Abstract
Endothelial nitric oxide synthase (eNOS) uncoupling is a mechanism that leads to endothelial dysfunction. Previously, we reported that shear stress-induced release of nitric oxide in vessels of aged rats was significantly reduced and was accompanied by increased production of superoxide (18, 27). In the present study, we investigated the influence of aging on eNOS uncoupling. Mesenteric arteries were isolated from young (3 mo) and aged (24 mo) C57 BL/6J mice. The expression of eNOS protein in young vs. aged mice was not significantly different. However, the aged mice had remarkable increases in the ratio of eNOS monomers to dimers and Nω-nitro-l-arginine methyl ester-inhibitable superoxide formation. The level of nitrotyrosine in the total protein and precipitated eNOS of aged vessels was increased compared with that in young vessels. HPLC analysis indicated a reduced level of tetrahydrobiopterin (BH4), an essential cofactor for eNOS, in the mesenteric arteries of aged mice. Quantitative PCR results implied that the diminished BH4 may result from the decreased expressions of GTP cyclohydrolase I and sepiapterin reductase, enzymes involved in BH4 biosynthesis. When isolated and cannulated second-order mesenteric arteries (∼150 μm) from aged mice were treated with sepiapterin, acetylcholine-induced, endothelium-dependent vasodilation improved significantly, which was accompanied by stabilization of the eNOS dimer. These data suggest that eNOS uncoupling and increased nitrosylation of eNOS, decreased expressions of GTP cyclohydrolase I and sepiapterin reductase, and subsequent reduced BH4 bioavailability may be important contributors of endothelial dysfunction in aged vessels.
Keywords: tetrahydrobiopterin, nitrotyrosine, superoxide
a number of studies reported that, in various diseases, such as atherosclerosis and diabetes, the function of endothelial nitric oxide (NO) synthase (eNOS) is altered, and that it produces superoxide instead of NO. This alteration is referred to as “eNOS uncoupling” and is linked to an increased monomerization of the enzyme (15, 32). When endothelial cells were treated with peroxynitrite, a product of NO and superoxide, the reduction in eNOS activity was associated with disruption of eNOS dimers (33). On the other hand, tetrahydrobiopterin (BH4) has been identified as a critical cofactor for the production of NO. Two molecules of BH4 bind to each eNOS dimer and facilitate electron transfer for l-arginine oxidation. When BH4 is limited, by decreasing its synthesis or increasing its oxidation, eNOS becomes uncoupled, and superoxide is produced (20, 21). It has also been demonstrated that BH4 preserves eNOS dimerization and improves endothelial function (6). Thus BH4 availability is essential for normal endothelial function.
Aging of blood vessels is a risk factor for the development of cardiovascular diseases. However, the cellular and molecular mechanisms underlying the aging of blood vessels remain unclear. Our laboratory has demonstrated previously that, in mesenteric arteries of aged rats, flow-induced dilation and shear stress-induced release of NO were reduced and associated with decreased eNOS activity, increased superoxide production, and decreased antioxidant capacity (27). The increased superoxide in aged vessels was contributed mostly by NADPH oxidase, xanthine oxidase, and eNOS itself, which decreased NO bioavailability, leading to endothelial dysfunction (18). It is controversial whether BH4 content is altered in aged blood vessels. In large conduit vessels, neither the concentration of BH4 nor the ratio of reduced BH4 to the oxidation products was different between young and aged mice (3). In skeletal muscle arterioles of aged rats, BH4 content was reduced, and supplementation of BH4 restored endothelium-dependent, flow-induced dilation (12). However, whether the reduced BH4 in aged microvessels is associated with eNOS uncoupling needs to be further elucidated. The present study, therefore, investigated the molecular mechanism leading to endothelial dysfunction in aged mice, including alterations in the stability of the eNOS dimer, oxidation of eNOS, bioavailability of BH4, the effect of supplementation by sepiapterin, and the expression level of genes related to BH4 biosynthesis in mesenteric arteries.
MATERIALS AND METHODS
Animal.
Male C57 black/6J mice (age: 3 and 24 mo for young and aged groups, respectively) were utilized in the study. Experimental protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conform to the current guidelines of the National Institutes of Health and the American Physiological Society for the care and use of laboratory animals.
Western blot analysis.
Equal amounts of total protein from mesenteric arteries of young and aged mice were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins fractionated on the denaturing gels were transferred to nitrocellulose membranes, which were probed with antibodies to eNOS (BD Transduction Laboratories, Franklin Lakes, NJ), nitrotyrosine (Upstate, Charlottesville, VA), and β-actin (Sigma-Aldrich, St. Louis, MO). Immunoreactive bands were detected with an appropriate second antibody and visualized with a chemiluminescence kit (Pierce, Rockford, IL).
