Abstract
4-Hydroxy-2-nonenal (HNE), a major racemic product of lipid peroxidation, preferentially reacts with cysteine residues to form a stable HNE-cysteine Michael addition adduct possessing three chiral centers. Here, to gain more insight into sulfhydryl modification by HNE, we characterized the stereochemical configuration of the HNE-cysteine adducts and investigated their stereoselective formation in redox-regulated proteins. To characterize the HNE-cysteine adducts by NMR, the authentic (R)-HNE- and (S)-HNE-cysteine adducts were prepared by incubating N-acetylcysteine with each HNE enantiomer, both of which provided two peaks in reversed-phase high performance liquid chromatography (HPLC). The NMR analysis revealed that each peak was a mixture of anomeric isomers. In addition, mutarotation at the anomeric center was also observed in the analysis of the nuclear Overhauser effect. To analyze these adducts in proteins, we adapted a pyridylamination-based approach, using 2-aminopyridine in the presence of sodium cyanoborohydride, which enabled analyzing the individual (R)-HNE- and (S)-HNE-cysteine adducts by reversed-phase HPLC following acid hydrolysis. Using the pyridylamination method along with mass spectrometry, we characterized the stereoselective formation of the HNE-cysteine adducts in human thioredoxin and found that HNE preferentially modifies Cys73 and, to the lesser extent, the active site Cys32. More interestingly, the (R)-HNE- and (S)-HNE-cysteine adducts were almost equally formed at Cys73, whereas Cys32 exhibited a remarkable preference for the adduct formation with (R)-HNE. Finally, the utility of the method for the determination of the HNE-cysteine adducts was confirmed by an in vitro study using HeLa cells. The present results not only offer structural insight into sulfhydryl modification by lipid peroxidation products but also provide a platform for the chemical analysis of protein S-associated aldehydes in vitro and in vivo.
Lipid peroxidation in tissue and in tissue fractions represents a degradative process, which is the consequence of the production and the propagation of free radical reactions primarily involving membrane polyunsaturated fatty acids and has been implicated in the pathogenesis of numerous diseases, including atherosclerosis, diabetes, cancer, and rheumatoid arthritis, as well as in drug-associated toxicity, post-ischemic reoxygenation injury, and aging (1). The peroxidative breakdown of polyunsaturated fatty acids has also been implicated in the pathogenesis of many types of liver injury and especially in the hepatic damage induced by several toxic substances. Lipid peroxidation leads to the formation of a broad array of different products with diverse and powerful biological activities. Among them is a variety of different aldehydes (2). The primary products of lipid peroxidation, lipid hydroperoxides, can undergo carbon-carbon bond cleavage via alkoxyl radicals in the presence of transition metals giving rise to the formation of short chain, unesterified aldehydes, or a second class of aldehydes still esterified to the parent lipid. These reactive aldehydic intermediates readily form covalent adducts with cellular macromolecules, including protein, leading to disruption of important cellular functions. The important agents that give rise to the modification of protein may be represented by α,β-unsaturated aldehydic intermediates, such as 2-alkenals, 4-hydroxy-2-alkenals, and 4-oxo-2-alkenals (3, 4).
4-Hydroxy-2-nonenal (HNE),2 among the reactive aldehydes, is a major product of lipid peroxidation and is believed to be largely responsible for the cytopathological effects observed during oxidative stress (2, 5). HNE exerts these effects because of its facile reactivity with biological materials, particularly the sulfhydryl groups of proteins. The reaction of HNE with sulfhydryl groups leads to the formation of thioether adducts that further undergo cyclization to form cyclic hemiacetals (2). Although HNE also forms Michael adducts with the imidazole moiety of histidine residues and the ϵ-amino group of lysine residues (5), the formation of thiol-derived Michael adducts, stabilized as the cyclic hemiacetal, is considered to constitute the main reactivity of HNE, because of the nucleophilic potential of the sulfhydryl group compared with those of the imidazole and amine groups. However, because of the lack of specific and reliable methods for the determination of HNE-cysteine adducts, no study has so far quantitatively demonstrated their formation in proteins.
Because HNE generated in lipid peroxidation is a racemic mixture of 4R- and 4S-enantiomers (6), the HNE Michael adducts, possessing three chiral centers at C-2, C-4, and C-5 in the tetrahydrofuran moiety (Fig. 1A), are composed of at least eight isomers. In our previous study (7), we characterized the configurational isomers of an HNE-histidine adduct by NMR spectroscopy and by molecular orbital calculations, and we found that the configuration of the tetrahydrofuran ring could affect the electron delocalization features, which contribute to the stability of the adduct. Moreover, we raised monoclonal antibodies against (R)-HNE- and (S)-HNE-histidine adducts and observed differential cellular distributions of these adducts in vivo. Balogh et al. (8) recently characterized the stereochemical configurations of the HNE-glutathione adduct by NMR experiments in combination with simulated annealing structure determinations. Despite these studies, however, the stereoselectivity of the HNE Michael addition adducts generated in proteins remains to be fully explored. In this study, to gain further structural insight into sulfhydryl modification by the lipid peroxidation product, we characterized the stereochemical configuration of the HNE-N-acetylcysteine adducts by NMR spectroscopy. In addition, we adapted a pyridylamination-based method for fluorescent labeling of the HNE-cysteine adducts, using 2-aminopyridine (2-AP) and sodium cyanoborohydride (NaCNBH3), and successfully analyzed the individual (R)-HNE- and (S)-HNE-cysteine adducts by reversed-phase HPLC following acid hydrolysis. Furthermore, using the pyridylamination method along with mass spectrometry, we characterized the stereoselective formation of the HNE-cysteine adducts in human thioredoxin (Trx).
FIGURE 1.
Reaction of cysteine residue with HNE. A, formation of the HNE-cysteine Michael adduct, possessing three chiral centers (asterisks). B, reaction of N-acetylcysteine with enantioisomeric HNE. The reactions were performed as described under “Experimental Procedures.” AU, absorbance units.
