Abstract
Calcineurin is a Ca2+/calmodulin-dependent protein phosphatase that induces myocardial growth in response to several physiological and pathological stimuli. Calcineurin inhibition, induced either via cyclosporine or genetically, can decrease myocardial hypertrophy secondary to pressure overload without affecting left ventricular (LV) systolic function. Since hypertrophy can also affect LV diastolic function, the goal of this study was to examine the effects of chronic pressure overload (2 wk aortic banding) in transgenic (Tg) mice overexpressing Zaki-4β (TgZ), a specific endogenous inhibitor of calcineurin, on LV diastolic function. As expected, in the TgZ mice with calcineurin inhibitor overexpression, aortic banding reduced the degree of LV hypertrophy, as assessed by LV weight-to-body weight ratio (3.5 ± 0.1) compared with that in non-Tg mice (4.6 ± 0.2). LV systolic function remained compensated in both groups with pressure overload. However, the LV end-diastolic stress-to-LV end-diastolic dimension ratio, an index of diastolic stiffness and LV pressure half-time and isovolumic relaxation time, two indexes of isovolumic relaxation, increased significantly more in TgZ mice with aortic banding. Protein levels of phosphorylated phospholamban (PS16), sarco(endo)plasmic reticulum Ca2+-ATPase 2a, phosphorylated ryanodine receptor, and the Na+/Ca2+ exchanger were also reduced significantly (P < 0.05) in the banded TgZ mice. As expected, genetic calcineurin inhibition inhibited the development of LV hypertrophy with chronic pressure overload but also induced LV diastolic dysfunction, as reflected by both impaired isovolumic relaxation and increased myocardial stiffness. Thus genetic calcineurin inhibition reveals a new mechanism regulating LV diastolic function.
Keywords: hypertrophy, diastole, hemodynamics
left ventricular (LV) hypertrophy (LVH) is the major compensatory mechanism in response to pressure or volume overload in the heart. There are few adverse effects of mild to moderate LVH, but more severe LVH is thought to be deleterious because of limitations in subendocardial coronary reserve (12, 13, 27) and alterations in LV function, particularly in isovolumic relaxation. Since the calcineurin pathway is thought to be a key mechanism mediating the development of cardiac hypertrophy, it is not surprising that several studies have suggested that the inhibition of this pathway could be an important approach for clinical therapy (4, 14, 18, 20, 24). However, the prior work in this field has not determined whether this therapeutic approach might invoke the precise adverse effects the therapy is designed to correct, e.g., alterations in LV diastolic function.
Calcineurin is a Ca2+/calmodulin-activated serine/threonine phosphatase that is activated by sustained elevations in intracellular Ca2+ and that plays a significant role in cardiac hypertrophy as a sensing molecule which links alterations with Ca2+ handling and the genetic program of hypertrophic growth (18). The hypertrophy process is initiated by the dephosphorylation of transcription factors of the nuclear factor of activated T cells (NFAT) family. It is presumed that the translocation of activated NFAT3 to the nuclei leads to genetic reprogramming and initiation of the hypertrophic transcriptional response. A cardiac-specific overexpression of calcineurin in transgenic (Tg) mice was shown to induce profound hypertrophy characterized by a two- to fourfold increase in the heart size, which rapidly progresses to dilated heart failure (23, 24, 28). On the other hand, calcineurin inhibition blunted hypertrophy, further demonstrating the involvement of calcineurin in this process. In this sense, several studies have shown that pharmacological (15, 24, 26) or genetic calcineurin inhibition (10, 20) can regress hypertrophy without affecting LV systolic function. However, the extent to which inhibiting calcineurin affects LV diastolic function is not established, despite its importance in LVH (7) and its contribution to the development of cardiac failure (1).