Low-temperature SDS-PAGE (LT-PAGE) was performed for detection of eNOS dimers using reported procedures (19). Briefly, total proteins, mentioned above, were incubated in 1 × Laemmli buffer without 2-mercaptoethanol at 37°C for 5 min. The samples were then subjected to SDS-PAGE with 6% gel. Gels and buffers were equilibrated at 4°C before electrophoresis, and the buffer tank was placed in an ice bath during electrophoresis to maintain the temperature of the gel <15°C. Subsequent to LT-PAGE, the gels were transferred, and the blots were probed as routine Western blot. A control sample treated with 2-mercaptoethanol was used as an eNOS monomer control. In separate experiments, LT-PAGE was performed to detect eNOS monomer/dimer in vessels of young and aged mice in control and after incubation with sepiapterin (0.1, 1, and 10 μM; Sigma-Aldrich), a precursor of BH4, for 2 h.
Detection of superoxide production.
Superoxide production in vessels of young and aged mice was assayed using dihydroethidium (DHE) and an HPLC/fluorescence detector-based assay to determine 2-hydroxyethidium (2-EOH), a superoxide-induced oxidative product of DHE (14, 30). First-order mesenteric arteries were isolated and perfused with MOPS buffered-physiological salt solution (MOPS-PSS) composed of (in mM) 124 NaCl, 5 KCl, 2 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 5 glucose, 2 pyruvate, 0.02 EDTA, and 3 MOPS at 37°C. Intravascular pressure was maintained constant at 80 mmHg. DHE (10 μM) was then administrated intraluminally, with or without Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10−4 M, 30 min; Sigma-Aldrich) pretreatment. After 1-h incubation, extra DHE was washed from the vessels. Vessels were then removed, pulverized in liquid nitrogen, and homogenized in a 1:1 mixture of acetonitrile and water. After a centrifugation, the supernatant was collected for HPLC analysis; the remaining tissues were dissolved in 1 N NaOH for protein determinations with the Bio-Rad protein assay. In separate experiments, superoxide formation in aged vessels was assessed, using DHE, in control and after incubation with sepiapterin (1 μM) for 2 h, with or without l-NAME.
Twenty microliters of samples or 2-EOH standards were separated by a HPLC system (PU-2080 Plus, Jasco) with a C-18 reverse-phase column (Beckman Ultrasphere ODS, 5 μm, 4.6 × 250 mm). The mobile phase was composed of 37% acetonitrile and 0.1% trifluoroacetic acid and run at a flow rate of 1 ml/min. The fluorescent signal of 2-EOH was detected at 480 nm (excitation) and 580 nm (emission) with a fluorescence detector (FP2020 Plus, Jasco). 2-EOH standard was synthesized from potassium nitrosodisulfonate, as previous described (31). Standard curves of 2-EOH (0.3–10 pmol) were generated and used to calculate superoxide production of vessels as picomoles per milligram protein in response to 1-h incubation with 10 μM DHE.
Measurement of biopterin.
Measurement of biopterin was performed by fluorometric HPLC analysis. BH4 levels were determined by a differential oxidation method described previously (5, 16). Briefly, total proteins were isolated from mesenteric arteries of individual mice with lysis buffer (50 mM Tris·HCl, pH 7.4, 1 mM DTT, and 1 mM EDTA, containing 0.1 μM neopterin as an internal recovery standard). After protein concentrations were determined, proteins were then removed by adding 10% of a 1:1 mixture of 1.5 M HClO4 and 2 M H3PO4 to the extracts, followed by centrifugation. To determine total biopterins [BH4, 7,8-dihydrobiopterin (BH2), biopterin] by acid oxidation, iodine solution (1% iodine in 2% KI solution) was added into protein-free supernatants to a final concentration of 10%. To determine BH2 and biopterin by alkali oxidation, iodine solution plus 1 M NaOH was added to the extract (10% each). Samples were incubated at room temperature for 1 h in the dark. Alkaline-oxidation samples were then acidified with 20% of 1 M H3PO4. Iodine was reduced by adding 5% of fresh ascorbic acid (20 mg/ml). HPLC was performed using a Symmetry C18 reverse-phase column (Waters) with a methanol-water (5:95 vol/vol) mobile phase run at 1 ml/min. Fluorescence detection (350 nm excitation, 450 nm emission) was performed using an FP-2020 plus detector. Biopterin in mesenteric arteries of each mouse was measured individually. BH4 amounts were calculated by subtracting BH2 + biopterin from total biopterins, which were expressed as picomoles per milligram protein.
RT-PCR and quantitative real-time PCR.
Total RNA was extracted from mesenteric arteries using TRIzol solution (Sigma-Aldrich, St. Louis, MO) and then treated with RNase-free DNase. Total RNA (2 μg) was reverse transcripted into cDNA by Moloney murine leukemia virus reverse transcriptase (Promaga, Madison, WI), with a reverse transcriptase negative control reaction for each sample. PCR reaction was performed with Taq polymerase (GoTaq Flexi) from Promaga, using GAPDH as an internal control in the same reaction tube. Quantitative PCR analysis was performed to compare relative quantities of gene expression by employing a LightCycler system (Roche Diagnostics), according to the manufacturer's instruction and a QuantiTect SYBR Green PCR kit (Qiagen). The primers were designed in-house (Table 1) and synthesized by Fisher Scientific customer services. Quantification is reported, using proprietary software, as cycle threshold (Ct). A relative quantification method (ΔΔCt) was used to evaluate the expression of each gene (22). Mean values were calculated from triplicate PCR reactions. Results from at least four animals per group were used to produce means and SE. Ct values were determined after adjustment of the baseline and were normalized and standardized on the basis of GAPDH.
