Abstract
TMP21 has been shown to be associated with the γ-secretase complex and can specifically regulate γ-cleavage without affecting ϵ-mediated proteolysis. To explore the basis of this activity, TMP21 modulation of γ-secretase activity was investigated independent of ϵ-cleavage using an amyloid-β precursor proteinϵ (APPϵ) construct which lacks the amyloid intracellular domain domain. The APPϵ construct behaves similarly to the full-length precursor protein with respect to α- and β-cleavages and is able to undergo normal γ-processing. Co-expression of APPϵ and TMP21 resulted in the accumulation of membrane-embedded higher molecular weight Aβ-positive fragments, consistent with an inhibition of γ-secretase cleavage. The APPϵ system was used to examine the functional domains of TMP21 through the investigation of a series of TMP21-p24a chimera proteins. It was found that chimeras containing the transmembrane domain bound to the γ-secretase complex and could decrease γ-secretase proteolytic processing. This was confirmed though investigation of a synthetic peptide corresponding to the TMP21 transmembrane helix. The isolated TMP21 TM peptide but not the homologous p24a domain was able to reduce Aβ production in a dose-dependent fashion. These observations suggest that the TMP21 transmembrane domain promotes its association with the presenilin complex that results in decreased γ-cleavage activity.
Alzheimer disease is a progressive neurodegenerative disorder and the most common cause of dementia worldwide. One of the main histopathological hallmarks in Alzheimer disease is senile plaque, an extracellular protein deposit composed primarily of the amyloid β-peptide (Aβ),5 which is generated by sequential cleavages of the amyloid-β precursor protein (APP) by β- and γ-secretase activities (1). Cleavage by γ-secretase occurs within the APP transmembrane domain at several sequences, such as the γ-site, to produce Aβ40/42, and the ϵ-site, which results in the release of the cytosolic amyloid intracellular domain (AICD) (2). AICD is targeted to the nucleus and regulates transcription of several genes including KAI1 (3, 4), glycogen synthase kinase 3 (4–6), neprilysin (7, 8), p53 (9), epidermal growth factor receptor (10), LRP1 (11), Tip60, APP, or β-site APP-cleaving enzyme (BACE) (4).
Several lines of evidence have shown that γ/ϵ-secretase activity requires multimeric membrane protein complexes, the presenilin complexes, which are composed of at least four components: presenilin (PS1 or PS2), nicastrin (NCT), anterior pharynx-defective phenotype 1 (APH-1), and presenilin enhancer 2 (PEN-2) (12–16). All four components appear to be essential for γ/ϵ-secretase activity (17–19). NCT and APH-1 form an initial subcomplex to which full-length PS1 or PS2 and PEN-2 are added (18, 20, 21). During maturation of the presenilin complexes, nicastrin is glycosylated, and presenilins are cleaved into N- and C-terminal fragments which remain associated as a heterodimer in the active high molecular weight complex (20, 22). In these complexes presenilins have been proposed to serve as the catalytic subunit (23), whereas the large ectodomain of NCT plays an essential role in substrate recognition (24, 25).
It has been demonstrated that TMP21, a member of p24 cargo protein with homology to p24a, binds the presenilin complexes and acts as a modulator resulting in the selective suppression of γ- but not ϵ-secretase cleavage (26). TMP21 small interfering RNA suppression enhances the production of Aβ, whereas overexpression or the ablation of TMP21 does not affect AICD production. In this context TMP21 has no effect on p53 or NEP, which are two target genes of AICD activation (27).
In the current study, the effects of TMP21 on γ-secretase activity were investigated independent of ϵ-cleavage using an APPϵ construct which lacks the AICD domain (terminates at Aβ49). The APPϵ fragment behaves similarly to the full-length precursor protein with respect to α and β cleavages and is able to undergo γ-processing, which is sensitive to inhibition by DFK167 (28). Co-expression of APPϵ and TMP21 resulted in the accumulation of membrane-embedded higher molecular weight Aβ-positive fragments, consistent with an inhibition of γ-secretase cleavage. This reporter system was used to determine the functional domain of TMP21 through the investigation of a series of TMP21-p24a chimera proteins. In addition, a synthetic peptide corresponding to the TMP21 transmembrane helix but not the p24a TM domain was able to reduce Aβ secretion in a dose-dependent fashion. These observations indicate that the transmembrane domain of TMP21 is a primary site of action, and its physical association with the presenilin complex can alter γ-cleavage.
EXPERIMENTAL PROCEDURES
Mutagenesis and cDNA Constructs
TMP21 and p24a tagged at the C-terminal with the FLAG epitope were cloned in pcDNA4 as previously described (26). Chimera constructs between TMP21 and p24a cDNA were obtained following a three-step PCR protocol as previously described (36), and then all constructs were confirmed by DNA sequencing and cloned in pcDNA4 vector with the FLAG epitope at the C terminus. APPϵ constructs containing a stop codon at the end of the transmembrane domain of human APP (isoforms corresponding to 695 or 751) were cloned in pcDNA3 as previously described (28). The wild-type APP constructs (APP WT isoforms 695 or 751) were cloned into pcDNA3.
Cell Culture, Transfection, and Treatment
Native HEK293 cells or stably transfected HEK293 cells expressing the Swedish APP mutant (APPSwe) were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum. Transient transfections were performed in native HEK293 cells by using Lipofectamine 2000 (Invitrogen) or FuGENE 6 (Roche Applied Science) according to the manufacturer's instructions, and then cells were collected 24–48 h after transfection.
