Abstract
Mutations in adenomatous polyposis coli (APC) protein is a major contributor to tumor initiation and progression in several tumor types. These mutations affect APC function in the Wnt-β-catenin signaling and influence mitotic spindle anchoring to the cell cortex and orientation. Here we report that the mitotic anchoring and orientation function of APC is regulated by cyclin A/cdk2. Knockdown of cyclin A and inhibition of cdk2 resulted in cells arrested in mitosis with activation of the spindle assembly checkpoint. The mitotic spindle was unable to form stable attachments to the cell cortex, and this resulted in the spindles failing to locate to the central position in the cells and undergo dramatic rotation. We have demonstrated that cyclin A/cdk2 specifically associates with APC in late G2 phase and phosphorylates it at Ser-1360, located in the mutation cluster region of APC. Mutation of APC Ser-1360 to Ala results in identical off-centered mitotic spindles. Thus, this cyclin A/cdk2-dependent phosphorylation of APC affects astral microtubule attachment to the cortical surface in mitosis.
Adenomatous polyposis coli (APC)5 was initially identified as a tumor suppressor in familial colon cancers. It is a regulator of Wnt-β-catenin signaling and thereby regulates progression into the cell cycle, but also has Wnt-independent mitotic roles in spindle anchoring and kinetochore function (1–3). These latter functions of APC are mediated through its ability to bind microtubules and the end-binding protein, EB1 (2). Loss or mutation of APC has been demonstrated to increase chromosomal instability, although whether this is through its Wnt-dependent or independent functions is unclear (3). The mitotic defects caused by APC mutation and depletion are characterized by an inability to locate the center of the cell and failure of chromosomal alignment (4). It was also associated with a loss of normal spindle orientation in small intestinal crypts of APCMin/+ mice (5), suggesting that disruption of the normal mitotic functions of APC are likely to be major contributors to the chromosomal instability observed.
APC interaction with EB1 is regulated by phosphorylation of its C-terminal domain by cyclin B/cdk1 in mitosis (6, 7). The majority of APC mutations occurs in a region from codons 1,000 to 1,500 called the mutation cluster region (MCR) and result in truncations of the C-terminal half of the protein, which includes the β-catenin, microtubule, and EB1 binding sites of APC (1, 2). Depletion of either APC or EB1 produce almost identical mitotic defects, indicating their interaction is critical to normal spindle formation (4, 8). However, expression of various truncation mutants across the MCR revealed interesting differences the spindle defects observed, suggesting that this role of APC in spindle function is not solely due to interaction with EB1 (4). Progression into mitosis is regulated by cyclin B/cdk1, but the timing of its activation is regulated by cyclin A/cdk2 (9–12), which in turn is regulated by the dual specificity phosphatase cdc25B in G2 phase (13). Cyclin A is destroyed at prometaphase (14) suggesting that its activity is required for not only entry into mitosis but during the early part of mitosis itself. The majority of substrates identified for cyclin A/cdk2 are nuclear, where the majority of cyclin A/cdk2 is localized in G2, but reports also suggest that cyclin A is capable of localizing to both the cytoplasm and centrosomes (9, 14, 15), thus there are likely to be additional substrates for this complex in the cytoplasmic compartment. In vitro studies using Xenopus extracts have demonstrated that cyclin A/cdk is capable of increasing microtubule nucleation at the centrosomes (16). Thus it is likely that cyclin A in association with its cdk partner has roles in not only promoting entry into mitosis but also in establishing mitosis, possibly by influencing the mitotic machinery.
We have used siRNA to knockdown cyclin A2, the major cyclin A isoform in somatic cells, and cdk2 inhibitors to examine the role of the G2 phase cyclin A/cdk2 complex in cell cycle progression. We demonstrate that knockdown of cyclin A delayed progression through mitosis and activation of the spindle assembly checkpoint. Spindle anchoring was also defective, a phenotype identical to APC-truncating mutants. We demonstrate that cyclin A/cdk2 binds to APC in late G2 phase/early mitosis and phosphorylates Ser-1360, and that the lack of this phosphorylation of APC results in identical mitotic defects to the absence of cyclin A/cdk2.
MATERIALS AND METHODS
Cell Culture and Synchronization
The cell lines, human cervical cancer cell line HeLa, HeLa cells stably expressing GFP-H2B or Cherry-tubulin as described previously (17), U2OS osteosarcoma cells, and primary neonatal foreskin fibroblasts (NFF) were cultured as described previously (9). Cells were synchronized with a single thymidine block as described previously (9, 18). Cells were collected for FACS analysis of DNA content as previously described (9, 18). For immunoblotting, cells were lysed analyzed as previously described (9, 17). Proteins were probed with the indicated antibodies and detected by enhanced chemiluminescent detection.
siRNA
For siRNA-mediated ablation of cyclin A, cells were transfected with three siRNAs using a scrambled control as described previously (9). Cdc25B siRNA was purchased from Dharmacon. To generate a cyclin A expression clone, which was resistant to A1 siRNA, site-directed mutagenesis was carried out on pCherry-Cyclin A as per manufacturer's instructions (Stratagene). HeLa cells were transfected with the pCherry-resistant cyclin A construct as previously described. siRNA knockdown of cyclin A was carried out at 24-h post-transfection.
Immunofluorescence Microscopy
Cells grown on poly-l-lysine-coated coverslips were treated as required and fixed in methanol at −20 °C. The cells were treated with 0.1% saponin/2% bovine serum albumin/PBS for 1 h at room temperature, before probing with the primary antibodies indicated in the figure legends in 2% bovine serum albumin/PBS at room temperature for 60 min; after washing in PBS, the cells were incubated with the appropriate secondary antibody conjugated with either fluorescein isothiocyanate (FITC) or Cy3. DNA was counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) at 1 μg/ml. Confocal images were obtained on the Zeiss LSM 510 Meta confocal or the Zeiss Apoptome microscope (Zeiss, Goettingen, Germany) as indicated. Epi-fluorescence microscopy was carried out using a Zeiss Axioskop 2 plus microscope and a Zeiss Axiocam HRm camera. The following antibodies used in this study were purchased from the indicated commercial sources: rabbit polyclonal cyclin A and cdc25B (Santa Cruz Biotechnology), α-tubulin, and Aurora B and EB1 (BD Biosciences), MAD2 (Covance), and APC (Abcam). Polyclonal cyclin B1, cdk2, and cdk1 antibodies were made in-house as previously reported (13, 18). To examine astral microtubules (MT), cells were fixed as follows: cells were first washed with PBS followed by MT stabilization buffer (80 mm Pipes pH 6.8, 3 mm EGTA, 1 mm MgCl2 in PBS) for ∼3 s at room temperature. This was replaced with 3% paraformaldehyde into which 1% glutaraldehyde was added after ∼3 s and incubated for 10 min at 37 °C. Cells were then incubated twice with 1 mg/ml NaBH4 in PBS for 2–7 min at 37 °C, washed with PBS, and stored at 4 °C in PBS.
