Abstract
It is well known that the strength of cardiac contraction is dependent on the cycle length, evidenced by the force-frequency relationship (FFR) and the existence of postrest potentiation (PRP). Because the contractile strength of the steady-state FFR and force-interval relationship involve instant intrinsic responses to cycle length as well as slower acting components such as posttranslational modification-based mechanisms, it remains unclear how cycle length intrinsically affects cardiac contraction and relaxation. To dissect the impact of cycle length changes from slower acting signaling components associated with persisting changes in cycle length, we developed a novel technique/protocol to study cycle length-dependent effects on cardiac function; twitch contractions of right ventricular rabbit trabeculae at different cycle lengths were randomized around a steady-state frequency. Patterns of cycle lengths that resulted in changes in force and/or relaxation times can now be identified and analyzed. Using this novel protocol, taking under 10 min to complete, we found that the duration of the cycle length before a twitch contraction (“primary” cycle length) positively correlated with force. In sharp contrast, the cycle length one (“secondary”) or two (“tertiary”) beats before the analyzed twitch correlated negatively with force. Using this protocol, we can quantify the intrinsic effect of cycle length on contractile strength while avoiding rundown and lengthiness that are often complications of FFR and PRP assessments. The data show that the history of up to three cycle lengths before a contraction influences myocardial contractility and that primary cycle length affects cardiac twitch dynamics in the opposite direction from secondary/tertiary cycle lengths.
Keywords: force-frequency relationship, postrest potentiation
modulation of contractile force can occur through either processes that are immediate (intrinsic) and/or processes that involve prolonged exposure to a given stress or situation and act via posttranslational modification. To elucidate processes that modify contractile performance, we need to be able to distinguish the effects of these contributors in a qualitative and quantitative manner. The strength of myocardial contraction is governed by the combined effect of intracellular calcium transient and properties of the myofilaments. The force-frequency relationship (FFR) is a major regulator of contraction and relaxation based on changes in the cycle length: an increase in heart rate (i.e., a decrease in cycle length) is converted into greater contractile force and faster relaxation (5). The underlying molecular mechanism involves both intrinsic properties of the calcium handling system and posttranslational modifications to key calcium handling and myofilament proteins (3, 29). In patients with heart failure, a blunted or negative FFR is one of the common hallmarks of cardiac dysfunction (20, 23), and thus understanding the physiological basis of how the cycle length-based contractility is regulated in normal myocardium would be a logical prerequisite to unravel how this relationship is impacted in heart failure.
The calcium transient amplitude plays an important role in the increase in contractile strength that is observed when frequency is changed. Consecutive cycle lengths that are shorter result in an increase in time-averaged intracellular calcium concentration, which results in an increase in sarcoplasmic reticulum (SR) load. The increase in SR load results in a greater amount of calcium released upon stimulation. The SR's intrinsic response to a change in the ionic balance after a change in frequency probably accounts for much of the modulation of contractility observed: when the calcium concentration in the cytosol is high, the SR ATPase works at a faster rate; i.e., its activity is driven by the cytosolic calcium concentration. Consistent elevation of time-averaged calcium also is thought to activate signaling mechanisms that result in posttranslational modifications to key calcium handling and myofilament proteins that can have additional positive inotropic effects. One example is the activation of CaMKII by average increases in intracellular calcium; although its involvement in regulating the FFR remains controversial and has not been found by all investigators, several studies have shown that CaMKII is activated with an increase in frequency and can alter calcium handling proteins (21) and myofilament proteins (27). It has been shown that there may be several targets for CaMKII that get phosphorylated with a change in frequency (9), whereas additional calcium-dependent kinases, such as myosin light chain kinase or protein kinase G, also may be activated. Because intrinsic responses to cycle length may show directionally similar effects on contractile force to those due to the impact of signaling cascades, it is impossible to differentiate the magnitude of each effect by studying changes that occur after a switch in steady-state frequency.
The aim of this study was to develop a protocol that potentially could isolate the effect of cycle length of the previous beat(s) on cardiac contraction and relaxation from the signaling-induced alterations of contractility. By using a stimulation protocol in which cycle length changes are randomized around a central baseline frequency, we are not evoking persistent changes in the cycle length that could lead to the sufficient impact of “slow” effects on contractile force by signaling-induced processes to have a functional significance. Hence, we can functionally isolate the effects of cycle length itself on cardiac contractile strength. Using this protocol, we observed that the length of the cycle preceding a stimulated beat positively correlates with myocardial contractile strength, whereas both cycle lengths preceding that one negatively correlate with contractile force. We conclude that the effect of cycle length involves a balance of negative and positive inotropic intrinsic myocardial properties that are exerted on future beats.
METHODS
Tissue isolation.
