Abstract
MAPKs are evolutionarily conserved immune regulators. MAPK phosphatases (MKPs) that negatively regulate MAPK activities have recently emerged as critical players in both innate and adaptive immune responses. MKP-1, also known as DUSP1, was previously shown to negatively regulate innate immunity by inhibiting pro-inflammatory cytokine production. Here, we found that MKP-1 is necessary in T cell activation and function. MKP-1 deficiency in T cells impaired the activation, proliferation, and function of T cells in vitro, associated with enhanced activation of JNK and reduced NFATc1 translocation into the nucleus. Consistently, MKP-1−/− mice were defective in anti-influenza immunity in vivo and resistant to experimental autoimmune encephalomyelitis. Our results thus demonstrate that MKP-1 is a critical positive regulator of T cell activation and function and may be targeted in treatment of autoimmune diseases.
Introduction
The MAPK2 pathways, including ERK, JNK, and p38 kinase in mammals, are evolutionarily conserved immune regulators (1). The biological outcome of MAPKs is determined by the magnitude and duration of their activation. The activation of MAPKs requires dual phosphorylation of Thr and Tyr residues in their activation loop. By dephosphorylating both Thr and Tyr residues, members of the MKP family serve as major negative regulators of MAPK activities. Recently, several MKPs have emerged as important players in both innate and adaptive immune responses through mouse genetic studies. For instance, we previously reported that MKP-5 (DUSP10) regulates innate and adaptive immune responses (2). In innate immunity, MKP-5 acts as a negative regulator in pro-inflammatory cytokine production. In adaptive immune responses, although MKP-5 is required for naïve CD4+ T cell activation and proliferation, it serves as a negative regulator of CD4+ and CD8+ T cell effector functions. Another MKP family member, PAC1 (DUSP2), has been shown to be a JNK phosphatase, too (3). A deficiency of PAC1 results in enhanced JNK activity and unexpectedly impaired ERK and p38 activation. PAC1 is a positive regulator of inflammatory cytokine expression in innate immunity, and its deficiency protects mice from inflammatory arthritis (3).
MKP-1 (DUSP1) is the first mammalian MKP identified, and its expression is induced by growth factors in mice and by stress in humans (4, 5). MKP-1 was originally shown to dephosphorylate ERK and to have a negative effect on cell proliferation (6, 7). Further study using primary MEFs derived from mice lacking MKP-1 demonstrated that this protein is required to inactivate p38 and JNK but not ERK in response to stress (8). In addition, MKP-1-deficient MEFs exhibit reduced cell growth and enhanced cell death compared with wild-type MEFs (8). More recently, the role of MKP-1 in innate immunity has been studied by multiple groups (9–12). MKP-1 was shown to act as a negative regulator of JNK and p38 but not ERK in macrophages and dendritic cells (DCs) (9, 10, 12). Moreover, MKP-1-deficient macrophages and DCs produce increased levels of both pro-inflammatory (including tumor necrosis factor-α and IL-6) and anti-inflammatory (IL-10) cytokines. MKP-1-deficient mice exhibit hypersensitivity to lipopolysaccharide-induced septic shock (10, 12) and develop more severe disease in a mouse model of rheumatoid arthritis compared with wild-type mice (11). MKP-1 is therefore considered as a crucial negative regulator of the innate immune response (13).
However, the function of MKP-1 in adaptive immune responses remains unclear. In this study, we demonstrate that MKP-1 KO T cells are defective in T cell activation and proliferation, associated with enhanced JNK activation and reduced nuclear NFATc1 expression. MKP-1 deficiency results in reduced Th1 and Th17 but not Th2 effector function. Consistent with in vitro results, antigen-specific T cells are defective in activation, proliferation, and function in the absence of MKP-1 in vivo. As a consequence, MKP-1 KO mice are resistant to EAE diseases and are impaired in anti-influenza viral infection. Collectively, our results demonstrate that MKP-1 is necessary in the activation of adaptive immune responses.
EXPERIMENTAL PROCEDURES
Mice
MKP-1 KO mice were provided by Dr. Anton Bennett (Yale University) with the permission of Bristol-Myers Squibb Co. (8, 14). OT-I and OT-II TCR transgenic mice were purchased from The Jackson Laboratory. All mice were maintained in the animal care facility at The University of Texas M. D. Anderson Cancer Center.
Peptides and Tetramers
Peptides SIINFEKL, OVA323–339 (ISQAVHAAHAEINEAGR), MOG35–55 (MEVGWYRSPFSRVVHLYRNGK), NP366–374 (ASNENMETM), PA224–233 (SSLENFRAYV), and NP311–325 (QVYSLIRPNENPAHK) were synthesized by SynBioSci Corp. (Livermore, CA). Phycoerythrin-conjugated MHC class I peptide tetramers specific for NP366–374/Db (DbNP366 tetramer) were generated by the NIAID MHC Tetramer Core Facility.
In Vitro T Cell Assays
Naïve CD4+ and CD8+ T cells were purified from lymph nodes and spleens of mice by fluorescence-activated cell sorting based on the CD4+CD62LhiCD44lo and CD8+CD62LhiCD44lo surface phenotypes. To analyze the effects of MKP-1 on T cell activation and proliferation, T cells were incubated with different concentrations of plate-bound anti-CD3 antibody or anti-CD3 plus anti-CD28 antibodies. IL-2 production by T cells was measured by ELISA (Pharmingen) 24 h after T cell activation. Cell proliferation was determined 72 h after incubation with [3H]thymidine in the last 8 h. To examine the role of MKP-1 in effector function, naïve CD4+ T cells were cultured under Th1, Th2, or Th17 conditions as described previously (15). After 4 days of differentiation, cells were washed and treated with 3 μg/ml plate-bound anti-CD3 antibody for cytokine measurement or treated with PMA and ionomycin (Sigma) in the presence of GolgiPlug (BD Biosciences) for intracellular cytokine staining. For assay of AICD, naïve CD4+ T cells were incubated with plate-bound anti-CD3 and anti-CD28 antibodies for 4 days in the presence of 30 units/ml human IL-2. Dead cells were removed using LSM® lymphocyte separation medium (MP Biomedicals, Solon, OH). Subsequently, cells were reactivated with 3 μg/ml plate-bound anti-CD3 antibody for 48 h. AICD was determined by staining cells with fluorescein isothiocyanate-conjugated annexin V and propidium iodide (BD Biosciences). Samples were analyzed by flow cytometry.