Table 1.
PCR primers
| Gene | GenBank Accession No. | Primer Sequence | Annealing Temperature, °C | PCR Product, bp |
|---|---|---|---|---|
| GCH1 | L09737 | F: CCA TCA CAG AAG CCT TGC AGC CT | 55–57 | 133 |
| R: TCC CGG AAC ACG CCC AGC AT | ||||
| 6-Pts | AF061880 | F: TTG ATC ACA AGA ACT TGG ACC T | 51–55 | 170 |
| R: TCC TTT ATA GAC TAC GAT GTT GTT | ||||
| SR | S77493 | F: GAC AAT GAC ATG CAG CAG TT | 54–55 | 174 |
| R: ATA GAA GTC CAC ATG GGC TC | ||||
| GAPDH | M32599 | F: CACTCTTCCACCTTCGATGCC | 55 | 240 |
| R: CTGGGATGGAAATTGTGAGGG |
GCH1, GTP cyclohydrolase I; 6-Pts, 6-pyruvoyl tetrahydropterin synthase; SR, sepiapterin reductase; F, forward; R, reverse.
Fluorescence microscopy and immunoprecipitation.
Immunostaining of frozen sections of mesenteric arteries was performed. Briefly, sections were fixed in 4% paraformaldehyde, treated with 0.1% Triton X-100 on ice, and then washed three times with ice-cold PBS. After blocking with 2% bovine serum albumin in PBS for 15 min on ice, the slides were incubated with the first antibody (mouse IgG for eNOS or rabbit IgG for nitrotyrosine) for 1 h, followed by incubation with the appropriate second antibodies (linked with FITC and Cy3, respectively) for 30 min. Finally, the slides were stained with 4',6-diamidino-2-phenylindole (1 μg/ml, DAPI, Fluka). Microscopy was performed on a fluorescence microscope (BX51WI, Olympus), and images were captured using a digital camera (CoolSNAP, Photometrics).
Immunoprecipitation was performed with eNOS antibody (1 μg/sample) and protein A/G beads. Five hundred-microgram proteins, isolated from mesenteric arteries of three mice, were incubated overnight in RIPA buffer with the antibody and beads. The immuno-complexes were then subjected to SDS-PAGE, followed by Western blot analysis with anti-nitrotyrosine antibody.
Acetylcholine-induced vessel dilation.
Similar to our laboratory's previous studies (27, 29), second-order mesenteric arteries were isolated from young and aged mice. Arteries were cannulated in a vessel chamber and perfused with MOPS-PSS at 37°C. Intravascular pressure was maintained constant at 80 mmHg. Changes of the internal diameter of vessels were measured with a video caliper. After 1 h of stabilization, due to an insufficient myogenic constriction in mice mesenteric arteries, phenylephrine (PE; 10−8–10−7 M) was used to preconstrict vessels to obtain a basal tone of ∼50% of their maximal diameter. Endothelium-dependent dilations to ACh (10−11–10−5 M) were assessed in control and after inhibition of NO synthesis with l-NAME, or after treatment with sepiapterin (10−6 M, 30 min). Endothelium-independent dilations to acidified NaNO2 (10−11–10−6 M) were detected after l-NAME treatment. The initial diameter of vessels, measured when intraluminal pressure was first increased to 80 mmHg and the temperature of perfusion solution was increased to 37°C, was used as the maximal diameter, which was not different from the passive diameter (PD) when vessels were incubated in Ca2+-free MOPS-PSS. Changes in diameter, in response to the agonist, were expressed as percentage of PD.
Statistical analysis.
Data are expressed as means ± SE. Statistical significance was calculated by Student's t-test and by repeated measures of two-way ANOVA, followed by Tukey-Kramer multiple-comparison test. Significance level was taken at P < 0.05.
RESULTS
Increased monomer-to-dimer ratio and uncoupling of eNOS in mesenteric arteries of aged mice.
