Abstract
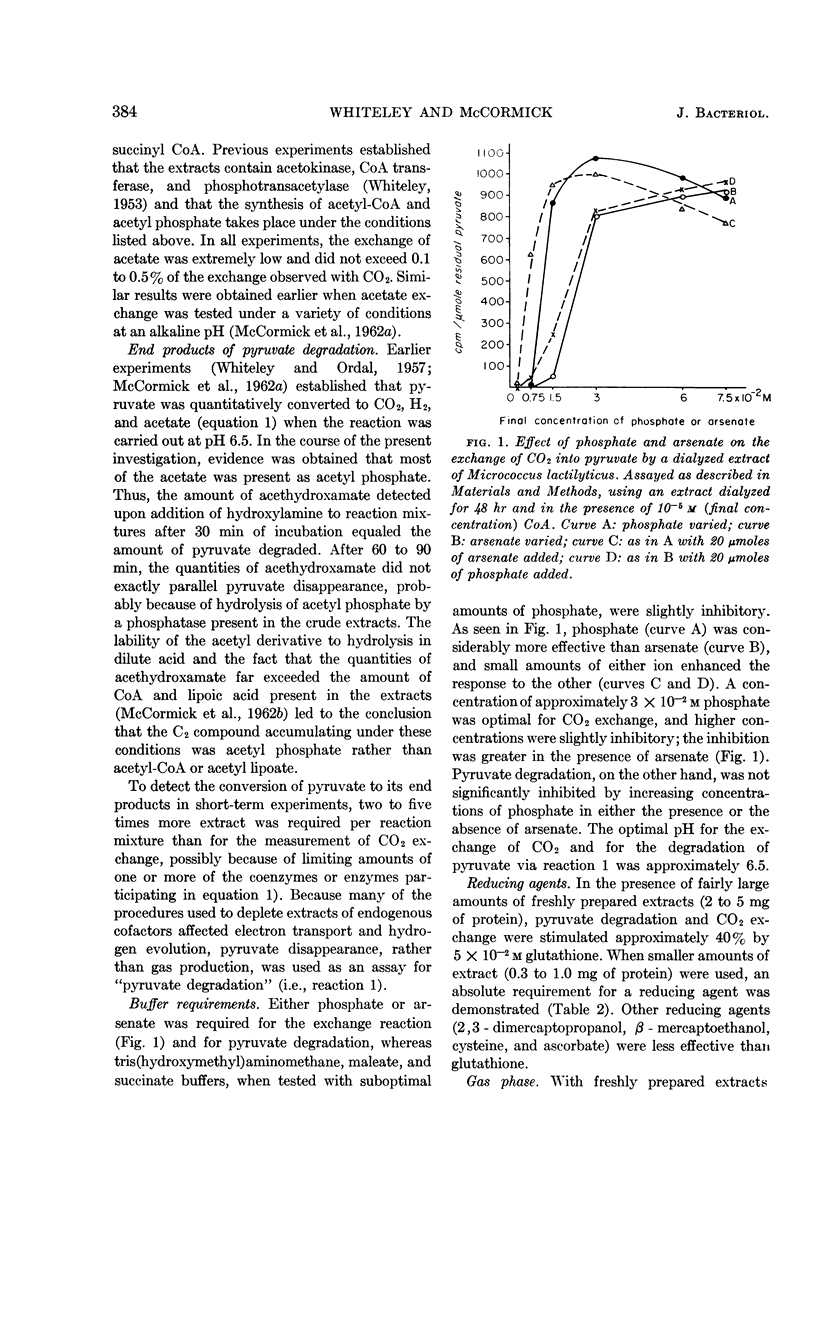
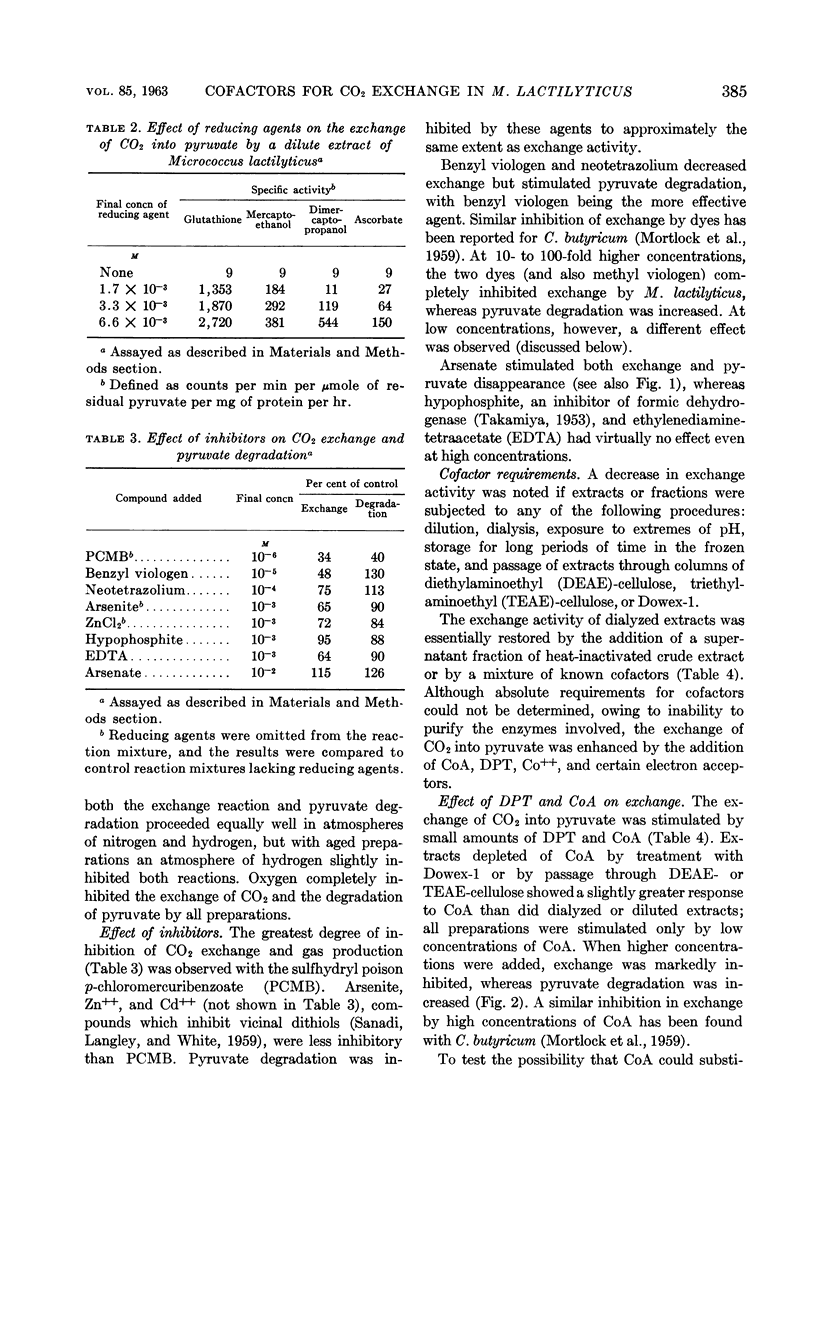
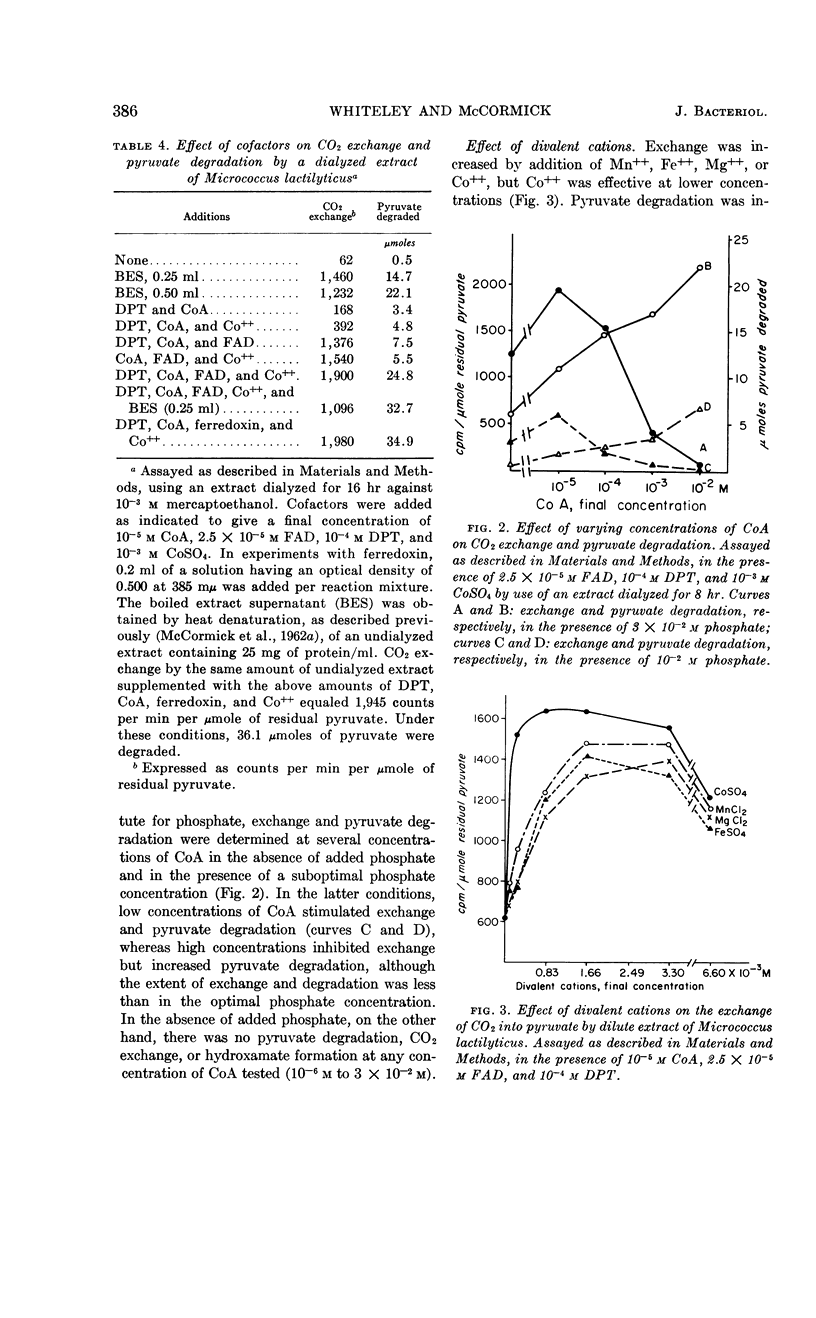
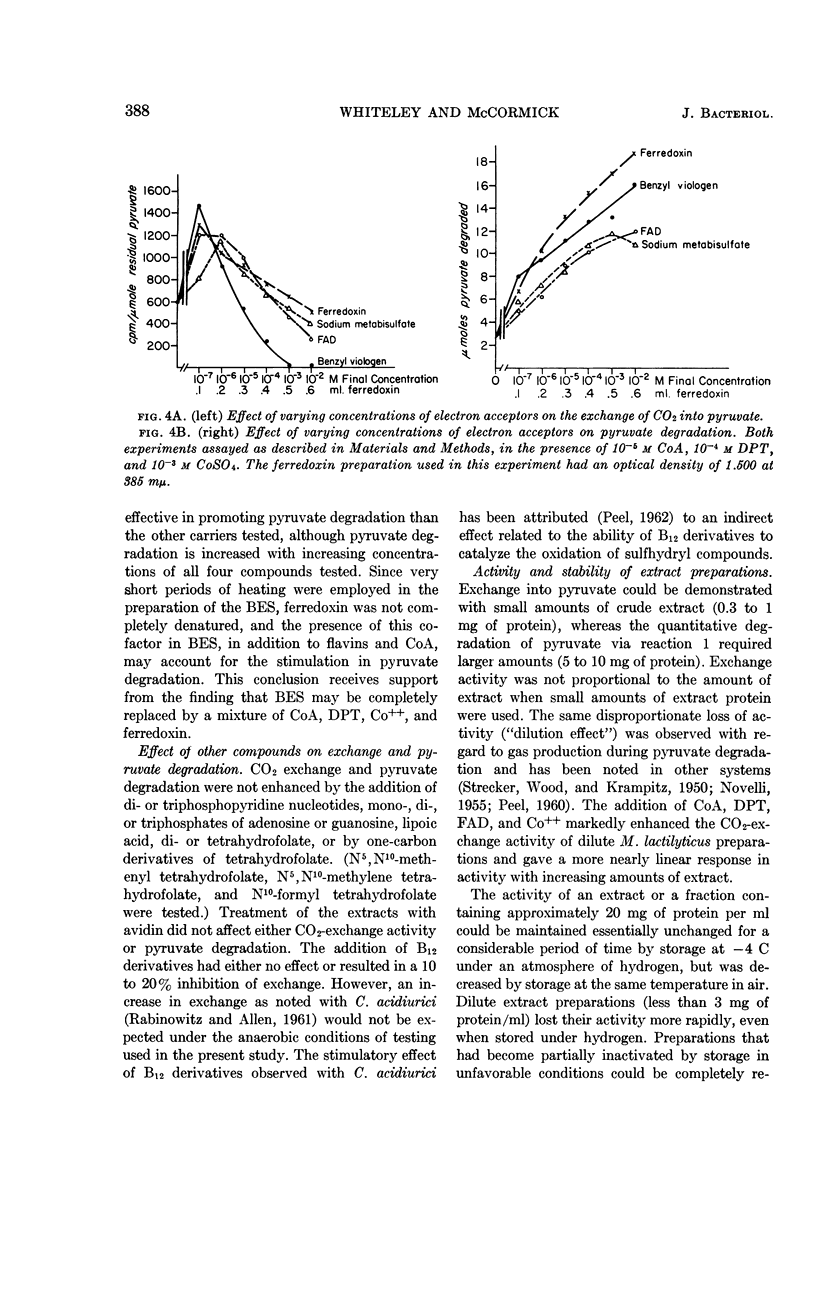
Whiteley, H. R. (University of Washington, Seattle) and N. G. McCormick. Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J. Bacteriol. 85:382–393. 1963.—At an acid pH, extracts of Micrococcus lactilyticus (Veillonella alcalescens) catalyze the oxidative decarboxylation of pyruvate to carbon dioxide, hydrogen, and acetyl phosphate, and the rapid exchange of carbon dioxide into the carboxyl group of pyruvate. These reactions take place only under anaerobic conditions and require phosphate (or arsenate), a reducing agent, diphosphothiamine, coenzyme A, an electron acceptor (ferredoxin, flavins, dyes, or certain inorganic anions), and a divalent cation (Co++> Mn++ > Mg++ > Fe++). High concentrations of coenzyme A and electron acceptors stimulate pyruvate breakdown but inhibit CO2 exchange. Exchange is also inhibited by p-chloromercuribenzoate but not by arsenite. Extracts