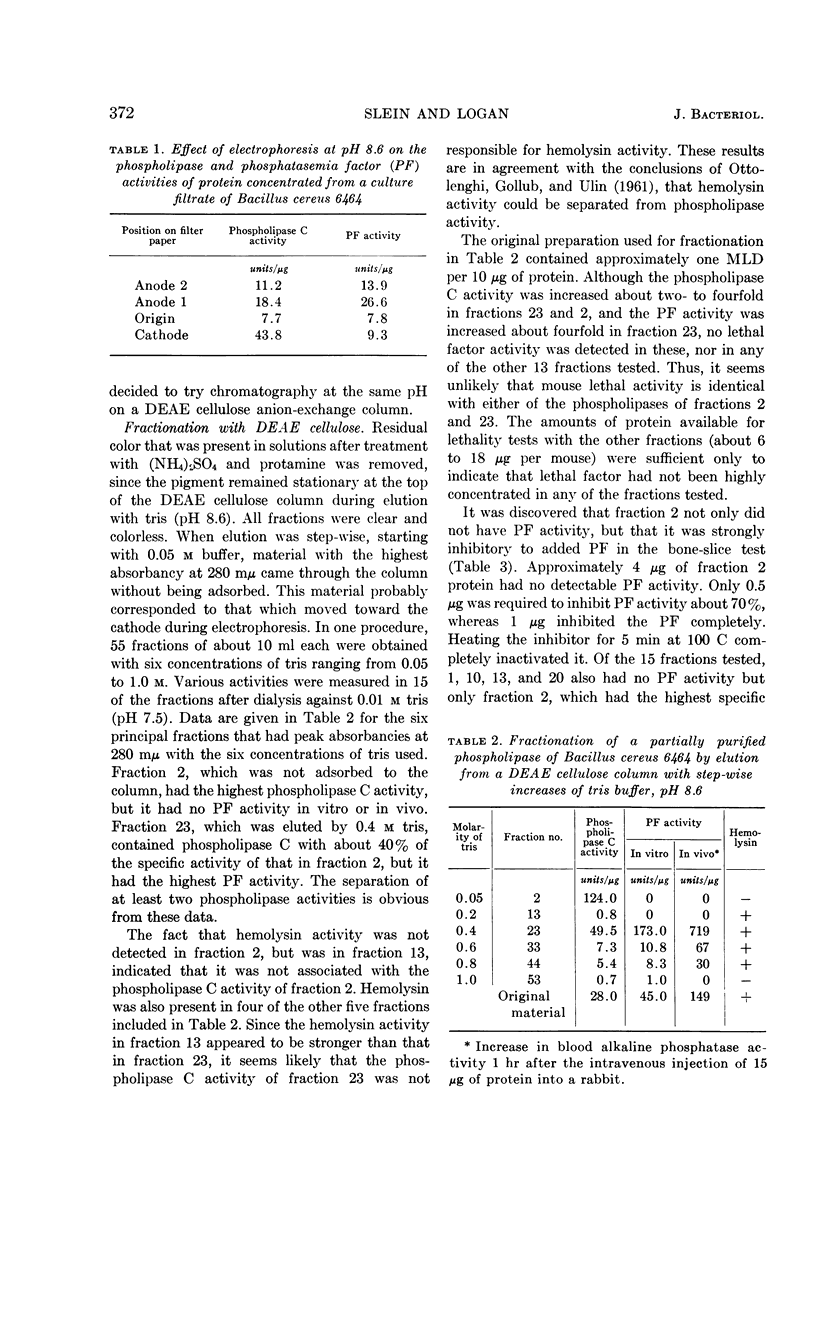
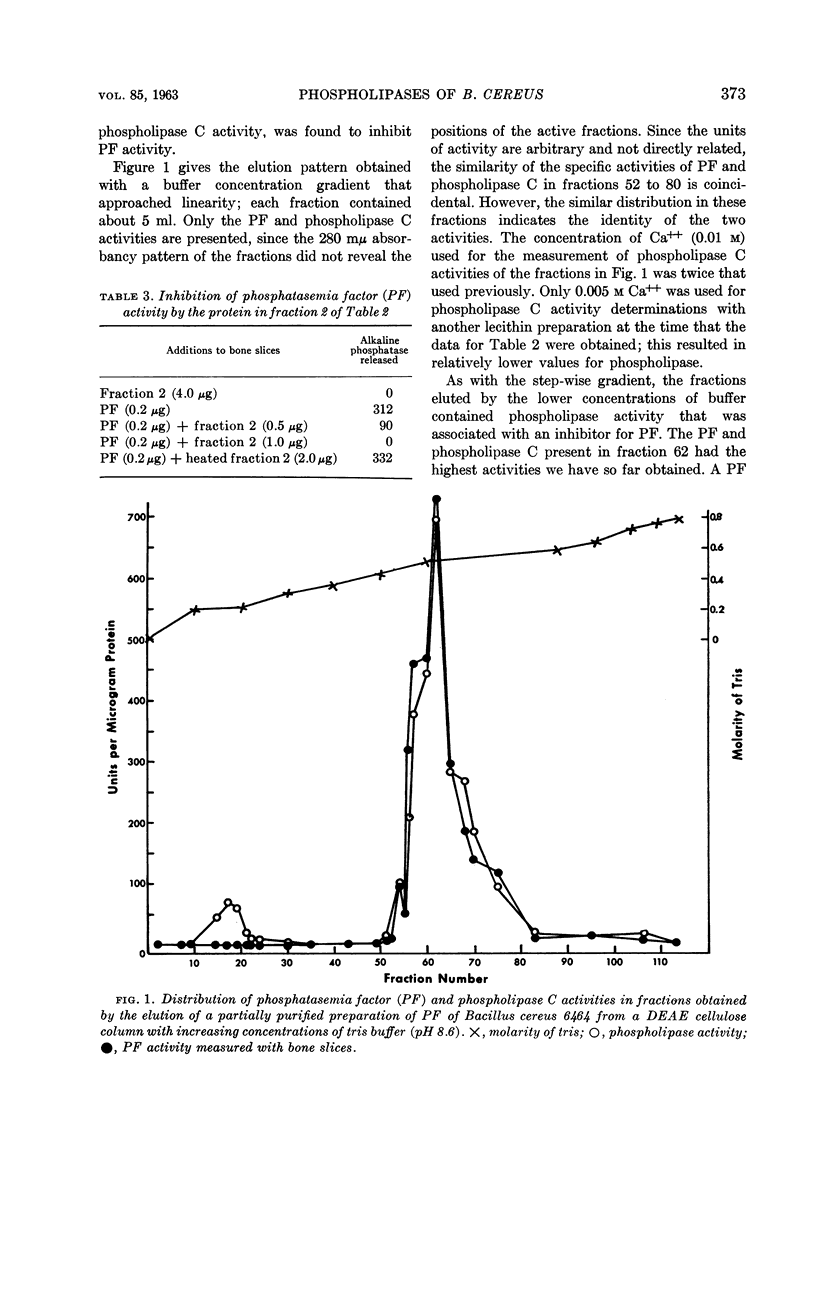
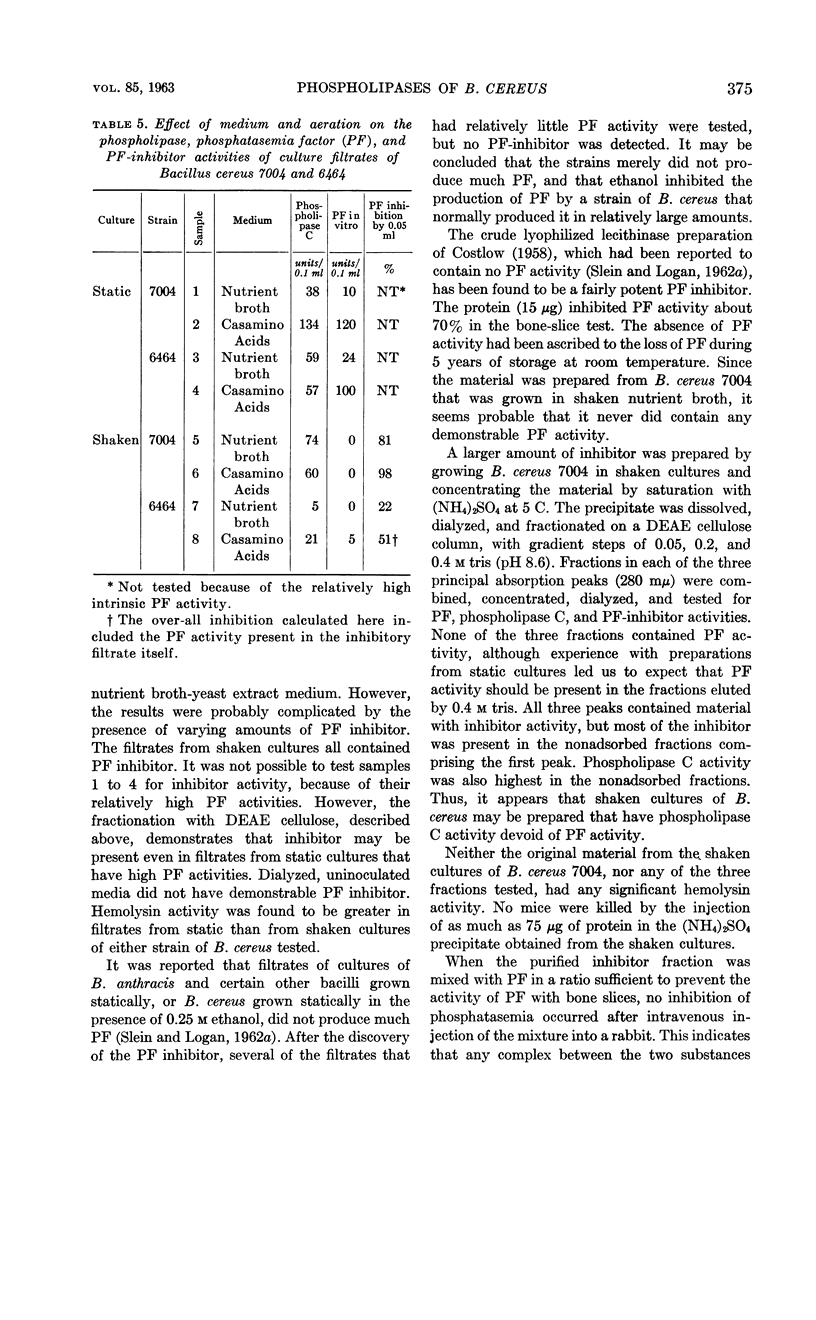
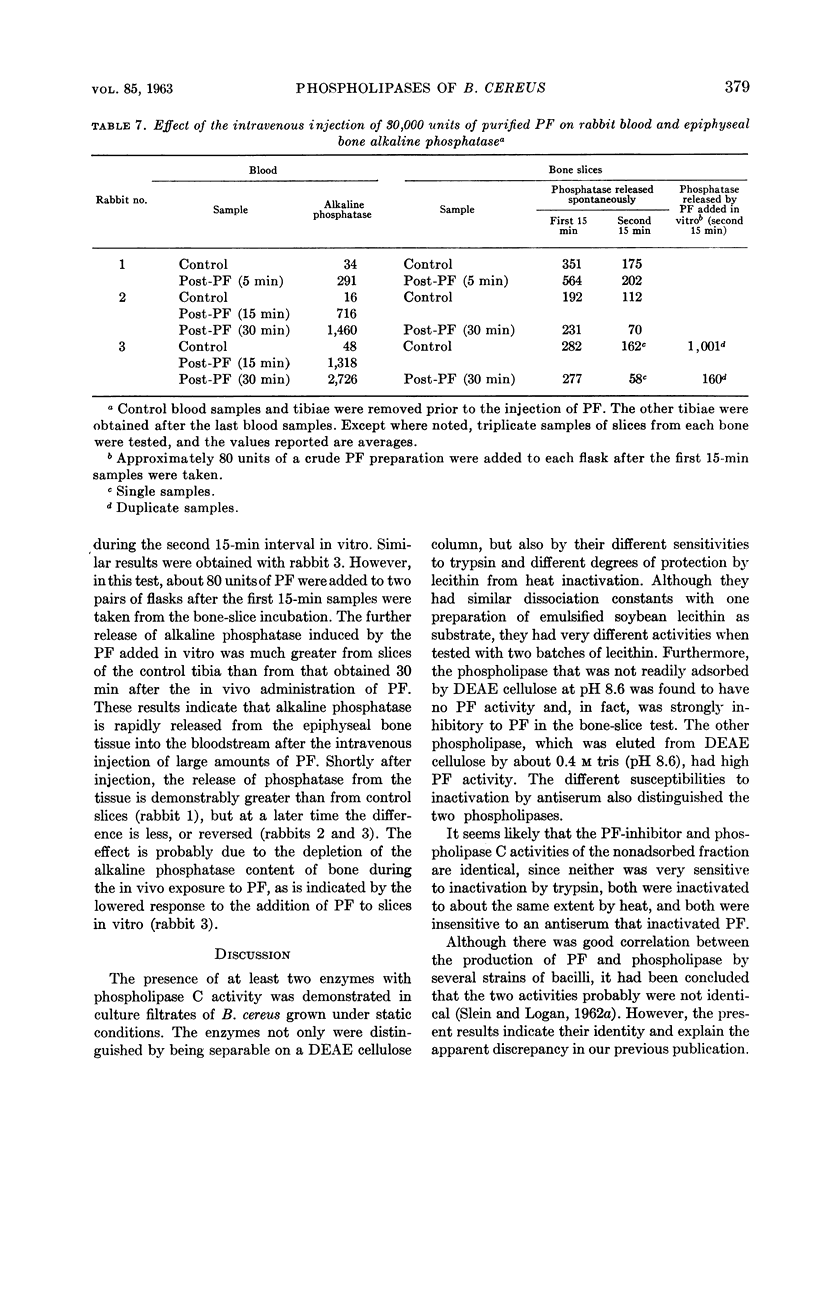
Abstract
Slein, Milton W. (U.S. Army Chemical Corps Biological Laboratories, Fort Detrick, Frederick, Md.) and Gerald F. Logan, Jr. Partial purification and properties of two phospholipases of Bacillus cereus. J. Bacteriol. 85:369–381. 1963.—Culture filtrates of Bacillus cereus contain a phosphatasemia factor (PF) that markedly increases blood alkaline phosphatase after intravenous injection into animals, and that releases alkaline phosphatase from epiphyseal bone slices in vitro. Fractionation of culture filtrates