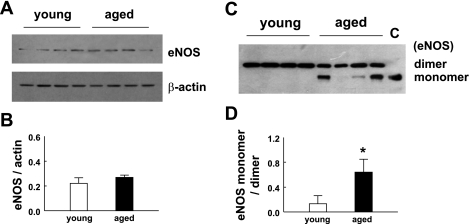
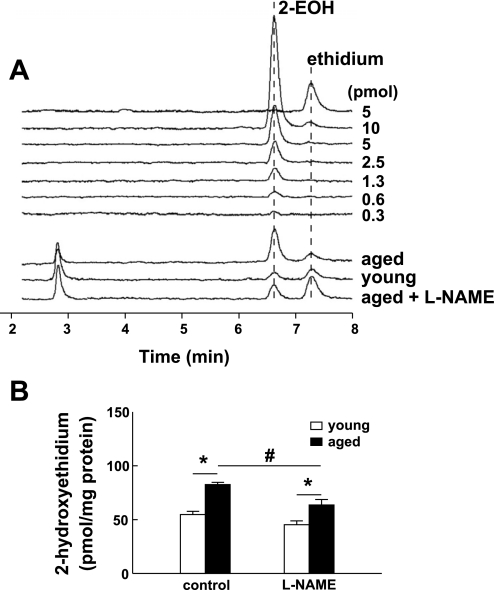
To determine whether endothelial dysfunction in aged vessels is related to decreased eNOS expression, total proteins of mesenteric arteries from young and aged mice were analyzed by Western blot. There was no significant difference in eNOS expression of mesenteric arteries between young and aged groups (Fig. 1, A and B). Since uncoupling of eNOS has been linked to endothelial dysfunction, with or without changes in eNOS expression, the same protein lysates and antibody used in Fig. 1A were used to perform LT-PAGE to determine the level of eNOS monomers in vessels of young and aged mice. As shown in Fig. 1C, a significant difference in the formation of eNOS monomer was exhibited between young and aged mice. The eNOS from young mice was shown to be dimers, whereas the eNOS from aged mice revealed a pronounced monomer band. The ratio of eNOS monomer to dimer was significantly higher in aged compared with young mice (Fig. 1D). We further analyzed intracellular superoxide formation in isolated, cannulated, and pressurized mesenteric arteries. 2-EOH was quantitatively measured with an HPLC/fluorescence detector system. Examples of recorded fluorescent signals and retention times for 2-EOH and ethidium standards and samples are shown in Fig. 2A. Figure 2B shows summarized data. Superoxide formation in vessels of aged mice was increased significantly compared with that of young mice. Inhibition of eNOS with l-NAME did not affect superoxide formation in young mice, but significantly decreased superoxide formation in aged mice. However, in the presence of l-NAME, superoxide formation in aged vessels was still significantly greater than that in young vessels. These data indicate that eNOS uncoupling contributes significantly to superoxide formation in aged vessels.
Fig. 1.
A: representative Western blots of endothelial nitric oxide synthase (eNOS; top) and β-actin (bottom). Total proteins were obtained from mesenteric arteries of young and aged mice. The expression of β-actin was used as a loading control. B: the densitometric ratio of eNOS and β-actin expressions in young and aged groups. Data present the mean of two blots with eight samples (mice) in each group. C: the same protein samples as in A were subjected to low-temperature SDS-PAGE (LT-PAGE) to assess eNOS dimers and monomers of young and aged mice. Lane C was a 2-mercaptoethanol denatured protein sample to indicate the molecular weight of the eNOS monomer. D: densitometric ratio of eNOS monomer and dimer. Data present the mean of three blots with eight samples (mice) in each group. *Significant increase compared with young mice, P < 0.05.
Fig. 2.
Superoxide formation in isolated and pressurized mesenteric arteries of young and aged mice. Vessels were incubated with dihydroethidium (DHE; 10 μM) for 60 min. Fluorescence intensity of 2-hydroxyethidium (2-EOH) was determined by an HPLC/fluorescence detector system. A: recorded fluorescent signals and retention times of 2-EOH and ethidium from young and aged vessels in control and after inhibition of NO synthesis with Nω-nitro-l-arginine methyl ester (l-NAME). B: summarized data of superoxide production in young and aged vessels (n = 5 per group). Data were normalized as picomoles of 2-EOH per milligram of protein for a reaction time of 60 min. *Significant (P < 0.05) difference between the 2 groups. #Significant (P < 0.05) difference between l-NAME-treated vessels of aged mice vs. control groups.
Decreased availability of BH4 in mesenteric arteries of aged mice.
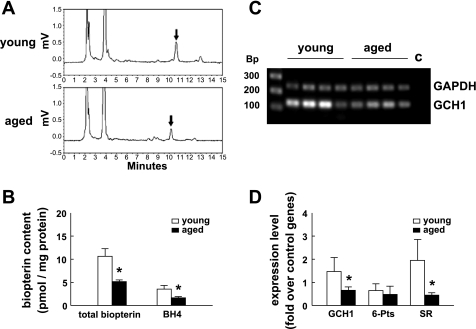
To determine whether loss of BH4 contributed to eNOS monomerization and uncoupling, both biopterin and BH4 content in mesenteric arteries was measured (Fig. 3A). Summary data (Fig. 3B) show that the level (pmol/mg protein) of biopterin and BH4 in vessels of aged mice was approximately one-half or less than one-half of that in young mice; the differences were statistically significant. Compared with total biopterin in mesenteric arteries of young mice, the level of BH4 decreased from 33% in young mice to 15% in aged mice. This reduction was statistically significant.
