Abstract
ADP-ribosylation is one of the favored modes of cell intoxication employed by several bacteria. Clostridium difficile is recognized to be an important nosocomial pathogen associated with considerable morbidity and attributable mortality. Along with its two well known toxins, Toxin A and Toxin B, it produces an ADP-ribosylating toxin that targets monomeric actin of the target cell. Like other Clostridial actin ADP-ribosylating toxins, this binary toxin, known as C. difficile toxin (CDT), is composed of two subunits, CDTa and CDTb. In this study, we present high resolution crystal structures of CDTa in its native form (at pH 4.0, 8.5, and 9.0) and in complex with ADP-ribose donors, NAD and NADPH (at pH 9.0). The crystal structures of the native protein show “pronounced conformational flexibility” confined to the active site region of the protein and “enhanced” disorder at low pH, whereas the complex structures highlight significant differences in “ligand specificity” compared with the enzymatic subunit of a close homologue, Clostridium perfringens iota toxin. Specifically in CDTa, two of the suggested catalytically important residues (Glu-385 and Glu-387) seem to play no role or a less important role in ligand binding. These structural data provide the first detailed information on protein-donor substrate complex stabilization in CDTa, which may have implications in understanding CDT recognition.
Clostridium difficile infection is a major problem as a healthcare-associated infection. The bacterium causes nosocomial, antibiotic-associated diarrhea and pseudomembranous colitis in patients treated with broad spectrum antibiotics (1–3). Elderly patients are most at risk from these potentially life-threatening diseases, and incidents of hospital infection have increased dramatically over the last 10 years.
Strains of C. difficile produce a variety of virulence factors, notable among which are several protein toxins: Toxin A, Toxin B (4–6), and, in some strains, the binary toxin CDT,3 which is similar to Clostridium perfringens iota toxin and Clostridium botulinum C2 toxin (7–9). Toxins A and B are large protein cytotoxins that play a key role in the pathology of infection and most probably are involved in the gut colonization process. Outbreaks of C. difficile infection have been reported with Toxin A-negative/Toxin B-positive strains, and a recent report (10) suggests that Toxin B plays a major role in the disease pathology. Little is presently known about the contribution of the binary toxin to C. difficile infection.
CDT binary toxin belongs to the family of actin-specific ADP-ribosylating toxin (ADPRT) (for a recent review see Ref. 11), composed of two independently produced components: a transport component of 99 kDa (CDTb) that facilitates translocation of the enzymatic component of 49 kDa (CDTa) into the target cell that is capable of transferring ADP-ribose group of NAD/NADPH to monomeric actin molecules in target cells (9, 12, 13). This irreversible modification of G-actin at Arg-177 (8, 14) blocks its polymerization and thus formation of the polymeric F-actin, which results in disruption of crucial F-actin-G-actin equilibrium in the cell. This leads to a collapse of cell cytoskeleton and subsequently results in excessive fluid loss from the cell (15), rounding of the cell (16), increased vascular permeability (17), and finally cell death.
