Abstract
The mTOR (mammalian target of rapamycin) promotes growth in response to nutrients and growth factors and is deregulated in numerous pathologies, including cancer. The mechanisms by which mTOR senses and regulates energy metabolism and cell growth are relatively well understood, whereas the molecular events underlining how it mediates survival and proliferation remain to be elucidated. Here, we describe the existence of the mTOR splicing isoform, TORβ, which, in contrast to the full-length protein (mTORα), has the potential to regulate the G1 phase of the cell cycle and to stimulate cell proliferation. mTORβ is an active protein kinase that mediates downstream signaling through complexing with Rictor and Raptor proteins. Remarkably, overexpression of mTORβ transforms immortal cells and is tumorigenic in nude mice and therefore could be a proto-oncogene.
Introduction
The mammalian target of rapamycin (mTOR)5 is a central regulator of an evolutionary conserved signaling pathway that controls cellular metabolism, autophagy, growth, and proliferation (1–3). mTOR belongs to a family of the phosphoinositide 3-kinase-related kinases (PIKKs), which also includes ATR, ATM, DNA-PK, SMG1, and TRRAP. Similar to other PIKK family members, mTOR contains a protein kinase domain at the C terminus and a long stretch of protein-protein interaction modules within its N terminus. These include HEAT (Huntingtin, elongation factor 3, protein phosphatase 2A, and TOR1) repeats and FAT (FRAP, ATM, and TRRAP) domain. There is also a short FAT domain at the C terminus (FATC), whose function is critical for mTOR kinase activity. mTOR differs from other PIKK family members by the presence of an FRB (FKBP12/rapamycin binding) domain that mediates the interaction with FKBP12/rapamycin inhibitory complex. mTOR is found in cells in two distinct multiprotein complexes, termed mTORC1 and mTORC2. The best characterized partners of mTOR in mTORC1 include a substrate-presenting protein Raptor and mLst8 (also known as GβL). The presence of another substrate-presenting protein, Rictor, and Sin1 defines mTORC2 along with mTOR and mLst8.
mTORC1 integrates growth factor signaling with amino acid- and energy-sensing pathways to regulate cell growth through downstream effectors, 4EBP1 and S6K1. The function of mTORC2 is not well understood, and so far, the strongest association is with the PI3K-PKB/Akt signaling as it directly phosphorylates/activates PKB/Akt.
Deregulation of the mTOR signaling pathway has been associated with numerous pathologies, including diabetes, inflammation, and cancer (4–6). In contrast to yeasts that have two tor genes (tor 1 and tor 2), there is only one gene encoding mammalian tor (7). The diversity of the mTOR-mediated signaling is conferred by two multiprotein complexes, mTORC1 and mTORC2, whose regulatory components and downstream effects mirror in part the signaling mediated in yeasts by TOR1 and TOR2 pathways (8, 9).
Here, we provide evidence of existence of the mTOR-splicing isoform, mTORβ, which lacks most of its protein-protein interaction modules, HEAT and FAT, but retains domains responsible for FRB, protein kinase activity, and regulation (RD and FATC). Importantly, mTORβ could form complexes in vivo with Raptor and Rictor, which are known companions of full-length mTOR (mTORα). Also, it readily phosphorylates characterized mTORα substrates, S6K1, PKB/Akt, and 4EBP1, in vitro. In contrast to mTORα, mTORβ has the potential to shorten considerably the G1 phase of the cell cycle and to stimulate cell proliferation. Significantly, overexpression of mTORβ transforms immortal cells and is tumorigenic in nude mice. Our studies suggest that the regulation of cell proliferation via the mTOR pathway could be mediated by mTORβ, which acts as a proto-oncogene and therefore could be a candidate for future anticancer drug discovery.
EXPERIMENTAL PROCEDURES
Plasmid Construction, Small Interfering RNA, and Expression Studies
For expression in mammalian cells, the full-length cDNA for mTORβ was cloned into expression vector pcDNA3.1(+) (Invitrogen) with the N-terminal FLAG or Myc tag. mTORβ KD with the substitution E514G (analog of E2357G in TORα was generated with the use of a site-directed mutagenesis kit (Stratagene). Transient transfections of cells were carried out with the use of ExGene 500 reagent (Fermentas). The C-terminal region of S6K1 (His-S6K1C, amino acids 332–502) was cloned into pET24a plasmid (Novagene). The expression and affinity purification of His-S6K1C were carried out in BL21 DE3 cells using nickel-nitrilotriacetic acid-agarose (Qiagen). pCMV/FLAG-mTORα plasmid was kindly provided by Prof. K. Yonezawa. Myc-mTOR, Myc-Rictor, HA-Raptor, and HA-GβL expressing plasmids were obtained from Dr. D. Sabatini through AddGene. The Myc-tagged mTOR cDNA from pRK-5/Myc-mTOR was subcloned into pcDNA3.1(+) vector.