All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of The Ohio State University. Male, New Zealand White rabbits (2–3 kg, n = 21) were given 5,000 U/kg heparin and anesthetized with 50 mg/kg pentobarbital sodium, both intravenously. Sufficient analgesia was determined by the complete absence of pain reflexes. The chest was opened via bilateral thoracotomy, and the heart was rapidly excised and retrogradely flushed in a modified Langendorff apparatus with a cardioplegic Krebs-Henseleit (KH) solution containing 20 mM 2,3-butanedione monoxime (BDM). BDM was added to prevent cutting injury and has been shown to be reversible (19). The right ventricle was opened, and thin, uniform trabeculae were carefully dissected and mounted in a small custom-made muscle bath as described previously (14, 31). The cardioplegic KH solution was replaced with a normal KH solution, and the muscle was stimulated at 1 Hz, at 37°C, for 20 min or until contractile parameters had stabilized. This temperature is physiological for most large mammals, although the rabbit generally has a slightly higher body temperature. However, for several technical reasons, we performed the experiments at 37°C, which is near physiological for the rabbit.
Assessment of force-interval relationship.
In the first set of experiments, we investigated the standard protocol used to assess postrest potentiation. After the muscle had equilibrated in the setup, stimulation was halted for 1, 2, 4, 8, 15, 30, 60, or 120 s. After each rest period, the muscle was allowed to stabilize. The amplitude of the first twitch after the rest period was analyzed for force-interval relationship behavior.
In the second set of experiments, the rest durations were changed so that they encompassed the in vivo rate of the rabbit, and the baseline frequency was set within the in vivo range. In addition to additional periods of rest, the second protocol included shorter-than-steady-state intervals. From a baseline beat-to-beat interval of 350 ms, the effect of a single beat with a beat-to-beat interval of 250, 300, 400, 450, 500, 700, 1,000, 2,000, 3,000, 4,000, 10,000, and 20,000 ms was tested.
Variable cycle length simulation protocol.
To ensure that the effects of cycle length could be distinguished from signaling-induced events and thus isolated to the greatest extent possible, we used a stimulation protocol that allowed cycle length to change around a baseline such that the variations in cycle length did not exert changes in the average duration over a multisecond time window. In addition, care was taken to choose physiological conditions of baseline stimulation rate (2.85 Hz, cycle length of 350 ms) and temperature (37°C) such that the resulting findings would be physiologically relevant. First, the average frequency over time would have to be constant to avoid any time-averaged calcium changes. Second, the cycle lengths chosen to study would need to be in the physiological range and temperature of the species we studied (rabbit). Finally, the cycle lengths would need to be chosen randomly to ensure no repeatable pattern would exert an effect on twitch dynamics.
Next, the muscle was subjected to an 8-min variable cycle length protocol. To encompass most of the rabbit's physiological range of heart rates, cycle lengths chosen were 250, 300, 350, 400, and 450 ms (corresponding to 2.2–4 Hz, covering the majority of the in vivo range for the rabbit). The muscle was stimulated to contract with one of the five chosen cycle lengths randomly selected as the time between beats for a total of 1,200 contractions. Thus, when viewed over a small time window (a few seconds, not long enough to exert the slow contractile response that generally takes 10 or more seconds to develop), the muscles were stimulated at an average frequency of ∼2.85 Hz (350-ms cycle length) with a random pattern, eliminating any long-term effect of changes in frequency. Analyzing 1,200 contractions allowed for the analysis of up to 3 cycle lengths preceding a contraction, since for each condition there were about 10 examples that could be averaged per muscle to reduce variability.
Data analysis.
Data were analyzed using custom-written software (LabVIEW). The algorithm was designed to recognize patterns of three consecutive cycle lengths. This was done for all 125 combinations of 3 cycle lengths. We named these cycle lengths primary, secondary, and tertiary, with primary being the immediate previous twitch cycle (Fig. 3), secondary the one before the primary, and tertiary the one before the secondary. If a data set was sufficiently long, the impact of “quaternary” was investigated as well. With the data categorized, we determined the effect of each cycle length on twitch dynamics. Correlation coefficients (R2) and slopes were calculated for developed force vs. primary cycle length (with constant secondary), developed force vs. secondary cycle length (with constant primary), and developed force vs. tertiary cycle length (with constant primary and secondary). The same was done for relaxation time to 90% of peak (RT90). Along with the correlation coefficients, twitch parameters of twitches with the same cycle length signature were averaged to quantify the total effect of each cycle length. The average forces allowed for quantification of cycle length impact on force and relaxation. All data are means ± SE, unless stated otherwise.