Little is known about CDT structure, cellular receptor, and mechanism of cell entry. To provide a structural basis of the understanding of CDT function, we have embarked on the structural analysis of CDT components. Such information would be invaluable for the rational design of therapeutic strategies. As a first step, we have determined high resolution crystal structures of recombinant CDTa in native form (at different pH states, named CDTa-4.0, -8.5, and -9.0) as well as in complex with ADP-ribose donors NAD and NADPH (at pH 9.0). For comparison purposes we make use of native CDTa structure at pH 9.0 (CDTa-9.0). Here we report the detailed molecular interactions underlying the mode of recognition of its substrate and the conformational flexibility exhibited by CDTa.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
The details of cloning, expression, and purification of the two individual components of CDT (i.e. CDTa and CDTb) will be described in detail elsewhere.4 Briefly, the cDNA clone of CDTa was purchased from GENEART (Germany). The DNA sequence coding for active CDTa (45-kDa protein without the signal peptide) was PCR-amplified and cloned in pMAL-P2X vector. Isopropyl β-d-thiogalactoside (Melford)-induced expression of maltose-binding protein-CDTa fusion protein in Escherichia coli was carried out in Terrific Broth medium with 1× Terrific Broth salts, 0.5% glucose, and 100 μg/ml ampicillin at 16 °C for 4 h. Cells were lysed in buffer A (20 mm NaCl and 5 mm CaCl2 in 50 mm Tris-HCl, pH 8.0) using a French press, and cell debris was removed by centrifugation. Clear supernatant was loaded on a Q-Sepharose anion exchange column equilibrated with buffer A, and bound protein was eluted from the column with 10% of buffer B (1 m NaCl and 5 mm CaCl2 in 50 mm Tris-HCl, pH 8.0) in buffer A. MBP tag was cleaved by factor Xa (Novagen)-mediated proteolytic cleavage at 20 °C. Tag cleaved protein was dialyzed against buffer C (20 mm NaCl in 50 mm Tris-HCl, pH 8.0) and again passed through a Q-Sepharose column equilibrated with buffer C. Purified CDTa was collected in column flow through. Purified CDTa protein was concentrated to 10 mg/ml and stored at −80 °C.
X-ray Crystallography
A total of 480 different crystallization conditions were screened with the help of Phoenix protein crystallization robot (Art Robbins Instruments) in sitting drop vapor diffusion method at 16 °C. After optimization, the final diffraction quality crystals of native CDTa were grown in three different conditions (Table 1) by streak seeding the drops. Reservoir solution was added to equal volume of the protein at 4 mg/ml concentration and allowed to equilibrate for 60–90 min at 16 °C. Equilibrated drops were streak seeded with thin plate crystals grown previously under identical conditions. To grow CDTa crystals in complex with NAD and NADPH, 100 mm of ligand solution was added to protein at 5 mg/ml and diluted with concentration buffer (20 mm NaCl in 50 mm Tris-HCl, pH 8.0) in such a manner that the final concentration of ligand was 10 mm and that of protein was 4 mg/ml. Crystallization trials were set up under conditions containing 20% polyethylene glycol 1500 in 0.1 m malonate imidazole boric acid buffer pH 9.0 (Table 1) by streak seeding as described for native CDTa above.
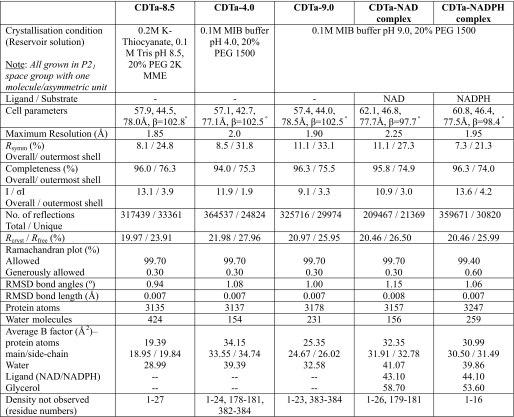
TABLE 1.
X-ray crystallographic data
Rsymm = ΣhΣi[|Ii(h) − <I(h)>|/ΣhΣiIi(h)], where Ii is the ith measurement, and <I(h)> is the weighted mean of all measurements of I(h). Rcryst = Σh|Fo − Fc|/ΣhFo, where Fo and Fc are the observed and calculated structure factor amplitudes of reflection h, respectively. Rfree is equal to Rcryst for a randomly selected 5–10% of reflections not used in the refinement.
All of the high resolution diffraction data sets were collected at Diamond Light Source (Oxon, UK) on stations IO2 and IO3. No cryoprotectant was used for the native crystals (at pH 4.0, 8.5, and 9.0), whereas 20% glycerol was used for CDTa-NAD and CDTa-NADPH complex crystals (Table 1). For each data set 200 images were collected by using a Quantum 4 CCD detetector (ADSC Systems). Raw data images were indexed and scaled with the HKL2000 software package (18). All of the crystals belonged to P21 space group with one molecule of CDTa per asymmetric unit. Data reduction was carried out by using the CCP4 program TRUNCATE (19) (Table 1).