MAPK and NFATc1 Analysis
For analysis of ERK and p38 activation, naïve CD4+ T cells were activated either with 50 ng/ml PMA and 500 ng/ml ionomycin or with 5 μg/ml plate-bound anti-CD3 antibody with or without 2.5 μg/ml anti-CD28 antibody for different times. Cell lysates prepared as described previously (16) were subjected to Western blot analysis with anti-phospho-JNK, anti-phospho-ERK, and anti-phospho-p38 antibodies (Cell Signaling Technology, Danvers, MA). The signal was detected with ECL reagent (Pierce). For NFATc1 analysis, naïve CD4+ cells were stimulated with 5 μg/ml plate-bound anti-CD3 antibody and 2.5 μg/ml anti-CD28 antibody for different times. Nuclear fractions were prepared as described previously (17). NFATc1 protein was detected by Western blot analysis using anti-NFATc1 antibody (Santa Cruz Biotechnology).
Chicken OVA Immunization
WT and MKP-1 KO mice were immunized as described (18) with chicken OVA protein (Sigma) emulsified in CFA at the base of the tail. On day 8, the immunized mice were killed, and three mice from each group were analyzed individually for their immune responses. Splenocytes were stimulated with SIINFEKL or OVA323–339 peptide to measure IL-2 production, T cell proliferation, and effector cytokine production. Sera were examined for OVA-specific antibodies.
Influenza Viral Infection and Analysis
Influenza virus A/Puerto Rico/8/34 (PR8, H1N1) was purchased from Charles River Laboratories. Mice (6–12 weeks) were anesthetized by intraperitoneal injection with ketamine and infected intranasally with 13 viral hemagglutinating units of PR8 influenza virus. Mice were monitored daily, and weight loss was recorded. Lymphocytes were collected from BAL and lungs as described previously (19). To compare NP366–374/Db-specific CD8+ T cells between WT and KO mice, phycoerythrin-conjugated DbNP366 tetramer staining was performed for 30 min at room temperature, followed by incubation with allophycocyanin-conjugated anti-CD8 antibody at 4 °C. Samples were analyzed by flow cytometry. Data were analyzed using FlowJo software (TreeStar Inc.). To analyze influenza virus-specific T cell responses, leukocytes from BAL and lungs were activated with 20 ng/ml PA224–233 or 20 μg/ml NP311–325 overnight, respectively. GolgiPlug was added during the last 6 h of incubation. Cells were permeabilized with a Cytofix/Cytoperm kit (BD Biosciences) and analyzed for the expression of IFNγ in CD4+ and CD8+ cells. The culture supernatant was collected. IFNγ concentration was determined by ELISA.
EAE Induction and Analysis
EAE was induced in WT and MKP-1 KO mice with MOG35–55 peptide as we described previously (20). Briefly, mice were immunized twice with the peptide in CFA, followed by two subsequent treatments with pertussis toxin 1 day after each immunization. The mice were observed daily for clinical signs and scored on a scale of 0–5 with gradations of 0.5 for intermediate scores: 0, no clinical signs; 1, loss of tail tone; 2, wobbly gait; 3, hind limb paralysis; 4, hind limb and forelimb paralysis; 5, death. Preparation and stimulation of mononuclear cells from brain and spinal tissues were done as described (20).
Adoptive Transfer and EAE Induction
Cells from lymph nodes and spleens of WT and MKP-1 KO mice were incubated with anti-CD4 microbeads, and CD4+ T cells were purified by an autoMACS sorter (Mitenyi Biotec, Auburn, CA). 5 × 106 cells were injected in the tail veins of Rag1−/− recipient mice. After 24 h, Rag1−/− recipients were used for EAE induction and analysis as described above.
Statistical Analysis
Statistical analysis was calculated with an unpaired Mann-Whitney test and STATISTICA software (StatSoft, Inc., Tulsa, OK). p values ≤0.05 were considered significant.
RESULTS
MKP-1 Regulates JNK Activities in T Cells
MAPKs have previously been shown to regulate T cell development (21). When we examined different thymocyte subpopulations, no difference in their percentages were detected in WT and MKP-1 KO mice (supplemental Fig. S1). Furthermore, the ratios of CD4+ and CD8+ mature T cells in the spleens and lymph nodes from WT and KO mice were comparable (supplemental Fig. S1). Although we have not examined the repertoire of TCR in peripheral T cells, MKP-1 appears to be dispensable for T cell development. It is possible that a deficiency of MKP-1 in T cell development can be compensated by other MKP family members.