Membrane Fraction Preparation and Immunoprecipitation
HEK293 cells were harvested and homogenized in buffer A (5 mm HEPES, pH 7.4, 1 mm EDTA, 0.25 m sucrose, protease inhibitor mixture from Roche Applied Science). The homogenate was clarified by centrifugation at 1000 × g for 5 min at 4 °C, and the supernatant was centrifuged at 100,000 × g for 1 h at 4 °C. For immunoprecipitation of the intracellular Aβ long species, cell pellets or membrane pellets were homogenized in radioimmune precipitation assay buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton, 0.5% deoxycholate, 0.1% SDS, protease inhibitor mixture). Isolates of either total lysate (2.5 mg) or membrane fraction (800 μg) were diluted in 1 ml of radioimmune precipitation assay buffer. For immunoprecipitation of presenilin complexes, membrane pellets were homogenized in buffer B (25 mm HEPES, pH 7.4, 150 mm NaCl, 2 mm EDTA, 1% CHAPSO, protease inhibitor mixture). Isolates of the membrane fraction (800 μg) were diluted in 1 ml of buffer B to a final concentration of 0.5% CHAPSO. After preclearing with protein G-Sepharose 4 fast flow (GE Healthcare) for 1 h at 4 °C, lysates were subjected to immunoprecipitation with the appropriate antibody (a mouse monoclonal anti-Aβ 6E10 (from Covance) or a mouse monoclonal anti-FLAG (from Sigma) antibodies). Immunoprecipitants were recovered by overnight incubation at 4 °C with protein G-Sepharose. Beads were washed 3 times with radioimmune precipitation assay buffer or buffer B (0.5% CHAPSO) and once with phosphate-buffered saline.
SDS/PAGE and Western Blot Analyses
Whole cell lysates (total protein 50 μg), membrane lysates (protein 25–50 μg), and immunoprecipitated proteins were dissolved in SDS sample buffer, separated on Tris-Tricine gels 16% or Tris-glycine gels 4–20% (Invitrogen), and transferred to nitrocellulose membranes. Target proteins were visualized by enhanced chemiluminescence (ECL Amersham Biosciences) with the following antibodies: mouse monoclonal anti-Aβ 6E10 (Covance), anti-Aβ 4G8 (Covance), rabbit polyclonal anti-PS1-NTF, anti-NCT-Nt, anti-FLAG (from Sigma), and anti-APP CTF antibodies.
Peptide Synthesis and Assays
The TMP21 transmembrane domain sequence containing three N- and C-terminal lysine residues (KKK-VLYFSIFSMFCLIGLATWQVFYL-KKK) and the p24a transmembrane domain (KKK-VVLWSFFEALVLVAMTLGQIYYL-KKK) peptides were synthesized by standard Fmoc (N-(9-fluorenyl)methoxycarbonyl)-based solid phase methodology. The terminal lysines were added to increase peptide solubility. Peptides were purified by reverse phase high pressure liquid chromatography using water/acetonitrile mixtures buffered with 0.1% trifluoroacetic acid on a POROS 20R2 column. Stock solutions of both peptides were prepared in DMSO and added at the desired final concentrations (10 and 30 μg/ml) to the media of cultures of HEK293 cells stably expressing the Swedish variant of APP. Media was collected at different time points and Aβ40 levels were determined by ELISA.
Aβ40 ELISA and Cell-free Assays
Aβ40 levels were measured by ELISA assays using conditioned medium collected from HEK293 cells after a transient transfection or a treatment with synthetic peptide (TM-TMP21, TM-p24). The enzyme-linked immunoabsorbent assay ELISA was performed according to the manufacturer's instructions (BIOSOURCE. Determination of γ-secretase activity by cell-free assay was as previously described (26). Briefly, membrane fractions were isolated from HEK293 cells and incubated with recombinant FLAG-tagged APP-C100. TMP21 and p24a synthetic peptides solubilized in DMSO were added to the reaction mixture at the desired concentrations and Aβ40 generated by proteolysis was measured by ELISA.
Statistical Analysis
Statistical analyses were performed with PRISM software (Graph-Pad Software, San Diego. CA) by using the unpaired Student's t test for pairwise comparisons.
RESULTS AND DISCUSSION
Effects of TMP21 on γ-Secretase Processing in an APPϵ Reporter Assay
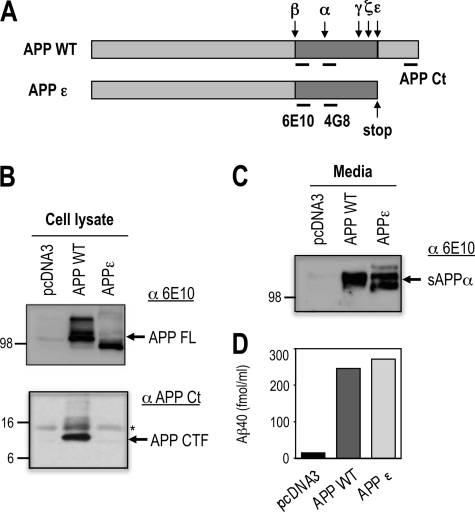
TMP21 is a type 1 transmembrane protein member of the p24 cargo-protein family that is involved in vesicular targeting and protein transport (29). In addition to its trafficking functions, TMP21 has been shown to specifically regulate proteolysis at the γ-secretase site, whereas no effects on ϵ-cleavage were observed (26, 27, 30). A C-terminal-truncated APP construct terminating at the ϵ-site was employed to allow investigation of TMP21 activity independent of ϵ-mediated cleavage (Fig. 1A). This APPϵ protein is recognized by an Aβ antibody targeting the N-terminal Aβ residues 1–17 (6E10) (Fig. 1B) and undergoes normal processing by α-, β-, and γ-secretase to produce similar levels of Aβ peptides and soluble APPα (sAPPα) fragment in the absence of AICD production (Fig. 1, C and D). Comparable findings have been previously reported which confirm that the APPϵ variant is comparable with the wild-type protein but permits examination of γ-secretase activities without any complicating factors associated with ϵ-related processing (28).
