A, top: ethidium bromide-stained RT-PCR gel; middle: HIF-1α mRNA abundance; bottom: EPO mRNA abundance. mRNA abundances are normalized to α-actin mRNA and presented relative to sham values. EPO mRNA values are plotted on logarithmic scale. B, top: typical immunoblots of HIF-1α, EPO, and actin loading control; middle: relative HIF-1α band densities; bottom: relative EPO band densities. In this figure and in Figs. 5–8, mean sham values are assigned a value of 1.0. Values are means ± SE; n = 8 experiments/group. *P < 0.001 vs. control; †P < 0.001 vs. sham.
Pyruvate-induced expression and release of EPO.
EPO mRNA was barely detectable in sham and control cardioplegia-treated left ventricular myocardium, but was strikingly increased (P < 0.001) 4 h after arrest with pyruvate-fortified cardioplegia (Fig. 3A). Indeed, EPO mRNA content increased ∼1,000-fold in pyruvate-treated vs. control and sham myocardium (Fig. 3A, bottom). Furthermore, EPO protein content increased by 55–60% (P < 0.001) 4 h after treatment with pyruvate cardioplegia vs. respective control cardioplegia and sham values (Fig. 3B, bottom).
The changes in EPO expression and content were associated with altered myocardial EPO balance. At pre-CPB baseline, EPO concentrations were similar in arterial and coronary sinus plasma, indicating essentially no cardiac EPO release before arrest. Coronary sinus-arterial EPO concentration difference increased in the pyruvate group throughout recovery, but fell to negative values in the control CPB group (Fig. 4). Thus augmented myocardial EPO content following pyruvate cardioplegia paralleled net EPO release into the coronary circulation, in contrast to myocardial net EPO uptake following control cardioplegia treatment.
Correlation: myocardial HIF-1α vs. EPO contents.
To interrogate further the proposal that HIF-1 mediates pyruvate enhancement of EPO expression, the correlation between myocardial HIF-1α and EPO contents was examined (Fig. 5A). Least squares linear regression analysis revealed a robust correlation (r2 = 0.82; P < 0.001) between the two protein contents, with pyruvate-treated myocardium (Fig. 5A, squares) containing the greatest amounts of both. Although not definitive proof of a cause-and-effect relationship, this correlation supports the proposal that pyruvate-enhanced HIF-1α increased myocardial EPO expression.
Myocardial EPO vs. water content.
The relationship between myocardial EPO and water contents at 4 h post-CPB was examined as an initial test of the proposal that pyruvate-induced EPO expression may limit myocardial injury. A statistically significant (P = 0.013) inverse correlation emerged between the two variables (Fig. 5B). Although the correlation is not particularly strong (r2 = 0.475), it does support the concept that increased EPO content in the pyruvate-treated myocardium may have contributed to the prevention of edema.
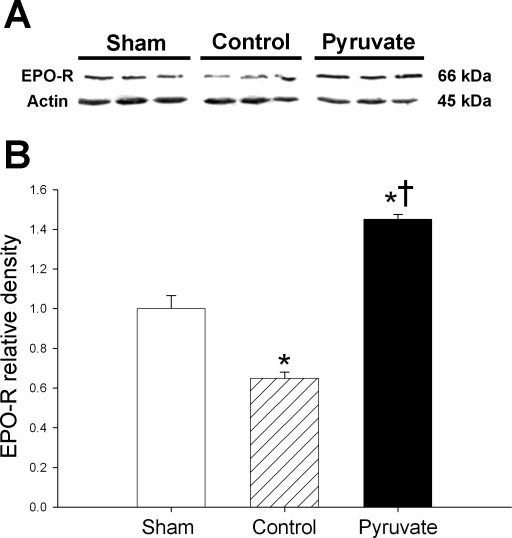
Myocardial EPO-R.
Myocardial content of EPO-R was analyzed by immunoblot (Fig. 6). EPO-R content fell 35% in control cardioplegia-treated vs. sham myocardium, but increased following pyruvate cardioplegia treatment. Thus CPB with control cardioplegia depleted myocardial EPO-R, but EPO-R content stabilized and even increased following treatment with pyruvate-fortified cardioplegia.