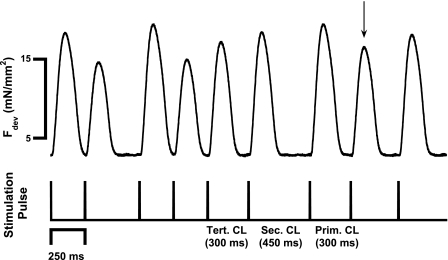
Fig. 3.
A representative chart section showing 3 s of a trabeculae being stimulated using the variable CL protocol. The 3 CLs, primary (Prim), secondary (Sec), and tertiary (Tert), are labeled for the twitch denoted with the arrow. This twitch was then categorized according to its previous CL signature.
RESULTS
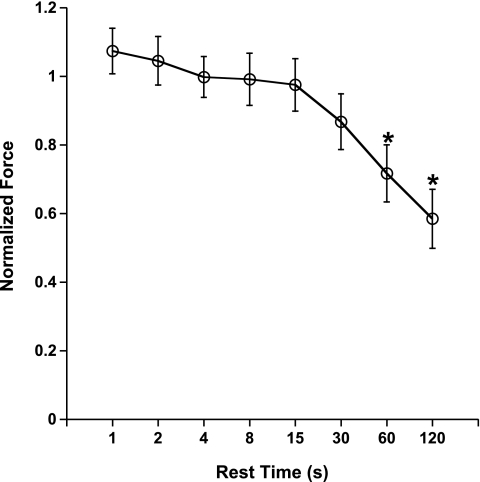
First, we assessed the effect of the force-interval relationship in rabbit myocardium using a protocol used previously by others (22). In Fig. 1, the postrest potentiation/decay of rabbit trabeculae (n = 7) is shown. From a baseline frequency of 1 Hz, at body temperature, very little postrest potentiation was observed. Only at the shortest interval, 1 s, was a nonsignificantly small amount of potentiated force developed (P = 0.32), whereas at rest periods of 60 s and longer, a significant postrest decay was observed (P < 0.05).
Fig. 1.
Force-interval relation in right ventricular rabbit trabeculae shows little potentiation at a baseline frequency of 1 Hz at 37°C. Average developed force (Fdev) at 1 Hz was 14.5 ± 1.3 mN/mm2. At rest periods of 60 s or longer, postrest decay of contractile force was observed. Data are means ± SE (n = 7). *P < 0.05.
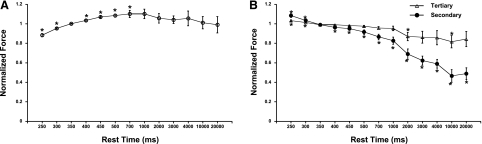
To further investigate the effect of cycle length, we proceeded to study the impact of a single rest interval from steady state in a more physiological setting for the rabbit. First, we elevated the base frequency to 2.85 Hz, which falls in the physiological heart rate range for the rabbit. Second, we employed much shorter rest intervals that can actually occur in vivo; the often used rest intervals of 1 s or more have little physiological relevance in the rabbit or smaller rodent, since their typical in vivo resting heart rate generally does not allow for resting intervals longer than 400 ms in the rabbit or longer than ∼100 ms in mice. In addition to the shorter rest intervals, we also included two intervals that were shorter than the baseline frequency and thus technically were not postrest durations but could be considered “premature beats.” In Fig. 2A, the actively developed force is depicted as a fraction from baseline, charted as a function of rest intervals between 250 ms and 20 s. In this subsecond domain, rather than the seconds domain usually investigated, there is a significant positive force-interval relationship; between 250 and 700 ms of cycle length interval, the longer the interval is, the more force is developed. Again, at rest intervals of 1 s or longer, no significant gain or loss of force was observed. This experiment indicates that the majority of single-beat cycle length modulation of contractile force generation takes place in the actual heart rate range domain of the rabbit. In Fig. 2B, we show that right after the postinterval twitch, contractile force rapidly decayed back to steady-state levels. The twitch right after the postinterval twitch was stronger than steady state for a rest interval shorter than steady state, whereas for all rest intervals exceeding steady state, the second twitch was significantly weaker. The second twitches after the postinterval twitch were still stronger if the resting interval was shorter than steady state, but for any interval that was longer than steady state, the third twitch after the rest period was not significantly different from steady state. The third twitch after the postinterval twitch was not different from steady state at any condition.
Fig. 2.
A: using an expanded spectrum of cycle length (CL), including shorter than steady state, potentiation of the postinterval twitch is observed at a baseline CL of 350 ms for CLs shorter than 700 ms. At longer CLs, 1–20 s, no significant potentiation or decay was observed. Fdev at the baseline CL of 350 ms was 40.9 ± 2.0 mN/mm2. B: the second twitch after the rest interval (secondary) was significantly stronger when the interval was shorter than steady state and was significantly weaker with longer intervals. The third twitch (tertiary) was slightly but significantly stronger for the 2 shortest intervals but was not significantly different from steady state at any interval longer than steady state. Data are means ± SE (n = 7). *P < 0.05 compared with the steady-state CL of 350 ms before the tested CL.