EXPERIMENTAL PROCEDURES
Materials
N-Acetyl-l-cysteine was obtained from Sigma. Human recombinant Trx was produced by a method described previously (9). Thiol determination of Trx using 5,5′-dithiobis-2-nitrobenzoic acid gave 4.5 sulfhydryl residues per Trx protein (mol/mol) (data not shown). Sequence grade-modified trypsin was obtained from Promega. 2-AP, bovine serum albumin (fatty acid-free), dithiothreitol, and iodoacetamide were obtained from Wako Pure Chemical Industries, Ltd. The stock solutions of HNE were prepared by the acid treatment (1 mm HCl) of HNE dimethylacetal, which was synthesized according to the procedure of De Montarby et al. (10). Enantioisomeric HNEs, (R)-HNE and (S)-HNE, were prepared by the enzymatic resolution of racemic HNE (11) and purified by chiral phase HPLC on a ChiralPak AS column (0.46 × 25 cm) (Daicel Chemical Industries, Ltd., Osaka, Japan) eluted with hexane/2-propanol/trifluoroacetic acid (90:10:0.01, by volume), at a flow rate of 1.0 ml/min. The elution profiles were monitored by UV absorbance at 224 nm. The concentrations of racemic and enantioisomeric HNE stock solutions were determined by the measurement of UV absorbance at 224 nm (12).
Preparation of HNE-N-Acetylcysteine Adducts and NMR Characterization
The reaction mixture (10 ml) containing 40 mm N-acetylcysteine was incubated with 20 mm (R,S)-HNE, (R)-HNE, or (S)-HNE in 50 mm sodium phosphate buffer (pH 7.2). After incubation for 24 h at 37 °C, the reaction mixtures were analyzed with reverse phase HPLC on a Develosil ODS-HG-5 column (4.6 × 250 mm inner diameter) (Nomura Chemicals, Aichi, Japan) eluted with a linear gradient of acetonitrile/water/trifluoroacetic acid (90:10:0.01, by volume) (solvent A), acetonitrile/trifluoroacetic acid (100:0.01, by volume) (solvent B) (time = 0–5 min, 100% A; 60 min, 0% A), at a flow rate of 1.0 ml/min. The elution profiles were monitored by absorbance at 200–400 nm. NMR spectra were obtained by an AMX2-600 (600 MHz for 1H) spectrometer (Bruker, Tsukuba). Chemical shifts (δ) of 1H NMR are given in parts per million (ppm) relative to the internal standard peak at δ 3.75 (dioxane) for D2O solutions. Coupling constants (J) are in Hz. Chemical shifts (δ) of 13C NMR are given in ppm relative to the internal standard peak at δ 69.3 (dioxane) for D2O solutions.
HPLC Analysis of Pyridylaminated HNE-Cysteine Adducts
The HNE-N-acetylcysteine adducts (1 mm) were treated with 340 mm 2-AP and 90 mm NaCNBH3 for 24 h at 37 °C. The pyridylaminated HNE-N-acetylcysteine adducts were hydrolyzed in vacuo with 6 n HCl for 24 h at 110 °C. The hydrolysates were then concentrated, dissolved in distilled water, and then analyzed by reverse phase HPLC on a Sunniest C18 column (4.6 × 250 mm inner diameter, ChromaNik, Japan). The samples were eluted with a gradient of water containing 0.1% trifluoroacetic acid (solvent A) and acetonitrile containing 0.1% trifluoroacetic acid (solvent B), (time = 0–40 min, 95 to 60% A; 40–45 min, 60 to 0% A), at a flow rate of 0.8 ml/min. The elution profiles were monitored by absorbance at 230 nm and by fluorescence intensity (excitation, 315 nm; emission, 380 nm).
Pyridylamination of HNE-treated Trx
Trx (1 mg/ml) in 50 mm sodium phosphate buffer (pH 7.2) was incubated with 1 mm HNE for 5–30 min at 37 °C. The mixture was then applied to a PD-10 column (Sephadex G-25), equilibrated in PBS, to separate protein-bound HNE from the free aldehyde. For determination of the HNE-cysteine adducts by the pyridylamination method, the reactions were terminated by incubating with 340 mm 2-AP and 16 mm NaCNBH3 for 24 h at 37 °C. After the incubation, they were extensively dialyzed against PBS to remove a large amount of the free probe. The pyridylaminated samples were hydrolyzed in vacuo with 6 n HCl for 24 h at 110 °C. The hydrolysates were concentrated, dissolved in distilled water, and then analyzed by HPLC for the pyridylaminated HNE-cysteine adducts.
Peptide Mapping and Mass Spectrometric Analysis
The pyridylaminated protein samples (1 mg/ml) were reduced with 10 mm dithiothreitol in 50 mm ammonium bicarbonate buffer (pH 8.0) for 30 min at 56 °C and then carbamidomethylated with 50 mm iodoacetamide in the dark for 30 min at room temperature. Dithiothreitol and iodoacetamide were removed by centrifugal filtration using YM-3 microcon centrifugal filter devices. The protein samples were digested with modified trypsin in 100 μl of 50 mm Tris-HCl buffer (pH 8.8) at 37 °C for 18 h using an enzyme/substrate ratio of 1:100 (w/w). Peptide samples were analyzed by reverse phase HPLC on a Sunniest C18 column (4.6 × 250 mm inner diameter, ChromaNik, Japan). The samples were eluted with a gradient of water containing 0.1% formic acid (solvent A) and acetonitrile containing 0.08% formic acid (solvent B), (time = 0–3 min, 90% A isocratic; 3–40 min, 90 to 60% A; 40–50 min, 60 to 50% A; 50–60 min, 50 to 20% A; 60–70 min, 20 to 0% A) at a flow rate of 0.8 ml/min. The elution profiles were monitored by absorbance at 215 nm and by fluorescence intensity (Ex., 315 nm; Em., 380 nm). The matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) and MALDI-MS/MS were acquired using an Applied Biosystems 4700 proteomics analyzer (Applied Biosystems, Foster City, CA) with time-of-flight/time-of-flight (TOF/TOF) ion optics; α-cyano-4-hydroxycinnamic acid (Bruker Daltonics, Billerica, MA) was used as the matrix.
Modeling Analysis
The (R)-HNE, (S)-HNE, and HNE-cysteine Michael adduct molecules were generated and energy-minimized using the Dundee PRODRG Server (13). The positions of the (R)-HNE, (S)-HNE, and HNE-cysteine Michael adduct were adjusted using Coot (14) and MolFeat (FiatLux Co., Japan) to avoid steric clash and to correct the direction of C-3 toward its target thiol group of Cys32. The surface electric potential was calculated from the coordinate of the human Trx structure (Protein Data Bank code 1ERW, reduced-form human Trx) using the program MolFeat.