FIGURE 1.
Characterization of the APPϵ construct. A, schematic representation of the wild-type human amyloid-β precursor protein (APPWT) and its cleavage sites by the indicated secretases. APPϵ corresponds to the N-terminal fragment of APP generated by ϵ-cleavage (APPϵ). 6E10 antibody recognizes the 1–17 sequence of Aβ, 4G8 antibody recognizes the 17–24 sequence of Aβ, and APP C-terminal antibody recognizes the cytoplasmic tail. B, HEK293 cells were transiently transfected with APPWT or APPϵ cDNA as described and analyzed by Western blot for detection of APP full-length (APP FL) or the APP C-terminal fragment (APP CTF) using different APP antibodies (6E10, APP Ct). The APP-CTF-directed antibody does not label APPϵ, which lacks the C terminus. C, medium of HEK293 cells transfected with APPWT or APPϵ cDNA were analyzed by immunoblotting to detect the secreted extracellular fragment soluble APPα using 6E10 antibody. D, level of secreted Aβ40 peptide were analyzed by ELISA from medium of HEK293 cells transfected with APPWT or APPϵ cDNA.
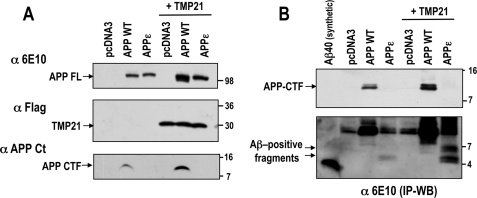
Co-transfection of APP-WT or APPϵ with C-terminal FLAG-tagged TMP21 resulted in a slight increase in expression levels of full-length proteins (Fig. 2A). A similar minor increase was observed for APP-CTF immunoreactivity in cells overexpressing APP-WT protein. This finding is consistent with previous investigations that demonstrated dual effects of TMP21 on γ-secretase activity and APP trafficking (26, 27, 30). However, unique effects in γ-cleavage were observed in cells co-expressing the APPϵ construct with TMP21. Immunoprecipitation and reprobing with an anti-Aβ antibody (6E10 N-terminal epitope) revealed the expected APP-CTF for the wild-type protein (Fig. 2B, short exposure, top panel). Upon longer exposure, two intracellular Aβ-reactive bands were observed in cells expressing APPϵ at higher molecular weights than are seen for the synthetic Aβ40 peptide (Fig. 2B, long exposure, bottom panel). These Aβ-reactive bands accumulated in the cells co-expressing TMP21. These bands were only observed after immunoprecipitation followed by Western blotting, suggesting that they are a small percentage of the total Aβ-related species.
FIGURE 2.
TMP21-induced accumulation of intracellular Aβ-positive fragments in cells expressing APPϵ. A, HEK293 cells were transiently co-transfected with TMP21-FLAG and APPWT or APPϵ cDNA, then analyzed by Western blot for detection of full-length APP (APP FL), the APP C-terminal fragment (APP CTF), or TMP21-FLAG protein. B, intracellular Aβ species were immunoprecipitated using 6E10 antibody (IP WB) in HEK293 cells transiently co-transfected with TMP21-FLAG along with APP-WT or APPϵ cDNA, then detected by Western blot using 6E10 antibody (short exposure, top panel). Note the high molecular weight intracellular Aβ at ∼5 kDa recognized by 6E10 antibody in cells overexpressing APPϵ and Aβ-positive bands at ∼6.5 and 5 kDa protected by co-transfection with TMP21 (longer exposure, bottom panel). Synthetic Aβ40 peptide was used as a molecular weight marker and positive control.
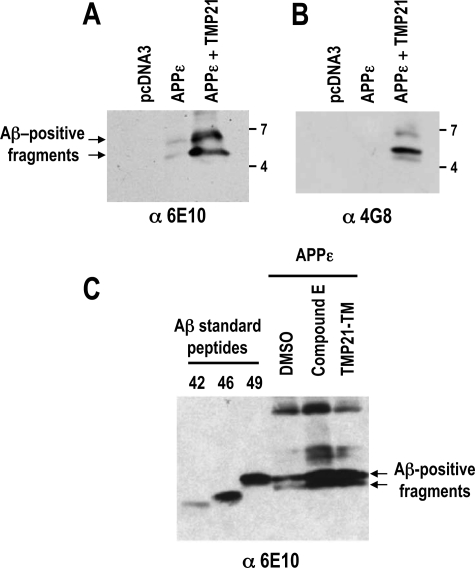
The appearance of these bands suggests that after extracellular cleavage (β,β′, or α cleavage) the γ-secretase is impaired by TMP21 expression, leading to unprocessed intracellular Aβ species terminating at the ϵ-site (corresponds to Aβ residue 49). The molecular weights of these TMP21-induced bands are in the range of β-to-ϵ Aβ (residues 1–49; molecular mass, 5.3 kDa) and possibly a β′-to-ϵ fragment (residues 11–49; molecular mass, 4.1 kDa). These fragments also contained the Aβ N terminus as shown by their reactivity to different antibodies. Aβ-positive species were isolated by immunoprecipitation from cells expressing APPϵ and probed with antibodies recognizing Aβ residues 1–17 (6E10) and 17–24 (4G8). The higher molecular weight bands induced by TMP21 expression were detected by both antibodies and were not seen in control mock-transfected cells (Fig. 3, A and B). These data suggest that the bands are produced by normal cleavage at or near the β-secretase site and their higher molecular weight as compared with Aβ-(1–40) would be consistent with impaired γ-processing in the presence of TMP21. The molecular weights of the bands are comparable with that of a synthetic Aβ 1–49 peptide, which indicates that the Aβ-positive fragments would be consistent with an incompletely processed Aβ-containing APPϵ fragments (Fig. 3C). Similar bands were also observed in cells expressing the APPϵ isoform when treated with the γ-secretase inhibitor, Compound E, which supports the possibility that they are generated by impaired γ-processing (Fig. 3C).