Time Lapse Microscopy
Time lapse experiments were performed using HeLa cells, and HeLa cells stably expressing GFP-H2B or Cherry-tubulin as previously described (9, 17). To maintain cells in mitosis for an extended period of time, a non-destructible form of cyclin B, TAT-ΔN86 cyclin B, was added to the cells as previously described (19).
Production of APC Mutants and GST Fusion Proteins
The APC fragment 1089–1370 was produced by PCR from full-length APC (pEF-myc-APC, gift from M. Faux, Ludwig Institute, Australia) and subcloned into pGEX-2T. Serine 1100 and/or serine 1360 were mutated to alanines by PCR in the pGEX fusion constructs and confirmed by sequencing. GST fusion products were expressed and purified as previously described (18). To produce full-length APC S 1360A mutant, the mutant fragment of GEX-APC-(1011–1470) was PCR-amplified then subcloned back into full-length APC (pEF-myc-APC) using unique restriction sites.
Kinase Assays
To evaluate cyclin A-specific kinase activity and GST-APC-(1011–1470)-associated kinase, cells were lysed for 30 min at 4 °C with agitation in lysis buffer with 0.25 m NaCl. Equal amounts of protein (300–500 μg per sample) were immunoprecipitated with either anti-cyclin A and 30 μl of protein A-Sepharose or GST-APC bound to glutathione-Sepharose overnight at 4 °C. The precipitates were washed three times with NETN buffer and once with KB buffer (20 mm Tris, pH 7.5, 1 mm dithiothreitol) before incubating in 30 μl of RC buffer (20 mm Tris, pH 7.5, 15 mm MgCl2, 0.5 mg/ml histone H1, 5 μCi [γ-32P]ATP per sample) for 15 min at 30 °C. The labeled proteins were resolved on 10% SDS-PAGE and quantitated by phosphorimaging. The cdk inhibitor, roscovitine and the cdk2 inhibitor RO-03-9099 were used at a concentration of 0.5 μm.
Colony Formation Assays
HeLa cells were transfected with siRNA A1 as previously described, 48-h post-transfection, the medium was gently removed and replated into fresh dishes to determine the number of viable cells present in the medium. The parental plate was harvested to determine levels of cyclin A by immunoblotting. Colonies were allowed to grow for 10 days, fixed in methanol/acetic acid (3:1) stained with crystal violet (0.4 mg/ml), and examined.
RESULTS
siRNA Depletion of Cyclin A Delays Progression through Mitosis
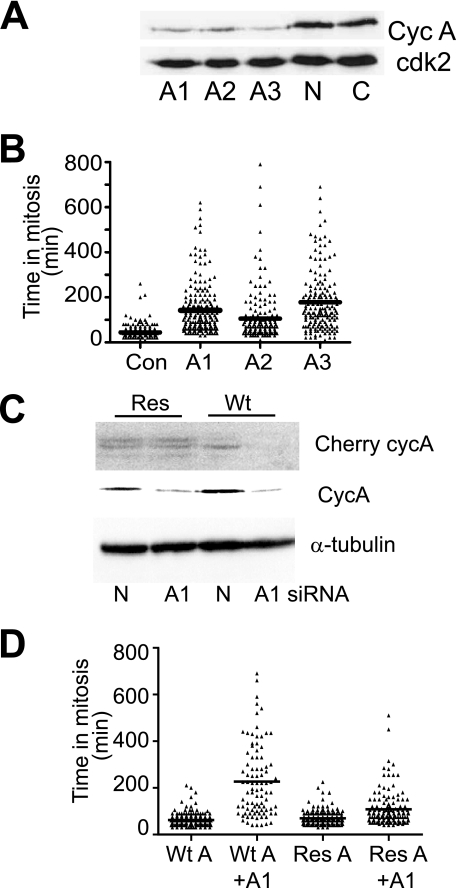
We have developed three independent cyclin A-specific siRNAs that reduced cyclin A protein levels (9) (Fig. 1A). This depletion was evident for up to 72 h after siRNA transfection (supplemental Fig. S1). The transfection efficiency was determined to be >90% when FITC-conjugated siRNA was used. Cyclin A depletion in asynchronous cultures increased the proportion of mitotic cells 24-h post-transfection, as determined by FACS analysis of MPM2 antibody staining of mitotic cells; 3.6% of cyclin A-depleted cells were in mitosis compared with 1.6% in controls. To further examine the effects of cyclin A knockdown on mitotic progression, cyclin A siRNA-transfected HeLa cells were synchronized in early S phase by thymidine block-and-release, and progress through the cell cycle was followed by time lapse microscopy. Depletion of cyclin A with any of the three cyclin A siRNAs consistently resulted in an extension in the duration of mitosis (Fig. 1B). HeLa cells transfected with the nonsense siRNA (Con) had a mean duration of 44 min in mitosis compared with 142 min in cyclin A knockdown cells (results for A1 siRNA knockdown). A proportion of cyclin A siRNA-treated cells had relatively normal length mitoses, which are likely to be cells where depletion failed.
FIGURE 1.
Cyclin A knockdown results in an extension in mitosis. A, HeLa cells were transfected with 50 nm three separate cyclin A siRNAs, A1, A2, and A3 or nonsense (N) siRNA, or untreated control (C), and harvested at 24 h after transfection. Whole cell lysates were immunoblotted for cyclin A and cdk2. B, HeLa cells were transfected as in A, and 16 h post-transfection cells were followed with time lapse microscopy. Cells were scored for mitosis; over 100 cells were counted for each treatment, with the data representing the mean of at least three experiments. C, HeLa cells were transfected with WT or resistant pCherry-cyclin A, and 24 h later were transfection with either cyclin A siRNAs A1 or nonsense siRNA (N). Cells were harvested at 16-h post-siRNA transfection, and lysates immunoblotted for Cherry-cyclin A, endogenous cyclin A and α-tubulin. D, HeLa cells were treated as in C, and at 16-h post-siRNA transfection, cells were followed by time lapse microscopy. Cells were scored for mitosis; >90 cells were counted for each treatment, with the data representing the mean of three experiments.