Therefore, to address the effects of the inhibition of LVH by calcineurin on LV diastolic function, it would be more desirable to inhibit the calcineurin pathway genetically. To this end, the genetic inhibition of calcineurin was achieved using Tg mice that overexpressed ZAKI-4β (TgZ) (also designated as MCIP2 or DSCR1L1), a specific endogenous calcineurin inhibitor (17, 21). Accordingly, the objective of this study was to assess the effects of the genetic inhibition of calcineurin on the development of LVH, on LV systolic and LV diastolic function, and on Ca2+ regulatory proteins in TgZ and NTg mice with chronic pressure overload due to aortic stenosis. Diastolic function was assessed both on isovolumic relaxation and myocardial stiffness.
MATERIALS AND METHODS
Tg mice.
TgZ mice were generated in an FVB background using the α-myosin heavy chain promoter (courtesy of Dr. J. Robbins, University of Cincinnati) to achieve cardiac-specific expression.
Experimental groups.
Four experimental groups were performed: non-Tg (NTg) and TgZ sham-operated mice and NTg and TgZ mice with 2 wk of transverse aortic banding (n = 8–18 in each group). All protocols concerning animal use were approved by the Institutional Animal Care and Use Committee at the New Jersey Medical School. The transverse thoracic aorta between the innominate artery and left common carotid artery was constricted using a 30-gauge needle and a 7-0 nylon suture with the aid of a dissecting microscope and under anesthesia (11, 22, 32–34). After removal of the needle, the aorta remained constricted. Aortic constriction was performed intraperitoneally using a mixture of ketamine (0.065 mg/g), xylazine (0.013 mg/g), and acepromazine (0.002 mg/g) for anesthesia.
Cardiac catheterization.
Two weeks after aortic banding, closed-chest catheterization was performed. Two high-fidelity catheter tip transducers (1.4 Fr, Millar) were used: one was inserted into the right carotid artery and carefully advanced to the LV, and the other into the left femoral artery and abdominal aorta, respectively. The pressures in the LV and abdominal aorta were measured simultaneously to calculate the pressure gradient. The first derivative of LV pressure was used as an isovolumic index of systolic function. After the hemodynamic study, the mice were then euthanized and the heart and lungs were dissected and weighed. Half of the LV tissue was frozen in liquid nitrogen, and the other half was fixed in 10% formalin.
Echocardiography.
Mice were anesthetized using 12 μl/g body wt of 2.5% filtered Avertin (Sigma-Aldrich), and echocardiography was performed using ultrasonography (Acuson Sequoia C256; Siemens Medical Solutions). A 13-MHz linear ultrasound transducer was used. We took two-dimensional-guided motion mode measurements of LV internal diameter from more than three beats and averaged the measurements. The LV end-diastolic (LVED) dimension (LVEDD) was measured at the time of the apparent maximal LVEDD, whereas LV end-systolic dimension was measured at the time of the most anterior systolic excursion of the posterior wall. Ejection fraction was also calculated and used as an ejective index of systolic function.
Indexes of diastolic function.
Diastolic function was assessed by indexes derived from the curve of LV pressure and dimensions and also using echocardiography Doppler data.
End-diastolic LV global circumferential wall stress was calculated using a cylindrical model: Stress = 1.36[(LVEDP·LVEDD)/(2·LVEDWT)], where LVEDP is LV end-diastolic pressure and LVEDWT is LVED wall thickness.
The LVED stress-to-diameter ratio was assessed to measure diastolic stiffness.
LV pressure half-time (T1/2), an isovolumic relaxation index, was calculated from the LV pressure curve.
The E wave, A wave, E-wave-to-A-wave ratio, and the duration of the isovolumic relaxation time were estimated by the Doppler-echo study.
Histology.
In each group, the hearts were separated and processed for histological analysis. They were cut and stained with hematoxylin and eosin and picrosirius red. The interstitial collagen concentration in the septum and LV free wall was assessed in the slides stained with the picrosirius red technique. Digital image software (Image ProPlus 5.0) bound to a Leitz light microscope was used with a ×20 objective, counting a total of 50 fields per heart. The percentage of collagen in each assessed region was calculated by adding the areas corresponding to collagen, divided by the addition of all the areas corresponding to myocytes plus the areas of collagen tissue.