Reagents, Antibodies, and Cell Cultures
FLAG tag, HA tag, and β-actin antibodies were purchased from Sigma. The N-terminal mTOR antibody was from Santa Cruz Biotechnology, and anti-Raptor antibody was from Millipore. The F11 anti-mTOR antibody was described previously (10). mTOR C-terminal rabbit polyclonal and phosphospecific antibodies to Ser473 Akt, Thr308 Akt, Thr389 S6K1, Ser65 and Thr37/Thr46 4EBP1 were purchased from Cell Signaling. NIH 3T3 fibroblasts and derived stable cell lines overexpressing RasG12/C40 effector-specific double mutant were kindly provided by Dr. J. Downward. Human embryonic kidney (HEK 293), human breast carcinoma (MCF-7), COS7 (monkey kidney fibroblast), and human liver carcinoma (HepG2) cells were obtained from ATCC.
RNA Purification and RT-PCR
Total RNA was purified from HEK 293, MCF-7, and HepG2 cell lines using the SV Total RNA Isolation System (Promega). The RT-PCR was performed using SuperScriptTMIII one-step RT-PCR Platinum Taq HiFi kit (Invitrogen) The RT-PCR was performed on 2 μg of total RNA according to the manufacturer's recommendations using a panel of specific primers for mTOR (S1, ATGCTTGGAACCGGACCTGCCG; S2, CAATGTGAGCGTCCTGCAGAAGA; AS1, TACCAGAAAGGGCACCAGCCAAT; AS2, TTTGGACAGATCCTCAGTGACCT). The PCR fragments were gel-separated, cloned, and sequenced. Specific fragments of glyceraldehyde-3-phosphate dehydrogenase and β-actin were amplified and used as loading and quality controls of first-strand DNA.
Northern Blot Analysis
The membrane-containing poly(A)+ RNA samples from various human tissues were purchased from OriGene. The Northern blot analysis was performed using a DIG Northern Starter kit (Roche Diagnostics). The mTOR DIG-labeled RNA probe was generated by using a 750-bp PCR product, corresponding to the C-terminal coding region of mTOR (5530–6430 bp) as a template for the in vitro transcription reaction with dioxigenin-11-UTP. The β-actin probe was supplied by the manufacturer (Roche Diagnostics).
Immunoprecipitations
HEK 293 cells were washed with ice-cold phosphate-buffered saline and extracted with lysis buffer containing 10 mm Tris-HCl (pH 7.5), 150 mm NaCl, 0.3% (v/v) CHAPS, 5 mm EDTA, 50 mm sodium fluoride, 10 mm sodium pyrophosphate, 1 mm sodium orthovanadate, and a mixture of protease inhibitors (Roche Applied Science). Whole cell extracts were centrifuged at 10,000 × g for 20 min at 4 °C. Endogenous or transiently expressed proteins were immunoprecipitated with corresponding antibodies immobilized on protein A-Sepharose beads (GE Healthcare) for 3 h at 4 °C. The immune complexes were then washed three times with lysis buffer and twice in wash buffer 2 (50 mm HEPES (pH 7.5) and 150 mm NaCl), and proteins were eluted by boiling 8 min in 1× PAGE loading buffer. When immunoprecipitates were used for in vitro kinase assay, beads were washed twice with lysis buffer, once with wash buffer 1 (lysis buffer complemented with 300 mm KCl), once with wash buffer 2, and once with kinase buffer (25 mm HEPES-KOH (pH 7.4), 50 mm KCl, 20% glycerol, 10 mm MgCl2, 4 mm MnCl2, 1 mm dithiothreitol).
To investigate the eIF4E·4EBP1 complex formation in mTORβ-, mTORα-, or EGFP-overexpressing cell lines, appropriate protein extracts were incubated with m7GTP-Sepharose (GE Healthcare) followed by immunoblotting with antibodies against eIF4E or 4EBP1.
In Vitro Kinase Assay
mTOR in vitro kinase assay was performed as published previously (11). Briefly, HEK 293 cells were transfected with pcDNA 3.1/Myc-mTORα or pcDNA 3.1/Myc-mTORβ. Two days later, transiently expressed proteins immunoprecipitated with anti-Myc antibody. The kinase assays with S6K1 and 4EBP1 were performed in 40 μl at 30 °C for 60 min and contained about half of the washed mTOR beads, 1 μg of a 4EBP1 (Calbiochem) or 1.0 μg of recombinant His-S6K1C, 25 mm HEPES-KOH (pH 7.4), 50 mm KCl, 20% glycerol, 10 mm MgCl2, 4 mm MnCl2, 1 mm dithiothreitol, and 100 μm ATP and 5 μCi of [γ-32P]ATP. Kinase reactions were resolved by SDS-PAGE and analyzed by phosphorimaging. The levels of immunoprecipitated Myc-mTORα and Myc-mTORβ were measured by Western blotting with anti-Myc. For kinase assays with PKB/Akt as a substrate, transiently expressed Myc-mTORα or Myc-mTORβ were immunoprecipitated from HEK 293 cells as described above. The reactions were performed in 40 μl at 35 °C for 40 min in buffer containing 1 μg of a recombinant inactive His-Akt (Upstate), 25 mm HEPES (pH 7.5), 100 mm potassium acetate, 1 mm MgCl2, and 300 μm ATP and 7.5 μCi of [γ-32P]ATP. Kinase reactions were stopped by adding 5× sample buffer.