Fig. 6.
Erythropoietin receptors (EPO-R). A: typical immunoblots of EPO-R and actin. B: actin-normalized EPO-R band densities. Proteins were measured in myocardium sampled 4 h after CPB with control (hatched bars) or pyruvate-fortified (solid bars) cardioplegia, or at the corresponding sham time point (open bars). Values are means ± SE; n = 8 experiments/group. *P < 0.01 vs. sham; †P < 0.001 vs. control.
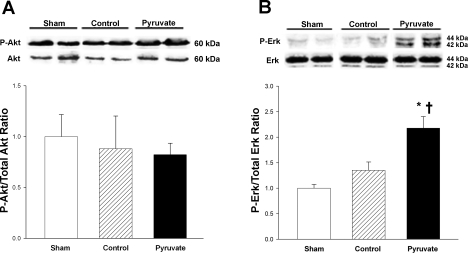
Total and phosphorylated Akt and ERK.
The impact of CPB ± pyruvate treatment on activities of the EPO-responsive protein kinases, Akt and ERK, was assessed by measuring their phosphorylation (Fig. 7). Myocardial total Akt and ERK contents were unaltered by control or pyruvate-enriched CPB. Similarly, neither control nor pyruvate-fortified CPB affected Akt phosphorylation vs. sham (Fig. 7A). On the other hand, although control CPB did not alter ERK phosphorylation, pyruvate-fortified cardioplegia administration during CPB increased myocardial ERK phosphorylation at 4 h post-CPB by 61 and 118% vs. the respective control cardioplegia and sham values (Fig. 7B). These results indicate that pyruvate administration during CPB enhanced subsequent activation of ERK but not Akt.
Fig. 7.
Akt and ERK phosphorylation. Top: typical immunoblots of phosphorylated and total Akt (A) and ERK (B). Bottom: ratios of actin-normalized P-Akt/total Akt and P-ERK/total ERK (arbitrary units). Proteins were measured in myocardium sampled 4 h after CPB with control (hatched bars) or pyruvate-fortified (solid bars) cardioplegia, or at the corresponding sham time point (open bars). Values are means ± SE; n = 8 experiments/group. *P < 0.05 vs. sham; †P < 0.05 vs. control.
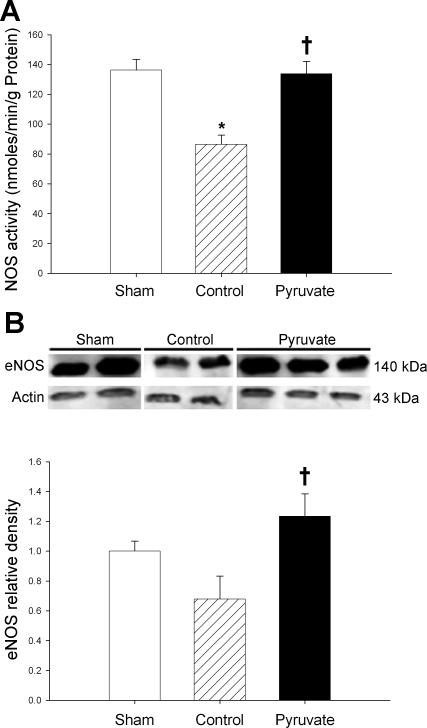
NOS.
Phosphorylated ERK activates eNOS, which produces cytoprotective nitric oxide. NOS activity and eNOS content fell in parallel, by 35 and 32%, respectively, in control cardioplegia-arrested vs. sham myocardium (Fig. 8). Pyruvate-fortified cardioplegia maintained NOS activity and eNOS content at the respective sham values. Thus pyruvate-induced enhancements of EPO, EPO-R, and P-ERK were associated with preservation of myocardial eNOS following CPB.
Fig. 8.