Western blot analysis.
Western blots were performed for phospholamban (PLB), phospho-PLB (Ser16) (PS16), sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a), phospho-ryanodine receptor (Ser2808), Na+/Ca2+ exchanger, and protein phosphatase 1 (PP1). Other noncontractile proteins like vinculin, paxillin, desmin, tallin, and β-tubulin were also evaluated using total protein lysates prepared from homogenized LVs of hearts. Membrane fractions were separated from total lysates by centrifugation at 100,000 g for 45 min. The membrane pellet was then suspended in extraction buffer. The proteins were separated on 8% SDS-PAGE, transferred to nitrocellulose, and probed with primary antibody. The secondary antibody was goat anti-rabbit coupled to horseradish peroxidase. The blots were developed with enhanced chemiluminescence and scanned, and the band densities were measured and expressed in arbitrary units. Protein kinase A (PKA) activity was determined by StressXpress PKA Kinase Activity Assay kit.
NFAT assays.
NFAT-luciferase reporter mice (Tg-NFAT-Luc) were generated on an FVB background (30). Heart homogenates (10 μg) from NFAT-Luc Tg mice were lysed in Reporter lysis buffer (Promega) and analyzed for Luc activity. The Luc activity was measured using the luciferase assay system (Promega) and OPTOCOMP I (MGM Instruments).
Statistical analysis.
All data are presented as means ± SE. For statistical analysis of the data from multiple groups, ANOVA was used and comparisons between groups were made using Student-Newman-Keuls test. P < 0.05 was taken as a minimal level of significance.
RESULTS
After 2 wk of aortic stenosis, the LV aortic pressure gradient was similar in the NTg and TgZ mice (97 ± 5 vs. 90 ± 4 mmHg). However, the LVH, LV weight-to-body weight ratio (mg/g), was greater in the NTg animals (4.6 ± 0.2) compared with TgZ (3.5 ± 0.1) (P < 0.05). Table 1 shows systolic function and hemodynamic data. The maximal first derivative of LV pressure and LV ejection fraction were not reduced in both banded groups, demonstrating a preserved LV systolic function. LVEDD was not increased in either group.
Table 1.
LV systolic function and hemodynamics
| NTg Sham | TgZ Sham | NTg Aortic Banded | TgZ Aortic Banded | |
|---|---|---|---|---|
| LVEF, % | 70±1.1 | 72±0.7 | 69±0.8 | 67±0.9 |
| LV dP/dt, mmHg/s | 7,756±443 | 8,000±328 | 9,659±714 | 8,798±518 |
| LVSP, mmHg | 92±2.3 | 91±2.3 | 168±5.5† | 163±3.9† |
| MAP, mmHg | 77±2.0 | 76±1.9 | 59±3.4† | 63±1.9† |
| HR, beats/min | 445±16 | 470±10 | 472±20 | 493±22 |
| LVEDD, mm | 3.56±0.07 | 3.64±0.04 | 3.56±0.04† | 3.58±0.04 |
| LV/BW | 2.9±0.0 | 2.7±0.1 | 4.6±0.2† | 3.5±0.1*† |
| Lung weight/BW | 5.2±0.2 | 5.9±0.1 | 7.8±0.4† | 6.7±0.2 |
Values are means ± SE; n = 10–18 mice. LV, left ventricular; NTg, nontransgenic; TgZ, transgenic mice overexpressing Zaki-4β; LVEF, LV ejection fraction; dP/dt, first derivitive of LV pressure; LVSP, LV systolic pressure; MAP, mean arterial pressure; HR, heart rate; LVEDD, LV end-diastolic dimension; BW, body weight.
P < 0.05, TgZ banded vs. NTg banded;
P < 0.05, aortic banded vs. sham operated.