Immunoblot Analysis
Immunoblot analysis was performed as described previously (10). The antigen·antibody complexes were detected using the ECL system (Millipore). When immunoblots had to be reprobed, the membranes were initially stripped (Restore Western Stripping Reagent; Pierce) and incubated with another type of primary antibody.
Subcellular Fractionation
Subcellular fractionation of MCF-7 cells was performed using ProteoExtract extraction kit (Calbiochem). mTORα and mTORβ were immunoprecipitated from generated fractions using anti-mTOR C-terminal antibody. Immune complexes were resolved by SDS-PAGE and immunoblotted with anti-mTOR N-terminal antibody. Anti-4EBP1, Hsp60, and c-Jun antibodies were used as controls for cytoplasmic, membrane, and nuclear fractions, respectively.
Stable Cell Line Production and Cell Proliferation Assay
Stable cell lines overexpressing wild type and mTORβ KD, EGFP, and wild-type mTORα were produced by transfecting linearized pcDNA 3.1/FLAG-mTORβ WT, pcDNA 3.1/FLAG-mTORβ KD, pEGFP C1, and pcDNA3.1/Myc-mTORα WT vectors into HEK 293 cells. Cell lines were selected for 10 days on 800 μg/ml Geneticin. NIH 3T3 cell lines stably transfected with mTORβ were generated using linearized pcDNA4-TO/FLAG-mTORβ vector, and transfected cells were selected on 200 μg/ml zeocin for 7 days.
For cell proliferation assay, HEK 293 cells expressing mTORα WT, mTORβ WT, mTORβ KD, or EGFP were seeded into 12-well plate (1000 cells/well) and grown under standard conditions. Cell numbers were then calculated every day over the next 5 days using the CASY Cell Counter Analyzer System. Growth curves for each cell line were calculated using data from at least six independent experiments and are presented as the mean ± S.D.
For testing the effect of rapamycin on proliferation of HEK 293 cells expressing mTORα WT, mTORβ WT, or EGFP, cells were seeded into a 96-well plate in four repeats (250 cells/well) and grown in the absence or presence of rapamycin (5, 10, 15, or 20 nm) for 4 days. Cell numbers were then measured in each well by resazurin-based assay (Cell Titer Blue; Promega) as recommended by the manufacturer.
Cell Cycle Analysis
The cell cycle status was analyzed using flow cytometry. HEK 293 stable cell lines overexpressing mTORα, mTORβ, or EGFP were pulse-labeled with 10 μm bromodeoxyuridine (BrdUrd) for 30 min and chased every 2 h for 24 h. Cells were scraped, washed with phosphate-buffered saline, and fixed in 70% ethanol at 4 °C for 16 h. Prior to analysis, cells were stained with propidium iodide in solution containing 100 μg/ml propidium iodide, 100 μg/ml RNase A, and anti-BrdUrd antibody as described previously (12). Green fluorescence was recorded as a measure of anti-BrdUrd antibody binding (BrdUrd incorporation). Red fluorescence was recorded as a measure of propidium iodide binding (DNA content). Bivariate distributions of cells showing incorporation of BrdUrd versus DNA content were obtained with a BD LSR II flow cytometer (BD Biosciences). A minimum of 5000 BrdUrd-positive cells in each treatment was analyzed. The percentage of labeled cells in G1, S, and G2 at each time point was plotted over a 24-h period to determine the length of each cell cycle phase.
Colony Forming Assay
To monitor the capacity of mTOR stable cell lines to grow in semisolid medium in vitro, cells were transferred to 2 ml of complete Dulbecco's modified Eagle's medium containing 0.7% low melting agarose. 103 cells were seeded into 35-mm dishes containing a 2-ml layer of solidified 1.2% low melting agarose in complete medium. Colonies were stained 3 weeks later with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and counted using Quantity One software (Bio-Rad). The results are presented as the mean ± S.D. of five independent experiments, with p ≥ 0.05.
Xenograft Studies in Nude Mice
Parental NIH 3T3 cells or generated NIH 3T3 cells stably expressing mTORβ or the RasG12/C40 effector-specific double mutant were injected (5 × 106 cells/site in 200 μl) subcutaneously into both flanks of (MF1 nu/nu) nude mice, using a 25-gauge needle. Tumor formation and growth were monitored every 2–3 days as described (13). All experiments were in compliance with the United Kingdom Coordinating Committee on Cancer Research Guidelines for the welfare of Animals in Experimental Neoplasia.