rapidly lose the ability to mediate the exchange reaction after passage through diethylaminoethyl- or triethylaminoethyl-cellulose or Dowex-1; this loss in activity may be prevented by adding a reducing agent and the above cofactors. The exchange of CO2 and formate by M. lactilyticus is compared.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARLSON G. L., BROWN G. M. The natural occurrence, enzymatic formation, and biochemical significance of a hydroxyethyl derivative of thiamine pyrophosphate. J Biol Chem. 1961 Jul;236:2099–2108. [PubMed] [Google Scholar]
- DELAVIER-KLUTCHKO C. [Intervention of biotin in the clastic reaction of pyruvate by Clostridium saccharobutyricum]. C R Hebd Seances Acad Sci. 1961 Mar 13;252:1681–1683. [PubMed] [Google Scholar]
- Foubert E. L., Douglas H. C. Studies on the Anaerobic Micrococci: I. Taxonomic Considerations. J Bacteriol. 1948 Jul;56(1):25–34. [PMC free article] [PubMed] [Google Scholar]
- GOEDDE H. W., INOUYE H., HOLZER H. [Conversion of "active acetaldehyde" (alpha-hydroxyethyl thiamine pyrophosphate) into acetyl coenzyme A with pyruvate oxidase]. Biochim Biophys Acta. 1961 Jun 10;50:41–44. doi: 10.1016/0006-3002(61)91057-5. [DOI] [PubMed] [Google Scholar]
- HOLZER H., BEAUCAMP K. [Detection and characterization of alpha-lactylthiamine pyrophosphate ("active pyruvate") and alpha-hydroxyethylthiamine pyrophosphate ("active acetaldehyde") as intermediate products of pyruvate decarboxylation by pyruvate decarboxylase from brewer's yeast]. Biochim Biophys Acta. 1961 Jan 15;46:225–243. doi: 10.1016/0006-3002(61)90747-8. [DOI] [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- MORTLOCK R. P., VALENTINE R. C., WOLFE R. S. Carbon dioxide activation in the pyruvate clastic system of Clostridium butyricum. J Biol Chem. 1959 Jul;234(7):1653–1656. [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS I. : General Properties of the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):887–898. doi: 10.1128/jb.83.4.887-898.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS II. : Studies of Cofactors in the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):899–906. doi: 10.1128/jb.83.4.899-906.