To demonstrate that this mitotic delay was specifically due to cyclin A depletion, cyclin A fused with Cherry fluorescent protein (Wt Cyclin A) was mutated to generate a clone resistant to the A1 siRNA (Res Cyclin A, Fig. 1C). Transfection of Wt Cyclin A into HeLa cells had little effect on transit through mitosis, while cyclin A knockdown using A1 siRNA in Wt Cyclin A-expressing cells increased the length of mitosis to an average of 227 min (Fig. 1D, p < 0.0001), similar to the effect of cyclin A depletion in parental HeLa cells (Fig. 1B). Expression of siRNA resistant Res Cyclin A in HeLa cells again had little effect on mitosis, while knockdown of the endogenous cyclin A and not the Res Cyclin A by siRNA A1 significantly rescued the mitotic delay normally observed (Fig. 1D, p < 0.0001). Together, the ability of three separate siRNA to induce a mitotic delay which was rescued by re-introduction of an siRNA-resistant form of cyclin A provides convincing evidence that the mitotic delay was consequence of the lack of cyclin A.
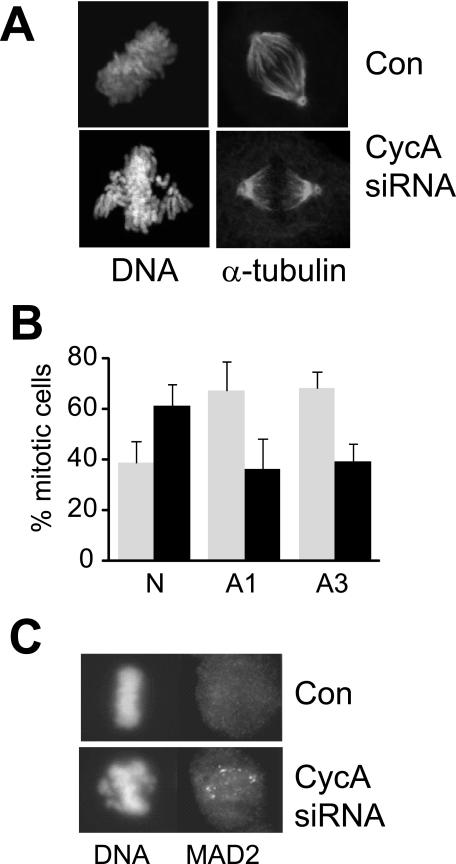
Examination of the mitotic cells in cyclin A siRNA-treated cultures by immunofluorescence analysis of DNA and microtubules revealed normal bipolar spindles but a failure of some chromosomes to congress to the metaphase plate (Fig. 2A). Quantitation of proportion of cells with formed bipolar spindles and lagging chromosomes revealed an increase from 38 to 67% (p < 0.01; Fig. 2B). Using MAD2 staining as a marker of spindle assembly checkpoint activity (20) revealed that chromosomes that had failed to congress were stained for MAD2, indicating activation of the spindle checkpoint (Fig. 2C, lower panel), while cells without lagging chromosomes had no MAD2 staining (Fig. 2C, upper panel). This indicated activation of the spindle assembly checkpoint in response to retarded chromosome congression. Examination of other regulators of chromosome congression, the chromosomal passengers Aurora B, INCENP, survivin, and Borealin (21) revealed their normal accumulation at the centromeres was unaffected by cyclin A depletion (data not shown). Examination of cells at extended times after cyclin A knockdown revealed no increase in cells with multiple or fractured nuclei, indicators of spindle checkpoint failure. Time lapse microscopy of cyclin A knockdown in HeLa cells expressing GFP-H2B confirmed that cells delayed in mitosis, and did not initiate anaphase and telophase until correct chromosome alignment was achieved, demonstrating that the spindle checkpoint was intact.
FIGURE 2.
Cyclin A depletion activates the spindle assembly checkpoint. A, HeLa cells were transfected with control (upper panel) and cyclin A A1 siRNA (lower panel), synchronized with a single thymidine block and harvested in mitosis. Cells were stained for DNA and α-tubulin, and mitotic cells imaged 24-h post-transfection. B, HeLa cells transfected with either scrambled (N), cyclin A siRNAs A1 or A3 were fixed and stained for DNA and microtubules. The percentage of mitotic cells containing formed bipolar spindles and normal metaphase plates (black bars), or bipolar spindles with lagging chromosome migration (gray bars) was assessed. The data are the average of triplicate determinations counting >100 mitotic cells in each experiment. C, cyclin A knockdown HeLa cells were harvested at 24 h after transfection and stained for MAD2 and DNA. A metaphase and prometaphase cell are shown.
Cyclin A Depletion Causes Dynamic Rotation of the Mitotic Spindle
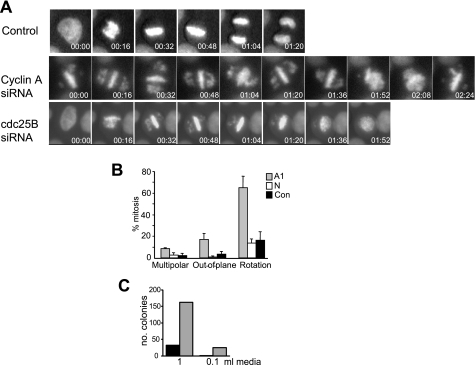
Time lapse microscopy of GFP-H2B HeLa cells revealed further mitotic abnormalities. Normally, the metaphase plate orientates so that the lateral movement of the dividing plate in anaphase is along the longest axis of the cell, parallel to the plane of the culture dish (22). This may involve a limited rotation of the metaphase plane in an axis perpendicular to the culture dish (Fig. 3A, Control). In cyclin A knockdown cells, the incomplete metaphase plate rotated dynamically within the cell (Fig. 3A, cyclin A siRNA, and supplemental movie S2). In some cases the condensed DNA appeared as a disk, suggesting that it had rotated to be parallel to the culture dish rather than its normal perpendicular orientation (Fig. 3A, cdc25B siRNA, 01:52). In addition, siRNA knockdown of cdc25B (supplemental Fig. 2A), the activator of G2 phase cyclin A/cdk2, produced similar rotation of the metaphase plate (Fig. 3A, cdc25B siRNA), indicating that lack of cyclin A/cdk2 activity was responsible for the rotation observed. Rotation of the metaphase plate was not simply a consequence of the extended time in mitosis, as introduction of a non-degradable form of cyclin B1 did not result in metaphase rotation during the resulting prolonged mitosis (data not shown).