RESULTS AND DISCUSSION
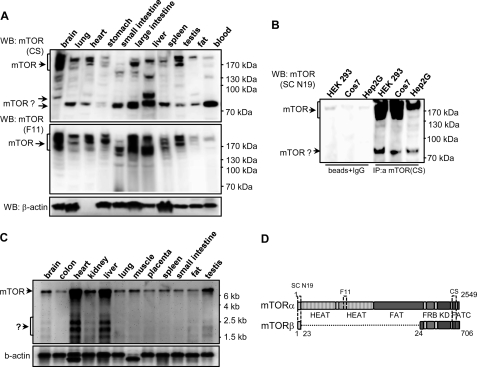
To investigate the molecular components of the mTOR signaling pathway and to define the mechanisms of its regulation, we generated monoclonal antibodies specific for mTOR. One of them, clone F11, efficiently recognized endogenous mTOR in various immunological assays (10). When F11 and a commercial antibody (mTOR rabbit polyclonal from Cell Signaling) were used to analyze the expression of mTOR in rat tissues, each showed a similar pattern of immunoreactive bands corresponding to full-length mTOR, whereas a distinct band of ∼80 kDa was only observed with Cell Signaling antibody (Fig. 1A). The 80-kDa protein was also detected in Western blot analysis of human tissues probed with Cell Signaling, but not F11, antibodies (supplemental Fig. 1F).
FIGURE 1.
Identification of a novel mTOR splicing isoform in mammalian cell lines and tissues. A, immunoblot analysis of the mTOR expression in rat tissues is shown. Protein extracts (30 μg) were probed with the C-terminal mTOR polyclonal antibodies from Cell Signaling (mTOR-CS, top panel) and F11 mTOR monoclonal antibody (mTOR-F11, middle panel). The membrane was stripped and reprobed with anti-β-actin antibody (bottom panel). The positions of the mTOR immunoreactive bands are indicated by arrows. WB, Western blotting. B, the 80-kDa mTOR immunoreactive protein is not the product of proteolytic degradation. The extracts of HEK 293, COS7, and Hep2G cells were immunoprecipitated with the C-terminal mTOR antibody (mTOR-CS), and eluted proteins were probed with the N-terminal mTOR antibody (N19). Protein A-Sepharose beads coupled with a nonspecific antibody were used as a negative control. C, Northern blot analysis reveals the presence of several potential mTOR transcripts in human tissues. Details of specific probes and blotting conditions are described under Experimental Procedures. The blot was initially probed with the 3′ mTOR probe (upper panel) and then reprobed for β-actin expression (lower panel). D, domain organization of the full-length mTORα and the mTORβ splicing isoform.
To prove that it is not the product of mTOR degradation but a potential splicing form, we immunoprecipitated mTOR from cell lines with the C-terminal mTOR antibody (Cell Signaling) and analyzed the immune complexes with N19 antibody (Santa Cruz Biotechnology), which recognizes the N terminus of mTOR (Fig. 1B). As a result, full-length mTOR as well as the 80-kDa protein were specifically immunoprecipitated from HEK 293, COS7, and Hep2G cells. Furthermore, the immune complexes of anti-mTOR CS from MCF-7 and HEK 293 cells were probed with F11 mTOR antibody that does not recognize the 80-kDa protein in rat tissue extracts (supplemental Fig. 2A). In this experiment, the 80-kDa protein is only observed in immunoblots with SC N19, but not with the F11 antibodies. These results suggest that the 80-kDa protein possesses both the N- and the C-terminal regions of mTOR and is not a product of mTOR degradation.
Northern blot analysis of human tissues with a probe corresponding to the 3′-coding region of mTOR provided further evidence for the existence of potential mTOR splicing variants (Fig. 1C). In addition to the 8.5-kb transcript, equivalent to the full-length mTOR mRNA, several clearly defined bands in the region of 1.7–3.4-kb were also observed, especially in heart and liver. To isolate a cDNA clone corresponding to the 80-kDa splicing variant, we employed RT-PCR screening of total RNA from MCF-7, HEK 293, and HepG2 cell lines with a panel of mTOR-specific primers (supplemental Fig. 1A). Sequence analysis of specifically amplified DNA fragments in three cell lines allowed us to identify PCR products that contained a potential mTOR splice fusion (supplemental Fig. 1, B–E). The identified splicing variant of mTOR, which we termed mTORβ, has an open reading frame for a protein of 706 residues, consisting of 23 amino acids from the mTORα N terminus, a short stretch of the FATN domain, FRB region, protein kinase and FATC domains (Fig. 1D). Bioinformatic searches of DNA data bases uncovered two expressed sequence tag clones (dbj BP286361.1 and dbj BP287435.1) with the same fusion sequence, demonstrating further the existence of a second mTOR-splicing isoform. Notably, the search revealed only 10 expressed sequence tag clones with sequences corresponding to the N-terminal region of mTOR (covering the splicing junction) and two of them encoded mTORβ.