Formation of HNE-Cysteine Adducts in HeLa Cells Exposed to HNE
HeLa cells were cultivated in Dulbecco's modified Eagle's medium supplemented with 5% heat-inactivated fetal bovine serum, 2 mm glutamine, and 100 units/ml penicillin/streptomycin. The cells were cultured at 37 °C in an incubator with 95% humidified atmosphere containing 5% CO2. The Trx activity was measured by the insulin reduction assay (15). To analyze the HNE-cysteine adducts, the HeLa cells were treated with 0–50 μm HNE for 30 min. The cells were then washed twice with PBS, lysed in lysis buffer (4% (w/v) CHAPS, 9 m urea, 40 mm Tris (base), and centrifuged at 10,000 rpm for 5 min at 4 °C; the supernatant was pyridylaminated, and the HNE-cysteine adducts were determined by HPLC analysis following acid hydrolysis.
For determination of HNE-cysteine adducts using the pyridylamination method in combination with SDS-PAGE, total cell lysates from the HNE-treated HeLa cells were pyridylaminated and separated by SDS-PAGE. Protein bands from Coomassie-stained gels were then selected, excised manually, and treated in vacuo with 6 n HCl for 24 h at 110 °C to hydrolyze proteins in gels. The hydrolysates were concentrated, dissolved in distilled water, and analyzed by HPLC for the pyridylaminated HNE-cysteine adducts.
RESULTS
Reactions of N-Acetylcysteine with Racemic and Enantioisomeric HNE
Because of the introduction of new chiral centers at C-2, C-4, and C-5 during the reaction, the HNE-cysteine adducts are composed of at least eight configurational isomers (Fig. 1A). To prepare individual (R)-HNE- and (S)-HNE-cysteine adducts, N-acetylcysteine was incubated with (R)- and (S)-HNE enantiomers, respectively. As shown in Fig. 1B, the reversed-phase HPLC demonstrated that the reaction of N-acetylcysteine with (R)-HNE in sodium phosphate buffer (pH 7.2) for 24 h at 37 °C mainly gave two products, R1 and R2, with relative amounts of 1:1. A similar HPLC profile was observed in the reaction of N-acetylcysteine with (S)-HNE, providing S1 and S2. The liquid chromatography-MS analysis of these peaks gave a pseudomolecular ion peak at m/z 320 (M + H)+ (data not shown), which could be expected from the Michael addition-type HNE-cysteine adducts, suggesting that they all represent the products derived from the Michael addition of the sulfhydryl group to the C-3 of HNE.
Stereochemical Configuration of HNE-Cysteine Adducts
To characterize the stereochemical configurations of HNE-N-acetylcysteine adducts, the four products (R1, R2, S1, and S2) obtained from the reaction of N-acetylcysteine with HNE enantiomers were analyzed by 600 MHz NMR. The assignment of the proton signals and stereochemistry could be done by analyzing 1H-1H-correlation spectroscopy (COSY), 1H-detected multiple-bond connectivity, 1H-detected multiple quantum coherence, and the nuclear Overhauser effect spectroscopy (NOESY) spectrum recorded in D2O. The COSY spectrum of S1 suggested that the peak was a mixture of anomeric isomers (Fig. 2). Similarly, other products (R1, R2, and S2) also appeared to contain a pair of anomeric isomers (supplemental Tables SI and SII). Identification of the proton signals of the tetrahydrofuran moiety in R1, R2, S1, and S2 follows from the analysis of the NOESY spectra collected in D2O. In S1, the proton signals at 4.31 and 4.10 ppm, which were assigned to the methine protons at C-5, displayed NOE cross-peaks to the methine proton at C-4, although there were weaker NOE cross-peaks in S2 (supplemental Fig. S1), suggesting that the configurations at C-4 of S1 and S2 are S and R, respectively. The absolute configurations at C-2 and C-3 were determined by the NOE connectivity between the C-2 methine proton and C-3 methylene protons (H-3a and H-3b). These data suggest that S1 is a mixture of 2R,4S,5S and 2S,4S,5S anomeric isomers, and S2 is a mixture of 2S,4R,5S and 2R,4R,5S anomeric isomers. Based on the assignment of these signals, the relative amounts (%) of 2R,4S,5S, 2S,4S,5S, 2S,4R,5S, and 2R,4R,5S anomeric isomers in D2O were found to be 1, 5, 3, and 3, respectively. In a manner similar to the assignment of the (S)-HNE-cysteine adducts, R1 and R2 were determined to be a mixture of 2S,4R,5R and 2R,4R,5R anomeric isomers and a mixture of 2R,4S,5R and 2S,4S,5R anomeric isomers, respectively. In addition, the relative amounts (%) of the (R)-HNE-cysteine configurational isomers (2S,4R,5R/2R,4R,5R/2R,4S,5R/2S,4S,5R = 1:5:3:3) were exactly comparable with those of the (S)-HNE-cysteine isomers. Fig. 3 summarizes the configurations of R1, R2, S1, and S2 from the HNE-N-acetylcysteine adducts.
FIGURE 2.
Portion of a 1H-1H-COSY spectrum in S1. The spectrum was acquired in D2O. The cross-peaks permit the assignment of resonances of the tetrahydrofuran moiety and indicate the presence of a pair of diastereomers.
FIGURE 3.
Chemical structures of the configurational isomers of the (R,S)-HNE-N-acetylcysteine adduct.
Mutarotation at the Anomeric Center of HNE-Cysteine Adducts
The hemiacetal ring opens and reforms to give products with different configurations at the anomeric center. This equilibration occurs with all reducing saccharides and is accompanied by a change in optical rotation. This process of equilibrating the ratios of two anomers in carbohydrates is known as mutarotation. It can be assumed that the anomers in the four products (R1, R2, S1, and S2) also interconvert via an open chain form (reversible formation of internal hemiacetal linkage). We indeed observed the NOE cross-peaks (Fig. 4A) between the anomeric isomers (2R,4S,5S and 2S,4S,5S) in the NOESY analysis of S1. Similar cross-peaks were also observed in the NOESY analysis of three other products (R1, R2, and S2). Mutarotation of S1 in solution at equilibrium is shown in Fig. 4B.
FIGURE 4.
Portion of a NOESY spectrum in S1. A, NOE cross-peak between the anomeric isomers (2R,4S,5S and 2S,4S,5S). B, mutarotation of S1 in solution at equilibrium.