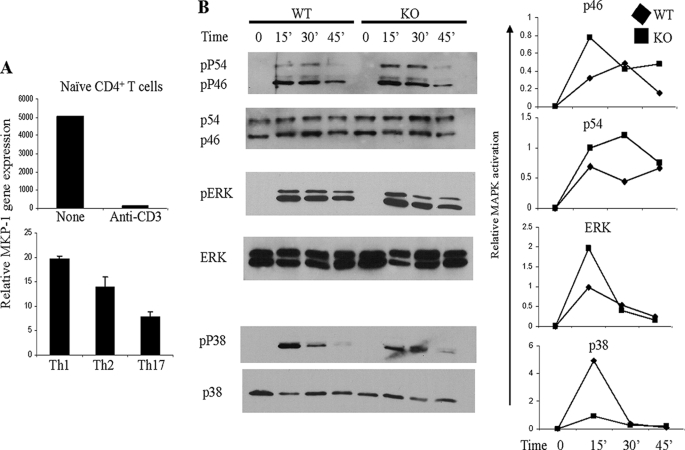
To investigate the possible roles of MKP-1 in T cell responses, we first examined its mRNA expression in naïve CD4+ T cells and effector Th cells, including Th1, Th2, and Th17 cells, using quantitative reverse transcription-PCR. MKP-1 was highly expressed in naïve CD4+ T cells (Fig. 1A). Its expression was dramatically down-regulated after 3 h of anti-CD3 stimulation. MKP-1 expression was observed in all effector Th subsets, with the highest expression in Th1 cells and the lowest expression in Th17 cells (Fig. 1A).
FIGURE 1.
Regulation of MAPK activation by MKP-1 in T cells. A, naïve CD4+ T cells were activated with or without anti-CD3 antibody. In vitro differentiated Th1, Th2, or Th17 cells were activated with anti-CD3 antibody. MKP-1 gene expression was determined by real-time reverse transcription-PCR. B, naïve CD4+ T cells were activated with PMA and ionomycin for the indicated times. Cells were lysed, and cell extracts were probed with antibodies to phospho (p)-p46 and phospho-p54 isoforms of JNK, total JNK, phospho-ERK, ERK, phospho-p38, and p38. Relative MAPK activation was determined by the levels of phospho-MAPKs normalized to their total protein expression. The data are representative of four separate experiments.
To examine the function of MKP-1 in T cells, we first assayed MAPK activation in WT and MKP-1 KO CD4+ T cells in response to PMA and ionomycin activation. As shown in Fig. 1B, the activation of JNK in both WT and MKP-1 KO cells was induced after 15 min of PMA and ionomycin stimulation (Fig. 1B). However, MKP-1 KO cells had enhanced activation of p46 JNK isoforms at 15 and 45 min and of p54 JNK at 15 and 30 min after PMA and ionomycin stimulation (Fig. 1B), indicating that MKP-1 is a negative regulator of JNK in CD4+ T cells. ERK activation in naïve CD4+ T cells from both WT and KO mice was undetectable without stimulation but was induced after 15 min of stimulation with PMA and ionomycin. The activation of ERK in both WT and KO cells exhibited similar kinetics; however, enhanced ERK activation in MKP-1 KO cells was detected at 15 min after PMA and ionomycin stimulation (Fig. 1B), indicating that MKP-1 might serve as a negative regulator of ERK in CD4+ T cells. Unlike ERK and JNK, the activation of p38 in MKP-1 KO CD4+ T cells was reduced compared with that in WT cells after 15 min of PMA and ionomycin activation (Fig. 1B). Similar results were obtained with WT and MKP-1 KO CD4+ T cells after TCR activation (data not shown). Therefore, in T cells, MKP-1 is a negative regulator of JNK and perhaps ERK but not p38.
MKP-1-deficient T Cells Are Defective in Their Activation
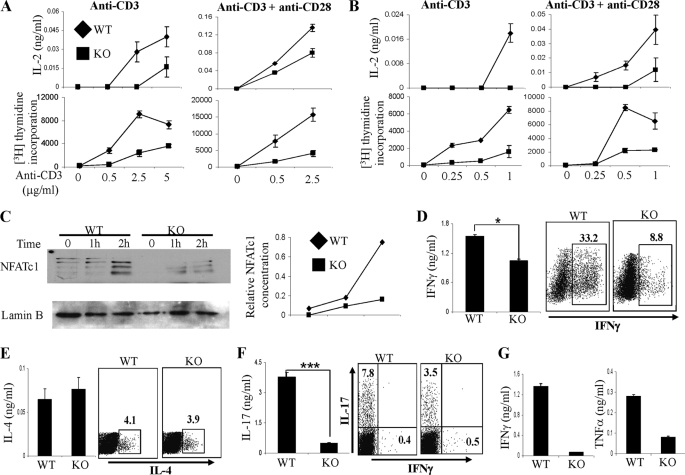
To examine the role of MKP-1 in CD4+ T cell activation and proliferation, WT and MKP-1 KO naïve CD4+ T cells were stimulated with plate-bound anti-CD3 antibody with or without anti-CD28 antibody. IL-2 concentration in the culture supernatant was determined by ELISA. We found that upon stimulation with anti-CD3 antibody alone or with anti-CD28 antibody, the production of IL-2 by MKP-1 KO CD4+ T cells was substantially lower than that by WT cells (Fig. 2A). Furthermore, MKP-1 KO CD4+ T cells exhibited impaired proliferation compared with WT cells. Similarly, we found that MKP-1 KO CD8+ T cells also produced lower levels of IL-2 and exhibited greatly reduced proliferative responses compared with WT cells in response to stimulation with anti-CD3 antibody alone or with anti-CD28 antibody (Fig. 2B). These results indicate that MKP-1 is required for T cell activation and proliferation.
FIGURE 2.
MKP-1 regulates T cell activation and effector function in vitro. A, naïve CD4+ T cells were activated with different concentrations of anti-CD3 antibody in the presence or absence of anti-CD28 antibody, and their IL-2 production and [3H]thymidine uptake were measured. B, IL-2 production and [3H]thymidine incorporation were measured in WT and KO CD8+ T cells in response to different concentrations of anti-CD3 antibody in the presence or absence of anti-CD28 antibody. C, nuclear fractions were prepared from WT and KO naïve CD4+ T cells. NFATc1 expression was examined by Western blot analysis. D–F, naïve CD4+ T cells from WT and KO mice were differentiated into Th1 (D), Th2 (E), and Th17 (F) cells, followed by anti-CD3 stimulation before ELISA assay and intracellular cytokine staining. *, p < 0.05; **, p < 0.005. G, purified naïve CD8+ T cells were activated with plate-bound anti-CD3 and anti-CD28 antibodies in the presence of IL-2 for 5 days. Cells were harvested and washed three times with RPMI 1640 complete medium, followed by restimulation with plate-bound anti-CD3 antibody for 24 h. Cytokine concentrations in the culture supernatant were measured by ELISA. The data are representative of three separate experiments.