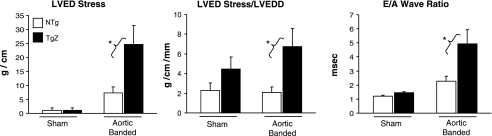
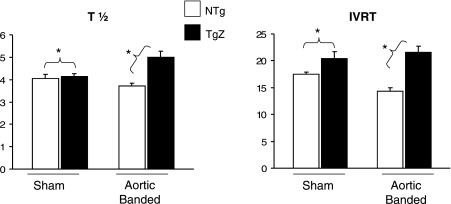
Although LVEDD was similar in both groups, LVED pressure rose significantly more (P < 0.05) in the TgZ group with aortic banding than in the NTg group (10.1 ± 2.13 vs. 3.7 ± 0.8 mmHg), resulting in an increased LVED wall stress and increased myocardial stiffness, as reflected by the LVED stress-to-LVEDD ratio and the E-wave-to-A-wave ratio, a Doppler index of diastolic function (Fig. 1). T1/2, an isovolumic relaxation index derived from the LV pressure curve, and isovolumic relaxation time, an isovolumic relaxation index derived from Doppler, both also consistently increased in the TgZ group with and without aortic banding (Fig. 2). These data suggest that calcineurin genetic inhibition caused diastolic dysfunction which affected not only myocardial stiffness but isovolumic relaxation as well.
Fig. 1.
Left ventricular (LV) end-diastolic (LVED) stress, LVED stress-to-LVED dimension (LVEDD) ratio (LVED stress/LVEDD), and the E-wave-to-A-wave (E/A wave) ratio are shown in nontransgenic (NTg) mice and transgenic (Tg) mice overexpressing Zaki-4β (TgZ) with and without (sham operated) aortic banding. In the Tg group with aortic banding, the 2 indexes of LV diastolic function were impaired, whereas LV diastolic dimensions did not change, resulting in increases in LV diastolic stress and diastolic stiffness. *P < 0.05, TgZ with aortic banding vs. NTg with aortic banding.
Fig. 2.
T1/2 and IVRT are shown in NTg and TgZ mice with and without (sham operated) aortic banding. In the Tg group with aortic banding, the 2 indexes show an impairment of LV isovolumic relaxation. *P < 0.05, TgZ with aortic banding vs. NTg with aortic banding. IVRT, isovolumic relaxation time; T1/2, LV pressure half-time.
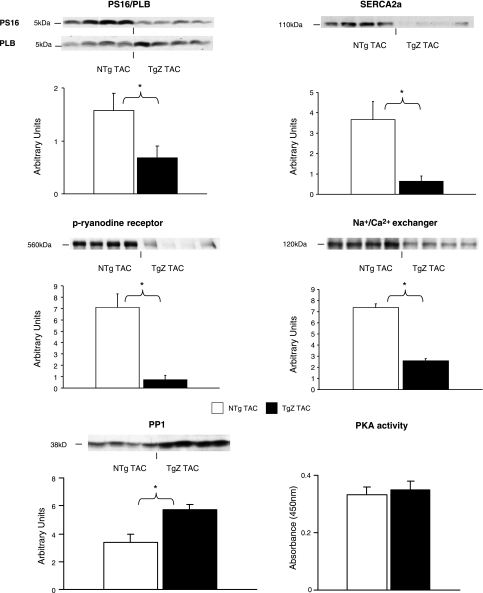
The total collagen did not change significantly with aortic banding in either group. The noncontractile proteins vinculin, paxillin, desmin, tallin, and β-tubulin were measured by Western blot analysis, and the levels were not different in the two groups (data not shown). PLB phosphorylation, reflected by the ratio of PS16 to PLB, was decreased (P < 0.05) in aortic-banded TgZ mice. The protein levels for SERCA2a, the Na+/Ca2+ exchanger, and phosphorylated ryanodine receptor were also decreased in aortic-banded TgZ mice (Fig. 3). As noted in Table 2, the protein levels for SERCA2a, the ratio of PS16 to PLB, and the Na+/Ca2+ exchanger were also significantly reduced in sham-operated TgZ mice. In addition, PP1 expression and PKA activity, which could be involved in PLB phosphorylation, were examined in the two groups. As shown in Fig. 3, PP1 was increased significantly (P < 0.05) in TgZ mice with aortic banding, but PKA activity showed no difference between the two groups.