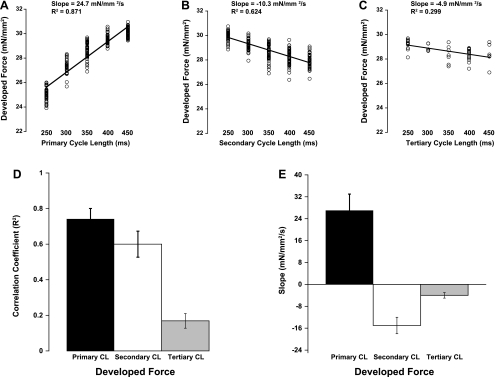
By randomizing the shortest five beat-to-beat intervals using the previous protocol, we set out to determine the dynamic influence of beat duration on contractile force. Figure 3 shows a representative chart section depicting nine consecutive contractions of one experiment where beat-to-beat duration was randomly forced on the muscle. The stimulation pattern was randomly determined, with each cycle length selected from one of the five options (250, 300, 350, 400, or 450 ms). It can be seen in Fig. 3 that developed force was very different between twitches of different previous cycle length signatures. The stimulation cycles named are those for the twitch designated with the arrow. When all these twitches were analyzed, they were categorized according to their previous three cycle lengths. For this protocol, for several minutes a random cycle length was used and data were collected. The relationships between cycle length and developed force obtained with this protocol are shown in Fig. 4. Developed force from a representative experiment is shown in Fig. 4, A–C. We found a steep positive relationship between primary cycle length and developed force when comparing twitches with the same secondary cycle length (example in Fig. 4A is with a 350-ms secondary cycle length). The opposite relationship was observed between the secondary cycle length and developed force at a constant primary cycle length (350 ms in example). The tertiary cycle length (with constant primary and secondary cycle length both at 350 ms) also showed a negative correlation with force much like the secondary cycle length but with a shallower slope. Figure 4, D and E, shows the average correlation coefficients and slopes, respectively, for each cycle length. The R2 values for primary and secondary cycle length were significantly higher than that of tertiary cycle length.
Fig. 4.
A–C show representative plots of the 3 different CLs vs. Fdev. A shows the effect of primary CL (all with a 350-ms secondary CL); B shows the effect of secondary CL (all with a 350-ms primary CL); and C shows the effect of tertiary CL (all with a 350-ms primary and secondary CL). D shows the average correlation coefficient (R2) for all 7 experiments, displaying the tightest relationships between primary and secondary CL. Data are means ± SE. E shows the average slopes of these linear fits to display the positive correlation of Fdev with primary CL and negative correlation for secondary and tertiary CL. All data in D and E are significantly different from zero. All effects of slope were significant (P < 0.05).
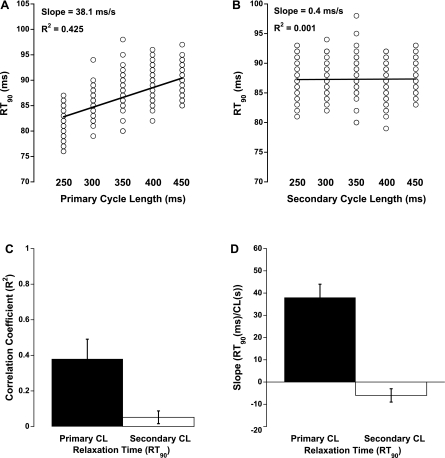
Relationships between cycle length and relaxation time (RT90) displayed weaker correlations compared with developed force. Figure 5, A and B, shows representative relationships of primary cycle length (with constant secondary) and secondary cycle length (with constant primary) to RT90. A weakly positive correlation was observed with RT90 and primary cycle length, i.e., a slower relaxation with increased primary cycle length, whereas secondary cycle length showed virtually no correlation with RT90. Likewise, tertiary cycle length displayed no relationship with RT90 (data not shown).
Fig. 5.
Effect of CL on relaxation time. A and B show representative plots of primary CL (with 350-ms secondary CL) and secondary CL (with 350-ms primary CL), respectively, vs. relaxation time to 90% of peak (RT90). C and D show the average correlation coefficients and slopes of all 7 experiments, respectively. No effect of the tertiary CL on relaxation was observed (data not shown).