FIGURE 3.
Intracellular Aβ-positive fragments are generated by impaired γ-processing and migrate at the level of Aβ1–49 synthetic peptides. Intracellular Aβ species were immunoprecipitated from membrane fractions using 6E10 antibody (6E10) in HEK293 cells transiently co-transfected with APPϵ in the presence and absence of TMP21-FLAG, then detected by immunoblotting using 6E10 (A) or 4G8 (B) antibodies. C, HEK293 cells were transfected with APPϵ in the presence or absence of TMP21-TM, then treated or not with Compound E (100 nm). Intracellular Aβ species were then immunoprecipitated and detected using 6E10 antibody. Both TMP21-TM overexpression and Compound E treatment led to an accumulation of Aβ-positive fragment. Synthetic Aβ standard peptides were used as molecular weight markers.
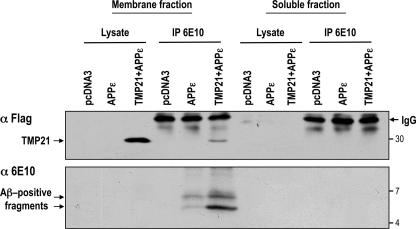
The fact that these fragments contain the hydrophobic terminus of Aβ was supported by fractionation studies. Membrane and soluble cytosolic fractions from cells expressing APPϵ with or without TMP21 were isolated, and the Aβ-positive species were immunoprecipitated. It should be noted that APPϵ is recovered in the membrane fraction, in agreement with the demonstration that APPϵ behaved as a membrane-bound type 1 protein as does βAPP (28). Probing with anti-Aβ (6E10) revealed the presence in the membrane fraction but not in the soluble fraction of the high molecular weight, presumably unprocessed fragments that were induced by TMP21 expression (Fig. 4). These findings support the fact that the fragments are membrane-embedded and, therefore, likely contain the APP transmembrane domain terminating at the ϵ-cleavage site (i.e. Aβ49).
FIGURE 4.
TMP21-induced Aβ species are associated with the lipid bilayer. HEK293 cells were transiently transfected with the APPϵ vector in the presence or absence of wild-type TMP21. Empty vector (pcDNA3) was used as a negative control. Membrane and cytosolic soluble fractions were prepared and probed with anti-FLAG antibodies for TMP21 detection or immunoprecipitated (IP) using anti-Aβ 6E10 and analyzed for detection of intracellular Aβ species. Both TMP21 and Aβ positive fragments were embedded in the membrane. No TMP21 or Aβ-species were detected in the soluble fraction.
Taken together, these findings indicate that in the absence of any rate-limiting ϵ-cleavage, TMP21 has a unique effect on γ-secretase activity, resulting in the accumulation of intracellular membrane-embedded Aβ-related species. These results are reminiscent of recent reports describing an accumulation of intracellular longer Aβ species when γ-secretase activity is inhibited at the γ site but not at the ϵ site (31–33). TMP21-mediated γ-secretase inhibition was seen only with the APPϵ construct, and no significant level of membrane-embedded Aβ-reactive fragments was observed with the co-expression of APP-WT and TMP21. This suggest that one of the main targets for TMP21 may be the membrane-embedded Aβ fragment (residues 1–49) that is generated after release of the AICD cytoplasmic tail. In addition, this finding provides a reporter assay to investigate which domains are responsible for its activity.
Identification of TMP21 Functional Domains
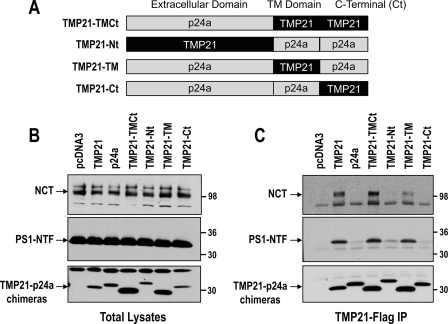
The TMP21-related and homologous cargo protein, p24a, was found to be inactive with respect to regulating γ-secretase activity (26). Therefore, p24a protein was used to create a series of chimera proteins to identify which domains of TMP21 modulate γ-secretase activity as seen in the APPϵ reporter assay. Chimeras are all FLAG-tagged and included substitution of the transmembrane (TM) and C terminus of TMP21 into p24a (TMP21-TMCt), replacement of the p24a N terminus with the corresponding extracellular domain of TMP21 (TMP21-Nt), replacement of p24a TM with TMP21 (TMP21-TM), and a final construct in which the TMP21 C terminus was inserted into full-length p24a (TMP21-Ct) (Fig. 5A).
FIGURE 5.
TMP21 interacted with PS-complex through its transmembrane domain. A, schematic representation of TMP21-p24a chimera FLAG-tagged proteins indicating the domains (extracellular, TM, and C terminus) of the p24a template, which were replaced with the corresponding TMP21 sequences. B, HEK293 cells were transiently transfected with TMP21, p24a, or chimera proteins. Membrane fractions were prepared and analyzed by Western blot for detection of NCT, PS1-NTF, and FLAG-tagged proteins. C, FLAG-tagged wild-type TMP21-p24a and chimeras were immunoprecipitated (IP) from membrane fractions and analyzed by immunoblotting for detection of NCT, PS1 N-terminal fragment (PS1-NTF), and the FLAG-tagged proteins. Note that only wild-type TMP21 and chimera FLAG proteins containing the transmembrane domain of TMP21 were associated with both NCT and PS1-NTF.