Fig. 3.
The ratio of PS16 to phospholamban (PLB), sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a), phospho-ryanodine receptor (p-ryanodine receptor), and the Na+/Ca2+ exchanger are shown. There is a decrease in all the Ca2+-handling proteins in the TgZ group with aortic banding. Protein phosphatase 1 (PP1) was increased significantly in the TgZ group with aortic banding. There was no difference in PKA activity between the 2 groups. *P < 0.05, TgZ with aortic banding vs. NTg with aortic banding (n = 4 to 5/group). TAC, transverse aortic constriction.
Table 2.
Ca2+-handling proteins
| NTg Sham | TgZ Sham | |
|---|---|---|
| PS16/phospholamban | 2.2±0.3 | 0.6±0.1* |
| SERCA2a | 9.0±1.4 | 3.2±0.6* |
| Phospho-ryanodine receptor | 6.5±1.2 | 4.1±1.4 |
| Na+/Ca2+ exchanger | 5.6±0.6 | 2.4±0.2* |
Values are means ± SE (in arbitrary units); n = 5 to 6 mice/group. SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase 2a.
P < 0.05 vs. sham operated.
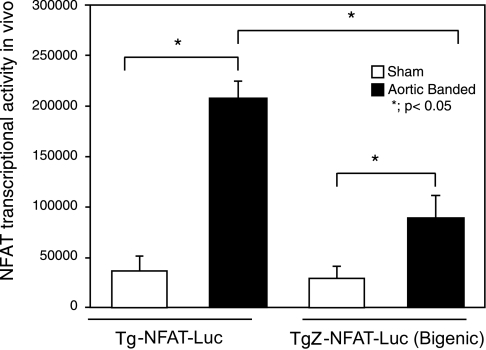
Transcriptional activity of NFAT is one of the most reliable indicators of the calcineurin activity in the heart in vivo (30). To evaluate the effect of ZAKI-4β upon the calcineurin activity in vivo hearts, TgZ mice were crossed with Tg-NFAT-Luc mice, which harbor an NFAT-Luc reporter gene, and the mice were then subjected to aortic banding for 2 wk. Aortic banding-induced increases in NFAT-Luc activity were significantly attenuated in TgZ-NFAT-Luc mice compared with Tg-NFAT-Luc mice, suggesting that calcineurin activity is significantly attenuated in the presence of ZAKI-4β (Fig. 4).
Fig. 4.
Nuclear factor of activated T cells (NFAT)-luciferase reporter mice (Tg-NFAT-Luc) were crossed with TgZ mice. Tg-NFAT-Luc and TgZ-NFAT-Luc (Bigenic) mice were subjected to either sham operation or transverse aortic banding. The Luc activity was measured from heart homogenates and normalized by the protein content. Values are means ± SE; n = 4/group.
DISCUSSION
In the present study we evaluated the effects of 2 wk of chronic pressure overload induced by aortic banding in mice with Tg inhibition of calcineurin on LVH and systolic and diastolic function. Calcineurin is a major mechanism mediating the development of LVH (10, 15, 20, 23, 24, 26, 28), and the inhibition of calcineurin has been shown to reduce LVH without affecting LV systolic function (10, 20). Our study confirmed these results, demonstrating significantly less hypertrophy in the banded TgZ mice without a compromise of LV systolic function. Although LVH is one of the major causes of LV diastolic function leading to diastolic heart failure (1, 7), few studies have examined the role of calcineurin on LV diastolic function. Surprisingly, despite reduced hypertrophy, which should protect against diastolic dysfunction and the preservation of LV systolic function in the banded Tg mice with inhibition of calcineurin, both components of LV diastolic function were compromised severely; i.e., relaxation was impaired and myocardial stiffness was significantly increased. The increase in myocardial stiffness was characterized by significant increases in LVED pressure and LVED wall stress without an increase in LVEDDs. Mitral inflow E-wave-to-A-wave ratio, an indirect index of stiffness, was also increased with banding in TgZ mice. The impairment of isovolumic relaxation was also measured by T1/2 using a high-fidelity micromanometer. The impaired diastolic function with pressure overload due to calcineurin inhibition has not been observed previously. Yamamoto et al. (31) found that both an angiotensin II type I receptor antagonist and calcineurin inhibition protected against the development of LVH, but the angiotensin II type I receptor antagonist also reduced myocardial stiffness in hypertensive rats with LVH, whereas the calcineurin inhibitor did not exhibit this positive effect on LV diastolic function. Semeniuk et al. (23) found that inhibition of LVH with cyclosporine in mice with overexpressed calcineurin in the heart did not reverse either LV systolic or diastolic dysfunction. The lack of effect could have been due to the inability to inhibit all of the effects of the overexpressed calcineurin, since cyclosporine was administered later, or due to the toxic effects of cyclosporine (15).