TCR and CD28 signaling leads to the induction and activation of several transcription factors that regulate T cell activation and proliferation. Among them, members of the NFAT (nuclear factor of activated T cells) family play important roles in peripheral T cell activation and effector function (22, 23). We have shown that the nuclear accumulation of NFATc1, an essential step for its function, is negatively regulated by JNK1 (17) and that JNK1 directly phosphorylates NFATc1 (24). To examine the regulation of NFATc1 by MKP-1, we examined NFATc1 protein expression in the nuclei of WT and KO CD4+ T cells after anti-CD3 and anti-CD28 stimulation. We found that the nuclear accumulation of multiple isoforms of NFATc1 proteins, which are generated from alternative splicing (22), was greatly increased in WT cells after 2 h of stimulation (Fig. 2C). In contrast, the nuclear accumulation of NFATc1 in KO cells was minimal, indicating that MKP-1 is required for NFATc1 nuclear translocation in response to TCR and CD28 activation. Because NFATc1 is required in T cell proliferation (23, 25), greatly reduced NFATc1 nuclear accumulation in MKP-1-deficient cells is likely the underlying reason for their poor proliferation.
Previously, we reported that JNK1-deficient T cells exhibit increased proliferation associated with reduced AICD (17). To investigate the regulation of AICD by MKP-1, WT and MKP-1 KO CD4+ T cells activated with anti-CD3 and anti-CD28 antibodies were restimulated with anti-CD3 antibody to induce apoptosis. We found that whereas ∼30% of WT cells underwent apoptosis, >60% of KO cells did (supplemental Fig. S2). KO cells contained ∼5 times more single annexin V-positive cells (early apoptotic cells) and 2 times more propidium iodide-positive annexin V-positive cells (late apoptotic cells) compared with WT cells (supplemental Fig. S2), indicating that MKP-1 is a negative regulator of AICD in CD4+ T cells.
Upon activation, naïve CD4+ T cells differentiate into different lineages of effector cells, such as Th1, Th2, and Th17. To study the role of MKP-1 in the regulation of Th cell differentiation and function, WT and MKP-1 KO naïve CD4+ T cells were cultured under Th1, Th2, or Th17 conditions in vitro with exogenous IL-2 to normalize their proliferation. After 4 days of differentiation, effector Th cells were restimulated with anti-CD3 antibody to examine the secretion of lineage-specific cytokines by ELISA. We found that both MKP-1 KO Th1 and Th17 cells produced significantly reduced amounts of IFNγ and IL-17, respectively, whereas MKP-1 KO Th2 cells produced similar amounts of IL-4 compared with WT cells (Fig. 2, D–F). We further confirmed these observations by intracellular cytokine staining. KO naïve CD4+ T cells differentiated under both Th1 and Th17 conditions had lower percentages of IFNγ- and IL-17-producing cells, respectively, compared with WT cells (Fig. 2, D and F). On the other hand, both WT and MKP-1 KO T cells exhibited similar percentages of IL-4-producing cells when differentiated under Th2 conditions (Fig. 2E). These results demonstrated that MKP-1 is required for Th1 and Th17 differentiation and function.
Similar to CD4+ T cells, naïve CD8+ T cells, in response to TCR-mediated activation, differentiate into effector CD8+ T cells that acquire effector functions, including IFNγ and tumor necrosis factor-α cytokine production. To investigate the role of MKP-1 in CD8+ T cell effector function, WT and MKP-1 KO effector CD8+ T cells were activated in vitro in the presence of exogenous IL-2 before restimulation with anti-CD3 antibody. As shown in Fig. 2G, MKP-1 deficiency resulted in a profound defect in CD8+ T cell effector cytokine production, indicating that MKP-1 is a critical regulator of CD8+ T cell effector function. Together, these results demonstrated that MKP-1 is required for T cell activation and proliferation and T cell effector function.
MKP-1-deficient Mice Are Defective in Antigen-specific T Cell Responses in Vivo
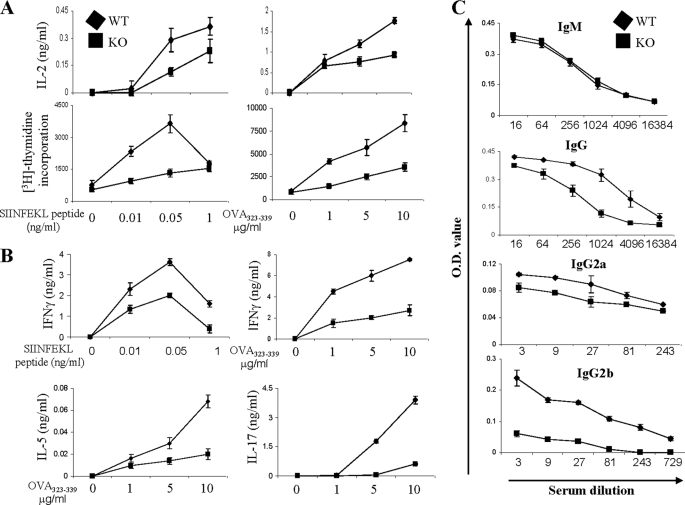
To investigate the regulation of antigen-specific T cell responses by MKP-1 in vivo, both WT and MKP-1 KO mice were immunized with chicken OVA protein in CFA, and on day 8 after immunization, splenocytes were isolated and restimulated with various concentrations of MHC class I-restricted SIINFEKL or MHC class II-restricted OVA323–339 peptide to assess antigen-specific CD8+ or CD4+ T cell responses, respectively. Upon restimulation with either peptide, MKP-1 KO cells produced reduced amounts of IL-2 and exhibited significantly reduced proliferation compared with WT cells (Fig. 3A). Effector cytokine production measurement revealed that KO cells also produced lower amounts of IFNγ compared with WT cells upon SIINFEKL peptide stimulation (Fig. 3B). In response to MHC class II-restricted OVA323–339 peptide restimulation, KO cells also produced much lower levels of Th1 (IFNγ), Th2 (IL-5), and Th17 (IL-17) cytokines compared with WT cells (Fig. 3B).