Fluorescent Labeling of HNE-Cysteine Adducts
Because of the lack of a specific and reliable method for determination of the HNE-cysteine adducts, no study has so far quantitatively demonstrated their formation in the HNE-modified proteins. Hence, we attempted to establish a method for the detection of HNE-cysteine adducts. Our strategy for the fluorescent labeling of HNE-cysteine adducts in proteins is illustrated in Fig. 5. The method is based on the fact that HNE forms Michael addition adducts with specific amino acid residues possessing a cyclic hemiacetal ring, which upon reaction with 2-AP in the presence of NaCNBH3 can be converted to the pyridylaminated derivatives. It was anticipated that the HNE-cysteine adducts after the pyridylamination were resistant to the process of acid hydrolysis. To test the validity of this procedure, we first attempted to detect the adducts in the hydrolyzed samples of authentic HNE-N-acetylcysteine adduct. As shown in Fig. 6, the pyridylamination of the HNE-cysteine adduct followed by acid hydrolysis gave two products in the reverse phase HPLC analysis. Based on the observations that the pyridylamination of the authentic (R)-HNE- and (S)-HNE-cysteine adducts gave main peaks at 27 and 28 min, respectively, these products were identified to be the pyridylaminated (R)-HNE- and (S)-HNE-cysteine adducts, respectively. However, we were unable to further separate the diastereomers of the individual (R)-HNE- and (S)-HNE-cysteine adducts (data not shown).
FIGURE 5.
Strategy for the fluorescent labeling of HNE-cysteine adducts in proteins.
FIGURE 6.
HPLC analysis of pyridylaminated HNE-cysteine adducts. The purified HNE-N-acetylcysteine adducts (1 mm) were treated with 340 mm 2-AP and 90 mm NaCNBH3 for 24 h at 37 °C. The pyridylaminated HNE-cysteine adducts were analyzed by a reverse phase HPLC as described under “Experimental Procedures.”
Stereoselective Formation of HNE-Cysteine Adducts in Human Trx
To investigate the formation of HNE-cysteine adducts in proteins, we utilized Trx, the major redox-regulated protein present in the mitochondria and cytosol (16). It has been shown that Trx is sensitive to HNE (17), and the redox residues (Cys32 and Cys35) in Trx represent primary targets for HNE modification (18). When Trx (1 mg/ml) was incubated with 0.5 mm HNE in 50 mm sodium phosphate buffer (pH 7.2) at 37 °C, selective loss of cysteine was observed (supplemental Fig. S2, panel A). In addition, accompanied by the loss of cysteine residues, the enzyme activity of Trx linearly decreased to 30% of the initial value after 30 min (supplemental Fig. S2, panel B). To further examine the selective modification of the Trx cysteine residues, the HNE-treated Trx and cysteine-blocked Trx for 5 min were analyzed by MALDI-TOF MS. As shown in supplemental Fig. S3 (panel A), the analysis of the native Trx revealed a peak of m/z 11738. When Trx was incubated with 0.5 mm HNE in 50 mm sodium phosphate buffer (pH 7.2) for 5 min at 37 °C, some adducted Trx subunits were observed (m/z 11,894), as well as the peak (m/z 12,050), corresponding to the addition of one to two molecules of HNE per Trx. However, the cysteine-blocked Trx (m/z 12,023, corresponding to the alkylation of five cysteine residues in Trx) was resistant to the HNE adduction (supplemental Fig. S3, panel B). These results suggest that HNE exclusively binds to the active site cysteine residues of Trx and forms adduct, most probably the Michael addition-type HNE-cysteine adducts.
To characterize the formation of the HNE-cysteine adducts in Trx, the HNE-treated and untreated Trx were pyridylaminated, hydrolyzed, and analyzed by reverse phase HPLC. As shown in Fig. 7A, the incubation of Trx with 1 mm HNE for 0–30 min at 37 °C resulted in the formation of both (R)-HNE- and (S)-HNE-cysteine adducts. Of interest, Trx exhibited a preference for the adduct formation with (R)-HNE in the presence of both enantiomers. The concentrations reached about 3.0 and 1.5 molecules of the (R)-HNE- and (S)-HNE-cysteine adducts per protein molecule, respectively, after 30 min of incubation (Fig. 7B). Such stereoselective formation of HNE-cysteine adducts was not observed in other sulfhydryl proteins, including glyceraldehyde-3-phosphate dehydrogenase.3 These data and the observation that, upon reaction with racemic HNE, N-acetylcysteine generated almost equal amounts of the (R)-HNE- and (S)-HNE-cysteine adducts (Fig. 6) suggested the unique stereoselectivity of Trx cysteine residues toward HNE.
FIGURE 7.
Stereoselective formation of HNE-cysteine adducts in Trx. Trx treated with 1 mm HNE for 0–30 min at 37 °C was pyridylaminated and hydrolyzed. The pyridylaminated HNE-cysteine adducts in the hydrolyzed samples were analyzed by reverse phase HPLC as described under “Experimental Procedures.” A, HPLC analysis of the (R)-HNE- and (S)-HNE-cysteine adducts. HNE-Cys, the hydrolyzed sample of pyridylaminated authentic HNE-N-acetylcysteine adduct. The symbols R and S represent (R)-HNE- and (S)-HNE-cysteine adducts, respectively. B, time-dependent formation of (R)-HNE- and (S)-HNE-cysteine adducts in the protein.
Identification of Target Cysteines in Human Trx
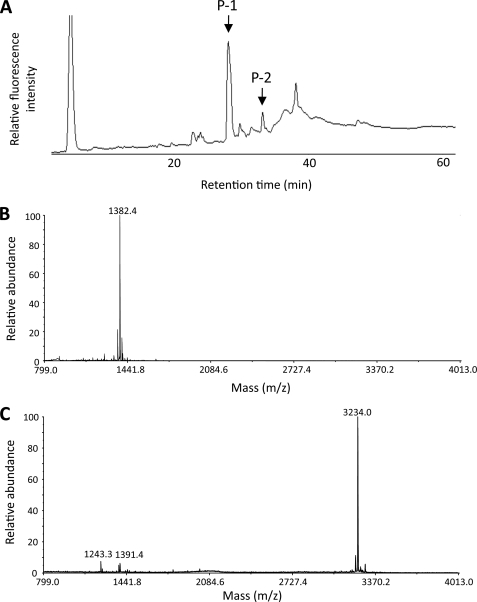
Based on the preferential formation of the (R)-HNE-cysteine adducts, we further characterized the stereochemistry of individual HNE-cysteine adducts generated in the HNE-modified Trx. Trx (1 mg/ml) in 50 mm sodium phosphate buffer (pH 7.2) was incubated with 1 mm HNE at 37 °C. To identify a hypersensitive cysteine, the incubation time was fixed to 5 min. Trx after treatment with and without HNE was pyridylaminated, carbamidomethylated, and digested with trypsin. The resulting peptides were then separated by reverse phase HPLC as described under “Experimental Procedures.” The peptide map demonstrated the appearance of one major fluorescent product (P-1) and several minor fluorescent products, including P-2, from the HNE-treated Trx (Fig. 8A), whereas the native Trx provided no fluorescent products (data not shown). These peptides were further purified and subjected to MALDI-TOF MS. P-1 and P-2 gave a peak of m/z 1382.4 (Fig. 8B) and 3234.0 Da, respectively (Fig. 8C). It was speculated that, relative to the calculated masses of the unmodified peptides, these mass values corresponded to the sequences Cys73–Lys81 (Mr of 1149.41) and Tyr9–Lys36 (Mr of 2944.35), respectively, i.e. an increased mass of 235 Da corresponds to the addition of pyridylaminated HNE per peptide for P-1 and an increased mass of 292 Da corresponds to the addition of pyridylaminated HNE (235 Da) and a carbamidomethyl group (57 Da) per peptide for P-2. The reason for the incomplete digestion at Lys21 in P-2 remains unclear.