FIGURE 3.
Cyclin A and cdc25B knockdown results in mitotic spindle rotation. A, time lapse images of cells undergoing mitosis in control, cyclin A siRNA (A1) or cdc25B siRNA-transfected GFP-H2B HeLa cells were taken at the indicated times. Time is given as hours/minutes for the start of mitosis. B, quantitation of effects on metaphase plate dynamics in control (Con), nonsense (N), and A1 siRNA-transfected HeLa cells. The data are from at least three independent experiments, counting >100 cells for each experiment. C, HeLa cells were transfected with nonsense or A1 cyclin A siRNA, and 48-h post-transfection, the indicated volumes of culture medium were removed and replated. The data are from duplicate determinations.
Confocal microscopy of cyclin A-depleted cells confirmed the rotation of the metaphase plate and spindle. Sectioning through control mitotic cells showed both spindle poles in the same section or within 3 μm in the vertical plane (supplemental Fig. S3, Control), whereas in cyclin A knockdown cells, the spindle poles were commonly found in Z-sections separated by >10 μm, and in some cells the spindle was oriented with the poles perpendicular to the plane of the culture dish (supplemental Fig. S3, Cyclin A siRNA). Quantification of mitotic abnormalities revealed that cyclin A knockdown resulted in more than 65% of the mitotic cells demonstrating rotation of the metaphase plate compared with only 15% of control cells (Fig. 3B).
A consequence of this spindle rotation was that 10% of cell divisions occurred out-of-the plane of the dish in cyclin A knockdown cells compared with less than 3% out-of-plane division in controls (Fig. 3B). In the cyclin A knockdown cells the few lagging chromosomes often formed a secondary metaphase plate at an angle to the primary plate (supplemental Fig. S2B). These asymmetrical multipolar spindles were observed in 10% of cyclin A knockdown cells, whereas the controls contained <3% symmetrical multipolar spindles commonly observed in HeLa culture. Similar proportions of each mitotic phenotype were observed with all three cyclin A siRNA (supplemental Fig. S4A), and the mitotic defects and spindle rotation were rescued with expression of siRNA-resistant Res cyclin A (supplemental Fig. S4B).
A consequence of out-of-plane division was that cells detached from the culture plate. To assess the relative frequency of this in control and cyclin A depleted cultures, colony formation assays on the medium collected from cultures transfected with cyclin A or nonsense siRNA and replated. Medium from cyclin A siRNA-treated cells produced >10-fold more colonies (Fig. 3C), confirming the elevated level of out-of-plane divisions observed in these cells.
Cyclin A Depletion Results in Failure of Centering of the Mitotic Spindle
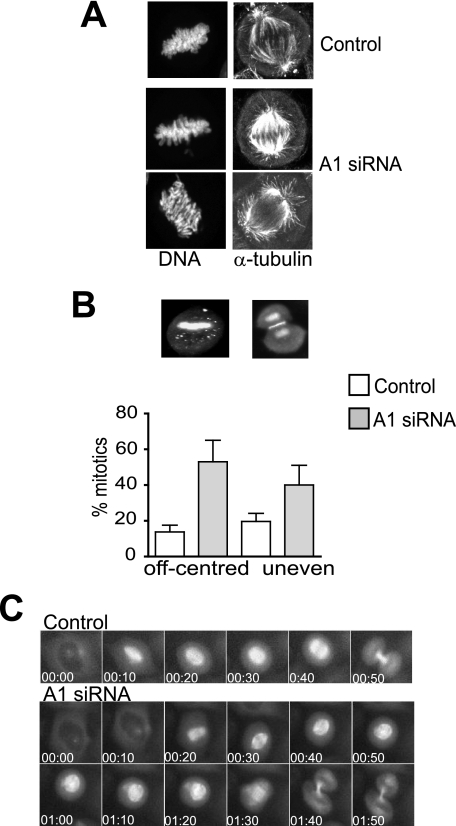
The mitotic spindle in cyclin A-depleted cells appeared intact although the astral microtubules appeared less directed in their contacts with the cell cortex. In controls, the astral microtubules make perpendicular contact with the cortex, whereas they appeared to fail to make direct contact with the cortex in the cyclin A-depleted cells, even where the spindle pole was in very close proximity to the cell cortex (Fig. 4A). There was also an increase in the proportion of cells where the mitotic spindle was off-centered in the cyclin A knockdown cells (55% compared with 15% in controls; Fig. 4B), most likely a direct consequence of the failure of the astral microtubules in contacting the cell cortex. Cyclin A depletion in U2OS and primary cultures of neonatal foreskin fibroblasts (NFF) with either A1 or A3 siRNA revealed similar elevated levels of off-centered spindles (supplemental Fig. S5A). This was also observed in cells undergoing anaphase (Fig. 4A), resulting in off-centered cytokinesis generating daughter cells of unequal size in 43% of these cells (Fig. 4B). To assess this in live cells, HeLa cells expressing Cherry-tubulin were examined by time lapse microscopy. Not only was it evident that the cyclin A knockdown cells spent longer in mitosis, but the mitotic spindle also moved dramatically within the cells, which frequently resulted in uneven cytokinesis (Fig. 4C). When cyclin A/cdk2 kinase activity was inhibited by the specific cdk2 inhibitor RO-09-3003 (9, 23) in G2 phase, a similar increase in the proportion of off-center mitotic spindles and uneven cytokinesis was evident (supplemental Fig. S5B).
FIGURE 4.
Cyclin A knockdown results in failure of spindle centering. A, control and cyclin A knockdown HeLa cells were fixed 24-h post-transfection and stained for DNA and α-tubulin. The outline of the cell shapes and position of the cortex are visible in the α-tubulin stain. The cyclin A-depleted cells shown are in metaphase (top) and anaphase (bottom). B, quantitation of mitotic spindle abnormalities, off-center metaphase plates and uneven cytokinesis, in HeLa cells transfected with cyclin A1 (A1) or scrambled siRNA (control) in three separate experiments. Images are of representative cells stained for DNA and INCENP to delineate the position of the midbody in cytokinesis. The data represent the mean of >100 cells in each treatment. C, time lapse microscopy images of cytokinesis in control or cyclin A siRNA (A1) transfected Cherry-tubulin HeLa cell taken at 10-min intervals.