FIGURE 3.
MKP-1 is required for antigen-specific T cell responses in vivo. WT and KO mice (n = 5) were immunized with OVA in CFA. On day 8, splenocytes were isolated and stimulated with peptide as indicated. A, IL-2 production and cell proliferation were measured after 1 and 3 days of stimulation, respectively. B, cytokine production was determined by ELISA after 3 days of stimulation. C, anti-OVA antibodies of different isotypes in sera from immunized mice were measured by ELISA. The data are representative of two independent experiments with similar results.
To examine if MKP-1 plays any role in T cell-dependent humoral responses, we examined anti-OVA antibodies in the sera of WT and KO mice. As shown in Fig. 3C, both WT and KO mice had similar levels of anti-OVA IgM. However, the levels of anti-OVA IgG, IgG2a, and IgG2b antibodies in the sera of KO mice were all significantly lower than those in WT mice (Fig. 3C), indicating that a deficiency of MKP-1 reduces T cell-dependent humoral responses. Together, these data demonstrated that MKP-1 is required for antigen-specific T cell responses in vivo.
MKP-1 Is Required for Anti-influenza T Cell Responses
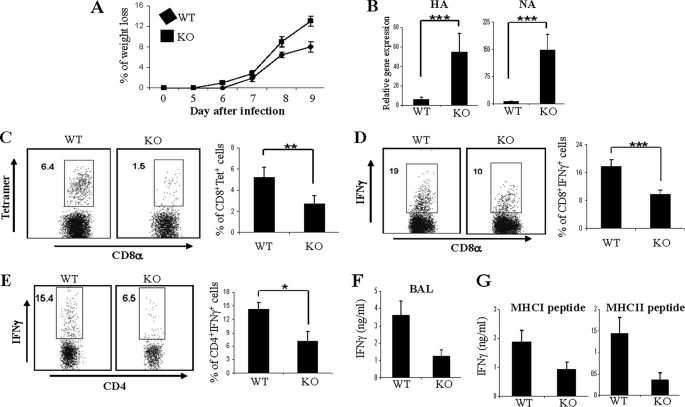
Adaptive immunity mediated by T cells plays a principal protective role in response to intracellular pathogen infection such as viral infection. Reduced T cell responses in MKP-1 KO mice following immunization in vivo suggest that MKP-1 may be important in host defenses to pathogens. To assess this, we employed influenza viral infection, in which both CD4+ and CD8+ T cell functions are critical to resolution (26, 27). We infected both WT and MKP-1 KO mice with influenza virus A/Puerto Rico/8/34 (PR8, H1N1) intranasally at a dose of 13 hemagglutinating units, which is nonlethal but causes severe disease in WT mice. At day 6 after influenza viral infection, WT mice started to lose weight, reaching the maximum weight loss by day 9 post-infection (∼8% of the original body weight) (Fig. 4A). In contrast, MKP-1 KO mice exhibited increased weight loss compared with WT mice in response to influenza viral infection, with a reduction as high as 13% of the original body weight by day 9 (Fig. 4A). To examine if MKP-1 KO mice have any defect in viral clearance, we examined the expression of hemagglutinin and neuraminidase genes of influenza virus in the lungs of WT and KO mice at days 1, 2, and 7 using real-time reverse transcription-PCR. We found that the expression of both hemagglutinin and neuraminidase genes in the lungs of MKP-1 KO mice was not different from that in WT mice on days 1 and 2 (supplemental Fig. S3), indicating that MKP-1 is dispensable for controlling viral growth at an early stage of infection. However, we found that on day 7, the expression of hemagglutinin and neuraminidase genes in the lungs of MKP-1 KO mice was significantly higher than that in the lungs of infected WT mice (Fig. 4B), indicating that MKP-1 KO mice are impaired in influenza viral clearance.
FIGURE 4.
MKP-1-deficient mice are defective in anti-influenza responses. WT and KO mice were infected with 13 hemagglutinating units of PR8 influenza virus. A, changes in body weight of WT and KO mice after infection were monitored daily. B, mice were killed 7 days after infection. Hemagglutinin (HA) and neuraminidase (NA) gene expression in the lungs of day 7 influenza-infected WT and KO mice was analyzed by real-time reverse transcription-PCR. C, virus-specific CD8+ T cells in BAL were determined by staining with DbNP366 tetramer and anti-CD8 antibody. D, IFNγ-producing CD8+ cells in BAL from day 7 influenza-infected WT and KO mice in response to PA224–233 peptide stimulation were analyzed by intracellular cytokine staining. E, IFNγ-producing CD4+ cells in BAL from day 7 influenza-infected WT and KO mice in response to NP311–325 peptide stimulation were analyzed by intracellular cytokine staining. F, IFNγ concentrations in BAL from day 7 influenza-infected WT and KO mice were measured by ELISA. G, IFNγ concentrations in culture supernatants of lung-infiltrated leukocytes from day 7 influenza-infected WT and KO mice in response to PA224–233 (MHCI) and NP311–325 (MHCII) peptide stimulation were determined by ELISA. The data are representative of four independent experiments with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
Both virus-specific CD4+ and CD8+ T cells are important in anti-influenza immunity and viral clearance (26, 27). It has been shown that the accumulation of both CD4+ and CD8+ T cells in nasal mucosa peaks on day 7 following PR8 viral challenge (28). To examine the regulation of anti-viral CD4+ and CD8+ T cell responses by MKP-1, on days 7 and 9 after infection, leukocytes from BAL and lungs of infected mice were stained with anti-CD4 and anti-CD8 antibodies. We found that BAL from MKP-1 KO mice contained significantly lower percentages of CD8+ T cells but increased CD4+ T cells compared with that from WT mice on both days 7 and 9 (supplemental Fig. S4A).