Fig. 3.
A: the extracts from total proteins of mesenteric arteries were analyzed by HPLC. Each sample (young or aged) was extracted from the same amount of total protein. The arrows indicate peaks of total biopterin (by acidic oxidation) that appeared at elution volumes of ∼10 ml. B: comparison of amounts of biopterin and tetrahydrobiopterin (BH4) in mesenteric arteries of young vs. aged mice. The error bars represent SE from 4 mice. C: the amounts of the gene GTP cyclohyrolase I (GCH1) expressed in young and aged mice were detected by RT-PCR. Bp, length in the base pair of the molecular marker. Lane C is a negative control, in which cDNA in the reaction was prepared without reverse transcriptase. GAPDH was used as a control gene. D: the results of quantitative PCR (n = 5 per group). The expression of each sample was normalized to GAPDH. SR, sepiapterin reductase; 6-Pts, 6-pyruvoyl tetrahydropterin synthase. *Significant difference from young mice, P < 0.05.
To understand the cause of BH4 deficiency in blood vessels of aged mice, GTP cyclohydrolase I (GCH1), 6-pyruvoyl tetrahydropterin synthase, and sepiapterin reductase (SR), genes involved in BH4 biosynthesis, were studied. GCH1 has been identified as the rate-limiting enzyme in the BH4 biosynthetic pathway. In Fig. 3C, the results show that, with GAPDH as an internal control, the level of GCH1 expression was lower in aged than in young mice. The expression levels of all three genes related to BH4 biosynthesis, GCH1, 6-pyruvoyl tetrahydropterin synthase, and SR were determined by quantitative PCR (real-time PCR). Figure 3D shows that the expression of GCH1 and SR was remarkably decreased in vessels of aged mice.
BH4 improved the endothelium-dependent dilation in vessels of aged mice.
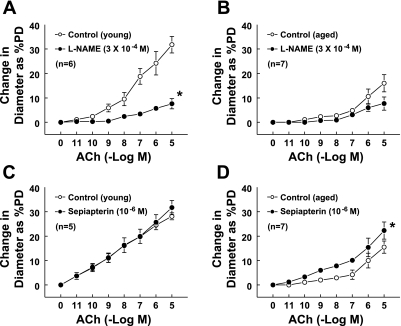
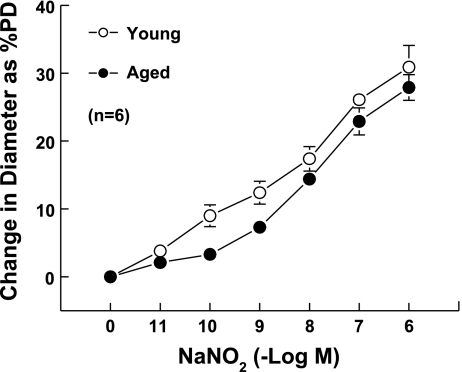
The potential beneficial effects of exogenous BH4 were studied on ACh-induced dilation in vessels of aged mice. The average PDs of mesenteric arteries of young and aged mice were 153.1 ± 16.6 and 175.0 ± 13.6 μm, respectively. After incubation with PE (10−8-10−7 M), the vessels constricted and stabilized at ∼50% of their PD. There were no significant differences in passive and PE-constricted diameters between the two groups. ACh-induced maximum dilation was significantly reduced in vessels of aged compared with young mice (control groups in Fig. 4, A and B). Maximum dilations to ACh in young and aged mice were 32.1 ± 1.4 and 16 ± 3.5% of PD, respectively. As shown in Fig. 4A, the vessels from young mice dilated gradually with increased concentrations of ACh, while the aged vessels (Fig. 4B) showed dilation only at higher concentrations (>10−7 M). Inhibition of NO synthesis by l-NAME significantly shifted the dilation curve downward (Fig. 4A), indicating that ACh-induced dilation in vessels of young mice is mainly mediated by NO, whereas, in aged vessels, l-NAME had little inhibitory effect on the dilation (Fig. 4B), further indicating that the reduced ACh-induced dilation is mainly due to a lack of NO. When the vessels were pretreated for 30 min with sepiapterin (10−6 M), a precursor of BH4, the dilation to ACh was not increased in young vessels (Fig. 4C), whereas it increased significantly in vessels from aged mice (repeated measures of two-way ANOVA). Also, the sensitivity of aged vessels to ACh increased significantly (Tukey-Kramer multiple-comparison test). At ACh concentrations of 10−9, 10−8, and 10−7 M, the dilations increased from 1.1, 2.3, and 2.7 to 6, 7.3, and 10.1% of PD (Fig. 4D), and the dilations became significantly different compared with the basal diameter. Dilations to acidified NaNO2 (10−11-10−6M) were not different in young and aged vessels (Fig. 5). Together, these data suggest that the reduced BH4 in aged vessels contributes partially to the endothelial dysfunction via an impaired eNOS/NO mechanism.