It is conceivable that the impaired LV diastolic function observed after 2 wk of banding in the TgZ group could reflect a more rapid LV systolic dysfunction and development of heart failure with banding in this group. This was not likely since the systolic LV function was almost identical in the two groups. Furthermore, we followed a subgroup of banded NTg and TgZ mice (n = 4–5/group) for 4 wk and found that LV systolic function declined similarly in the NTg and TgZ groups, e.g., LV ejection fraction (61 ± 0.8 vs. 61 ± 1.2%). Thus LV systolic function was maintained in both groups after 2 wk aortic banding and began to decline by similar amounts after 4 wk aortic banding.
The sarcoplasmic reticulum includes SERCA2a, a Ca2+-ATPase. These proteins regulate Ca2+ dynamics, through a Ca2+ release during contraction and Ca2+ sequestration during relaxation. During depolarization, a small amount of Ca2+ enters the cardiac cell through the L-type Ca2+ channels, which causes a localized increase in Ca2+ between the sarcolemma and the sarcoplasmic reticulum, which activates the Ca2+-release channel or ryanodine receptors to release Ca2+ into the cytosol. This process is termed “Ca2+-induced Ca2+ release” and is involved in excitation-contraction coupling. Relaxation is initiated by the uptake of Ca2+ into the sarcoplasmic reticulum by Ca2+-ATPase and SERCA2a, which are under the regulation of PLB, and to a lesser extent by the Na+/Ca2+ exchanger.
Although it is known that calcineurin affects Ca2+ handling and the regulation of SERCA and PLB (5, 16, 18, 19, 25), less is known how these proteins may regulate diastolic function with inhibition of calcineurin. In the current investigation we found that the impairment in isovolumic relaxation was associated with a decrease in PLB phosphorylation, as well as in the SERCA2a, ryanodine receptor phosphorylation, and the Na+/Ca2+ exchanger, which could be involved in the mechanism for the impaired relaxation. These findings were also detected in the TgZ sham-operated mice, which are consistent with the diastolic dysfunction found even in the absence of aortic banding. Interestingly, the data from Chu et al. (2), demonstrating increased PLB phosphorylation and SERCA protein levels in mice overexpressing calcineurin activity, are consistent with our data demonstrating reduced PLB phosphorylation and SERCA protein levels with calcineurin inhibition. In the present study we also observed a reduction in ryanodine receptor phosphorylation and in the Na+/Ca2+ exchanger protein levels. Thus, based on our data, we speculate that the regulation of diastolic Ca2+ by calcineurin may result from a complex interplay among the different proteins involved in controlling Ca2+ in cardiac cells. Previous studies also demonstrated that PP1 (6, 8, 9) and PKA (3, 29) are involved in PLB phosphorylation. Our data indicate that a reduction in PLB phosphorylation may be due to an upregulation of PP1, but PKA is not involved in PLB phosphorylation.
Although increased collagen can affect LV diastolic stiffness, we did not find an increase in collagen or other noncontractile proteins (data not shown) in the TgZ mice compared with NTg mice with aortic banding.