Initial phases for structure solution were obtained using the molecular replacement routines of the MOLREP program (20). For CDTa-8.5, the search model was derived from the coordinates of enzymatic component of iota toxin (21) (Protein Data Bank code 1GIQ). The resultant models were refined using REFMAC5 (22). Five percent of reflections were separated as Rfree set and used for cross-validation (23). After an initial round of rigid body refinement, rounds of restrained refinement with electron density map calculations and manual adjustments of model using COOT (24) were carried out. On the basis of Fo − Fc electron density, side chain atoms were omitted at some positions. Water molecules were added at positions where Fo − Fc electron density peaks exceeded 3 σ and potential hydrogen bonds could be made. Detailed refinement statistics for all of the structures are given in Table 1. A similar approach was adopted to solve other structures using the refined CDTa-8.5 structure as the starting model. Based on electron density interpretation, NAD and NADPH ligands and one glycerol molecule (from the cryoprotectant) were added in their respective complex structures, and further refinement was carried out. Model validation was conducted with the aid of programs PROCHECK (25) and MOLPROBITY (26). There were no residues in the disallowed region of the Ramachandran plot. The figures were drawn with PyMOL (DeLano Scientific, San Carlos, CA). The hydrogen bonds were verified with the program HBPLUS (27).
RESULTS AND DISCUSSION
Overall Structure
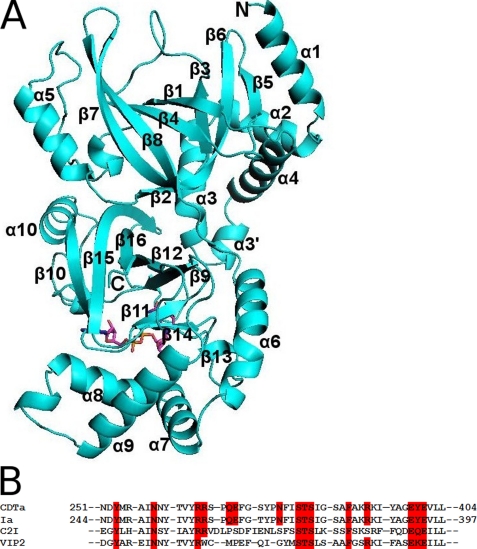
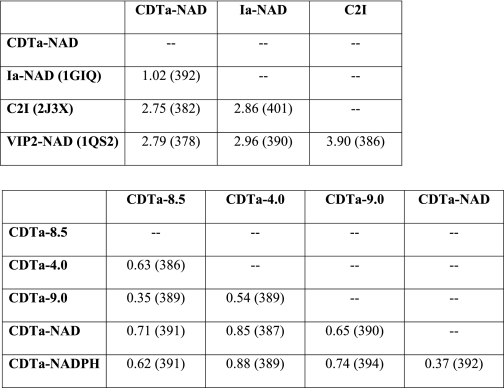
The C. difficile actin-ADPRT CDTa ADP-ribosylates all three isoforms of actin and shares 84% sequence identity with the enzymatic component of C. perfringens iota toxin (Ia) (the closest homologue) and 40% with the enzymatic component of C. botulinum C2 toxin. The high degree of sequence conservation (Fig. 1B) is reflected at the structural level; when comparing the three-dimensional structure of CDTa (present study, Fig. 1A) with those of previously reported crystallographic results on Ia (21), C. botulinum C2 toxin (28), and ADPRT component, VIP2 (vegetative insecticidal protein) (29) (Table 2), all of them are composed of two mixed α/β globular domains. For comparison purposes we restrict our discussion on CDTa with Ia.
FIGURE 1.