Fig. 4.
Concentration-response curves to ACh in second-order mesenteric arteries of young and aged mice. A and B were control vs. l-NAME treatment; C and D were control vs. sepiapterin treatment for young and aged groups, respectively. l-NAME or sepiapterin were preincubated with vessels for 30 min before additional response to ACh. PD, passive diameter. *P < 0.05 between the two curves via repeated measures of two-way ANOVA.
Fig. 5.
Concentration-response curves to acidified NaNO2 in vessels of young and aged mice. There is no significant difference between the two curves.
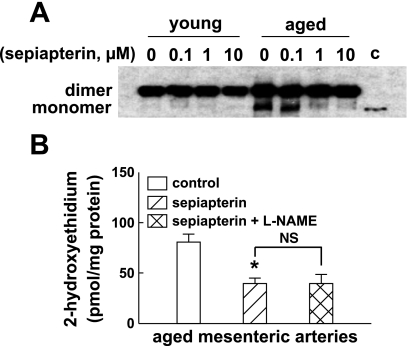
BH4 supplementation decreases the formation of monomers and uncoupling of eNOS in vessels of aged mice.
Figure 6A shows the effects of sepiapterin treatment (2 h) on eNOS dimer stabilization. The eNOS from mesenteric arteries of young mice was in dimer form and showed no changes after sepiapterin treatment. In aged mice, sepiapterin treatment increased the stability of the eNOS dimer. At concentrations of 1 and 10 μM, it remarkably reduced the formation of eNOS monomers. Sepiapterin treatment also significantly decreased superoxide production in aged vessels (Fig. 6B). In the presence of sepiapterin, l-NAME had no inhibitory role in superoxide production, indicating that the deficiency of BH4 in aged vessels contributes significantly to the eNOS uncoupling (Fig. 2).
Fig. 6.
A: the effect of sepiapterin in eNOS dimer stability. The total proteins of mesenteric arteries were isolated from young and aged mice, and Western blotting (LT-PAGE) was performed with anti-eNOS antibody. The arteries were treated with 0, 0.1, 1, and 10 μM sepiapterin for 2 h. Lane c indicates the molecular weight of eNOS monomer. B: superoxide formation in mesenteric arteries of aged vessels in control and after incubation with sepiapterin for 1 h and inhibition of eNOS with l-NAME (3 × 10−4 M). The method used to detect superoxide was the same as that shown in Fig. 2. *Significant difference from control, P < 0.05 (n = 5 per group). NS, no significant difference.
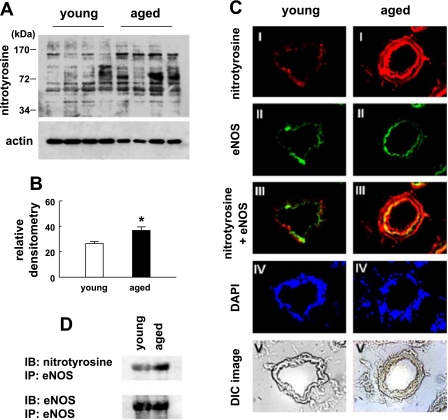
Elevated protein nitrotyrosine accompanied by eNOS uncoupling in aged mice.
As we showed, sepiapterin could only partially improve endothelium-mediated dilation in aged vessels. Therefore, we further assessed protein nitrotyrosine formation in mesenteric arteries of mice. Total proteins of mesenteric arteries from young and aged mice were subjected to Western blot analysis and detected by anti-nitrotyrosine antibody (Fig. 7A). The summary results show that the nitrotyrosine density was significantly higher in aged compared with young mice (Fig. 7B). Dual staining of nitrotyrosine and eNOS was performed on frozen sections of mesenteric arteries (Fig. 7C). The staining showed that the intensity of nitrotyrosine was greater in aged vessels, as indicated by the yellow color in panel III, which showed colocalization of nitrotyrosine and eNOS in the endothelial layer of the vessel wall. Furthermore, when eNOS protein was immunoprecipitated by monoclonal eNOS antibody and the immuno-complexes were subjected to Western blot analysis with anti-nitrotyrosine antibody, the isolated eNOS from vessels of aged mice contained more nitrotyrosine than that from vessels of young mice (Fig. 7D). Two blots of immunoprecipitated eNOS were performed, and the results were similar.
Fig. 7.
A: total proteins of mesenteric arteries from young and aged mice were analyzed by Western blotting with anti-nitrotyrosine antibody. The bottom panel is the same blot probed with β-actin as a loading control. B: summary data of densitometry of nitrotyrosine. Data are means of two Western blots. The amounts of nitrotyrosine were normalized to the expression of actin. *Significant differences from young mice, P < 0.05. C: immunostaining of nitrotyrosine and eNOS in sections of mesenteric arteries of young and aged mice. I, Cy3-labeled nitrotyrosine. II, FITC-labeled eNOS. III, merged images of I and II. IV, DAPI-stained nuclei. V, profile of vessel sections. DIC, differential interference contrast. D: eNOS protein was immunoprecipitated by monoclonal eNOS antibody. The immuno-complexes were then subjected to SDS-PAGE, followed by Western blot analysis with anti-nitrotyrosine antibody. The bottom panel indicates that equal amounts of eNOS were loaded. IB, immunoblotting; IP, immunoprecipitation.