In an additional control experiment, we verified that the average force of contraction remained unchanged during this protocol. We compared the average twitch developed force during randomized pacing with the steady-state force at the average cycle length of 350 ms. No changes in average contractile force were observed; during the randomized pacing, the force was 98 ± 1% of the initial force (n = 4 preparations), and after randomized pacing, it was 96 ± 3%. The small changes in force over time are attributed to the nonavoidable rundown of multicellular preparations in vitro over time. From this experiment we conclude that even if changes in the slow processes (i.e., posttranslational modification) already occur, they are not strong enough to impact contractile strength or speed of relaxation, indicating our protocol can functionally “isolate” intrinsic from slow processes.
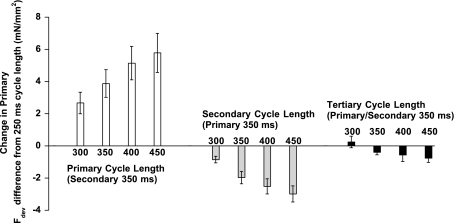
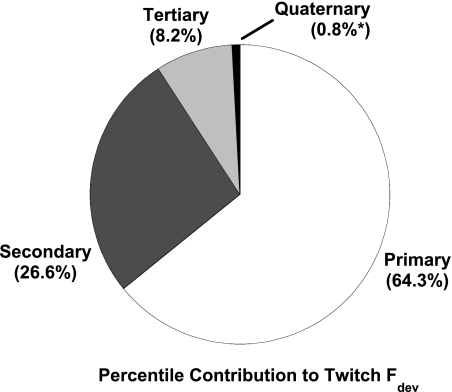
Figure 6 shows averaged developed force differences (from 250-ms cycle length) for twitches that displayed the same cycle length signature. For example, the first part of Fig. 6 shows the difference in developed force between each primary cycle length (with constant secondary cycle length at 350 ms) and developed force of the shortest primary cycle length (250 ms). Force is greater for the longest primary compared with the shortest primary cycle length by an average of ∼6 mN/mm2. Data for secondary (middle) and tertiary cycle length (right) for developed force also are shown in Fig. 6. In two additional experiments, we used a longer run (n = 2) and analyzed the existing data as well as the effect of the quaternary beat in data sets sufficiently long to get at least three records for each of 625 possible interval combinations. In none of these five experiments did we find any correlation of either force development or speed of relaxation and the quaternary cycle length, indicating the cycle length history is limited to three beats under the conditions present. Figure 7 shows the percent contribution each cycle length had on developed force on all records where up to the quaternary beats were analyzed (n = 5). This was calculated by determining the percentile difference between the short and long cycle length developed force for all four cycle lengths. The total absolute change from the short to the long cycle lengths were added together so that each individual change could be displayed as a percentage of the total. Primary cycle length exerted the most effect, with secondary and tertiary cycle length following, whereas the quaternary cycle length was <1% and not significant. This is in agreement with our data from the force-interval experiments, which only showed an influence of the rest interval on the first three twitches after a period of rest.
Fig. 6.
Average change in Fdev observed for each CL using the 250-ms CL as a baseline for primary CL (all with a constant 350-ms secondary CL), secondary CL (all with a constant 350-ms primary CL), and tertiary CL (all with a constant 350-ms primary and secondary CL).
Fig. 7.
Average contribution each CL exerted on the Fdev of a twitch. The data were obtained by determining the percent difference in force between the 250- and 450-ms CL for each of the 4 previous CLs. The percent differences were added together, and a percent contribution was calculated for each CL as a fraction of the total percent difference. The effect of the quaternary cycle length was not significant (n = 5 muscles).
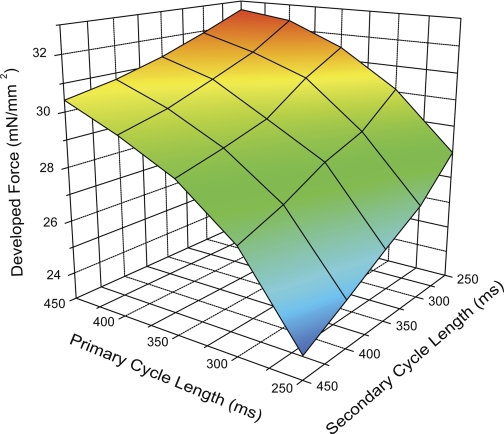
The interaction between the primary and secondary cycle length effects are shown in the three-dimensional plot of Fig. 8, where we show the developed force average from all seven experiments plotted against primary and secondary cycle length, with the points connected to create a plane. From this plot, we can see how each primary-secondary combination affected developed force. The tilt of the plane rightward reflects the percent contribution of the two cycle lengths shown in the pie chart in Fig. 7. It also shows that the cycle length combination that produces the most force is the short secondary cycle length followed by a long primary cycle length. Likewise, the lowest force comes from the longest secondary cycle length followed by the shortest primary cycle length.