Cyclin A/cdk2 Associates with and Phosphorylates APC in Late G2 Phase
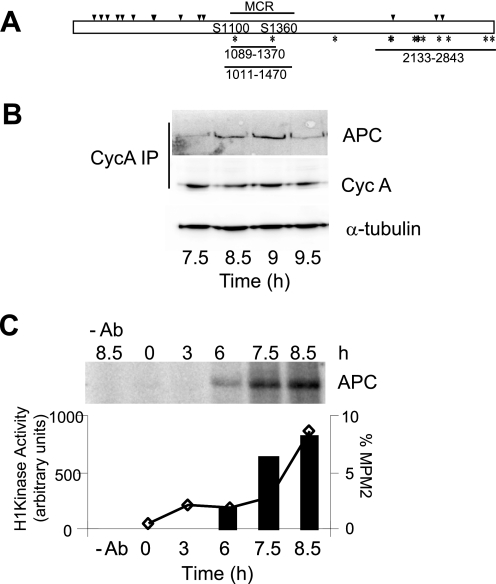
The spindle defects observed with knockdown of cyclin A or inhibition of the cyclin A/cdk2 activity are similar to those seen with disruption of the spindle-anchoring proteins, EB1 and APC. Mutation or depletion of these proteins compromise the ability of astral microtubules to make productive interactions with the cortical surface of the cell (4), although cyclin A depletion had no effect on either APC or EB1 levels (data not shown). Inspection of the amino acid sequences of EB1 and APC revealed that only APC contained both consensus cdk phosphorylation sites (13 sites, indicated with an asterisks in Fig. 5A) and cyclin A binding sites (14 sites, indicated with arrowheads). Cyclin A/cdk2 co-immunoprecipitated with APC from G2/M phase cells (Fig. 5B, 8.5–9 h post-thymidine release), although this appeared to be restricted to a short period during G/M transition as there was much reduced interaction at the 7.5- and 9-h time point, even though cyclin A and APC protein levels were relatively unchanged during this time course (Fig. 5B). This restricted period of interaction was observed in multiple experiments and appeared to correspond to late G2 phase. In vitro kinase assays performed using cyclin A immunoprecipitates from synchronized HeLa cells produced a phosphorylated band that co-migrated with APC (determined by immunoblotting the kinase reaction), which was not detected in control antibody precipitates from the peak fraction (8.5 h, Fig. 5C). High levels of cyclin A-associated APC phosphorylation occurred in late G2/early M phase (Fig. 5C, 7.5 and 8.5 h), denoted by the increasing proportion of MPM2 prior to a significant increase in the proportion of cells entering mitosis, assessed using MPM-2 staining (lower panel, Fig. 5C). This corresponded to the peak of cyclin A/cdk2 activity, which preceded entry into mitosis (supplemental Fig. S6).
FIGURE 5.
Cyclin A/cdk2 associates with and phosphorylates APC. A, representation of APC. The arrowheads represent the RXL cyclin A binding sites, and the asterisks represent the cdk phosphorylation sites. The Ser-1100 and Ser-1360 sites are indicated as are the GST-APC truncations used in the in vitro phosphorylation studies. The position of the mutation cluster region (MCR) is also indicated. B, cyclin A immunoprecipitates from HeLa cells harvested at times indicated post-thymidine release, immunoblotted for APC. The levels of cyclin A and α-tubulin (loading control) were determined by immunoblotting of the whole cell lysate. C, immunoprecipitated cyclin A complexes were assayed for kinase activity. The phosphorylated band was identified as APC by immunoblotting. The level of phosphorylation relative to control antibody (-Ab) is shown in the bar graph. The percentage of cells in mitosis was determined by MPM2 staining and represented in the line graph.
APC Ser-1360 Is Phosphorylated by Cyclin A/cdk2
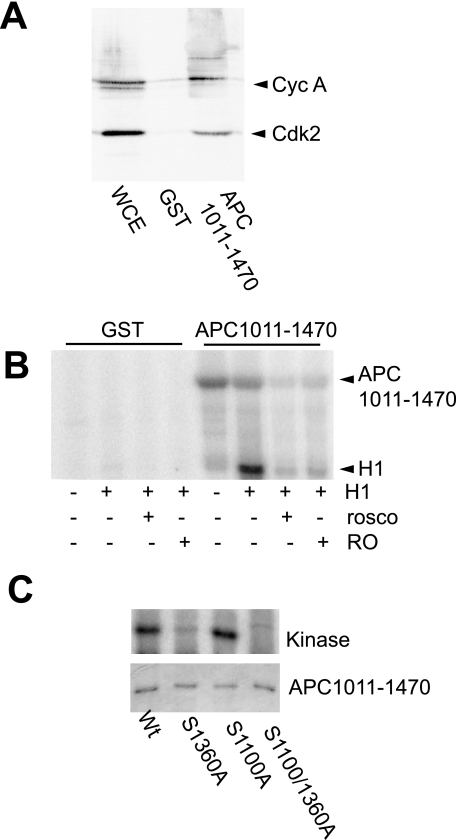
APC mutants with truncations between codons 850 and 1450 produce mitotic phenotypes similar to cyclin A depletion (4), and this region contained two potential cdk phosphorylation sites, Ser-1100 and Ser-1360. When a GST-APC fragment, APC-(1011–1470), was mixed with late G2 phase cell extracts, cyclin A/cdk2 bound to GST-APC-(1011–1470) but not to GST alone (Fig. 6A). No cyclin B was found to associate with APC-(1011–1470). In vitro kinase assay of GST-APC-(1011–1470)/cyclin A/cdk2 complex showed both the APC fragment and histone H1 were phosphorylated (Fig. 6B), and was sensitive to the cdk inhibitor roscovitine and the cdk2-specific inhibitor RO-09-3033, demonstrating that cyclin A/cdk2 was capable of binding to and phosphorylating APC-(1011–1470).
FIGURE 6.