The chimeras as well as wild-type TMP21 and p24a were transiently expressed in HEK293 cells, and no effects were observed on the levels of γ-secretase components on the levels of nicastrin maturation or PS1 processing (Fig. 5B). The chimeras all expressed at reasonable levels, suggesting that they were not misfolded or rapidly degraded. Two of the constructs, TMP21-TMCt and TMP21-TM, were found at slightly higher levels as compared with wild-type TMP21 and may, therefore, be well tolerated by the cells. Co-immunoprecipitation studies were conducted for the FLAG-tagged proteins, which successfully pulled down all of the chimeras (Fig. 5C). FLAG-tagged wild-type TMP21 and p24a were used as controls, and as previously reported, TMP21 but not p24a interacts with mature NCT and the PS1-NTF (Fig. 5C). However, significant differences were observed between the chimeras. Association with mature NCT and the PS1-NTF were only found for constructs containing the TM domain of TMP21 (TMP21-TMCt and TMP21-TM; Fig. 5C). In contrast, no binding was observed for chimera containing the N- or C-terminal fragments of TMP21. These observations indicate that the transmembrane domain of TMP21 is essential for its physical interaction with the γ-secretase complex and may be the primary site of action.
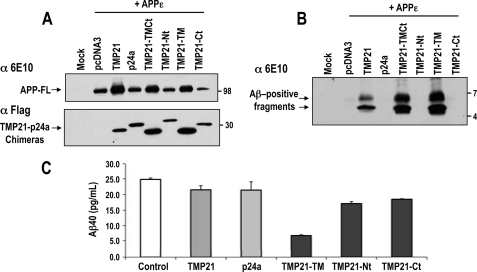
To investigate their functional aspects, TMP21 chimeras were co-transfected with APPϵ. Full-length APPϵ was found to be expressed at higher levels for cells co-transfected with wild-type TMP21 or the chimeras TMP21-TMCt or TMP21-TM, containing the transmembrane domain (Fig. 6A). This phenomenon may be the result of the effect of TMP21 on APP trafficking as noted previous (30). Examination of the intracellular Aβ-containing fragments by immunoprecipitation followed by Western blotting revealed that the two higher molecular weight species were only seen for the chimeras containing the TMP21 transmembrane domain (TMP21, TMP21-TMCt, and TMP21-TM, Fig. 6B). This is consistent with the observed interactions of the chimeras with PS1 and NCT which were dependent upon the TMP21 TM domain. The relative intensity of Aβ-positive fragments observed in cells expressing TMP21-TMCt and TMP21-TM chimeras was higher than for the wild-type TMP21. This could be because of slightly higher levels of the chimeras, as FLAG immunoreactivities were more pronounced (Fig. 6A). Alternatively, this may reflect the fact that the chimeric proteins were more readily or efficiently incorporated into the γ-secretase complexes. Only a small proportion of the full-length TMP21 is incorporated into complexes, and therefore, replacement, for example, of the ectodomain in the chimeras may result in a more accessible conformation for secretase binding.
FIGURE 6.
Aβ production and effects of individual TMP21 domains. A, HEK293 cells were transiently co-transfected with TMP21-FLAG and APPϵ. Membrane fraction were prepared and analyzed by Western blot for detection of APP holoprotein (APP-FL) and TMP21-FLAG proteins. B, intracellular Aβ species were immunoprecipitated using 6E10 antibody in HEK293 cells transiently co-transfected with TMP21-FLAG and APPϵ and subsequently probed with the 6E10 antibody. Only TMP21 and chimera FLAG proteins containing the transmembrane domain of TMP21 resulted in the accumulation of the membrane-embedded higher molecular weight Aβ-positive fragments. C, HEK293 cells were co-transfected with APPϵ and TMP1-p24a chimeras, and Aβ40 levels were determined by ELISA. No changes were observed for full-length TMP21 or p24 and chimeras containing the extracellular N terminus (TMP21-Nt) or intracellular C terminus (TMP21-Ct) of TMP21. Significant reduction was observed for cells expressing the chimera containing the TMP21 transmembrane domain (TMP21-TM).
Appearance of the Aβ-positive fragments suggests that they may result from a decreased processing of the APP-CTF. It would, therefore, be predicted that a corresponding decrease in Aβ secretion would be seen under these conditions. However, a more complex response was associated with TMP21 and the chimera proteins with respect to Aβ production. Overexpression of full-length wild-type TMP21 and APPϵ did not result in any significant change in Aβ production (Fig. 6C). Levels were comparable with controls as well as cell transfected with the p24a homolog. This is consistent with other studies in whole cells where high levels of TMP21 were also shown to not have an appreciable effect on Aβ levels (26, 27). Therefore, the Aβ-positive fragments must represent only a small percentage of the total Aβ. This could be because of a limited incorporation of full-length TMP21 into mature complexes. Similar issues with incorporation into functional complexes are seen for NCT, which is also not readily assembled into active complexes and accumulates as the immature species.