Fig. 8.
A 3-dimensional plane representation of Fdev vs. primary and secondary CL. Note the location of the 4 points of the plane. The top and bottom points represent the greatest Fdev (long primary, short secondary) and the lowest Fdev (long secondary, short primary). The long primary, long secondary point (left) produces greater contractility than the short primary, short secondary point (right), supporting the data in Fig. 4 that showed a greater percent contribution for the primary over the secondary CL.
DISCUSSION
The randomized beat protocol we have first described can assess the impact of cycle length and cycle length history for a wide range of conditions in only 10 min. Previous protocols that worked from steady-state conditions and imposed shorter and longer beats from this steady-state condition require a return to steady state before the next intervention (1, 4, 24). As such, previously employed protocols would require much longer, up to 10 times the experimental time used in this study, to obtain a full data set. The life span of in vitro preparations using conventional muscle physiology is only a couple of hours due to rundown that unavoidably occurs, especially at body temperature, when not specifically cultured in a specialized setting (12, 13). This novel protocol can be used in the future in conjunction with fluorescence measurements in muscles, which at body temperature in rabbits is typically limited to 1 h or less due to loss of the indicator (29). Likewise, the short duration of the randomized beat protocol will allow for a comparison before and after an intervention in baseline parameters (i.e., length, baseline frequency, or presence of a drug or compound) within a time frame that rundown is minimal, and all data are collected at physiologically relevant temperature and frequency to maximize extrapolation of the results to the in vivo situation.
Besides development of this protocol, from the present study we can glean several new insights regarding cycle length impact on cardiac contractility. Using the randomized beat-interval protocol, we can assess the effect of cycle length on contractile force in a pseudo-steady-state system. We have shown in this study that 1) there exists a differential effect of previous cardiac cycle lengths on developed force of the myocardium; 2) the primary cycle length positively correlates with developed force, whereas the secondary and tertiary CL correlate negatively; and 3) a single cycle length exerts an inotropic effect for three consecutive beats.
Because of this random pacing protocol, twitch developed force widely varies between beats, but the average twitch force when assessed over a few seconds remains constant. Although we agree that the underlying processes, over large ranges, certainly are not linear, we observed that over the range of cycle lengths studied, the sum of the underlying properties behaves in a nearly pure linear fashion. The “loss of force” (long-long-short, for instance) of a potentiating combination is virtually equal to the gain with the “opposite” cycle length pattern (in this case, short-short-long). From this, we glean that the system within the range and conditions measured behaves functionally as a linear system. Second, posttranslational modifications that occur when cycle length is changed for a long time, such as in the steady-state FFR that can induce phosphorylation of myofilament proteins (31), either do not occur or occur to such a limited extent that they do not functionally impact on twitch force development. In addition, it has been shown that stretch-induced activation (2) or persistent diastolic pressure (8) can cause posttranslational modification of myofilaments after prolonged induction, but the average force data indicate that this does not occur when applied on a beat-to-beat basis where these stresses cycle around a mean. Moreover, despite cycle lengths as short as 250 ms, at no point was the muscle unable to fully relax. This implies the length of the muscle has returned to resting values between beats, and thus no elevated diastolic tension was observed further, indicating that posttranslational processes likely played no, or a nonsignificant, role in the outcome of the experiments.
We observed, in line with previous observations, that the main determinant of twitch force was the primary cycle length, i.e., the duration of rest before the beat. This observation is very similar to the so-called postrest potentiation; like a longer rest period, a longer cycle time results in greater force (15). In most studies, postrest potentiation is observed when a muscle (or cardiac myocyte) is allowed to rest for several seconds after being stimulated at a constant frequency for some time. In the present study, we have shown that a similar relationship is observed within the physiological twitch interval. Postrest potentiation/decay has mainly been used to describe dynamics of the SR and is generally not discussed as a regulator of contractility in normal physiology because its study is typically performed with rest periods between 2 s and 2 min, periods of rest that the heart normally is not exposed to. The basis of this force-interval relationship lies in the fact that the SR has more time to load with calcium, producing a greater calcium transient on the next twitch (24). The force-interval relationship has been shown to be species dependent (17) and has been used as an indicator for SR function in cardiomyopathy (12, 22). In the present study, we have shown that the effect of the force-interval relationship can be seen in muscle twitching at physiological frequency, and not only at the widely known seconds scale but at a temporal scale of a fraction of the baseline heart rate. Even at shorter than normal intervals (analogous to extrasystolic beats), the relationship between interval and developed force was observed. This is in agreement with data by Schouten et al. (24, 25), who examined the extrasystolic beat behavior in rat muscle at room temperature. Our data extend their conclusions, since they indicated that subsecond interpretation of their results could not be done. Thus, despite vastly different experimental settings, their data are in line with what we derived from the rabbit data under more physiological conditions and at physiological cycle length durations.