Cyclin A/cdk2 phosphorylates APC Ser-1360. A, whole cell lysates from G2/M phase HeLa cells were incubated with GST-APC-(1011–1470) or GST alone, the resulting complexes were washed, and immunoblotted for cyclin A and cdk2. WCE, whole cell extract. B, GST alone or GST-APC1011–1470 was used to bind kinase activity from G2/M phase HeLa cell lysates. The GST or GST-APC-(1011–1470) were subjected to in vitro phosphorylation assays of the GST or GST-APC fragment, and exogenously added histone H1. The kinase activity was also tested for inhibition by either 0.5 μm roscovitine (rosco) or 0.5 μm RO-09-3033 (RO). C, in vitro kinase assay of cyclin A/cdk2 bound to GST-APC-(1011–1470) (Wt), and the S1036A, S1100A, and S1100/1360A mutants of GST-APC-(1011–1470). The GST fusions were used to bind cyclin A/cdk2 from G2/M phase HeLa cell lysates as in A. Loading was determined by Coomassie Blue staining of the gel.
The two potential cdk2 phosphorylation sites in GST-APC-(1011–1470), Ser-1100 and Ser-1360 were mutated to alanine individually (S1100A, S1360A) or together (S1100/1360A) in GST-APC-(1089–1370). Serine 1360 was the sole site phosphorylated by cyclin A/cdk2 (Fig. 6C). Cyclin B/cdk1 phosphorylates APC at a number of sites on the C terminus (24). GST-APC-(2133–2843), covering the C-terminal cyclin B/cdk1 phosphorylation sites, bound cyclin A and cdk2 to a similar extent as GST-APC-(1089–1370) (wild type WT and S1360A), but only APC-(1089–1370) was phosphorylated by the associated kinase (supplemental Fig. S7).
APC S1360A Expression Mimics Cyclin A/cdk2 Depletion
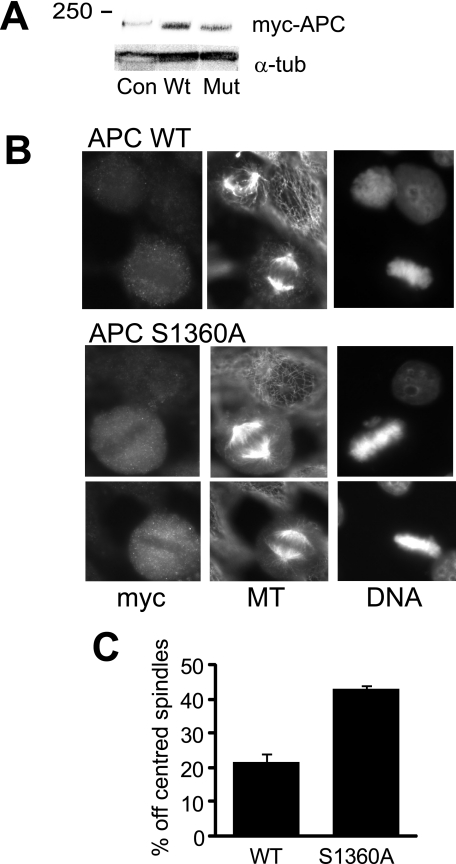
APC mutants have been demonstrated to have a dominant effect over the endogenous wild type protein (4, 5). Therefore full-length Myc-tagged APC wild type or the S1360A mutant were transfected into HeLa cells to determine whether lack of phosphorylation of APC Ser-1360 was responsible for the mitotic defects observed with cyclin A/cdk2 depletion (Fig. 7A). Overexpression of the wild-type APC had no effect on the proportion of off-centered spindles, with 20% off-centered, which was similar to normal level detected (compare Fig. 4B, Control with 7C WT). APC S1360A mutant resulted in a significant increase in the proportion of cells with off-centered spindles, which was double the proportion of similar phenotype in wild-type APC-overexpressing cells (Fig. 7). The off-centered spindles showed the same failure of astral microtubules attachment to the cell cortex as observed with cyclin A depletion (compare Figs. 4A and 7B). Some cells overexpressing APC mutant also contained lagging chromosomes, although when quantified the effect was not statistically significant. The mutation of APC S1360A had no obvious effects on the cytoplasmic localization of APC (Fig. 7B). Similar phenotypes were observed with overexpression of mutant APC in U2OS (supplemental Fig. S8A) and 293T cells (data not shown). Replating medium from transfection experiments revealed a >2-fold increase in the number of cells detached from culture plate and capable of forming colonies in the mutant APC-transfected cells, indicating that similar to cyclin A depletion, overexpression of mutant APC increased the proportion of cells undergoing out of plane division (supplemental Fig. S8B and Fig. 3C).
FIGURE 7.
APC S1360A mutation causes off-centered mitotic spindles. A, HeLa cells were transfected with either Myc-tagged APC wild type (WT) or S1360A mutant (Mut), or mock control (Con), and harvested 48 h after transfection. Lysates were immunoblotted for the Myc-tagged APC (myc-APC), which ran just ahead of the 250-kDa marker, and α-tubulin (α-tub) as a loading control. The Myc tag antibody detected a nonspecific band that ran just ahead of the Myc-APC band (the weak band detected in the control transfection lane). B, similarly transfected HeLa cells were fixed 48 h after transfection and stained for the Myc tag to identify overexpressing cells, α-tubulin for microtubules (MT) and DNA. C, quantitation of the percentage of off-centered spindles in APC-overexpressing cells. These data represent >1,000 Myc-tagged APC-expressing cells in three independent experiments.
DISCUSSION
We have demonstrated a role for cyclin A/cdk2 in early mitosis, and identified APC as a substrate involved in an unexpected mitotic function for this complex. Previous work from a number of laboratories has indicated that G2 phase cyclin A/cdk2 may have a critical role in the timing of entry into mitosis (9–11, 25). Cyclin A/cdk2 also has a role in S phase progression (26–28); depletion of cyclin A with RNAi had little effect on S phase (9–11), which was likely to be a consequence of the incomplete depletion achieved with these techniques. This indicates that the level of cyclin A required for S phase progression is much lower than requirement for G2/M progression. Increased cyclin A levels or stabilizing cyclin A delays mitotic progression, indicating that the timely destruction of cyclin A is necessary for normal progression through mitosis (14, 25). In Drosophila, cyclin A has been demonstrated to a mitotic role (29), although the exact nature of this role is unclear.