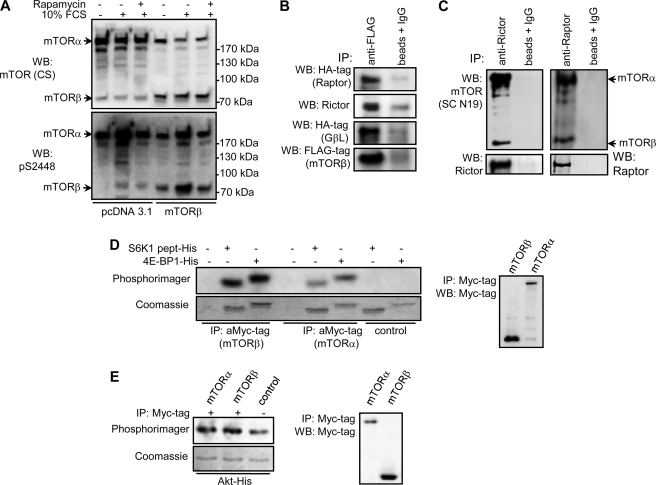
Ectopic expression of FLAG-mTORβ in HEK 293 cells revealed a protein of ∼80 kDa (Fig. 2A,upper panel) together with a band of endogenous mTORβ observed in HEK 293 cells transfected with pcDNA3.1. Stimulation of cells with serum or growth factors, such as insulin growth factor 1, is known to induce phosphorylation of mTORα at Ser2448 in a rapamycin-sensitive manner (14, 15). Both endogenous and ectopically expressed mTORβ are phosphorylated at the equivalent site, Ser605, in response to serum stimulation, and this phosphorylation is inhibited by pretreatment with rapamycin (Fig. 2A, lower panel).
FIGURE 2.
Regulation and downstream signaling of mTORβ. A, mTORβ is phosphorylated in response to serum in a rapamycin-sensitive manner. HEK 293 cells were transfected with pcDNA3.1 or pcDNA3.1/FLAG-mTORβ constructs. Two days later, cells were starved and serum-stimulated for 1 h in the presence or absence of rapamycin. Total cell lysates were probed with the mTOR-CS (upper panel) and pS2448 mTOR (lower panel) antibodies. WB, Western blotting. B, mTORβ forms specific complexes with Raptor, Rictor, and GβL in vitro. HEK 293 cells were transfected with pcDNA3.1/FLAG-mTORβ and pcRK/HA-Raptor, pcDNA3.1/FLAG-mTORβ and pRK/Myc-Rictor, or pcDNA3.1/FLAG-mTORβ and pcRK/GβL. Two days later, cell lysates were immunoprecipitated with anti-FLAG or nonspecific antibodies. Immune complexes were resolved by SDS-PAGE and immunoblotted with anti-FLAG, anti-HA, or anti-Rictor antibodies. C, specific association of endogenous Raptor and Rictor with mTOR splicing isoforms α and β. Endogenous Raptor and Rictor were immunoprecipitated (IP) with specific antibodies from HEK 293 cells. Co-precipitated mTORα and mTORβ were detected by immunoblotting with mTOR-CS antibody (upper panel). The membrane was then reprobed with anti-Raptor or anti-Rictor antibodies. D and E, S6K1, 4EBP1, and PKB/Akt are the substrates for mTORβ in vitro. Overexpressed mTOR splicing isoforms α and β were immunoprecipitated from HEK 293 cells using anti-Myc tag antibody. The in vitro kinase assays were carried out as described under Experimental Procedures.
Similarly, the phosphorylation of mTORα at Ser2448 and mTORβ at Ser605 was increased upon amino acid stimulation (supplemental Fig. 2B). Probing cell lysates with p(Ser)389 S6K1 and p(Thr)37/p(Thr)46 4EBP1 phosphospecific antibodies confirmed the activation of the mTOR pathway in MCF-7 cells in response to amino acid stimulation.
In cells, mTOR is associated predominantly with endoplasmic reticulum, Golgi, and mitochondria membranes, as well as the nucleus (16–18). Sequences located within the HEAT domains have been implicated in mediating mTORα membrane localization (16). Subcellular fractionation of MCF-7 cells, followed by immunoblotting with CS-mTOR antibody, as well as immunofluorescence analysis indicated that mTORβ is localized predominantly in the cytoplasm, consistent with the absence of HEAT domains (supplemental Fig. 2D).
Next, we asked whether mTORβ is capable of associating with known mTOR regulators (19–21). HEK 293 cells were transiently transfected with FLAG-mTORβ and Raptor-HA, Rictor-Myc, or Gβl-HA. As a result, despite mTORβ lacking HEAT repeats, exogenously expressed Raptor, Rictor, and GβL formed specific complexes with mTORβ in vitro (Fig. 2B) as well as in vivo with endogenous mTORβ, Raptor, and Rictor (Fig. 2C). These findings prompted us to test the ability of mTORβ to phosphorylate known physiological substrates for mTORα (11, 22). In vitro, mTORβ can phosphorylate S6K1, 4EBP1, and PKB/Akt (Fig. 2, D and E). The phosphorylation of specific sites on S6K1, 4EBP1, and PKB/Akt by mTORβ in in vitro kinase assay has been further confirmed by immunoblotting with phosphospecific antibodies (data not shown). Because yeast and mammalian TOR proteins function as dimers or oligomers (23), we excluded the presence of mTORα in mTORβ immunoprecipitates by showing that FLAG-mTORα and Myc-mTORβ do not oligomerize in vivo (supplemental Fig. 2E). Moreover, probing immune complexes with anti-mTOR (CS) antibody showed that endogenous mTORα is also not present in mTORβ immunoprecipitates (supplemental Fig. 2E). Taken together, the above results indicate that mTORβ, similarly to the full-length mTOR, has the ability to function through mTORC1 and mTORC2.