Nitric oxide synthase (NOS) activity and endothelial NOS (eNOS) content. A: NOS activities. B, top: typical immunoblot of eNOS and actin; bottom: actin-normalized eNOS contents. These variables were measured in myocardium sampled 4 h after CPB with control (hatched bars) or pyruvate-fortified (solid bars) cardioplegia, or at the corresponding sham time point (open bars). Values are means ± SE; n = 8 experiments/group. *P < 0.01 vs. sham; †P < 0.01 vs. control.
DISCUSSION
This study tested the hypothesis that administration of pyruvate-enriched cardioplegia to arrest the heart during CPB induced myocardial expression of EPO and activation of its signaling mechanisms 4 h later, a time at which arterial pressure was better maintained in the pigs administered pyruvate-enriched vs. conventional cardioplegia containing glucose as basal energy substrate. Compared with conventional cardioplegia, pyruvate-enriched cardioplegia 1) increased left ventricular myocardial contents of HIF-1α, EPO mRNA and protein, and EPO-R; 2) increased phosphorylation (i.e., activation) of ERK, a protein kinase implicated in EPO-induced cardioprotection (23, 25, 30); and 3) stabilized content and activity of the EPO-ERK effector, eNOS (5, 23). Another EPO-responsive kinase, Akt, was not activated by pyruvate cardioplegia. Thus pyruvate cardioplegia evoked selective activation of the EPO-ERK signaling pathway, an effect that persisted 4 h after pyruvate administration.
Pyruvate cardioplegia and EPO signaling.
Cardioplegia-induced cardiac arrest affords a quiescent field for cardiac surgical procedures and slows myocardial energy consumption. Nevertheless, interruption of coronary blood flow during CPB produces myocardial ischemia, which can cause functional cardiodepression for several hours post-bypass (3, 28). In a small, randomized phase I clinical trial, administration of pyruvate-fortified vs. conventional cardioplegia afforded more rapid postsurgical recovery of cardiac performance and hastened hospital discharge (27). This study sought to decipher mechanisms that may contribute to the favorable effects of pyruvate-enhanced CPB. Special attention was placed on the signaling cascade initiated by HIF-1 induction of EPO. Although the previously reported (14, 15) increase in myocardial ATP content and phosphorylation potential afforded by pyruvate-enriched cardioplegia had subsided by 4 h recovery, GSH redox state was still elevated, and major elements of the EPO cascade were enhanced. EPO mRNA expression, virtually undetectable in sham and control myocardium, was robust in pyruvate-treated myocardium, in which the α-subunit of EPO's transcription factor HIF-1 was stabilized.
The striking enhancement of EPO mRNA expression by pyruvate exceeded the more modest increases in myocardial EPO content and net EPO release. Conversely, sham and control cardioplegia-treated myocardium also contained appreciable amounts of EPO, despite the paucity of EPO mRNA. Circulating EPO may have contributed to myocardial EPO content in these groups, and sustained EPO release may have limited myocardial EPO accumulation following pyruvate treatment. Moreover, messenger RNA abundance is but one of several factors, including translational initiation, translational efficiency, and protein degradation, that determine steady-state tissue contents of specific proteins (24). Furthermore, mRNA synthesis and processing must occur before the encoded protein can be translated, so mRNA abundance may peak before protein content.
EPO-R, previously identified in rodent heart (7, 41), were detected in porcine myocardium. These receptors were depleted in myocardium arrested with control cardioplegia, but pyruvate-fortified cardioplegia prevented this decline. Pyruvate treatment also provoked phosphorylation of EPO's downstream kinase ERK and preserved myocardial content of eNOS, which ERK activates to produce cardioprotective nitric oxide (5, 22, 23). Thus pyruvate-fortified cardioplegia enhanced the EPO signaling cascade in left ventricular myocardium. This response may have been initiated by pyruvate stabilization of HIF-1α, an effect that was demonstrated in human cancer cell lines (19).