Here we demonstrate that specific depletion of cyclin A, depletion of its activator cdc25B, and selective cdk2 inhibitors produce the same aberrant mitotic phenotypes. This involves the unexpected and dynamic rotation of the metaphase plate, which results in increased out-of-plane divisions in these cells. This spindle rotation is the consequence of a lack of cyclin A/cdk2-dependent phosphorylation of APC on Ser-1360. Delayed migration of chromosomes to the metaphase plate was also observed in cyclin A-depleted cells, although this was a less common phenotype in all cell types examined; in neonatal foreskin fibroblasts the effect failed to reach statistical significance, although the trend to increased lagging chromosomes was retained. By contrast, similar levels of off centered and rotated spindles were observed in all cell lines. Overexpression of the APC mutant only weakly recapitulated the lagging chromosomes phenotype, indicating that whereas Ser-1360 phosphorylation was a strong contributor to spindle anchoring, it was a minor contributor to the lagging chromosome phenotype observed with APC mutant overexpression.
Cyclin A/cdk2-dependent phosphorylation of APC in its spindle-anchoring role must occur in the cytoplasm. Cyclin A is normally localized in the nucleus, but a small pool does translocate to the cytoplasm in late G2 phase (9, 30). This correlates with the transient interaction with and phosphorylation of APC observed in late G2 phase. Cyclin A/cdk2 is also localized to the centrosome in late G2 phase (9, 30), defining unexpected cytoplasmic roles for this normally nuclear complex in mitotic spindle function.
Many of the mitotic spindle defects observed with cyclin A/cdk2 inhibition phenocopy depletion of APC or overexpression of APC mutants. The mitotic arrest imposed by cyclin A/cdk2 inhibition is a consequence of activation of the mitotic spindle assembly checkpoint. The effects of APC depletion on initiating the spindle checkpoint are controversial, with some studies reporting mitotic arrest (8, 31) and another reporting no effect (4). There is also some controversy about the exact role of APC in the spindle assembly checkpoint with some studies indicating no effect on the checkpoint (8, 31), whereas others indicate that depletion of APC reduces checkpoint fidelity (32). The basis for this disagreement is not clear, although it may reflect cell type difference or the relative depletion of APC achieved in each case (3). APC also has a kinetochore function, and lagging chromosomes and less compact metaphase plates are features commonly observed with APC depletion and mutants (3, 8, 31, 32). The lack of cyclin A/cdk2-dependent phosphorylation of APC may contribute to the lagging chromosomes. However, the effect of cyclin A depletion on lagging chromosomes was much weaker than the spindle-centering defect in all three cell lines examined, and the relatively weak effect observed with overexpression of APC S1360A suggests that this modification may not be a major contributor to this function of APC.
Exactly how phosphorylation of APC Ser-1360 contributes to microtubule stabilization at the cortex is unclear at present. It has been shown that the cortical connections of the astral microtubules anchor the mitotic spindle and allow symmetrical cell division to occur (33). While there is no obvious defect in the astral microtubule nucleation or polymerization, the rotating metaphase plates and failure of the spindle to locate the center of the mitotic cell are evidence of a problem with their cortical anchoring. The data presented here indicate that cyclin A/cdk2-dependent phosphorylation of APC Ser-1360 is required for stable astral microtubule attachment to the cell cortex. This effect is similar to that reported for expression of APC1–1020 and APC1–850 (APCmin) mutants in cell lines and in vivo, whereas other truncation mutations ablate astral microtubule formation (4, 5). Another difference between these APC mutants and cyclin A depletion was the elevated rate of failed cytokinesis in the APC mutants. This would indicate that the Ser-1360 point mutation has a more subtle effect than truncation of the protein, and separates the lack of spindle attachment from cytokinesis defects observed with the truncations. A lack of stable astral microtubule attachment to the cell cortex imposes a mitotic arrest through a mechanism known as the spindle orientation checkpoint reported in fission yeast (34). This checkpoint utilizes many of the kinetochore checkpoint components, including Bub1 and Bub3, but is triggered by failure of stable astral microtubule binding to the cell cortex. APC also binds Bub1 and Bub3 in mitosis (35), suggesting that this checkpoint mechanism may also contribute to the mitotic delay we observe with inhibition of cyclin A/cdk2 phosphorylation of APC.
Interestingly, Ser-1360 is in the mutation cluster region (MCR) of APC (Fig. 5A), which is also the region binding to β-catenin (2). However, a number of lines of evidence suggest that it is unlikely that β-catenin signaling contributes to mitotic defects observed here. Mutation of the Wnt/β-catenin signaling pathway increased chromosomal bridges, markers of failure of mitosis (3, 36, 37), whereas inhibition of cyclin A/cdk2 did not. The timing of APC binding by cyclin A/cdk2 in late G2 phase would also preclude a significant β-catenin-dependent transcriptional effect. Additionally, the increased chromosomal instability with increased Wnt/β-catenin signaling required a number of cell division to be manifested, suggesting that the cyclin A/cdk2-dependent regulation of APC Ser-1360 is separate from β-catenin-dependent effects.
Another consequence of the mitotic defects with cyclin A/cdk2 inhibition is an increase in out-of-plane division, producing a daughter cell with little or no attachments to the substratum as mitotic exit was dissociated from normal spindle orientation. This allows the viable daughter cell to establish itself at a distance from its site of origin. Out-of-plane division may affect the distribution of cells in the epithelial layer, resulting in the loss of the normal organization. In a stratified epithelium such as in the colon polyps, expression of APCMin mutant influenced the orientation of mitotic spindles, which may contribute to tumor spread (5). APC has roles in defining cell polarity (38), and in cell migration and adhesion through its association with the cytoskeleton (1). The data presented here and elsewhere (4, 5) indicate that APC may potentially influence the symmetry of cell division by influencing the orientation of the mitotic spindle. Overexpression of cyclin A has been linked to poor prognosis in cancer (39). Our study suggests that heterozygous loss of cyclin A may affect the mitotic function of cyclin A and reduce its phosphorylation of APC. This would result in the reduction in cortical attachment of the mitotic spindle and the orientation and/or symmetry of division. In cells that require asymmetric division, this could have significant consequences in terms of the fate of the daughter cells produced, and in transformed cells this could promote the initial stage of progression from in situ cancer.
Supplementary Material
Acknowledgments
We thank Dr. Maree Faux for the APC constructs; Drs. Nicole den Elzen, Rose Boutros, Sherilyn Goldstone and members of the Gabrielli laboratory for critical reading of the manuscript and advice; and Huong Le for technical assistance.