A positive force frequency is observed in myocardium of humans, dogs, and rabbits as well as in rats and mice, albeit to a lesser extent in the latter two species (10, 16, 26). The vast majority of studies investigating the FFR focus on changes in force that have reached a steady state after a change in frequency. Since frequency can affect time-averaged calcium, specifically in larger mammals where a significant increase in diastolic calcium occurs at higher heart rates (29), it is likely that calcium-dependent signaling mechanisms play a role in modulating changes to twitch dynamics. Indeed, it can take up to 45 s after a change in frequency to observe a steady-state force (29). Huke and Bers (9) likewise showed that in isolated myocytes, a very rapid change in contractility is observed and that posttranslational modification of proteins is too slow to account for these effects but may contribute to the level of the new steady state at the higher frequency after a prolonged time. To isolate the effect of twitch cycle time per se from such slowly occurring changes, we used a pacing algorithm that allows for a constant average stimulation rate with variable changes in cycle length that occurred randomly. Analysis of the resulting force and cycle length fluctuations showed that shorter secondary and tertiary cycle lengths caused an increase in force. Thus the intrinsic mechanisms that respond to a change in cycle length can do so within a single cycle length. We also found that the secondary cycle length displayed a much larger effect on force than the tertiary. No evidence of impact on force or relaxation of quaternary beats was observed under the conditions used, which closely resembled the frequency range of the in vivo rabbit. Therefore, it can be concluded that cardiac muscle response to cycle length alone is both fast to develop and fast to decay.
We only observed changes in speed of relaxation with the primary cycle length, not with the secondary or tertiary. Under several conditions, force of contraction and speed of relaxation are correlated. Specifically, this holds true for steady-state conditions regarding changes in length; when a muscle has a higher preload, force of contraction increases, whereas the speed of relaxation decreases (11). In other regulatory mechanisms, such as the FFR, the force of contraction increases, whereas speed of relaxation increases (10). When we compare these regulatory mechanism, we conclude that force may often correlate with relaxation, but it is not necessarily actually coupled to relaxation (18), as can be observed in our data, where we show that cycle length per se drastically affects force of contraction but not necessarily the kinetics of relaxation.
In pilot experiments in rat myocardium (28), we observed that with fluctuations in cycle length, the calcium transient amplitude likewise fluctuated and correlated with force development. The fluctuations in calcium transient amplitude were, however, of lesser magnitude than those observed in the force tracings in our studies. Although species differences are likely responsible to a certain degree, this is more likely explained by the fact that the force-calcium relationship in cardiac muscle is very steep, with a Hill coefficient of ∼3–7 (6, 7). Thus, near the Kd of this relationship (pCa50), a minor change in calcium can be responsible for a much larger change in force, implying that the small changes in calcium concentration could be sufficient to cause significant force fluctuations with cycle length changes. Diastolic calcium concentration can decline further with a longer cycle length, and the cycle length duration thus may impact the minimal diastolic cytosolic calcium concentration. To what quantitative extent the diastolic or systolic calcium concentrations contribute to the underlying mechanism was deemed beyond the scope of this initial methodology study, but unraveling this mechanism could in future help us understand impairment of frequency-dependent activation in hypertrophic or otherwise diseased myocardium (30).
In conclusion, we observed that the history of the three previous cycle lengths exerts an inotropic “memory” of myocardial contraction under near-physiological conditions. The contributions of the last interval compared with the two beats preceding that last interval are opposite, and they contribute to contractile strength with decreasing importance as the cycle length is further removed from the current beat.
GRANTS
This investigation was supported by National Heart, Lung, and Blood Institute Grants R01 HL746387 and K02 HL83957 (to P. M. L. Janssen), American Heart Association Established Investigator Award 0740040 (to P. M. L. Janssen), and American Heart Association Ohio Valley affiliate Predoctoral Fellowship 0615288B (to K. D. Varian).
ACKNOWLEDGMENTS
We thank Anil Birdi for technical assistance.