DISCUSSION
In this study, we have investigated the mechanisms underlying endothelial dysfunction in mesenteric arteries of aged mice. We have provided sufficient evidence to demonstrate that reduced BH4 availability contributes to decreased eNOS dimer stability and increased eNOS uncoupling in small arteries of aged mice.
In various vascular diseases, endothelial dysfunction is characterized by a decrease in NO bioactivity, with a concomitant increase in superoxide formation, despite the observation that eNOS mRNA and protein levels are maintained or even increased. For example, eNOS protein expression was found to be increased, yet endothelial function was impaired in response to hyperglycemia (9), high blood pressure (4), or advanced age (7). Furthermore, eNOS overexpression was reported to accelerate the development of atherosclerosis in apolipoprotein E knockout mice (24), and eNOS gene therapy was shown to exacerbate ischemia-reperfusion injury in diabetes (13). In the present study, we also found that endothelial dysfunction in aged vessels was associated with a maintained level of eNOS protein expression (Fig. 1). Together, these findings indicate that alteration of eNOS itself can be a significant cause for endothelial dysfunction, and sufficient expression of eNOS protein alone does not guarantee bioavailability of NO.
Dimerization is required for eNOS catalytic function. In intact eNOS enzyme, the dimer catalyzes flavin-mediated electron transfer from one monomer to the heme of the other monomer. When sufficient substrate l-arginine and cofactor BH4 are present, intact eNOS dimers couple their heme and O2 reduction to the synthesis of NO. Early studies showed that BH4 stabilizes the dimeric formation of inducible NO synthase and permits the catalytic function of the enzyme (1, 2). Studies have further revealed the differences in the stability of dimerization and that eNOS exhibits the most dimer strength among all three NO synthases (25). However, the role of BH4 in eNOS dimer stability is still controversial. Using recombinant eNOS, studies have demonstrated that BH4 dose-dependently decreased superoxide production, increased NO production, and stabilized the enzyme in its dimeric form (28). Furthermore, when GCH1, the rate-limiting enzyme for BH4 biosynthesis, was transfected into endothelial cells, the augmented BH4 restored eNOS dimerization (5). However, other studies have shown that BH4-free eNOS existed in a monomer-dimer equilibrium, very similar to that observed with the BH4-reconstituted protein, although the activity of eNOS was inhibited in the absence of BH4 (26). Our results seem to favor the concept that BH4 plays an important role in the maintenance of eNOS dimerization in aged vessels. We have demonstrated that availability of both biopterin and BH4 was significantly reduced in mesenteric arteries of aged mice (Fig. 3B). In our experiments, there is clear evidence of a reduced BH4 biosynthesis, via a GCH1-related pathway (Fig. 3D), contributing to the reduced bioavailability of BH4 in aged vessels. The level of BH4 is also controlled by a BH4 salvage pathway. Studies have shown that an increased oxidative stress, induced by angiotensin II or in diabetes, reduces the expression of dihydrofolate reductase, a critical protein catalyzing BH4 regeneration from its oxidized form, BH2, which then results in a reduced NO bioavailability via an eNOS uncoupling mechanism (8, 23). Recent studies further revealed that a reduced BH4-to-BH2 ratio, via dihydrofolate reductase inhibition or knockdown, rather than absolute concentrations of BH4, exacerbated eNOS uncoupling (10, 11). Therefore, these mechanisms may contribute to the increased eNOS uncoupling in aged vessels. On the other hand, with increased oxidative stress in vascular aging, increased oxidation of BH4 could also be a significant cause for the reduced BH4 (20). With respect to the reduced BH4 content, the formation of eNOS monomers was found to be significantly increased (Fig. 1D). Conversely, when aged vessels were incubated with sepiapterin, the increased eNOS monomers were reversed to the dimeric form (Fig. 6A). Although, in the present study, we could not distinguish between the reduction of BH4 in the endothelium and smooth muscle of aged vessels, the role of sepiapterin in the reduction of eNOS-derived superoxide production and in the restoration of eNOS dimerization suggests that endothelial BH4 deficiency is one of the major causes leading to endothelial dysfunction in aged vessels.