The present findings confirm and extend those of Lee et al. (18), who reported pyruvate-induced ERK phosphorylation in parallel with suppression of proapoptotic caspases in human umbilical vein endothelial cells. Lee et al. ascribed these effects to pyruvate's enhancement of GSH redox state and oxidation of cytosolic NADH (18). In accordance with the findings of Lee et al., enhancement of myocardial GSH/GSSG persisted for 4 h after pyruvate-enriched CPB, in parallel with increased ERK phosphorylation.
Pyruvate stabilization of HIF-1α.
This study provides evidence of a second mechanism of pyruvate-induced ERK phosphorylation, evoked by HIF-1α stabilization and HIF-1-driven EPO expression. HIF-1 orchestrates gene expression and synthesis of an array of cytoprotective proteins, including EPO, EPO-R, and eNOS (17, 20, 38, 40); pyruvate cardioplegia increased myocardial content of each of these proteins, relative to control cardioplegia. Indeed, a striking direct correlation emerged between HIF-1α and EPO contents. HIF-1 transcriptional activity is modulated by the dynamic balance of constitutive expression vs. degradation of its α-subunit (4, 34, 40). In the presence of O2, Fe2+, and α-ketoglutarate, hydroxylation of two proline residues (402P, 564P) by prolyl hydroxylase (11) targets HIF-1α for proteosomal degradation, thereby suppressing HIF-1-dependent gene expression (4, 34).
Pyruvate cardioplegia increased HIF-1α protein but not mRNA, in accord with the concept that pyruvate enhances HIF-1α content by preventing its degradation, not by increasing its gene expression and synthesis. Pyruvate and its carboxylation product oxaloacetate directly suppress HIF-1α degradation by competing with the essential cofactor α-ketoglutarate for access to prolyl hydroxylase's catalytic core (8, 19). In cultured human cancer cells, pyruvate concentrations as low as 1 mM produced robust HIF-1α accumulation, which persisted at least 40 min after the pyruvate was removed (19). In addition, a recent study in rat cortical neurons revealed strong correlations between HIF-1α content and GSH redox state, i.e., GSH/GSSG (9). The authors proposed that increased GSH/GSSG could suppress activation of proteosomes by reactive oxygen species and thus preserve HIF-1α (9). Pyruvate-fortified cardioplegia increased myocardial GSH/GSSG in the porcine CPB model (14); in the present study, this effect persisted for 4 h after CPB and washout of cardioplegia. Collectively, inhibition of prolyl hydroxylase and proteosomes by pyruvate and its metabolic products, including oxaloacetate and GSH, could suppress HIF-1α degradation and thus augment HIF-1-induction of EPO, EPO-R, and eNOS.
EPO signaling and cardioprotection.
Classically a hematopoietic cytokine, EPO recently has been found to powerfully protect myocardium from ischemia-reperfusion injury (6, 7). EPO occupation of its receptor mobilizes signaling elements that phosphorylate and activate ERK and/or Akt (22, 23). These kinases stimulate eNOS to produce nitric oxide, which activates mitochondrial ATP-sensitive K+ (mito-KATP) channels (26). Although pyruvate's impact on mito-KATP channels is unknown, Kerr et al. (13) showed that pyruvate suppressed mitochondrial permeability transition in postischemic rat myocardium, a protective effect potentially mediated by mito-KATP channels (16). Moreover, Kang et al. (12) demonstrated that pyruvate suppression of apoptosis in H2O2-challenged bovine pulmonary artery endothelial cells required pyruvate entry into the mitochondria.
Limitations.
Definitive proof linking the EPO-ERK-eNOS mechanism to pyruvate-induced cytoprotection requires specific pharmacological, short interfering RNA, and/or transgenic strategies. Such approaches are impractical in the porcine CPB model due to its size and complexity, but could be applied to transgenic rodents or cultured cell systems. Pharmacological inhibitors or activators of signaling kinases or NOS also could be administered via cardioplegia.
This study was conducted in juvenile swine without evidence of cardiac disease. In clinical settings, CPB is used to facilitate invasive surgical procedures on diseased hearts, e.g., coronary artery revascularization or valve replacement. Such procedures were not a focus of this study and were not conducted on the arrested hearts.