REFERENCES
- 1.Burkhoff D, Yue DT, Franz MR, Hunter WC, Sunagawa K, Maughan WL, Sagawa K. Quantitative comparison of the force-interval relationships of the canine right and left ventricles.Circ Res 54:468–473, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Cazorla O, Szilagyi S, Le Guennec JY, Vassort G, Lacampagne A. Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction.FASEB J 19:88–90, 2005 [DOI] [PubMed] [Google Scholar]
- 3.DeSantiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban.J Mol Cell Cardiol 34:975–984, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Dumitrescu C, Narayan P, Cheng Y, Efimov IR, Altschuld RA. Phase I and phase II of short-term mechanical restitution in perfused rat left ventricles.Am J Physiol Heart Circ Physiol 282:H1311–H1319, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance.Eur J Pharmacol 500:73–86, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Gao WD, Backx PH, Azan-Backx M, Marban E. Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle.Circ Res 74:408–415, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Gwathmey JK, Hajjar RJ. Relation between steady-state force and intracellular [Ca2+] in intact human myocardium. Index of myofibrillar responsiveness to Ca2+.Circulation 82:1266–1278, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo C, Wu Y, Peng J, Siems WF, Campbell KB, Granzier H. Effect of diastolic pressure on MLC2v phosphorylation in the rat left ventricle.Arch Biochem Biophys 456:216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Huke S, Bers DM. Temporal dissociation of frequency-dependent acceleration of relaxation and protein phosphorylation by CaMKII.J Mol Cell Cardiol 42:590–599, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen PM, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium.J Mol Cell Cardiol 43:523–531, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen PML, Hunter WC. Force, not sarcomere length, correlates with prolongation of isosarcometric contraction.Am J Physiol Heart Circ Physiol 269:H676–H685, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Janssen PML, Lehnart SE, Prestle J, Hasenfuss G. Preservation of contractile characteristics of human myocardium in multi-day cell culture.J Mol Cell Cardiol 31:1419–1427, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Janssen PML, Lehnart SE, Prestle J, Lynker JC, Salfeld P, Just H, Hasenfuss G. The trabecula culture system: a novel technique to study contractile parameters over a multiday time period.Am J Physiol Heart Circ Physiol 274:H1481–H1488, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Janssen PML, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat.Am J Physiol Heart Circ Physiol 282:H499–H507, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Koch-Weser J, Blinks JR. The influence of the interval between beats on myocardial contractility physical factors in the analysis of the actions of drugs on myocardial contractility.Pharmacol Rev 15:601–652, 1963 [PubMed] [Google Scholar]
- 16.Layland J, Kentish JC. Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies.Am J Physiol Heart Circ Physiol 276:H9–H18, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Maier LS, Bers DM, Pieske B. Differences in Ca2+-handling and sarcoplasmic reticulum Ca2+ content in isolated rat and rabbit myocardium.J Mol Cell Cardiol 32:2249–2258, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Monasky MM, Varian KD, Davis JP, Janssen PM. Dissociation of force decline from calcium decline by preload in isolated rabbit myocardium.Pflügers Arch 456:267–276, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Mulieri LA, Hasenfuss G, Ittleman F, Blanchard EM, Alpert NR. Protection of human left ventricular myocardium from cutting injury with 2,3-butanedione monoxime.Circ Res 65:1441–1449, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure.Circulation 85:1743–1750, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency- dependent acceleration of relaxation and Ca2+ current facilitation.J Mol Cell Cardiol 42:196–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pieske B, Sutterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, Holubarsch C, Just H, Hasenfuss G. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling.J Clin Invest 98:764–776, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossman EI, Petrel RE, Chuddar KW, Peacetime V, 3rd, Janssen PM, Vaughan JP, Houser SR, Margulies KB. Abnormal frequency-dependent responses represent the pathophysiologic signature of contractile failure in human myocardium.J Mol Cell Cardiol 36:33–42, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Schouten VJ. Interval dependence of force and twitch duration in rat heart explained by Ca2+ pump inactivation in sarcoplasmic reticulum.J Physiol 431:427–444, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schouten VJ, van Deen JK, de Tombe P, Verveen AA. Force-interval relationship in heart muscle of mammals. A calcium compartment model.Biophys J 51:13–26, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stull LB, Leppo M, Marban E, Janssen PML. Physiological determinants of contractile force generation and calcium handling in mouse myocardium.J Mol Cell Cardiol 34:1367–1376, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics.J Physiol 558:927–941, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres CAA, Varian KD, Janssen PML. Variability in interbeat duration influences myocardial contractility in rat cardiac trabeculae.Open Cardiovasc Med J:96–100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varian KD, Janssen PM. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol 292:H2212–H2219, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Varian KD, Kijtawornrat A, Gupta SC, Torres CAA, Monasky MM, Hiranandani N, Delfin DA, Rafael-Fortney JA, Periasamy M, Hamlin RL, Janssen PM. Impairment of diastolic function by lack of frequency-dependent myofilament desensitization in rabbit right ventricular hypertrophy.Circ Heart Fail 2:472–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varian KD, Raman S, Janssen PML. Measurement of myofilament calcium sensitivity at physiological temperature in intact cardiac trabeculae. Am J Physiol Heart Circ Physiol 290:H2092–H2097, 2006 [DOI] [PubMed] [Google Scholar]