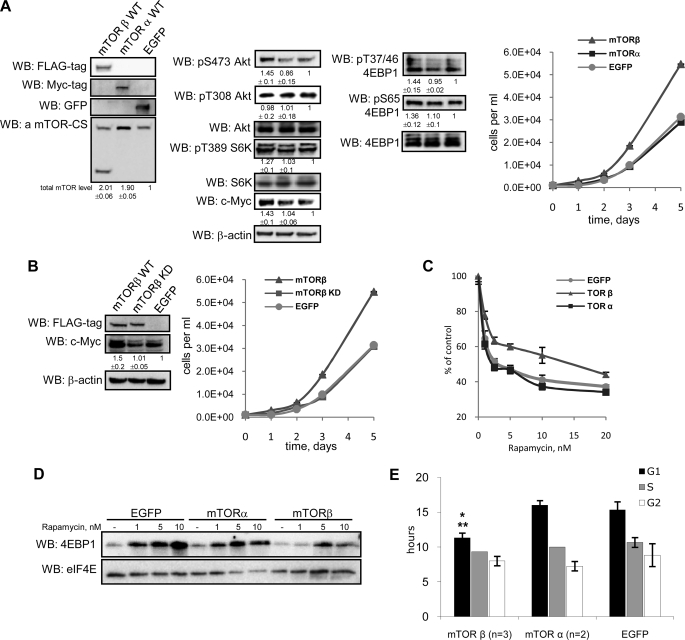
mTOR integrates intracellular and extracellular signals to regulate cell growth, proliferation, and survival. To elucidate the precise role of mTORβ, we generated HEK 293 stable cell lines that overexpress WT forms of mTORα, mTORβ, and EGFP. To avoid nonphysiological effects of exceedingly overexpressed exogenous proteins, we used in this study a mix of clones of stable cell lines with a relatively small overexpression of mTORα and mTORβ (Fig. 3A). The proliferative potential of HEK 293 cells stably overexpressing mTORβ was found to be significantly higher compared with mTORα or EGFP cells (Fig. 3A). To gain insight into the mechanism by which mTORβ controls cell proliferation, we assessed the phosphorylation status of known mTOR targets. The phosphorylation of Ser473 PKB/Akt, Thr389 S6K1 as well as Ser65 and Thr37/Thr46 in 4EBP1 was increased in mTORβ-expressing cells compared with mTORα or EGFP counterparts, whereas Thr308 PKB/Akt phosphorylation was the same (Fig. 3A). Consistent with these observations, we also observed an increase in c-Myc protein level, in cells overexpressing mTORβ, but not mTORα (Fig. 3A). Because the mTORα and mTORβ were overexpressed with different tags, total levels of endogenous and overexpressed mTORα/β were measured by probing with the CS antibody (Fig. 3A). Similar results were obtained with HEK 293 cells transiently overexpressing mTORα and mTORβ (supplemental Fig. 3).
FIGURE 3.
mTORβ isoform, but not mTORα, promotes cell proliferation and G1/S transition of the cell cycle. A, ectopic expression of mTORβ in HEK 293 cells induces cell proliferation. Immunoblotting of total cell lysates was carried out with the indicated antibodies. The protein levels were measured by densitometry, normalized to actin, and fold changes were calculated against EGFP-expressing stable cells. Data are means ± S.D. of four experiments. WB, Western blotting. B, mTORβ kinase activity is required for the induction of proliferation. Protein expression levels were measured as described in A. C and D, mTORβ-overexpressing cells are less sensitive to rapamycin. C, HEK 293 cells expressing mTORα, mTORβ, or EGFP were seeded into 96-well plate in four repeats (250 cells/well) and grown with or without increasing concentrations of rapamycin (1, 2.5, 5, 10, and 20 nm) for 4 days. Cell numbers were then measured in each well by resazurin-based assay. The proliferation curve is presented as percentages of non–rapamycin-treated points in each group. The data are the mean of three independent experiments ± S.D. D, precipitation of eIF4E·4EBP1 complex using m7GTP-Sepharose from mTORβ-, mTORα-, or EGFP-overexpressing cell lines incubated in the absence or presence (1, 5, or 10 nm) of rapamycin. E, the G1 phase of the cell cycle is regulated by the mTORβ isoform. BrdUrd pulse labeling was performed as described under Experimental Procedures. The duration of G1, S, and G2 phases of the cell cycle observed for each cell line is presented in the graph. Data are means ± S.D., p value ≤0.04 (*, against EGFP; **, against mTORα).
To find whether mTORβ kinase activity is required for the induction of proliferation, we generated HEK 293 cells overexpressing a KD form of mTORβ. The ability of the mTORβ KD mutant to stimulate cell proliferation was markedly reduced compared with the WT and correlated with the decrease in the substrate phosphorylation and the expression of c-Myc (Fig. 3B). It should be noted that the KD form of mTORβ associates with substrate-presenting proteins Raptor and Rictor as efficiently as the WT kinase (supplemental Fig. 2C).
Next, we examined the sensitivity of generated stable cell lines to rapamycin. In three independent experiments, we found that the proliferation of all examined cell lines is inhibited by rapamycin in a dose-dependent manner (Fig. 3C). However, we reproducibly observed that the proliferation of mTORβ-overexpressing cells is less sensitive to the inhibitory effect of rapamycin compared with cells expressing mTORα or EGFP.