A parallel change in eNOS monomerization (Fig. 1D) and uncoupling (Fig. 2) was found in aged vessels. Furthermore, these alterations could be restored in parallel by BH4 supplementation (Fig. 6). However, whether there is a causal link between eNOS monomerization and uncoupling in aged vessels needs to be further investigated. LT-PAGE has been used to detect eNOS dimerization in various in vitro experiments. So far, there is no direct evidence demonstrating the existence of eNOS monomers and the linkage between eNOS monomers and endothelial dysfunction in vivo. Use of l-NAME-inhibitable superoxide formation to evaluate eNOS uncoupling is still a practical approach in isolated vessels and has been demonstrated in response to hypertension (20) and advanced age (18). Nevertheless, the increased eNOS monomerization caused by BH4 deficiency is an important characteristic of vascular aging and is related to eNOS uncoupling and endothelial dysfunction. Thus, while eNOS monomerization may not be a cause of eNOS uncoupling, it can be considered one of the many consequences of the aging process.
Endothelial dysfunction develops during aging. In the present study, we demonstrated that ACh-induced arteriolar dilation was impaired in aged mice (Fig. 4). However, when the aged vessels were treated with exogenous BH4 (in our case, sepiapterin was used because of its permeability characteristics), ACh-induced endothelium-dependent dilation was significantly increased, concomitant with reduced eNOS monomer and eNOS-derived superoxide formation. These data establish an essential role of BH4 in the maintenance of endothelial function and support the notion that eNOS uncoupling is an important source of superoxide that leads to endothelial dysfunction in the process of vascular aging. BH4 supplementation increased forearm blood flow in response to ACh in elder subjects (17). A decreased BH4 bioavailability has also been demonstrated to be contributing to endothelial dysfunction in skeletal muscle arterioles of aged rats (12). Sepiapterin supplementation was able to partially restore flow-induced dilation in those vessels. Similarly, in our results, while sepiapterin reduced the formation of eNOS monomers and eNOS-derived superoxide production, it did not fully restore ACh-induced dilation in aged vessels, suggesting that BH4 deficiency-induced eNOS uncoupling is not solely responsible for the endothelial dysfunction in aged vessels. Indeed, increased oxidative stress contributed significantly to the endothelial dysfunction in carotid arteries of aged mice, and impaired ACh-induced relaxation could be normalized by supplementation of cell-permeable superoxide dismutase (3). We have found that increased superoxide production in aged mesenteric arteries was contributed to by eNOS, NADPH oxidase, and xanthine oxidase (18). In line with these findings, we reveal that the increased nitrotyrosine in total protein and eNOS in aged vessels (Fig. 7) may underlie additional mechanisms responsible for endothelial dysfunction in vascular aging.
In mesenteric arteries of aged rats, chronic increases in shear stress restored endothelial function via mechanisms of increased NO production, decreased superoxide production, and increased antioxidant capacity (29). Here, we further demonstrate that BH4 supplementation improves endothelial function in vascular aging as well. Thus efforts to increase NO bioavailability are essential for the maintenance of endothelial function in aged vessels.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-070653, HL-68813, and HL-43023.
REFERENCES
- 1.Abu-Soud HM, Loftus M, Stuehr DJ. Subunit dissociation and unfolding of macrophage NO synthase: relationship between enzyme structure, prosthetic group binding, and catalytic function. Biochemistry 34: 11167–11175, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Baek KJ, Thiel BA, Lucas S, Stuehr DJ. Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J Biol Chem 268: 21120–21129, 1993 [PubMed] [Google Scholar]
- 3.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287: H2448–H2453, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bouloumie A, Bauersachs J, Linz W, Scholkens BA, Wiemer G, Fleming I, Busse R. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension 30: 934–941, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Cai S, Alp NJ, McDonald D, Smith I, Kay J, Canevari L, Heales S, Channon KM. GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovasc Res 55: 838–849, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia 48: 1933–1940, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cernadas MR, Sanchez dM, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 83: 279–286, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 102: 9056–9061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 96: 25–28, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Crabtree MJ, Tatham AL, Al Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem 284: 1136–1144, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric oxide synthase coupling: relative importance of the de novo Biopterin synthesis vs. salvage pathways. J Biol Chem In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, Kevil CG, Champion HC, Lefer DJ. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res 99: 78–85, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem 102: 176–188, 1980 [DOI] [PubMed] [Google Scholar]
- 17.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 186: 390–395, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol 293: H1344–H1350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger HP, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and l-arginine in the formation of an SDS-resistant dimer. EMBO J 14: 3687–3695, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes 56: 118–126, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, Masada M, Yokoyama M. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest 110: 331–340, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panda K, Rosenfeld RJ, Ghosh S, Meade AL, Getzoff ED, Stuehr DJ. Distinct dimer interaction and regulation in nitric-oxide synthase types I, II, and III. J Biol Chem 277: 31020–31030, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Crespo I, Gerber NC, Ortiz de Montellano PR. Endothelial nitric-oxide synthase. Expression in Escherichia coli, spectroscopic characterization, and role of tetrahydrobiopterin in dimer formation. J Biol Chem 271: 11462–11467, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun 237: 340–344, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Yan C, Huang A, Kaley G, Sun D. Chronic high blood flow potentiates shear stress-induced release of NO in arteries of aged rats. Am J Physiol Heart Circ Physiol 293: H3105–H3110, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3: 8–21, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 11: 89–97, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817–826, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]