FIGURE 8.
Peptide mapping and MALDI-TOF MS analysis. Trx (1 mg/ml) treated with 1 mm HNE for 5 min at 37 °C was pyridylaminated, carbamidomethylated, and digested with trypsin. The resulting peptides were then separated by reverse phase HPLC as described under “Experimental Procedures.” A, HPLC analysis of the tryptic fragments from the HNE-treated Trx. B, MALDI-TOF MS analysis of the major fluorescent product P-1. C, MALDI-TOF MS analysis of the minor fluorescent product P-2.
To confirm the HNE modification sites, the tryptic fragments P-1 and P-2 were further analyzed by MALDI TOF/TOF tandem mass spectrometer. Fig. 9A shows the MS/MS spectrum of the [M + H]+ at m/z 1382.4 from P-1. The singly charged C-terminal fragment ions (y-series ions; y1 to y8) were observed in the MS/MS analysis. The b-series fragment ions b2 to b8 were observed to increase by 235 Da, indicating that the HNE modification site in the sequence is on Cys73. The MS/MS spectrum of the [M + H]+ at m/z 3234.0 from P-2 is shown in Fig. 9B. In the MS/MS analysis, the singly charged N-terminal fragment ions (b-series ions; b4 to b22) were observed. An increased mass of 57 Da was observed in the fragment ion y4. The fragment ions b24 and b25 were observed to increase by 235 Da, whereas the y-series fragment ions y5 to y23 were observed to increase by 292 Da, indicating the HNE modification site at Cys32 and the carbamidomethylation site at Cys35. Thus, MS/MS analyses identified Cys73 as the major and Cys32 as the minor targets of HNE in Trx.
FIGURE 9.
Product ion spectra of the tryptic fragments P-1 and P-2. A, MS/MS spectrum of the [M + H]+ at m/z 1382.4 from P-1. B, MS/MS spectrum of the [M + H]+ at m/z 3234.0 from P-2.
Stereochemistry of the HNE-Cysteine Adducts Generated at Cys32 and Cys73
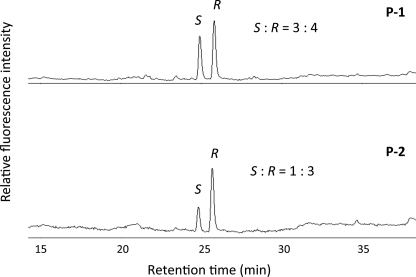
Finally, to characterize the stereochemistry of the HNE-cysteine adducts generated at Cys32 and Cys73, we hydrolyzed the tryptic fragments P-1 and P-2 and analyzed the (R)-HNE- and (S)-HNE-cysteine adducts by reverse phase HPLC. As shown in Fig. 10, the (R)-HNE- and (S)-HNE-cysteine adducts were detected in the hydrolysates of both peptide fragments in the ratio of 4:3 (P-1) and 3:1 (P-2). Thus, the (R)-HNE- and (S)-HNE-cysteine adducts were almost equally formed at Cys73, whereas Cys32 exhibited a remarkable preference for the adduct formation with (R)-HNE.
FIGURE 10.
HPLC analysis of the (R)-HNE- and (S)-HNE-cysteine adducts in the tryptic fragments P-1 and P-2. Both fragments were hydrolyzed, and the pyridylaminated HNE-cysteine adducts in the hydrolyzed samples were analyzed by reverse phase HPLC as described under “Experimental Procedures.” Chromatograms: upper, P-1; lower, P-2. The symbols R and S represent (R)-HNE- and (S)-HNE-cysteine adducts, respectively.
Modeling Analysis
To elucidate the mechanism of stereoselective formation of HNE-cysteine adducts in Trx, we performed a computational analysis. The conserved active site amino acids Trp31–Cys32–Gly33–Pro34–Cys35 form L-shaped hydrophobic pocket, and the residues Val59 and Met74 also participate in the hydrophobic interactions with Trx substrates (19–21). It can be expected that the hydrophobic HNE is trapped into this hydrophobic pocket. Cys32 is exposed to the solvent; however, the sulfhydryl group of Cys35 is buried behind Cys32 (Fig. 11A). The side chains of Cys62 and Cys69 are also buried at opposite ends of the α3 helix (Fig. 11B) and are surrounded by extensive acidic residues (Glu6, Asp60, Asp61, Asp64, Glu68, and Glu70). Thus, the steric hindrance and acidic environment may reduce their accessibility to HNE. On the other hand, because Cys73 is completely exposed to the solvent, both HNE enantiomers may therefore be freely accessible to the cysteine residue (Fig. 11A). In addition, the neighboring Lys72, facilitating the deprotonation of the thiol group to form a nucleophilic thiolate anion, may further potentiate the reactivity of Cys73 with HNE. These structural properties may be associated with the observation that the (R)-HNE- and (S)-HNE-cysteine adducts were almost equally formed at Cys73 (Fig. 10). Meanwhile, the partially exposed sulfur atom of Cys32 is located at the bottom of the L-shaped hydrophobic active site pocket. The modeling analysis of the HNE-cysteine Michael adducts adjusted to avoid steric clash shows that they can fit into the Trx active site (Fig. 11C). (R)-HNE can bind to the hydrophobic pocket in the same orientation of the HNE-cysteine adducts, having a proper direction toward its target thiol group of Cys32 (Fig. 11D), resulting in the predominant formation of the (R)-HNE-cysteine adduct. However, the configuration of the hydroxyl group in (S)-HNE disturbs the nucleophilic attack of Cys32 at the C-3 carbon of (S)-HNE (Fig. 11E).