A, ribbon representation of CDTa along with the secondary structure details. Bound NAD is shown in stick representation. B, amino acid sequence alignment of residues at the catalytic site for CDTa, Ia, C. botulinum C2 toxin (C2I), and VIP2.
TABLE 2.
Structural comparison of CDTa with known homologues (top) and structural comparison of different CDTa (bottom, present study)
The root mean square deviation values are shown in Å. The aligned length of the protein (number of Cα atoms) is shown in parentheses.
In CDTa, the N-terminal domain extends from residues 1 to 215, whereas the C-terminal domain is from residues 224–420. The two domains of the protein are linked by a loop that stretches from residue 216 to 223. As in other actin-ADPRTs, both domains adopt similar fold despite very low sequence identity between them. The N-domain consists of five α-helices and eight β-strands and is believed to interact with its translocation partner, i.e. CDTb. The C-domain of the protein also comprises five helices and eight strands and accommodates CDTa catalytic machinery (Fig. 1A) (following the secondary structure assignment as in Ia). The N- and C-domains are arranged almost perpendicular to each other but facing their clefts toward the same face. The active site is located in the cleft of C-domain (Fig. 1A). A few of the N-terminal residues of CDTa were found to be highly disordered (Table 1). The overall structure matches extremely well with that of Ia, except the ADP-ribosyl turn-turn (ARTT) loop (Table 2 and Fig. 2).
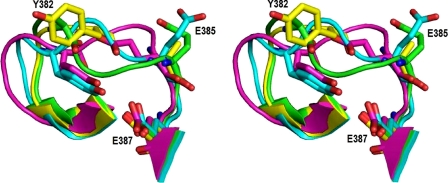
FIGURE 2.
Stereo view of ARTT loop in CDTa (native and NAD-bound form) and Ia (NAD-bound form). Green, CDTa-8.5; yellow, CDTa-9.0; cyan, CDTa-NAD; magenta, Ia-NAD. Residue numbering is according to CDTa (present structure). The corresponding residues in Ia are Tyr-375, Glu-378, and Glu-380.
Catalytic Site Cleft and Binding of NAD and NADPH
Amino acid residues that have been suggested essential for the ADP-ribosylating activity of Ia (Arg-295, Arg-296, Arg-352, Gln-300, Asn-335, Glu-378, and Glu-380) (30) are well conserved in CDTa (Arg-302, Arg-303, Arg-359, Gln-307, Asn-342, Glu-385, and Glu-387). Our present structural analysis has shown that both NAD and NADPH bind to the catalytic cleft of CDTa in a “closed conformation,” interacting with residues Arg-302, Arg-303, Arg-359, Gln-307, Asn-342, and Ser-345 (Fig. 3). This is in analogous to the structural observations made with Ia with the NAD molecule interacting with residues Glu-380, Arg-295, Arg-296, Gln-300, Arg-352, and Asn-335 (21) (Fig. 3B). Based on these observations it is interesting to note that in CDTa, Glu-387 (Glu-380 in Ia) does not seem to interact with either NAD or NADPH. However Ser-345 in CDTa seems to be an important residue in the catalytic site and makes direct interactions with the ligand in both NAD and NADPH complex structures. These observations point out that even between these two close homologues (CDTa and Ia), the mode of ligand recognition is significantly different (Table 3).
FIGURE 3.
A, active site cleft of CDTa. Green, CDTa-9.0; cyan, CDTa-NAD; magenta, CDTa-NADPH complex. B, NAD binding to CDTa (top panel) and Ia (bottom panel). On the left-hand side, a schematic representation of hydrogen bonding pattern is shown. On the right-hand side, surface representations of these residues are shown. Red, Arg; green, Gln; orange, Glu; blue, Ser; cyan, Asn.
TABLE 3.