For the chimera proteins, expression of constructs containing the N-terminal ectodomain (TMP21-Nt) or the intracellular cytoplasmic tail (TMP21-Ct) did not result in any appreciable changes in Aβ production (Fig. 6C). In contrast, expression of the chimera containing the TMP21 transmembrane domain (TMP21-TM) with APPϵ reduced Aβ levels by ∼65% (Fig. 6C). The double-chimera TMP21-TMCt also reduced Aβ levels below that of the TMP21-Nt and TMP21-Ct constructs, consistent with their binding to PS1 and NCT (data not shown). The ability of the TMP21-TM-containing chimera was a consistent observation which suggests that the transmembrane domain has a more pronounced effect than full-length TMP21. The reason for this is unclear, but it is possible that the other domains (N and C termini) regulate other functions such as trafficking or binding to the γ-secretase complex. Therefore, replacement of these domains may result in a more potent activity of the TMP21 transmembrane region.
TM-containing chimeras binding to the γ-complex may represent one of their mechanisms of action. However, the chimeras also consistently increased the levels of full-length APPϵ precursor. This could provide another explanation for the accumulation of the Aβ-positive fragments and possibly changes in Aβ levels. Based upon these observations, either of the two functional aspects of TMP21, inhibition of γ-secretase cleavage or changes in APP metabolism, may be responsible for the alterations in Aβ-related processing (26, 27, 30). To try and dissect these two functions, direct interaction of the TMP21 transmembrane domain to the γ-secretase complex and its ability to alter cleavage were investigated using synthetic peptides in cell culture and cell-free assays.
Effects of a Synthetic Transmembrane Domain on Aβ Processing
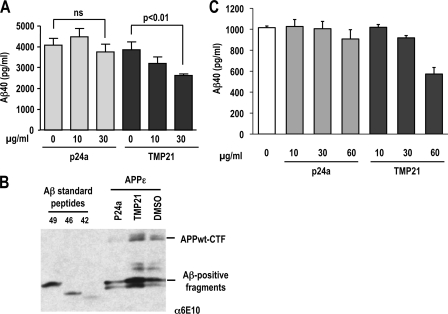
The TM domains of TMP21 and p24a were synthesized with three additional lysine residues attached at both the N and C termini to increase solubility. This approach has been used previously for investigations into the membrane insertion and structure of the APP transmembrane helix (34). The TMP21 TM peptide (KKK-VLYFSIFSMFCLIGLATWQVFYL-KKK) solubilized in DMSO was added at different concentrations to cells expressing the APP-Swedish variant, and Aβ40 secretion was measured in medium by ELISA analysis. After incubation for 8 h, cells exposed to 10 μg/ml (final media concentration) of the TMP21 peptide reduced Aβ40 by ∼17% (Fig. 7A). A dose response was observed at 30 μg/ml peptide which produced an ∼32% reduction in Aβ secretion. The corresponding p24a transmembrane peptide (KKK-VVLWSFFEALVLVAMTLGQIYYL-KKK) had no statistically significant effects at either 10 or 30 μg/ml (Fig. 7A). The reduction of Aβ levels was also not because of an inherently toxic effect of the TMP21 peptide that may have led to loss of production because of cell death. Viability assays indicated that no cell toxicity was associated with either the TMP21 or p24a peptides at the concentrations investigated (supplemental Fig. 1). The reduced Aβ40 trend for cells treated with the TMP21 peptide at longer conditioning time (e.g. 16 h) remained the same with comparable dose-dependent decreases for treatments with 10 and 30 μg/ml (data not shown). Treatment of cells expressing APPϵ with the TMP21-TM synthetic peptide demonstrated a similar increase in the antibody-positive fragments (Fig. 7B). These fragments were identical to those seen after transfection of the TMP21-TM construct or wild-type protein. No change in these antibody-positive fragments was observed after treatment with the comparable p24a-TM peptide or DMSO control (Fig. 7B). These observations suggest that, in addition to its effects on APP metabolism, TMP21 can directly alter γ-secretase activity. Given the specific ability of the synthetic TMP21 transmembrane domain to decrease Aβ40 production, it is likely that the peptide associates with the γ-secretase complex and reduces proteolysis. These observations are consistent with the effects of recombinant TMP21 on Aβ processing as shown previously (26).
FIGURE 7.
Synthetic transmembrane domain of TMP21 reduced Aβ40 secretion. A, HEK293 cells stably overexpressing APPsw were treated with the synthetic transmembrane domain of TMP21 or p24a (0, 10, 30 μg/ml), and levels of secreted Aβ40 peptide were analyzed by ELISA after 8 h of incubation. The synthetic peptide corresponding to the TMP21 transmembrane domain decreased Aβ40 processing and secretion in a dose-dependent manner. No significant effects were observed for the related p24a TM domain peptide. B, HEK293 cells were transfected with APPϵ, and 8 h before harvesting cells were treated with TMP21 or p24a transmembrane peptides (30 μg/ml) or a DMSO control. Intracellular Aβ species were immunoprecipitated and detected using 6E10 antibody. Treatment with the TMP21-TM peptide only resulted in the accumulation of Aβ-positive fragments. Synthetic Aβ standard peptides were used as molecular weight markers confirmed that the fragments had molecular weights corresponding to Aβ1–49 and related peptides. C, cell-free assays were conducted using membrane preparations from HEK293 cells with recombinant APP-C100 as the γ-secretase substrate. Synthetic peptides corresponding to transmembrane domains of TMP21 and p24a were added to final concentrations of 0, 10, 30, and 60 μg/ml to the reaction mixture.