FIGURE 11.
Electrostatic surface potential of human Trx (A and B) and putative interaction sites of HNE-cysteine Michael adducts (R)-HNE, and (S)-HNE (C–E). Blue and red surfaces represent positive and negative potentials, respectively.
Stereoselective Formation of HNE-Cysteine Adducts in HeLa Cells Exposed to HNE
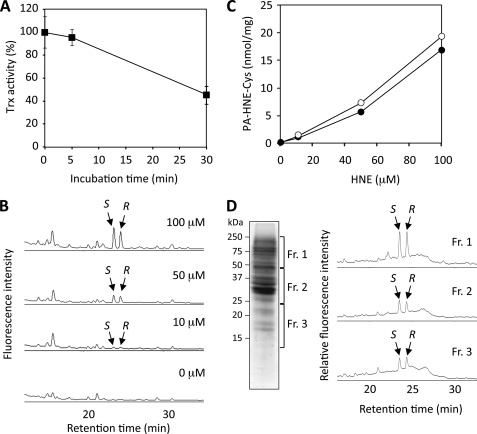
To demonstrate the utility of the method for the determination of the HNE-cysteine adducts, in vivo experiments using HeLa cells were carried out. When HeLa cells were exposed to HNE (50 μm) for 30 min, the Trx activity decreased to 40% of the initial value after 30 min (Fig. 12A). We then sought to detect the HNE-cysteine adducts in the cells exposed to HNE. As shown in Fig. 12B, the incubation with 0–100 μm HNE for 30 min resulted in the formation of both (R)-HNE- and (S)-HNE-cysteine adducts. The concentrations reached about 17 and 19 (mol/mg protein) of the (R)-HNE- and (S)-HNE-cysteine, respectively (Fig. 12C). In addition, using the pyridylamination method in combination with SDS-PAGE, we confirmed the presence of both (R)-HNE- and (S)-HNE-cysteine adducts in protein bands from Coomassie-stained SDS-polyacrylamide gels (Fig. 12D).
FIGURE 12.
Detection of HNE-cysteine adducts in HeLa cells exposed to HNE. A, changes in Trx activity. HeLa cells were exposed to HNE (50 μm) for 30 min. The Trx activity was measured by the insulin reduction assay. B and C, detection of the HNE-cysteine adducts in total cell lysates. HeLa cells were treated with 0–50 μm HNE for 30 min. The cells were then washed twice with PBS, lysed, and centrifuged at 10,000 rpm for 5 min at 4 °C; the supernatant was pyridylaminated, and the HNE-cysteine adducts were determined by HPLC analysis following acid hydrolysis. D, detection of the HNE-cysteine adducts using the pyridylamination method in combination with SDS-PAGE. Total cell lysates from the HNE-treated HeLa cells were pyridylaminated and separated by SDS-PAGE. Protein bands (Fr. 1–3) from Coomassie-stained gels (left panel) were then selected, excised manually, and treated in vacuo with 6 n HCl for 24 h at 110 °C to hydrolyze proteins in gels. The hydrolysates were concentrated, dissolved in distilled water, and analyzed by HPLC for the pyridylaminated HNE-cysteine adducts (right panel).
DISCUSSION
HNE, under physiological conditions, reacts most rapidly with sulfhydryl residues of proteins, resulting in the formation of the HNE-cysteine Michael adducts (22). Because of the introduction of new chiral centers at C-2, C-4, and C-5 during the reaction, these adducts have been suggested to be composed of at least eight configurational isomers. We previously showed that the HNE-N-acetylcysteine Michael adduct was mainly detected as two peaks upon reverse phase HPLC analysis and suggested the multiplicity of primary products in the HNE/N-acetylcysteine reaction (23). However, this previous work on the HNE-cysteine adducts has focused on racemic HNE, and therefore, the stereoselectivity remains to be fully explored. Because stereochemically distinct substrates and products have different biological effects, it is important to define the stereochemistry of the HNE-cysteine adducts. In this study, to gain structural insight into sulfhydryl modification by HNE enantiomers, we characterized the configurational isomers of the HNE-N-acetylcysteine adducts by NMR spectroscopy and observed the product stereoselectivity upon reaction of N-acetylcysteine with HNE enantiomers. In addition, during the course of the NOESY analysis, we observed unusual intermolecular NOE cross-peaks between two anomeric isomers (Fig. 3), due probably to the rapid interconversion (mutarotation) between the isomers. This finding suggests that the adduct may be in the equilibrium between the ring-opened and ring-closed structures. This structural property may be characteristic of the 4-hydroxy-2-alkenal-derived Michael adducts and important for understanding the biological impact on the formation of the adducts composed primarily of the four ring-opened and eight ring-closed structures.
To differentiate between various modes of carbonyl group formation, a method for the detection and quantification of protein carbonyl groups associated with the conjugation of protein sulfhydryl groups with lipid peroxidation products was previously developed (23). This method is based on the reduction of the adducts with NaB[3H]H4 to stable radioactive derivatives followed by cleavage of the thioether linkage upon treatment with Raney nickel. Although this procedure is not specific for the HNE-cysteine adducts, it can provide a means of determining the fraction of total free carbonyl groups introduced into proteins via reaction of α,β-unsaturated aldehydes with protein sulfhydryl groups. In later studies, both HNE-histidine and HNE-lysine Michael adducts generated in peptides and proteins were analyzed by HPLC following o-phthaldehyde derivatization (24–26). This method allowed quantitating the Michael addition-type HNE-histidine adducts and trace amounts of HNE-lysine adducts in Cu2+-oxidized low density lipoprotein (27). However, in terms of the HNE-cysteine adducts, no study has so far revealed that they are indeed formed in the HNE-modified proteins because of the lack of specific and reliable methods for the determination of the adducts. To analyze the HNE-cysteine adducts in protein, we adapted a reductive amination-based pyridylamination using 2-AP and NaCNBH3. This method was originally developed for detection of reducing sugars (28). Upon pyridylamination followed by acid hydrolysis of HNE-N-acetylcysteine, we detected two fluorescent products, which were identified to be the pyridylaminated (R)-HNE- and (S)-HNE-cysteine adducts (Fig. 6). It is noteworthy that these 2-AP derivatives of the HNE-cysteine adducts were stable against the acid hydrolysis using 6 n HCl (110 °C, 24 h). Thus, the mild derivatization conditions and derivatives resistant to acid hydrolysis permitted the reliable and accurate quantification of the HNE-cysteine adducts. The derivatives were fluorescent after acid hydrolysis and therefore could be determined sensitively and rapidly by fluorometric HPLC.