It has been shown that pulmonary CD8+ T cell response to primary intranasal PR8 influenza viral infection is directed predominantly against epitopes derived from the nucleoprotein and polymerase protein (NP366–374/Db and PA224–233/Db) (29). We further evaluated virus-specific CD8+ T cells in both BAL and lungs from WT and KO mice by DbNP366 tetramer staining. As shown in Fig. 4C and supplemental Fig. S4B, MKP-1 KO mice contained significantly lower percentages of DbNP366-specific CD8+ T cells in both BAL and lungs compared with WT mice. Furthermore, in response to the activation of another CD8+-specific viral peptide, PA224–233, cells from both BAL and lungs of MKP-1 KO mice contained significantly lower percentages of IFNγ-producing CD8+ T cells compared with WT mice (Fig. 4D and supplemental Fig. S4C). Although CD4+ T cells were not reduced in the lungs of MKP-1 KO mice, in response to the activation of NP311–325 peptide (an MHC class II-specific dominant epitope from a nucleoprotein of PR8 virus), MKP-1 KO mice had significantly lower percentages of IFNγ-producing CD4+ T cells in BAL (Fig. 4E) and lungs (data not shown) compared with WT mice. Consistent with reduced virus-specific CD8+ and CD4+ IFNγ-producing cells in BAL, we detected significantly lower concentrations of IFNγ in the BAL fluid from KO mice than in that from WT mice (Fig. 4F). Furthermore, when incubated with PA224–233 and NP311–325 peptides, respectively, supernatants of lung leukocytes from KO mice had lower concentrations of IFNγ than those from WT mice (Fig. 4G). Together, these results demonstrated that MKP-1 deficiency results in defective influenza virus-specific CD4+ and CD8+ T cell responses.
MKP-1 Is Required for Autoimmune Responses in Vivo
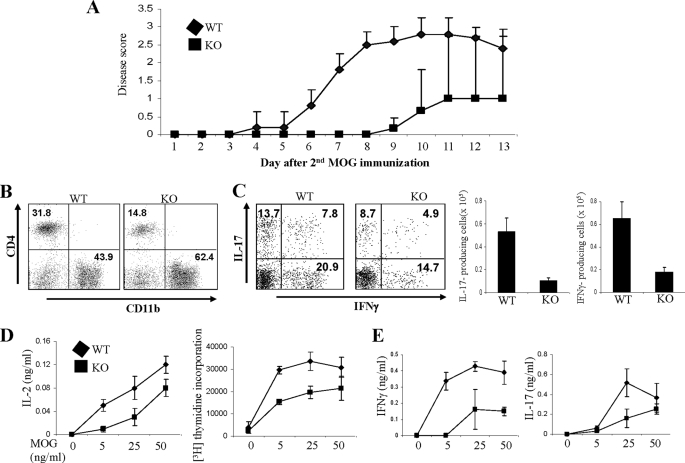
To examine the importance of MKP-1 in regulating autoreactive CD4+ T cell responses in vivo, we immunized both WT and MKP-1 KO mice with MOG35–55 peptide as we described previously (20) to induce EAE disease. We found that MKP-1 KO mice exhibited a consistently and considerably delayed onset of disease compared with WT mice (Fig. 5A). On day 3 after the second MOG immunization, WT mice started to develop the disease and reached scores of 2.5–3.0 by day 8, at which point MKP-1 KO mice did not show signs of the disease.
FIGURE 5.
MKP-1-deficient mice are resistant to EAE disease. A, WT and MKP-1 KO mice were immunized with MOG35–55 peptide to induce EAE. EAE disease was scored. B, mononuclear cells in the central nervous system were stained with anti-CD4 and anti-CD11b antibodies. C, central nervous system-infiltrated leukocytes were activated with PMA and ionomycin in the presence of GolgiPlug. IFNγ and IL-17 production by CD4+ cells was examined by intracellular cytokine staining. Total cytokine-producing cells were calculated and averaged. D and E, splenocytes from MOG-immunized WT and KO mice were restimulated with MOG35–55 peptide. IL-2 production and [3H]thymidine incorporation were determined (D). IFNγ and IL-17 concentrations in the culture supernatant were measured by ELISA (E). The data are representative of two independent experiments with similar results.
CD4+ T cells (particularly Th17 cells) play a critical role in the pathogenesis of EAE (30, 31). Consistent with the reduced EAE disease in MKP-1 KO mice, we detected reduced CD4+ T cell infiltration in the central nervous systems of diseased MKP-1 KO mice (Fig. 5B). Furthermore, central nervous system-infiltrating CD4+ T cells from MKP-1 KO mice expressed reduced levels of IL-17 and IFNγ compared with those from WT mice (Fig. 5C). Moreover, CD4+ T cells from the spleens of MKP-1 KO mice produced lower levels of IL-2, exhibited reduced proliferation, and produced reduced amounts of IL-17 and IFNγ compared with those of WT mice in response to MOG peptide restimulation (Fig. 5, D and E). Together, these results demonstrate that MKP-1 is required for autoreactive CD4+ T cell responses in vivo.