Potential hydrogen bond contacts with the ligands in CDTa
| Bonded residues (atoms) | CDTa-NAD |
CDTa-NADPH |
||
|---|---|---|---|---|
| Length | Angle | Length | Angle | |
| Å | ° | Å | ° | |
| Arg-302 (NH1)–NADPH (O1A) | 3.38 | 149.2 | ||
| Arg-302 (NH2)–NAD/NADPH (O1A) | 2.92 | 158.7 | 3.34 | 151.5 |
| Arg-303 (N)–NAD/NADPH (O7N) | 2.67 | 150.9 | 2.68 | 161.8 |
| Arg-303 (O)–NAD/NADPH (N7N) | 3.09 | 3.08 | ||
| Ser-345 (OG)–NAD/NADPH (O2D) | 3.12 | 139.1 | 3.0 | 148.9 |
| Asn-342 (OD1)–NAD/NADPH (N6A) | 3.06 | 2.98 | ||
| Arg-359 (NH1)–NAD/NADPH (O1N) | 2.71 | 164.1 | 2.40 | 154 |
| Arg-359 (NH2)–NAD/NADPH (O2N) | 2.82 | 154.0 | 2.89 | 127.8 |
| Gln-307 (N)–NAD/NADPH (O3X) | 2.48 | 160.9 | ||
| Gln-307 (NE2)–NAD (O3B)/NADPH (O1X) | 2.60 | 94.1 | 2.71 | 103.5 |
Ligand Binding and the ARTT Loop
It has been well established that in ADPRTs the ARTT loop is important for substrate binding and ADP-ribosylation even though the length of the loop varies among these molecules (for a review see Ref. 13). In CDTa, this loop (connecting strands β13 and β14) spans residues 377–387 and consists of two sharp turns (in Ia between residues 370–380) (21). The ARTT loop is associated with significant disorder and high conformational flexibility in all the three native structures of CDTa as observed from their electron density maps. However, upon ligand (NAD/NADPH) binding, the loop adopts a highly ordered structure (Fig. 2) associated with some critical changes in the orientation of side chains in the catalytic site when compared with Ia (see below).
The side chain of Tyr-382 (a conserved critical aromatic residue known to be important in ADPRTs) in the ARTT loop was disordered in CDTa-8.5 and CDTa-4.0 structures. However, clear electron density was visible in CDTa-9.0, CDTa-NAD, and CDTa-NADPH structures, and it adopts different orientation in the native and ligand-bound forms (Fig. 3A). In the ligand-bound structures, Tyr-382 is pointing inwards making a hydrogen bond interaction with Glu-387, an important catalytic site residue. Glu-385 adopts different orientation with respect to the corresponding residue Glu-378 in Ia that has been suggested to be crucial for transfer of ADP-ribose and has been shown to interact with Arg-177 of actin (31).
EXE and STS Motifs
In CDTa, residues Glu-385 and Glu-387 (that correspond to Glu-378 and Glu-380 in Ia) belong to EXE motif (part of the ARTT loop). Based on mutagenesis studies on Ia, it has been established that this motif has a definitive role in stabilizing substrate-enzyme complexes and catalysis (32). This motif is well ordered in all five CDTa structures and adopts a significantly different conformation from that observed by Tsuge et al. (21) in Ia. Glu-380 has been shown to interact directly with NAD in Ia-NAD complex, whereas Glu-378 and Glu-380 are at hydrogen bonding distance in Ia-NADPH complex structure (21). In CDTa, however, the structurally equivalent Glu residues (Glu-385 and Glu-387) are not involved in direct interaction with either NAD or NADPH. In addition, Glu-385 (corresponding to Glu-378 in Ia) adopts different orientations in CDTa. In Ia, the side chain of Glu-378 points toward the ligand binding cleft, whereas in all five CDTa structures presented here it points away from the cleft (Fig. 3A). This suggests that in CDTa, the EXE motif is perhaps not necessary for ligand binding and stabilization of the complex.