To study the observed differences at the molecular level, we analyzed the effect of rapamycin (0, 1, 5, and 10 nm) on complex formation between eIF4E and 4EBP1 in cells overexpressing mTORα, mTORβ, or EGFP. It is well established that treatment of cells with rapamycin increases the interaction between initiation factor eIF4E and translational inhibitor 4EBP1. Here, we used m7GTP-Sepharose to examine the state of eIF4E·4EBP1 complex in the presence or absence of rapamycin. The results presented in Fig. 3D demonstrate that treatment of examined cell lines with rapamycin induces eIF4E·4EBP1 complex formation in a dose-dependent manner. Notably, the induction of eIF4E·4EBP1 complex formation in response to rapamycin is significantly reduced in cells overexpressing mTORβ compared with mTORα or EGFP stable cell lines. These results are in agreement with the above findings (Fig. 3C), showing the growth of mTORβ-expressing cells being less sensitive to rapamycin compared with mTORα or EGFP cell lines.
To elucidate the mechanism by which mTORβ can stimulate cell proliferation, we explored its effect on the cell cycle progression. Flow cytometric analysis with use of pulse BrdUrd labeling of HEK 293 cells overexpressing mTORα, mTORβ, and EGFP (see Experimental Procedures) clearly indicated that the G1 phase in mTORβ cells is ∼4 h shorter than in mTORα and EGFP cells (Fig. 3E). At the same time, no significant differences in the length of the S and G2 phases were detected. The pattern was replicated in NIH 3T3 cells overexpressing WT mTORβ and EGFP (not shown). These findings are consistent with the induction of c-Myc expression in mTORβ cells, as c-Myc controls the expression of genes that participate in G1/S transition (24).
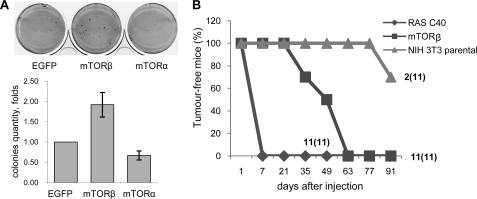
There is compelling evidence that mTOR is a critical downstream component of the Ras/PI3K/PKB pathway in tumorigenesis (25). The ability of mTORβ to shorten the cell cycle significantly and to stimulate cell proliferation prompted us to assess its oncogenic potential. Initially, we examined whether the overexpression of mTORα or mTORβ isoforms in HEK 293 cells is sufficient to induce colony formation in soft agar. We reproducibly observed from 1.5- to 2.5-fold more colonies originating from mTORβ-expressing cells compared with EGFP or mTORα cells (Fig. 4A).
FIGURE 4.
mTORβ transforms immortal cells and is tumorigenic in nude mice. A, anchorage-independent growth in soft agar of HEK 293 cells expressing mTORβ, mTORα, or EGFP. Stable cell lines were plated in a thin layer of agarose in culture medium. Three weeks later, colonies were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (left) and counted using Quantity One software, and fold changes were plotted (right). Data are means ± S.E. of four experiments. *, p = 0.01 against EGFP. B, NIH 3T3 cells overexpressing mTORβ are tumorigenic in nude mice. 5 × 106 parental NIH 3T3 cells and stable NIH 3T3 cells overexpressing mTORβ or RasG12/C40 double mutant were injected subcutaneously into nude mice. Tumor formation and growth were measured over time and are presented in the graph. The number of tumors grown/number of injections (in parentheses) is shown.
To find out whether the overexpression of mTORβ isoform alone can be a tumorigenic event, we performed xenograft studies in nude mice with parental cells or NIH 3T3 cells expressing either mTORβ or the RasG12/C40 effector-specific double mutant, which preferentially activates the PI3K pathway (26). The appearance and growth of tumors were monitored over a period of 3 months (Fig. 4B). Mice injected with cells expressing RasG12/C40 developed tumors rapidly, within 10 days. Significantly, the mTORβ-expressing cells gave rise to tumors in all injected mice, but their development and growth were delayed compared with RasG12/C40-derived tumors (Fig. 4B). Thus, the overexpression of mTORβ is sufficient to induce tumorigenicity of NIH 3T3 cells in nude mice. Immunoblot analysis of injected cell lines and the developed tumors revealed that tumorigenesis in nude mice induces the endogenous expression of the mTORβ-splicing isoform (supplemental Fig. 4). Alternative splicing has been implicated in regulating the expression of many oncogenes and tumor-suppressor isoforms. Recently, the splicing factor SF2/ASF was shown to act as an oncoprotein by controlling the alternative splicing of several signaling proteins, including the oncogenic form of S6K1 (27). Preliminary data show that mTORβ is an endogenous splicing target of SF2/ASF in NIH 3T3 cells.6 It remains to be investigated whether up-regulation of mTORβ expression is required for oncogenic transformation mediated by SF2/ASF or proto-oncogenes in the RAS/PI3K pathway.