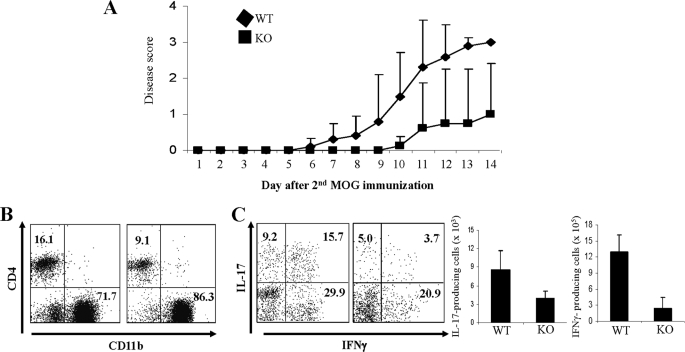
To investigate whether an intrinsic T cell defect results in the resistance of MKP-1 KO mice to EAE disease, we isolated CD4+ T cells from WT and MKP-1 KO mice and transferred 5 × 106 WT or KO cells into Rag1−/− mice, followed by immunization with MOG in CFA to induce EAE disease (32). On day 6 after the second MOG immunization, mice that received WT cells started to develop the disease, and all the mice in the group developed the disease with scores of 2.5–3.0 by day 11 (Fig. 6A). In contrast, mice that received MKP-1 KO CD4+ T cells did not develop the disease until day 10 after the second MOG immunization, and only 50% of the mice in this group developed mild disease with sores of 0.5–1.0 by day 14 (Fig. 6A). Consistent with the reduced EAE disease in mice that received KO cells, we detected reduced CD4+ T cell infiltration in the central nervous systems of diseased mice in this group (Fig. 6B). Furthermore, central nervous system-infiltrating CD4+ T cells from those mice expressed reduced levels of IL-17 and IFNγ compared with those from mice that received WT T cells (Fig. 6C). Together, these results demonstrated that an intrinsic defect in MKP-1 KO CD4+ T cells is responsible for the resistance of the mice to EAE disease.
FIGURE 6.
MKP-1 deficiency in CD4+ T cells causes resistance to EAE disease. CD4+ T cells were purified from WT and MKP-1 KO mice. 5 × 106 WT or KO cells were injected into Rag1−/− mice through the tail vein. 24 h after T cell transfer, Rag1−/− recipients (five mice in each group) were immunized with MOG35–55 peptide to elicit EAE. A, EAE disease was scored. B, mononuclear cells in the central nervous system were stained with anti-CD4 and anti-CD11b antibodies. C, central nervous system-infiltrated leukocytes were activated with PMA and ionomycin in the presence of GolgiPlug. IFNγ and IL-17 production by CD4+ cells was examined by intracellular cytokine staining. Total cytokine-producing cells were calculated and averaged. The data are representative of two independent experiments with similar results.
DISCUSSION
MKPs play critical roles in the regulation of MAPK activation. Gene KO studies of MKP-5, PAC1, and MKP-1 demonstrated that these molecules have a non-redundant function in innate immunity (2, 9–12). By differentially regulating MAPKs, they serve as negative (MKP-5 and MKP-1) or positive (PAC1) regulators of innate cytokine production. Our previous analysis of MKP-5 also demonstrated that MKPs regulate T cell priming and function (2). In the present study, we found that MKP-1 plays a critical role in adaptive immunity.
First, we found that MKP-1 is required to reduce JNK and possibly ERK but not p38 activation in T cells, which is different from the previous observations in macrophages and DCs (9, 10, 12). MKP-1 has been shown to target different MAPKs in different cell types and tissues. In macrophages, DCs, and primary MEFs, MKP-1 inactivates JNK and p38 but not ERK (8–10, 12). In MKP-1 KO white adipose tissues, the activation of ERK, JNK, and p38 is enhanced, whereas in MKP-1 KO mouse liver, the activation of JNK and p38 is enhanced and the activation of ERK is reduced compared with the WT control (12). One possible explanation for the different substrates of MKP-1 in different cells and tissues is the existence of several MKPs that have a redundant function in the regulation of the same MAPK. It is possible that ERK-specific phosphatases expressed in macrophages, DCs, MEFs, and liver, but not in adipocytes and T cells, and p38-specific phosphatases expressed in T cells compensate for the loss of MKP-1. Another possible explanation is the presence of regulatory mechanisms that regulate protein complex formation, such as those by scaffold proteins, which determine the substrate accessibility of MKP-1. For instance, the JIP-1 (JNK-interacting protein-1) scaffold protein has been shown to recruit MKP-7, a JNK phosphatase, into the assembled signaling complex to control JNK activation (33).
More importantly, in our study, we found that the absence of MKP-1 has profound effects on CD4+ and CD8+ T cell activation and effector function in vitro and in vivo. In general, ERK signaling is believed to promote cell proliferation and survival (34). On the other hand, JNK MAPKs inhibit CD4+ T cell activation and proliferation and promote apoptosis (17, 35). For instance, JNK1-deficient CD4+ T cells are hyperproliferative with decreased AICD (17). The nuclear accumulation of NFATc1, an important transcription factor for T cell activation and effector function, is increased in Jnk1−/− CD4+ T cells. CD4+ T cells lacking both JNK1 and JNK2 produce higher amounts of IL-2 and proliferate better than WT cells (35). Furthermore, JNK directly phosphorylates NFATc1 as a physiological substrate (24). Thus, the impaired T cell activation seen in MKP-1-deficient T cells is consistent with increased JNK activity. We detected reduced protein levels of NFATc1 in the nuclei of MKP-1 KO CD4+ T cells in response to TCR and CD28 signaling compared with those of WT cells. Because NFATc1 is required for proper T cell proliferation (23, 25), we believe that the regulation of NFATc1 nuclear translocation by MKP-1 is likely responsible for these defects of the MKP-1 KO T cells.