of B. cereus with N,N′-diethyl-aminoethyl cellulose results in the separation of two phospholipases, one that has PF activity and one that inhibits PF activity in vitro. Growth of shaken cultures favors accumulation of the inhibitor, whereas static cultures yield more PF. Lethality for mice and hemolysin activity do not appear to be associated with the phospholipase that inhibits PF. The relationship of the lethal and hemolysin factors to the phospholipase that produces phosphatasemia is not clear. The effects of heat, trypsin, lecithin, and antiserum on the phospholipases are reported. The intravenous injection of relatively large amounts of the purified PF resulted in the depletion of bone alkaline phosphatase.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTENBERN R. A. Lysogeny and toxinogeny in Bacillus cereus. Biochem Biophys Res Commun. 1962 Sep 25;9:109–112. doi: 10.1016/0006-291x(62)90097-9. [DOI] [PubMed] [Google Scholar]
- COSTLOW R. D. Lecithinase from Bacillus anthracis. J Bacteriol. 1958 Sep;76(3):317–325. doi: 10.1128/jb.76.3.317-325.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNKEL H. G., TISELIUS A. Electrophoresis of proteins on filter paper. J Gen Physiol. 1951 Sep;35(1):89–118. doi: 10.1085/jgp.35.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- MOLNAR D. M. Separation of the toxin of Bacillus cereus into two components and nonidentity of the toxin with phospholipase. J Bacteriol. 1962 Jul;84:147–153. doi: 10.1128/jb.84.1.147-153.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F. MECHANISM OF ACTION OF THE TOXIN OF BACILLUS ANTHRACIS II. : Alkaline Phosphatasemia Produced by Culture Filtrates of Various Bacilli. J Bacteriol. 1962 Feb;83(2):359–369. doi: 10.1128/jb.83.2.359-369.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAYSER K. A., COLOWICK S. P. Properties of crystalline hexokinase from yeast. III. Studies on glucose-enzyme interaction. Arch Biochem Biophys. 1961 Jul;94:169–176. doi: 10.1016/0003-9861(61)90025-x. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]