HIF-1-induced EPO expression and signaling was examined in myocardium sampled at 4-h recovery following CPB. This single time point might not have coincided with maximum pyruvate-evoked gene expression, protein content, or kinase activation. Of special interest for future studies is whether pyruvate-enhanced EPO signaling suppresses mechanisms of apoptosis and inflammation, thereby improving cardiac recovery in the days following CPB.
Implications.
This study reveals a novel cardioprotective mechanism of pyruvate, distinct from and more persistent than its enhancements of myocardial energy and antioxidant redox states (14, 15, 35). For the first time, a metabolic substrate is found capable of inducing EPO expression within the myocardium and activating EPO signaling. Intracoronary administration of pyruvate-enriched cardioplegia affords the opportunity to mobilize this novel, potentially cardioprotective mechanism.
GRANTS
This work was funded by grants from the National Heart, Lung, and Blood Institute (HL-71684), the Osteopathic Heritage Foundation (02-18-522), and University of North Texas Health Science Center (UNTHSC: 97001). M. G. Ryou and H. Gurji were supported by predoctoral fellowships from the UNTHSC Graduate School of Biomedical Sciences. This work was conducted in partial fulfillment of the requirements for the PhD degree for M. G. Ryou.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Boyle EM, Jr, Pohlman TH, Cornejo CJ, Verrier ED. Endothelial cell injury in cardiovascular surgery: ischemia-reperfusion. Ann Thorac Surg 62: 1868–1875, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Breisblatt WM, Stein KL, Wolfe CJ, Follansbee WP, Capozzi J, Armitage JM, Hardesty RL. Acute myocardial dysfunction and recovery: a common occurrence after coronary bypass surgery. J Am Coll Cardiol 15: 1261–1269, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev 17: 2614–2623, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol 100: 397–403, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108: 79–85, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci USA 100: 4802–4806, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalgard CL, Lu H, Mohyeldin A, Verma A. Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem J 380: 419–424, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S, Bragina O, Xu Y, Cao Z, Zhou B, Morgan M, Lin Y, Jiang BH, Liu KJ, Shi H. Glucose up-regulates HIF-1α expression in primary cortical neurons in response to hypoxia through maintaining cellular redox status. J Neurochem 105: 1849–1860, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Itoya M, Mallet RT, Gao ZP, Williams AG, Jr, Downey HF. Stability of high-energy phosphates in right ventricle: myocardial energetics during right coronary hypoperfusion. Am J Physiol Heart Circ Physiol 271: H320–H328, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem 74: 115–128, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kang YH, Chung SJ, Kang IJ, Park JHY, Bünger R. Intramitochondrial pyruvate attenuates hydrogen peroxide-induced apoptosis in bovine pulmonary artery endothelium. Mol Cell Biochem 216: 37–46, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kerr PM, Suleiman MS, Halestrap AP. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am J Physiol Heart Circ Physiol 276: H496–H502, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Knott EM, Ryou MG, Sun J, Heymann A, Sharma AB, Lei Y, Baig M, Mallet RT, Olivencia-Yurvati AH. Pyruvate-fortified cardioplegia suppresses oxidative stress and enhances phosphorylation potential of arrested myocardium. Am J Physiol Heart Circ Physiol 289: H1123–H1130, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Knott EM, Sun J, Lei Y, Ryou MG, Olivencia-Yurvati AH, Mallet RT. Pyruvate mitigates oxidative stress during reperfusion of cardioplegia-arrested myocardium. Ann Thorac Surg 81: 928–934, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Krolikowski JG, Bienengraeber M, Weihrauch D, Warltier DC, Kersten JR, Pagel PS. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: the role of mitochondrial KATP channels. Anesth Analg 101: 1590–1596, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Lam SY, Tipoe GL, Fung ML. Upregulation of erythropoietin and its receptor expression in the rat carotid body during chronic and intermittent hypoxia. Adv Exp Med Biol 648: 207–214, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Kang IJ, Bünger R, Kang YH. Enhanced survival effect of pyruvate correlates MAPK and NF-κB activation in hydrogen peroxide-treated human endothelial cells. J Appl Physiol 96: 793–801, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem 280: 41928–41939, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1α-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics 23: 79–88, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Mehlhorn U, Geissler HJ, Laine GA, Allen SJ. Myocardial fluid balance. Eur J Cardiothorac Surg 20: 1220–1230, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Merla R, Ye Y, Lin Y, Manickavasagam S, Huang MH, Perez-Polo RJ, Uretsky BF, Birnbaum Y. The central role of adenosine in statin-induced ERK1/2, Akt, and eNOS phosphorylation. Am J Physiol Heart Circ Physiol 293: H1918–H1928, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Miki T, Miura T, Tanno M, Nishihara M, Naitoh K, Sato T, Takahashi A, Shimamoto K. Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res Cardiol 102: 163–170, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Morgan HE, Beinlich CJ. Contributions of increased efficiency and capacity of protein synthesis to rapid cardiac growth. Mol Cell Biochem 176: 145–151, 1997 [PubMed] [Google Scholar]
- 25.Mudalagiri NR, Mocanu MM, Di Salvo C, Kolvekar S, Hayward M, Yap J, Keogh B, Yellon DM. Erythropoietin protects the human myocardium against hypoxia/reoxygenation injury via phosphatidylinositol-3 kinase and ERK1/2 activation. Br J Pharmacol 153: 50–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ockaili R, Emani VR, Okubo S, Brown M, Krottapali K, Kukreja RC. Opening of mitochondrial KATP channel induces early and delayed cardioprotective effect: role of nitric oxide. Am J Physiol Heart Circ Physiol 277: H2425–H2434, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Olivencia-Yurvati AH, Blair JL, Baig M, Mallet RT. Pyruvate-enhanced cardioprotection during cardiopulmonary bypass surgery. J Cardiothorac Vasc Anesth 17: 715–720, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Perrault LP, Menasché P. Preconditioning: can nature's shield be raised against surgical ischemic-reperfusion injury? Ann Thorac Surg 68: 1988–1994, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Quing M, Görlach A, Schumacher K, Wöltje M, Vazquez-Jimenez JF, Hess J, Seghaye MC. The hypoxia-inducible factor HIF-1 promotes intramyocardial expression of VEGF in infants with congenital cardiac defects. Basic Res Cardiol 102: 224–232, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Rafiee P, Shi Y, Su J, Pritchard KA, Jr, Tweddell JS, Baker JE. Erythropoietin protects the infant heart against ischemia-reperfusion injury by triggering multiple signaling pathways. Basic Res Cardiol 100: 187–197, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Ryou MG, Sun J, Oguayo KN, Manukhina EB, Downey HF, Mallet RT. Hypoxic conditioning suppresses nitric oxide production upon myocardial reperfusion. Exp Biol Med (Maywood) 233: 766–774, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Schmitt JP, Schröder J, Schunkert H, Birnbaum DE, Aebert H. Role of apoptosis in myocardial stunning after open heart surgery. Ann Thorac Surg 73: 1229–1235, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8: S62–S67, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Sharma AB, Knott EM, Bi J, Martinez RR, Sun J, Mallet RT. Pyruvate improves cardiac electromechanical and metabolic recovery from cardiopulmonary arrest and resuscitation. Resuscitation 66: 71–81, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Sharma AB, Sun J, Howard LL, Williams AG, Jr, Mallet RT. Oxidative stress reversibly inactivates myocardial enzymes during cardiac arrest. Am J Physiol Heart Circ Physiol 292: H198–H206, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Rafiee P, Su J, Pritchard KA, Tweddell JS, Baker JE. Acute cardioprotective effects of erythropoietin in infant rabbits are mediated by activation of protein kinases and potassium channels. Basic Res Cardiol 99: 173–182, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol 33: 968–979, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El-Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an AKT-dependent pathway. Biochem Biophys Res Commun 308: 990–994, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16: 1151–1162, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J 18: 1031–1033, 2004 [DOI] [PubMed] [Google Scholar]