Cell-free γ-secretase assays were also conducted in an effort to support these findings and investigate the activity of the synthetic TM peptides. Membrane fractions were prepared from HEK293 cells and incubated with the synthetic TM peptides at various concentrations. Recombinant C100 protein was added to the reaction mixture, and Aβ production was assayed by ELISA. Incubation of membranes with the TMP21 or p24a transmembrane peptides at 10 μg/ml did not have any detectable effect on Aβ levels (Fig. 7C). The TMP21 peptide had a modest effect at 30 μg/ml which resulted in approximately a 10% reduction in Aβ production (Fig. 7C). A more pronounced decrease in Aβ was observed for membranes incubated with 60 μg/ml of the TMP21 TM peptide which resulted in an ∼50% reduction in Aβ processing. This was specific for the TMP21 peptide, as no significant difference was observed for the corresponding p24a TM peptide (Fig. 7C).
Relatively higher concentrations of the TMP21 transmembrane peptide were required in the cell-free assay to produce the same decrease in Aβ processing as compared with the live cell cultures. This may be because of the fact that the level of the C100 substrate is much higher in the cell-free assay. Therefore, if the TMP21 binds to the γ-secretase complex as proposed, then the higher amount of substrate could compete for binding sites in the γ-complex. As a result, increased amounts of the TMP21 transmembrane peptide would be required to displace and/or compete with the C100 substrate.
Taken together, these observations have demonstrated that TMP21 expression has a unique effect on γ-secretase processing when examined in the absence of a possibly competing ϵ-mediated proteolysis. This was evident by the effects seen with the truncated APPϵ which led to the accumulation of Aβ-containing membrane-embedded fragments. Because the AICD sequence, which is lacking in APPϵ construct, contains the YENP motif, essential for APP internalization (35), it is possible to envisage that TMP21 acts on γ-secretase activity and upstream APP recycling and preferentially targets the membrane-embedded Aβ fragment released after the ϵ-cleavage. However, this remains speculative, and the exact site of the twin function of TMP21 in terms of γ-secretase modulation and APP metabolism has yet to be elucidated. The direct activity of TMP21 on γ-secretase appeared to be primarily mediated by its transmembrane domain. It is likely that specific residues are responsible for these effects, and further mutagenesis studies may shed more light on this issue. However, the ability of the TMP21 peptide to alter APP processing and Aβ production could represent a novel strategy for modulating amyloid production and its subsequent pathology.
Supplementary Material
This work was supported by grants from the Canadian Institutes of Health Research, Howard Hughes Medical Institute, Alzheimer Society of Ontario, The Ontario Research and Development Challenge Fund and the Canada Foundation for Innovation, the Fondation pour la Recherche Médicale, and the Conseil Général des Alpes Maritimes.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- Aβ
- amyloid β-peptide
- APP
- amyloid-β precursor protein
- AICD
- cytosolic amyloid intracellular domain
- PS
- presenilin
- TM
- transmembrane
- NCT
- nicastrin
- WT
- wild type
- CHAPSO
- 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonic acid
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- NTF
- N-terminal fragment
- Nt
- N terminus
- Ct
- C terminus
- ELISA
- enzyme-linked immunosorbent assay.
REFERENCES
- 1.Vardy E. R., Catto A. J., Hooper N. M. (2005) Trends Mol. Med. 11, 464–472 [DOI] [PubMed] [Google Scholar]
- 2.Weidemann A., Eggert S., Reinhard F. B., Vogel M., Paliga K., Baier G., Masters C. L., Beyreuther K., Evin G. (2002) Biochemistry 41, 2825–2835 [DOI] [PubMed] [Google Scholar]
- 3.Baek S. H., Ohgi K. A., Rose D. W., Koo E. H., Glass C. K., Rosenfeld M. G. (2002) Cell 110, 55–67 [DOI] [PubMed] [Google Scholar]
- 4.von Rotz R. C., Kohli B. M., Bosset J., Meier M., Suzuki T., Nitsch R. M., Konietzko U. (2004) J. Cell Sci. 117, 4435–4448 [DOI] [PubMed] [Google Scholar]
- 5.Kim H. S., Kim E. M., Lee J. P., Park C. H., Kim S., Seo J. H., Chang K. A., Yu E., Jeong S. J., Chong Y. H., Suh Y. H. (2003) FASEB J. 17, 1951–1953 [DOI] [PubMed] [Google Scholar]
- 6.Ryan K. A., Pimplikar S. W. (2005) J. Cell Biol. 171, 327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardossi-Piquard R., Petit A., Kawarai T., Sunyach C., Alves da Costa C., Vincent B., Ring S., D'Adamio L., Shen J., Müller U., St. George-Hyslop P., Checler F. (2005) Neuron 46, 541–554 [DOI] [PubMed] [Google Scholar]
- 8.Pardossi-Piquard R., Dunys J., Yu G., St. George-Hyslop P., Alves da Costa C., Checler F. (2006) J. Neurochem. 97, 1052–1056 [DOI] [PubMed] [Google Scholar]