Trx is an essential cofactor electron donor for ribonucleotide reductase but also has many other cellular functions, including regulation of transcription factors and apoptosis, and can act exogenously as a redox active growth factor (29, 30). Trx is also known to play important roles in the redox regulation of signal transduction and in cytoprotection against oxidative stress (31, 32). The catalytic activity of Trx resides in its active site where the two redox active cysteine residues (Cys32 and Cys35) undergo reversible oxidation/reduction. In addition to the conserved cysteine residues in the active site, three additional structural cysteine residues (Cys62, Cys69, and Cys73) are present in the structure of the human Trx. We here investigated the stereoselective formation of the HNE-cysteine adducts in HNE-treated Trx. Pyridylamination followed by mass spectrometry demonstrated the preferential formation of the HNE-cysteine adducts at Cys73 (Figs. 8 and 9). The result is consistent with the previous finding by Go et al. (17), showing that low concentrations of reactive aldehydes, such as acrolein, preferentially modify Cys73 of human Trx rather than Cys32 or Cys35 in the active site or the regulatory Cys62 or Cys69 sites. Because Cys73 is known to be structurally and functionally important (33), formation of HNE adducts at this site may be directly associated with the inactivation of Trx. Glutathiolation (34) and the experimental anticancer drug PX-12 (35, 36) indeed target Cys73, causing the inhibition of Trx activity. Cys73 is therefore likely to represent the most sensitive cysteine to the covalent binding with glutathione and electrophilic molecules. Furthermore, our preliminary experiment has shown that when Trx was treated with 0.5 mm HNE for 5 min, 38% of the Trx activity was lost. Thus, because Cys73 is the primary target of HNE within 5 min of incubation, it is likely that the modification of Cys73 by itself might be directly associated with the inactivation of Trx. Meanwhile, the previous study by Fang and Holmgren (18) demonstrated that HNE induced catalytic inhibition of Escherichia coli Trx by modification of active site cysteines. The preferential modification of the catalytic cysteines might be attributable to the fact that E. coli Trx contains two cysteines (Cys32 and Cys35) in the catalytic site and does not have the other three regulatory cysteines observed in mammalian Trx. The pyridylamination method also allowed us to characterize the nature of the HNE-cysteine adducts generated in HNE-treated Trx. The data showed that, upon incubation of Trx with HNE, the (R)-HNE-cysteine adducts were more preferentially formed than the (S)-HNE-cysteine adducts (Fig. 7). In addition, we characterized the stereochemistry of the HNE-cysteine adducts generated at Cys32 and Cys73 and found that, upon reaction with racemic HNE, Cys73 almost equally formed the (R)-HNE- and (S)-HNE-cysteine adducts, whereas Cys32 exhibited a remarkable preference for the adduct formation with (R)-HNE (Fig. 10). Trx has been shown to form a disulfide-linked dimer connected through Cys73 located on the surface close to the active site (21). Thus, it is reasonable that the surface-exposed cysteine equally reacted with both enantiomers. The identification of Cys73 as the major target of HNE also led us to the assumption that the cysteine could play a role as a primary sensor for electrophiles. On the other hand, although the mechanism for the distinct formation of (R)- and (S)-HNE-cysteine adducts at Cys32 is currently unknown, structural properties of the active site may underlie the observed preferential formation of (R)-HNE-cysteine adducts. As shown in Fig. 11, the active site is formed by a hydrophobic patch, consisting of residues Trp31, Val59, Ala66, and Met74 that create a hydrophobic pocket through which Trx interacts with substrates. Thus, the spatial distribution of these hydrophobic amino acid side chains in the active site of Trx is likely to play a major role in the correct positioning of the sulfur of Cys32, allowing the cysteine residue to react stereoselectively with (R)-HNE. In addition, there may be an interaction between the C-4-hydroxyl group of (R)-HNE and a symmetrically or enantiospecifically located residue at the active site, resulting in a distinct accessibility of the cysteine residue toward the HNE enantiomers. Further studies are required to understand the mechanism for the stereoselective reactivity of the cysteine residues toward the HNE enantiomers. Furthermore, the characterization of the biological consequences of the production of HNE-modified Trx merits immediate attention. With regard to this, Go et al. (17) have previously shown that microinjection of HNE-modified Trx stimulates monocyte adhesion to endothelial cells. Thus, chemical modification of Trx by HNE may stimulate critical early events of atherosclerosis.
In this study, we applied the pyridylamination method for the quantitative evaluation of the (R)-HNE- and (S)-HNE-cysteine adducts generated in the cells exposed to HNE (Fig. 12B). Moreover, in combination with SDS-PAGE, we successfully detected the adducts in protein bands from Coomassie-stained SDS-polyacrylamide gels (Fig. 12C). These data suggest that the pyridylamination followed by gel electrophoresis/mass spectrometry analysis could be a powerful method to identify and quantify target proteins in complex protein samples.
In summary, we characterized the configurational isomers of the HNE-cysteine adducts by NMR and determined the absolute configurations of these isomers by nuclear Overhauser effect analysis. In addition, by using the pyridylamination method, we successfully established the method for determination of the (R)-HNE- and (S)-HNE-cysteine adducts by reversed-phase HPLC following acid hydrolysis. Using this method along with mass spectrometry, we characterized the HNE-cysteine adducts generated in human Trx and demonstrated the stereoselective formation at the target cysteines. Although the detailed mechanism for the stereoselectivity remains unclear, the modification of these cysteines, especially one of the two active site cysteines, may be directly associated with the abolishment of its redox regulatory functions. Finally, the utility of the method for the determination of the HNE-cysteine adducts was confirmed by an in vitro study using HeLa cells. The present results not only offer structural insights into sulfhydryl modification by lipid peroxidation products but also provide a platform for the chemical analysis of protein S-associated aldehydes in vitro and in vivo.
Supplementary Material
This work was supported by a grant-in-aid for scientific research on innovative areas (Research in a Proposed Research Area) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K. U.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables SI and SII and Figs. S1–S3.