Amino acid residues Ser-345, Thr-346, and Ser-347 together constitute the STS motif in CDTa. The corresponding residues in Ia are Ser-338, Thr-339, and Ser-340. Previous mutational data on Ia showed that replacement of Ser-338 to Ala or Cys did not result in the complete loss of activity, suggesting that the hydroxyl group of Ser-338 is not essential for catalytic activity (32). However, its replacement to amino acids with larger side chains such as Phe results in complete loss of ADPase activity. Ser-345 in CDTa occupies the equivalent position of Ser-338 in Ia situated very close to active site cleft. Based on structural observation, it is clear that (as in Ia) replacement of Ser-345 with a larger residue would abrogate substrate binding. Furthermore in all CDTa structures, Ser-345 and Glu-387 form a strong hydrogen bond (2.4–2.7 Å), and Ser-345 makes a direct hydrogen bond with both NAD and NADPH in their respective complex structures. This is a significant difference observed based on the structural data from Ia, where Glu-380 makes direct interaction with the ligand rather than Ser-338. Based on these structural results, it is tempting to suggest that Ser-345 in CDTa appears to have a crucial role in ligand binding and perhaps in catalysis as speculated by Tsuge et al. (21).
Contribution from Other Important Residues
Glu-301 Tyr-246, Asn-255, and Phe-349 have also been suggested to play an important role in enzymatic activity of Ia (21). In CDTa Glu-308 (Glu-301 in Ia) does not seem to participate in the ligand binding directly but stays close to Arg-302 (Arg-295 in Ia), which interacts with the ligand directly (Fig. 3A). Replacement of Glu-301 to Ala in Ia resulted in complete loss of NADase and ARTase activity of enzyme (32). Our structural analysis shows that Glu-308 holds Arg-302 in position by hydrogen bonding to form an optimal interaction with the ligand. A similar role can be attributed for residues Tyr-253 and Asn-262 stabilized by a hydrogen bond (Tyr-246 and Asn-255 in Ia). Asn-262 further restricts the movement of Asn-342 (Asn-335 in Ia) and places Asn-342 optimally for interaction with NAD/NADPH. Phe-356 (Phe-349 in Ia) adopts similar orientation in all five CDTa structures. The side chain is relatively mobile in the three native structures. In the ligand-bound structures its orientation is rearranged and provides stacking interactions against the nicotinamide ring (Fig. 3A). This is further stabilized by Arg-303 through hydrogen bonding (similar observations were made with Ia). This network of interactions facilitates tight binding of the ligand at the active site.
pH Induced Catalytic Site Flexibility
To understand the active site flexibility in CDTa, the native structures were determined at three different pH levels: 4.0, 8.5, and 9.0. The crystallization conditions at pH 4.0 and 9.0 were identical (Table 1). All three structures superimpose well with an root mean square deviation of 0.63 Å (Table 2). However, clear “conformational flexibility” was observed among these structures in the active site. This was confined to the ARTT loop (between strands β13 and β14) and the loop between strand β9 and helix α10 (loop 304) (Fig. 4). This flexibility was more pronounced in the CDTa-4.0 structure, consistent with the analysis of crystallographic temperature factors, which provides an opportunity to obtain a relatively unbiased picture of the mobility of different parts of the structure. Indeed these regions adopt a more stable structure at a higher pH (e.g. 8.5 and 9.0) and NAD/NADPH complex structures of CDTa. Although this region is clearly influenced by the conditions required to obtain crystals, the innate flexibility may be important in translocation of enzymatic component (CDTa) into cytosol via receptor-mediated early endosomal pathway.
FIGURE 4.
A, orientation of ARTT loop in CDTa (native and NAD bound form). B, orientation of loop 304, which shows differences between CDTa-4 and other structures. Green, CDTa-8.5; yellow, CDTa9.0; magenta, CDTa-4.0; cyan, CDTa-NAD.