- 9.Alves da Costa C., Sunyach C., Pardossi-Piquard R., Sévalle J., Vincent B., Boyer N., Kawarai T., Girardot N., St. George-Hyslop P., Checler F. (2006) J. Neurosci. 26, 6377–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y. W., Wang R., Liu Q., Zhang H., Liao F. F., Xu H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10613–10618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q., Zerbinatti C. V., Zhang J., Hoe H. S., Wang B., Cole S. L., Herz J., Muglia L., Bu G. (2007) Neuron 56, 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogaev E. I., Sherrington R., Rogaeva E. A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T., et al. (1995) Nature 376, 775–778 [DOI] [PubMed] [Google Scholar]
- 13.Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., et al. (1995) Nature 375, 754–760 [DOI] [PubMed] [Google Scholar]
- 14.Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., Song Y. Q., Rogaeva E., Chen F., Kawarai T., Supala A., Levesque L., Yu H., Yang D. S., Holmes E., Milman P., Liang Y., Zhang D. M., Xu D. H., Sato C., Rogaev E., Smith M., Janus C., Zhang Y., Aebersold R., Farrer L. S., Sorbi S., Bruni A., Fraser P., St. George-Hyslop P. (2000) Nature 407, 48–54 [DOI] [PubMed] [Google Scholar]
- 15.Francis R., McGrath G., Zhang J., Ruddy D. A., Sym M., Apfeld J., Nicoll M., Maxwell M., Hai B., Ellis M. C., Parks A. L., Xu W., Li J., Gurney M., Myers R. L., Himes C. S., Hiebsch R., Ruble C., Nye J. S., Curtis D. (2002) Dev. Cell 3, 85–97 [DOI] [PubMed] [Google Scholar]
- 16.De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. (1998) Nature 391, 387–390 [DOI] [PubMed] [Google Scholar]
- 17.Kimberly W. T., LaVoie M. J., Ostaszewski B. L., Ye W., Wolfe M. S., Selkoe D. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 6382–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takasugi N., Tomita T., Hayashi I., Tsuruoka M., Niimura M., Takahashi Y., Thinakaran G., Iwatsubo T. (2003) Nature 422, 438–441 [DOI] [PubMed] [Google Scholar]
- 19.Edbauer D., Winkler E., Regula J. T., Pesold B., Steiner H., Haass C. (2003) Nat. Cell Biol. 5, 486–488 [DOI] [PubMed] [Google Scholar]
- 20.Gu Y., Chen F., Sanjo N., Kawarai T., Hasegawa H., Duthie M., Li W., Ruan X., Luthra A., Mount H. T., Tandon A., Fraser P. E., St. George-Hyslop P. (2003) J. Biol. Chem. 278, 7374–7380 [DOI] [PubMed] [Google Scholar]
- 21.Gu Y., Sanjo N., Chen F., Hasegawa H., Petit A., Ruan X., Li W., Shier C., Kawarai T., Schmitt-Ulms G., Westaway D., St. George-Hyslop P., Fraser P. E. (2004) J. Biol. Chem. 279, 31329–31336 [DOI] [PubMed] [Google Scholar]
- 22.Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M., Hardy J., Levey A. I., Gandy S. E., Jenkins N. A., Copeland N. G., Price D. L., Sisodia S. S. (1996) Neuron 17, 181–190 [DOI] [PubMed] [Google Scholar]
- 23.Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Nature 398, 513–517 [DOI] [PubMed] [Google Scholar]
- 24.Chen F., Yu G., Arawaka S., Nishimura M., Kawarai T., Yu H., Tandon A., Supala A., Song Y. Q., Rogaeva E., Milman P., Sato C., Yu C., Janus C., Lee J., Song L., Zhang L., Fraser P. E., St. George-Hyslop P. H. (2001) Nat. Cell Biol. 3, 751–754 [DOI] [PubMed] [Google Scholar]
- 25.Shah S., Lee S. F., Tabuchi K., Hao Y. H., Yu C., LaPlant Q., Ball H., Dann C. E., 3rd, Südhof T., Yu G. (2005) Cell 122, 435–447 [DOI] [PubMed] [Google Scholar]
- 26.Chen F., Hasegawa H., Schmitt-Ulms G., Kawarai T., Bohm C., Katayama T., Gu Y., Sanjo N., Glista M., Rogaeva E., Wakutani Y., Pardossi-Piquard R., Ruan X., Tandon A., Checler F., Marambaud P., Hansen K., Westaway D., St. George-Hyslop P., Fraser P. (2006) Nature 440, 1208–1212 [DOI] [PubMed] [Google Scholar]
- 27.Dolcini V., Dunys J., Sevalle J., Chen F., Guillot-Sestier M. V., St. George-Hyslop P., Fraser P. E., Checler F. (2008) Biochem. Biophys. Res. Commun. 371, 69–74 [DOI] [PubMed] [Google Scholar]
- 28.Lefranc-Jullien S., Sunyach C., Checler F. (2006) J. Neurochem. 97, 807–817 [DOI] [PubMed] [Google Scholar]
- 29.Blum R., Feick P., Puype M., Vandekerckhove J., Klengel R., Nastainczyk W., Schulz I. (1996) J. Biol. Chem. 271, 17183–17189 [DOI] [PubMed] [Google Scholar]
- 30.Vetrivel K. S., Gong P., Bowen J. W., Cheng H., Chen Y., Carter M., Nguyen P. D., Placanica L., Wieland F. T., Li Y. M., Kounnas M. Z., Thinakaran G. (2007) Mol. Neurodegener. 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi-Takahara Y., Morishima-Kawashima M., Tanimura Y., Dolios G., Hirotani N., Horikoshi Y., Kametani F., Maeda M., Saido T. C., Wang R., Ihara Y. (2005) J. Neurosci. 25, 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren Z., Schenk D., Basi G. S., Shapiro I. P. (2007) J. Biol. Chem. 282, 35350–35360 [DOI] [PubMed] [Google Scholar]
- 33.Zhao G., Mao G., Tan J., Dong Y., Cui M. Z., Kim S. H., Xu X. (2004) J. Biol. Chem. 279, 50647–50650 [DOI] [PubMed] [Google Scholar]
- 34.Gorman P. M., Kim S., Guo M., Melnyk R. A., McLaurin J., Fraser P. E., Bowie J. U., Chakrabartty A. (2008) BMC Neurosci. 9, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King G. D., Scott Turner R. (2004) Exp. Neurol. 185, 208–219 [DOI] [PubMed] [Google Scholar]
- 36.Grandori R., Struck K., Giovanielli K., Carey J. (1997) Protein Eng. 10, 1099–1100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.