In summary, in the present investigation we have found that TgZ mice, which inhibit calcineurin, develop less hypertrophy with chronic pressure overload with preserved LV systolic function, but with a clear impairment of diastolic function as reflected by isovolumic relaxation impairment and increased myocardial stiffness. This suggests that the inhibition of calcineurin in hearts subjected to pressure overload could represent a new mechanism for diastolic dysfunction resulting in an impaired relaxation and an increased myocardial stiffness, despite its concomitant action to reduce LVH.
GRANTS
This work was supported in part by National Institutes of Health Grants AG-027211, HL-033107, HL-059139, HL-069752, HL-069020, AG-023137, AG-014121, and HL-067724.
REFERENCES
- 1.Brutsaert DL. Diastolic heart failure: perception of the syndrome and scope of the problem. Prog Cardiovasc Dis 49: 153–156, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Chu G, Carr AN, Young KB, Lester JW, Yatani A, Sanbe A, Colbert MC, Schwartz SM, Frank KF, Lampe PD, Robbins J, Molkentin JD, Kranias EG. Enhanced myocyte contractility and Ca2+ handling in a calcineurin transgenic model of heart failure. Cardiovasc Res 54: 105–116, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Chu G, Kranias EG. Functional interplay between dual site phospholambam phosphorylation: insights from genetically altered mouse models. Basic Res Cardiol 97, Suppl 1: I43–I48, 2002 [DOI] [PubMed] [Google Scholar]
- 4.De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, Glascock BJ, Kimball TF, del Monte F, Hajjar RJ, Molkentin JD. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 98: 3322–3327, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degand I, Catty P, Talla E, Thines-Sempoux D, de Kerchove d'Exaerde A, Goffeau A, Ghislain M. Rabbit sarcoplasmic reticulum Ca2+-ATPase replaces yeast PMC1 and PMR1 Ca2+-ATPases for cell viability and calcineurin-dependent regulation of calcium tolerance. Mol Microbiol 31: 545–556, 1999 [DOI] [PubMed] [Google Scholar]
- 6.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res 61: 87–93, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Friedrich SP, Lorell BH, Rousseau MF, Hayashida W, Hess OM, Douglas PS, Gordon S, Keighley CS, Benedict C, Krayenbuehl HP. Intracardiac angiotensin-converting enzyme inhibition improves diastolic function in patients with left ventricular hypertrophy due to aortic stenosis. Circulation 90: 2761–2771, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Grote-Wessels S, Baba HA, Boknik P, El-Armouche A, Fabritz L, Gillmann HJ, Kucerova D, Matus M, Muller FU, Neumann J, Schmitz M, Stumpel F, Theilmeier G, Wohlschlaeger J, Schmitz W, Kirchhefer U. Inhibition of protein phosphatase 1 by inhibitor-2 exacerbates progression of cardiac failure in a model with pressure overload. Cardiovasc Res 79: 464–471, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Gupta RC, Mishra S, Yang XP, Sabbah HN. Reduced inhibitor 1 and 2 activity is associated with increased protein phosphatase type 1 activity in left ventricular myocardium of one-kidney, one-clip hypertensive rats. Mol Cell Biochem 269: 49–57, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Hill JA, Rothermel B, Yoo KD, Cabuay B, Demetroulis E, Weiss RM, Kutschke W, Bassel-Duby R, Williams RS. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. Preservation of systolic function. J Biol Chem 277: 10251–10255, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, Hong C, Yatani A, Avila J, Sadoshima J. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ Res 101: 1164–1174, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hittinger L, Mirsky I, Shen YT, Patrick TA, Bishop SP, Vatner SF. Hemodynamic mechanisms responsible for reduced subendocardial coronary reserve in dogs with severe left ventricular hypertrophy. Circulation 92: 978–986, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Hittinger L, Shannon RP, Bishop SP, Gelpi RJ, Vatner SF. Subendomyocardial exhaustion of blood flow reserve and increased fibrosis in conscious dogs with heart failure. Circ Res 65: 971–980, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Lim