C. Wakita, A. Yamazaki, and K. Uchida, unpublished data.
- HNE
- 4-hydroxy-2-nonenal
- 2-AP
- 2-aminopyridine
- COSY
- correlation spectroscopy
- HMBC
- 1H-detected multiple-bond connectivity
- MALDI-TOF MS
- matrix-assisted laser desorption and ionization time-of-flight mass spectrometry
- NOE
- nuclear Overhauser effect
- NOESY
- nuclear Overhauser effect spectroscopy
- NaCNBH3
- sodium cyanoborohydride
- Trx
- thioredoxin
- HPLC
- high pressure liquid chromatography
- MS/MS
- tandem mass spectrometry
- PBS
- phosphate-buffered saline
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
REFERENCES
- 1.Gutteridge J. M., Halliwell B. (1990) Trends Biochem. Sci. 15, 129–135 [DOI] [PubMed] [Google Scholar]
- 2.Esterbauer H., Schaur R. J., Zollner H. (1991) Free Radic. Biol. Med. 11, 81–128 [DOI] [PubMed] [Google Scholar]
- 3.Uchida K. (2000) Free Radic. Biol. Med. 28, 1685–1696 [DOI] [PubMed] [Google Scholar]
- 4.Marnett L. J., Riggins J. N., West J. D. (2003) J. Clin. Invest. 111, 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida K. (2003) Prog. Lipid Res. 42, 318–343 [DOI] [PubMed] [Google Scholar]
- 6.Schneider C., Tallman K. A., Porter N. A., Brash A. R. (2001) J. Biol. Chem. 276, 20831–20838 [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto M., Sibata T., Wasada H., Toyokuni S., Uchida K. (2003) J. Biol. Chem. 278, 5044–5051 [DOI] [PubMed] [Google Scholar]
- 8.Balogh L. M., Roberts A. G., Shireman L. M., Greene R. J., Atkins W. M. (2008) J. Biol. Chem. 283, 16702–16710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tagaya Y., Maeda Y., Mitsui A., Kondo N., Matsui H., Hamuro J., Brown N., Arai K., Yokota T., Wakasugi H. (1989) EMBO J. 8, 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Montarby L., Mosset P., Gree R. (1988) Tetrahedron Lett. 29, 3895 [Google Scholar]
- 11.Allevi P., Anastasia M., Cajone F., Ciuffreda P., Sanvito A. M. (1993) J. Org. Chem. 58, 5000–5002 [Google Scholar]
- 12.Schauenstein E., Taufer M., Esterbauer H., Kylianek A., Seelich T. (1971) Monatsh. Chem. 102, 517–529 [Google Scholar]
- 13.Schüttelkopf A. W., van Aalten D. M. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 14.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 15.Sasada T., Nakamura H., Ueda S., Sato N., Kitaoka Y., Gon Y., Takabayashi A., Spyrou G., Holmgren A., Yodoi J. (1999) Free Radic. Biol. Med. 27, 504–514 [DOI] [PubMed] [Google Scholar]
- 16.Deneke S. M. (2000) Curr. Top. Cell. Regul. 36, 151–180 [DOI] [PubMed] [Google Scholar]
- 17.Go Y. M., Halvey P. J., Hansen J. M., Reed M., Pohl J., Jones D. P. (2007) Am. J. Pathol. 171, 1670–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang J., Holmgren A. (2006) J. Am. Chem. Soc. 128, 1879–1885 [DOI] [PubMed] [Google Scholar]
- 19.Qin J., Clore G. M., Kennedy W. M., Huth J. R., Gronenborn A. M. (1995) Structure 3, 289–297 [DOI] [PubMed] [Google Scholar]
- 20.Qin J., Clore G. M., Kennedy W. P., Kuszewski J., Gronenborn A. M. (1996) Structure 4, 613–620 [DOI] [PubMed] [Google Scholar]
- 21.Weichsel A., Gasdaska J. R., Powis G., Montfort W. R. (1996) Structure 4, 735–751 [DOI] [PubMed] [Google Scholar]
- 22.Hartley D. P., Kroll D. J., Petersen D. R. (1997) Chem. Res. Toxicol. 10, 895–905 [DOI] [PubMed] [Google Scholar]
- 23.Uchida K., Stadtman E. R. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 5611–5615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida K., Stadtman E. R. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 4544–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida K., Stadtman E. R. (1993) J. Biol. Chem. 268, 6388–6393 [PubMed] [Google Scholar]
- 26.Szweda L. I., Uchida K., Tsai L., Stadtman E. R. (1993) J. Biol. Chem. 268, 3342–3347 [PubMed] [Google Scholar]
- 27.Uchida K., Toyokuni S., Nishikawa K., Kawakishi S., Oda H., Hiai H., Stadtman E. R. (1994) Biochemistry 33, 12487–12494 [DOI] [PubMed] [Google Scholar]
- 28.Hase S., Ikenaka T., Matsushima Y. (1978) Biochem. Biophys. Res. Commun. 85, 257–263 [DOI] [PubMed] [Google Scholar]
- 29.Holmgren A. (1985) Annu. Rev. Biochem. 54, 237–271 [DOI] [PubMed] [Google Scholar]
- 30.Arnér E. S., Holmgren A. (2000) Eur. J. Biochem. 267, 6102–6109 [DOI] [PubMed] [Google Scholar]
- 31.Yodoi J., Uchiyama T. (1992) Immunol. Today 13, 405–411 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H., Nakamura K., Yodoi J. (1997) Annu. Rev. Immunol. 15, 351–369 [DOI] [PubMed] [Google Scholar]
- 33.Watson W. H., Pohl J., Montfort W. R., Stuchlik O., Reed M. S., Powis G., Jones D. P. (2003) J. Biol. Chem. 278, 33408–33415 [DOI] [PubMed] [Google Scholar]
- 34.Casagrande S., Bonetto V., Fratelli M., Gianazza E., Eberini I., Massignan T., Salmona M., Chang G., Holmgren A., Ghezzi P. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9745–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan B. F., Runquist M., Raghunand N., Gillies R. J., Tate W. R., Powis G., Baker A. F. (2005) Clin. Cancer Res. 11, 529–536 [PubMed] [Google Scholar]
- 36.Kirkpatrick D. L., Kuperus M., Dowdeswell M., Potier N., Donald L. J., Kunkel M., Berggren M., Angulo M., Powis G. (1998) Biochem. Pharmacol. 55, 987–994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.