Mechanistic Implications
Currently available structural and biochemical data on ADPRTs, i.e. conservation of catalytic site apparatus and NAD binding, suggest a common catalytic mechanism, either SN1or SN2 type reaction. In the case of Ia, Arg-177 of actin or a water molecule has been suggested to act as nucleophile in a SN2 mechanism. In the structure of the Ia-actin complex, it has been shown that Arg-177 of actin is positioned at a considerably long distance (8.0 Å) from nicotinamide ring of NAD or Glu-378 (31), eliminating the possibility of a direct nucleophilic attack by Arg-177. It was suggested that a water molecule that is present near the nicotinamide mononucleotide ring (∼4.0 Å) could be a possible nucleophile. However, this water molecule could be modeled only in one of the two molecules in the asymmetric unit with a high temperature factor (21). In the case of CDTa in complex with either NAD or NADPH, there are at least two water molecules with reasonably good temperature factors near the ring. One of the water molecules seems to be important because it bridges NAD, Ser-345 and Tyr-253 in the complex. However, the nearest water molecule to the reaction center (C1D of NAD/NADPH) is at a distance of 5.45 and 4.25 Å away in the NAD and NADPH complexes, respectively, and makes the SN2 mechanism less preferred.
In the case of Actin-ADPRTs, the SN1 reaction would involve an isolated oxocarbenium intermediate stabilization in a pentacoordinate state with direct stabilizing electrostatic interactions from the catalytic glutamate with serine hydroxyl group. In Ia, the SN1 reaction mechanism has been proposed via two reaction intermediates where rotation of oxocarbanium ion has been suggested (31). In the Ia-actin complex structure, loop II of Ia (between α7 and α8) undergoes significant conformational changes, and Gly-249 directly interacts with Arg-177 of actin. These changes in the loop rearrange Tyr-246 and Tyr-251. Previous mutational studies of both of these residues have been shown to have adverse effects on NADase as well as ARTase activity (21). In CDTa, a similar SN1 mechanism could be followed. Based on the structural data, it is clear that Ser-345 interacts with both of the ligands directly surrounded by Glu-387, Tyr-353, and Tyr-358 (corresponding to Gly-380, Tyr-346, and Tyr-351 in Ia). We propose that in CDTa it is Ser-345 that stabilizes the oxocarbenium ion and facilitates its transfer to Tyr-358 following rotation. In addition our structural data show that the ARTT loop is not directly involved in ligand binding in the CDTa-NAD and CDTa-NADPH complexes and is free to rearrange itself further. It is tempting to suggest that once NAD is cleaved followed by the formation of oxocarbenium ion, further rearrangement of the ARTT loop could not be ruled out considering its high flexibility and presence of a large open cavity near the active site cleft (as observed in Ia-actin complex, 31). This could bring Glu-385 of CDTa into the reaction center to proceed with the transfer of ADP-ribose to Arg-177 of actin. However, this hypothesis must wait for further direct structural evidence of CDTa in complex with actin.
Acknowledgments
We thank the scientists at stations IO2 and IO3 of Diamond Light Source (Oxon, United Kingdom) and Nethaji Thiyagarajan for support during x-ray diffraction data collection.
The atomic coordinates and structure factors (codes 2wn4, 2wn5, 2wn6, 2wn7, and 2wn8) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
A. Sundriyal, A. K. Roberts, R. Ling, J. McGlashan, C. C. Shone, and K. R. Acharya, unpublished results.
- CDT
- C. difficile toxin
- CDTa
- C. difficile toxin domain a
- CDTb
- C. difficile toxin domain b
- Ia
- C. perfringens iota toxin domain a
- ADPRT
- ADP-ribosylating toxin
- ARTT
- ADP-ribosyl turn-turn.