This study identifies the mTORβ isoform, but not currently known mTORα, as a key component of the mTOR signaling responsible for coordinating cell cycle progression and cell proliferation. Furthermore, we provide evidence that mTORβ is an oncoprotein with the capacity to promote cell transformation and tumor maintenance and is, therefore, a novel therapeutic target for human cancer.
Supplementary Material
Acknowledgments
We thank P. Driscoll, D. Saggerson, J. Brockes, and N. Shaikh for critical comments on the manuscript and J. Downward for providing NIH 3T3 RasG12/C40 cells.
This work was supported in part by an International Association for the promotion of co-operation with scientists from the New Independent States of the former Soviet grant and the Atlantic Philanthropies/Ludwig Institute for Cancer Research Clinical Discovery Program (to I. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
G. Panasyuk and I. Nemazanyy, unpublished observation.
- mTOR
- mammalian target of rapamycin
- BrdUrd
- bromodeoxyuridine
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- SF2/ASF
- splicing factor 2/alternative splicing factor
- EGFP
- enhanced green fluorescent protein
- FRB
- FKBP12/rapamycin binding
- HA
- hemagglutinin
- KD
- kinase-dead
- PI3K
- phosphoinositide 3-kinase
- PIKK
- phosphoinositide 3-kinase-related kinase
- PKB
- protein kinase B
- RT
- reverse transcription
- WT
- wild type.
REFERENCES
- 1.Wullschleger S., Loewith R., Hall M. N. (2006) Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 2.Díaz-Troya S., Pérez-Pérez M. E., Florencio F. J., Crespo J. L. (2008) Autophagy 4, 851–865 [DOI] [PubMed] [Google Scholar]
- 3.Harris T. E., Lawrence J. C. (2003) Sci. STKE 212, re15. [DOI] [PubMed] [Google Scholar]
- 4.Guertin D. A., Sabatini D. M. (2007) Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 5.Dann S. G., Selvaraj A., Thomas G. (2007) Trends Mol. Med. 13, 252–259 [DOI] [PubMed] [Google Scholar]
- 6.Tee A. R., Blenis J. (2005) Semin. Cell Dev. Biol. 16, 29–37 [DOI] [PubMed] [Google Scholar]
- 7.Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. (2002) Mol. Cell 10, 457–468 [DOI] [PubMed] [Google Scholar]
- 8.Bhaskar P. T., Hay N. (2007) Dev. Cell 12, 487–502 [DOI] [PubMed] [Google Scholar]
- 9.Corradetti M. N., Guan K. L. (2006) Oncogene 25, 6347–6360 [DOI] [PubMed] [Google Scholar]
- 10.Nemazanyy I., Breus O., Gout I., Filonenko V., Panasyuk G. (2008) Hybridoma 27, 395–399 [DOI] [PubMed] [Google Scholar]
- 11.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 12.Gratzner H. G., Leif R. C., Ingram D. J., Castro A. (1975) Exp. Cell Res. 95, 88–94 [DOI] [PubMed] [Google Scholar]
- 13.El Emir E., Qureshi U., Dearling J. L., Boxer G. M., Clatworthy I., Folarin A. A., Robson M. P., Nagl S., Konerding M. A., Pedley R. B. (2007) Cancer Res. 67, 11896–11905 [DOI] [PubMed] [Google Scholar]
- 14.Sekuliæ A., Hudson C. C., Homme J. L., Yin P., Otterness D. M., Karnitz L. M., Abraham R. T. (2000) Cancer Res. 60, 3504–3513 [PubMed] [Google Scholar]
- 15.Reynolds T. H., 4th, Bodine S. C., Lawrence J. C., Jr. (2002) J. Biol. Chem. 277, 17657–17662 [DOI] [PubMed] [Google Scholar]
- 16.Liu X., Zheng X. F. (2007) Mol. Biol. Cell 18, 1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai B. N., Myers B. R., Schreiber S. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 4319–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X., Shu L., Hosoi H., Murti K. G., Houghton P. J. (2002) J. Biol. Chem. 277, 28127–28134 [DOI] [PubMed] [Google Scholar]
- 19.Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 20.Kim D. H., Sarbassov D. D., Ali S. M., Latek R. R., Guntur K. V., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2003) Mol. Cell 11, 895–904 [DOI] [PubMed] [Google Scholar]
- 21.Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 22.Burnett P. E., Barrow R. K., Cohen N. A., Snyder S. H., Sabatini D. M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 1432–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahara T., Hara K., Yonezawa K., Sorimachi H., Maeda T. (2006) J. Biol. Chem. 281, 28605–28614 [DOI] [PubMed] [Google Scholar]
- 24.Sears R., Nuckolls F., Haura E., Taya Y., Tamai K., Nevins J. R. (2000) Genes Dev. 14, 2501–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obaya A. J., Mateyak M. K., Sedivy J. M. (1999) Oncogene 18, 2934–2941 [DOI] [PubMed] [Google Scholar]
- 26.Joneson T., White M. A., Wigler M. H., Bar-Sagi D. (1996) Science 271, 810–812 [DOI] [PubMed] [Google Scholar]
- 27.Karni R., de Stanchina E., Lowe S. W., Sinha R., Mu D., Krainer A. R. (2007) Nat. Struct. Mol. Biol. 14, 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.