This work was supported in part by the National Health and Medical Research Council of Australia and the Cancer Council of Queensland.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- APC
- adenomatous polyposis coli
- FACS
- fluorescent-activated cell sorting
- PBS
- phosphate-buffered saline
- Pipes
- 1,4-piperazinediethanesulfonic acid
- GST
- glutathione S-transferase
- Wt
- wild type.
REFERENCES
- 1.Aoki K., Taketo M. M. (2007) J. Cell Sci. 120, 3327–3335 [DOI] [PubMed] [Google Scholar]
- 2.Näthke I. (2006) Nat. Rev. Cancer 6, 967–974 [DOI] [PubMed] [Google Scholar]
- 3.Rusan N. M., Peifer M. (2008) J. Cell Biol. 181, 719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green R. A., Wollman R., Kaplan K. B. (2005) Mol. Biol. Cell 16, 4609–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell C. M., Green R. A., Kaplan K. B. (2007) J. Cell Biol. 178, 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura M., Zhou X. Z., Lu K. P. (2001) Curr. Biol. 11, 1062–1067 [DOI] [PubMed] [Google Scholar]
- 7.Askham J. M., Moncur P., Markham A. F., Morrison E. E. (2000) Oncogene 19, 1950–1958 [DOI] [PubMed] [Google Scholar]
- 8.Draviam V. M., Shapiro I., Aldridge B., Sorger P. K. (2006) EMBO J. 25, 2814–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Boer L., Oakes V., Beamish H., Giles N., Stevens F., Somodevilla-Torres M., Desouza C., Gabrielli B. (2008) Oncogene. 27, 4261–4268 [DOI] [PubMed] [Google Scholar]
- 10.Fung T. K., Ma H. T., Poon R. Y. (2007) Mol. Biol. Cell 18, 1861–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong D., Pomerening J. R., Myers J. W., Gustavsson C., Jones J. T., Hahn A. T., Meyer T., Ferrell J. E., Jr. (2007) Curr. Biol. 17, 85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitra J., Enders G. H. (2004) Oncogene. 23, 3361–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstone S., Pavey S., Forrest A., Sinnamon J., Gabrielli B. (2001) Oncogene 20, 921–932 [DOI] [PubMed] [Google Scholar]
- 14.den Elzen N., Pines J. (2001) J. Cell Biol. 153, 121–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pines J., Hunter T. (1990) Nature 346, 760–763 [DOI] [PubMed] [Google Scholar]
- 16.Buendia B., Draetta G., Karsenti E. (1992) J. Cell Biol. 116, 1431–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens F. E., Beamish H., Warrener R., Gabrielli B. (2008) Oncogene 27, 1345–1354 [DOI] [PubMed] [Google Scholar]
- 18.Gabrielli B. G., De Souza C. P., Tonks I. D., Clark J. M., Hayward N. K., Ellem K. A. (1996) J. Cell Sci. 109, 1081–1093 [DOI] [PubMed] [Google Scholar]
- 19.Warrener R., Beamish H., Burgess A., Waterhouse N. J., Giles N., Fairlie D., Gabrielli B. (2003) Faseb J. 17, 1550–1552 [DOI] [PubMed] [Google Scholar]
- 20.Skoufias D. A., Andreassen P. R., Lacroix F. B., Wilson L., Margolis R. L. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4492–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vagnarelli P., Earnshaw W. C. (2004) Chromosoma 113, 211–222 [DOI] [PubMed] [Google Scholar]
- 22.O'Connell M. J., Walworth N. C., Carr A. M. (2000) Trends Cell Biol. 10, 296–303 [DOI] [PubMed] [Google Scholar]
- 23.Stead E., White J., Faast R., Conn S., Goldstone S., Rathjen J., Dhingra U., Rathjen P., Walker D., Dalton S. (2002) Oncogene. 21, 8320–8333 [DOI] [PubMed] [Google Scholar]
- 24.Trzepacz C., Lowy A. M., Kordich J. J., Groden J. (1997) J. Biol. Chem. 272, 21681–21684 [DOI] [PubMed] [Google Scholar]
- 25.Furuno N., den Elzen N., Pines J. (1999) J. Cell Biol. 147, 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. (1992) EMBO J. 11, 961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenblatt J., Gu Y., Morgan D. O. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 2824–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strausfeld U. P., Howell M., Descombes P., Chevalier S., Rempel R. E., Adamczewski J., Maller J. L., Hunt T., Blow J. J. (1996) J. Cell Sci. 109, 1555–1563 [DOI] [PubMed] [Google Scholar]
- 29.Knoblich J. A., Lehner C. F. (1993) EMBO J. 12, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pines J., Hunter T. (1991) J. Cell Biol. 115, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Ahmad S., Mao Y. (2007) J. Cell Biol. 178, 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dikovskaya D., Schiffmann D., Newton I. P., Oakley A., Kroboth K., Sansom O., Jamieson T. J., Meniel V., Clarke A., Näthke I. S. (2007) J. Cell Biol. 176, 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCartney B. M., McEwen D. G., Grevengoed E., Maddox P., Bejsovec A., Peifer M. (2001) Nat Cell Biol. 3, 933–938 [DOI] [PubMed] [Google Scholar]
- 34.Tournier S., Gachet Y., Buck V., Hyams J. S., Millar J. B. (2004) Mol. Biol. Cell 15, 3345–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan K. B., Burds A. A., Swedlow J. R., Bekir S. S., Sorger P. K., Näthke I. S. (2001) Nat. Cell Biol. 3, 429–432 [DOI] [PubMed] [Google Scholar]
- 36.Aoki K., Aoki M., Sugai M., Harada N., Miyoshi H., Tsukamoto T., Mizoshita T., Tatematsu M., Seno H., Chiba T., Oshima M., Hsieh C. L., Taketo M. M. (2007) Oncogene 26, 3511–3520 [DOI] [PubMed] [Google Scholar]
- 37.Hadjihannas M. V., Brückner M., Jerchow B., Birchmeier W., Dietmaier W., Behrens J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10747–10752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez C. (2007) Nat. Rev. Genet. 8, 462–472 [DOI] [PubMed] [Google Scholar]
- 39.Aaltonen K., Ahlin C., Amini R. M., Salonen L., Fjällskog M. L., Heikkilä P., Nevanlinna H., Blomqvist C. (2006) Br. J. Cancer 94, 1697–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.