HW, De Windt LJ, Mante J, Kimball TR, Witt SA, Sussman MA, Molkentin JD. Reversal of cardiac hypertrophy in transgenic disease models by calcineurin inhibition. J Mol Cell Cardiol 32: 697–709, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Meguro T, Hong C, Asai K, Takagi G, McKinsey TA, Olson EN, Vatner SF. Cyclosporine attenuates pressure-overload hypertrophy in mice while enhancing susceptibility to decompensation and heart failure. Circ Res 84: 735–740, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Mendoza I, Quintero FJ, Bressan RA, Hasegawa PM, Pardo JM. Activated calcineurin confers high tolerance to ion stress and alters the budding pattern and cell morphology of yeast cells. J Biol Chem 271: 23061–23067, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Mizuno Y, Kanou Y, Rogatcheva M, Imai T, Refetoff S, Seo H, Murata Y. Genomic organization of mouse ZAKI-4 gene that encodes ZAKI-4 alpha and beta isoforms, endogenous calcineurin inhibitors, and changes in the expression of these isoforms by thyroid hormone in adult mouse brain and heart. Eur J Endocrinol 150: 371–380, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369: 486–488, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 98: 3328–3333, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothermel BA, Vega RB, Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med 13: 15–21, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Sadoshima J, Montagne O, Wang Q, Yang G, Warden J, Liu J, Takagi G, Karoor V, Hong C, Johnson GL, Vatner DE, Vatner SF. The MEKK1-JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J Clin Invest 110: 271–279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semeniuk LM, Severson DL, Kryski AJ, Swirp SL, Molkentin JD, Duff HJ. Time-dependent systolic and diastolic function in mice overexpressing calcineurin. Am J Physiol Heart Circ Physiol 284: H425–H430, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Shimoyama M, Hayashi D, Takimoto E, Zou Y, Oka T, Uozumi H, Kudoh S, Shibasaki F, Yazaki Y, Nagai R, Komuro I. Calcineurin plays a critical role in pressure overload-induced cardiac hypertrophy. Circulation 100: 2449–2454, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Sulakhe PV, Vo XT, Morris TE, Pato MD, Khandelwal RL. Protein phosphorylation in rat cardiac microsomes: effects of inhibitors of protein kinase A and of phosphatases. Mol Cell Biochem 175: 109–115, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science 281: 1690–1693, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Vatner SF, Shannon R, Hittinger L. Reduced subendocardial coronary reserve. A potential mechanism for impaired diastolic function in the hypertrophied and failing heart. Circulation 81: III8–III14, 1990 [PubMed] [Google Scholar]
- 28.Vega RB, Bassel-Duby R, Olson EN. Control of cardiac growth and function by calcineurin signaling. J Biol Chem 278: 36981–36984, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Watanuki S, Matsuda N, Sakuraya F, Jesmin S, Hattori Y. Protein kinase C modulation of the regulation of sarcoplasmic reticular function by protein kinase A-mediated phospholamban phosphorylation in diabetic rats. Br J Pharmacol 141: 347–359, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K, Masuyama T, Sakata Y, Nishikawa N, Mano T, Yoshida J, Miwa T, Sugawara M, Yamaguchi Y, Ookawara T, Suzuki K, Hori M. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc Res 55: 76–82, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest 112: 1395–1406, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhai P, Galeotti J, Liu J, Holle E, Yu X, Wagner T, Sadoshima J. An angiotensin II type 1 receptor mutant lacking epidermal growth factor receptor transactivation does not induce angiotensin II-mediated cardiac hypertrophy. Circ Res 99: 528–536, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zhai P, Gao S, Holle E, Yu X, Yatani A, Wagner T, Sadoshima J. Glycogen synthase kinase-3alpha reduces cardiac growth and pressure overload-induced cardiac hypertrophy by inhibition of extracellular signal-regulated kinases. J Biol Chem 282: 33181–33191, 2007 [DOI] [PubMed] [Google Scholar]