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVELLI G. D. The exchange of H14COOH with the carboxyl group of pyruvate by Clostridium butylicum and Micrococcus lactilyticus. Biochim Biophys Acta. 1955 Dec;18(4):594–596. doi: 10.1016/0006-3002(55)90170-0. [DOI] [PubMed] [Google Scholar]
- PEEL J. L. The breakdown of pyruvate by cell-free extracts of the rumen micro-organism LC. Biochem J. 1960 Mar;74:525–541. doi: 10.1042/bj0740525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ J. C., ALLEN E. H. Role of vitamin B12 derivatives in reactions of pyruvate. Fed Proc. 1961 Dec;20:962–966. [PubMed] [Google Scholar]
- SANADI D. R., LANGLEY M., WHITE F. alpha-Ketoglutaric dehydrogenase. VII. The role of thioctic acid. J Biol Chem. 1959 Jan;234(1):183–187. [PubMed] [Google Scholar]
- VALENTINE R. C., JACKSON R. L., WOLFE R. S. Role of ferredoxin in hydrogen metabolism of Micrococcus lactilyticus. Biochem Biophys Res Commun. 1962 Jun 4;7:453–456. doi: 10.1016/0006-291x(62)90334-0. [DOI] [PubMed] [Google Scholar]
- WHITELEY H. R., ORDAL E. J. Fermentation of alpha keto acids by Micrococcus aerogenes and Micrococcus lactilyticus. J Bacteriol. 1957 Sep;74(3):331–336. doi: 10.1128/jb.74.3.331-336.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J Biol Chem. 1955 Aug;215(2):637–643. [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the phosphoroclastic reaction of Clostridium butyricum. J Biol Chem. 1953 Dec;205(2):755–765. [PubMed] [Google Scholar]
- WOOLFOLK C. A., WHITELEY H. R. Reduction of inorganic compounds with molecular hydrogen by Micrococcus lactilyticus. I. Stoichiometry with compounds of arsenic, selenium, tellurium, transition and other elements. J Bacteriol. 1962 Oct;84:647–658. doi: 10.1128/jb.84.4.647-658.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R. The Mechanism of Propionic Acid Formation by Succinate Decarboxylation: I. The Activation of Succinate. Proc Natl Acad Sci U S A. 1953 Aug;39(8):772–779. doi: 10.1073/pnas.39.8.772. [DOI] [PMC free article] [PubMed] [Google Scholar]