REFERENCES
- 1.Lyerly D. M., Krivan H. C., Wilkins T. D. (1988) Clin. Microbiol. Rev. 1, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McFarland L. V., Stamm W. E. (1986) Am. J. Infect. Control. 14, 99–109 [DOI] [PubMed] [Google Scholar]
- 3.Knoop F. C., Owens M., Crocker I. C. (1993) Clin. Microbiol. Rev. 6, 251–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. (1985) Infect. Immun. 47, 349–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Just I., Selzer J., Wilm M., von Eichel-Streiber C., Mann M., Aktories K. (1995) Nature 375, 500–503 [DOI] [PubMed] [Google Scholar]
- 6.Voth D. E., Ballard J. D. (2005) Clin. Microbiol. Rev. 18, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. (1986) Nature 322, 390–392 [DOI] [PubMed] [Google Scholar]
- 8.Vandekerckhove J., Schering B., Bärmann M., Aktories K. (1987) FEBS Lett. 225, 48–52 [DOI] [PubMed] [Google Scholar]
- 9.Barth H., Aktories K., Popoff M. R., Stiles B. G. (2004) Microbiol. Mol. Biol. Rev. 68, 373–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyras D., O'Connor J. R., Howarth P. M., Sambol S. P., Carter G. P., Phumoonna T., Poon R., Adams V., Vedantam G., Johnson S., Gerding D. N., Rood J. I. (2009) Nature 458, 1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Q., Barbieri J. T. (2008) Annu Rev. Microbiol. 62, 271–288 [DOI] [PubMed] [Google Scholar]
- 12.Popoff M. R., Rubin E. J., Gill D. M., Boquet P. (1988) Infect. Immun. 56, 2299–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holbourn K. P., Shone C. C., Acharya K. R. (2006) FEBS J. 273, 4579–4593 [DOI] [PubMed] [Google Scholar]
- 14.Mauss S., Chaponnier C., Just I., Aktories K., Gabbiani G. (1990) Eur. J. Biochem. 194, 237–241 [DOI] [PubMed] [Google Scholar]
- 15.Simpson L. L. (1982) J. Pharmacol. Exp. Ther. 223, 695–701 [PubMed] [Google Scholar]
- 16.Reuner K. H., Presek P., Boschek C. B., Aktories K. (1987) Eur. J. Cell Biol. 43, 134–140 [PubMed] [Google Scholar]
- 17.Ohishi I. (1983) Infect. Immun. 40, 336–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 19.The CCP4 Suite: Programs for Protein Crystallography (1994) Acta Crystallogr. Sect. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 20.Vagin A., Teplyakov A. (1997) J. Appl. Crystallogr. 30, 1022–1025 [Google Scholar]
- 21.Tsuge H., Nagahama M., Nishimura H., Hisatsune J., Sakaguchi Y., Itogawa Y., Katunuma N., Sakurai J. (2003) J. Mol. Biol. 325, 471–483 [DOI] [PubMed] [Google Scholar]
- 22.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. Sect. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 23.Brünger A. T. (1992) Nature 355, 472–475 [DOI] [PubMed] [Google Scholar]
- 24.Emsley P., Cowtan K. (2004) Acta Crystallogr. Sect. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 25.Laskowski A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 26.Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) Nucleic Acids Res. 35, 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald I. K., Thornton J. M. (1994) J. Mol. Biol. 238, 777–793 [DOI] [PubMed] [Google Scholar]
- 28.Schleberger C., Hochmann H., Barth H., Aktories K., Schulz G. E. (2006) J. Mol. Biol. 364, 705–715 [DOI] [PubMed] [Google Scholar]
- 29.Han S., Craig J. A., Putnam C. D., Carozzi N. B., Tainer J. A. (1999) Nat. Struct. Biol. 6, 932–936 [DOI] [PubMed] [Google Scholar]
- 30.van Damme J., Jung M., Hofmann F., Just I., Vandekerckhove J., Aktories K. (1996) FEBS Lett. 380, 291–295 [DOI] [PubMed] [Google Scholar]
- 31.Tsuge H., Nagahama M., Oda M., Iwamoto S., Utsunomiya H., Marquez V. E., Katunuma N., Nishizawa M., Sakurai J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7399–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagahama M., Sakaguchi Y., Kobayashi K., Ochi S., Sakurai J. (2000) J. Bacteriol. 182, 2096–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]