Of note, enhanced JNK activation in MKP-5-deficient T cells leads to defective CD4+ T cell activation and proliferation (2). It thus appears that MKP-1 and MKP-5 have a similar function in early T cell activation. Consistent with this idea, a deficiency of MKP-1 or MKP-5 results in the resistance of the mice to MOG-induced EAE disease (2). These results point out the importance of MKPs in autoimmune responses in vivo. However, MKP-1 and MKP-5 appear to have differential function in effector T cell function: whereas MKP-1 is required in the generation of Th1, Th17, and CD8+ T cell responses, MKP-5 is a negative regulator of CD4+ and CD8+ T cell effector function. Why this is the case will require further investigation.
In summary, we show here that MKP-1 is required for adaptive immunity and for T cell activation and function. The regulatory roles of MKP-1 in EAE and influenza viral infection suggest that inhibition of MKP-1 expression or function in self-destructive T cells may be beneficial in the treatment of T cell-mediated autoimmune diseases such as multiple sclerosis, whereas maintaining proper or enhancing MKP-1 function should be taken into consideration when developing therapeutic methods to boost T cell responses in infectious diseases and cancer.
Supplementary Material
Acknowledgments
We thank Dr. Anton Bennett and Bristol-Myers Squibb Co. for MKP-1 KO mice and the entire Dong laboratory for help and discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant AI050761 (to C. D. and R. I. N.). This work was also supported by grants from the Center for Targeted Therapy of the M. D. Anderson Cancer Center (to C. D.), an NCI training grant (to J. R.), an American Heart Association scientist development grant (to R. I. N.), and a Leukemia and Lymphoma Society scholar award and a Trust fellowship from the M. D. Anderson Cancer Center (to C. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated protein kinase
- JNK
- c-Jun N-terminal kinase
- MKP
- MAPK phosphatase
- MEFs
- mouse embryonic fibroblasts
- DCs
- dendritic cells
- IL
- interleukin
- KO
- knock-out
- EAE
- experimental autoimmune encephalomyelitis
- TCR
- T cell receptor
- OVA
- ovalbumin
- MOG
- myelin oligodendrocyte glycoprotein
- NP
- nucleoprotein
- PA
- polymerase
- MHC
- major histocompatibility complex
- ELISA
- enzyme-linked immunosorbent assay
- PMA
- phorbol 12-myristate 13-acetate
- AICD
- activation-induced cell death
- WT
- wild-type
- CFA
- complete Freund's adjuvant
- BAL
- bronchoalveolar lavage
- IFNγ
- interferon-γ.
REFERENCES
- 1.Dong C., Davis R. J., Flavell R. A. (2002) Annu. Rev. Immunol. 20, 55–72 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Blattman J. N., Kennedy N. J., Duong J., Nguyen T., Wang Y., Davis R. J., Greenberg P. D., Flavell R. A., Dong C. (2004) Nature 430, 793–797 [DOI] [PubMed] [Google Scholar]
- 3.Jeffrey K. L., Brummer T., Rolph M. S., Liu S. M., Callejas N. A., Grumont R. J., Gillieron C., Mackay F., Grey S., Camps M., Rommel C., Gerondakis S. D., Mackay C. R. (2006) Nat. Immunol. 7, 274–283 [DOI] [PubMed] [Google Scholar]
- 4.Charles C. H., Abler A. S., Lau L. F. (1992) Oncogene 7, 187–190 [PubMed] [Google Scholar]
- 5.Keyse S. M., Emslie E. A. (1992) Nature 359, 644–647 [DOI] [PubMed] [Google Scholar]
- 6.Sun H., Charles C. H., Lau L. F., Tonks N. K. (1993) Cell 75, 487–493 [DOI] [PubMed] [Google Scholar]
- 7.Noguchi T., Metz R., Chen L., Mattéi M. G., Carrasco D., Bravo R. (1993) Mol. Cell. Biol. 13, 5195–5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J. J., Bennett A. M. (2005) J. Biol. Chem. 280, 16461–16466 [DOI] [PubMed] [Google Scholar]
- 9.Hammer M., Mages J., Dietrich H., Servatius A., Howells N., Cato A. C., Lang R. (2006) J. Exp. Med. 203, 15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q., Wang X., Nelin L. D., Yao Y., Matta R., Manson M. E., Baliga R. S., Meng X., Smith C. V., Bauer J. A., Chang C. H., Liu Y. (2006) J. Exp. Med. 203, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salojin K. V., Owusu I. B., Millerchip K. A., Potter M., Platt K. A., Oravecz T. (2006) J. Immunol. 176, 1899–1907 [DOI] [PubMed] [Google Scholar]
- 12.Chi H., Barry S. P., Roth R. J., Wu J. J., Jones E. A., Bennett A. M., Flavell R. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 2274–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Shepherd E. G., Nelin L. D. (2007) Nat. Rev. Immunol. 7, 202–212 [DOI] [PubMed] [Google Scholar]
- 14.Dorfman K., Carrasco D., Gruda M., Ryan C., Lira S. A., Bravo R. (1996) Oncogene 13, 925–931 [PubMed] [Google Scholar]
- 15.Angkasekwinai P., Park H., Wang Y. H., Wang Y. H., Chang S. H., Corry D. B., Liu Y. J., Zhu Z., Dong C. (2007) J. Exp. Med. 204, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurieva R. I., Duong J., Kishikawa H., Dianzani U., Rojo J. M., Ho I., Flavell R. A., Dong C. (2003) Immunity 18, 801–811 [DOI] [PubMed] [Google Scholar]
- 17.Dong C., Yang D. D., Wysk M., Whitmarsh A. J., Davis R. J., Flavell R. A. (1998) Science 282, 2092–2095 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Chung Y., Bishop C., Daugherty B., Chute H., Holst P., Kurahara C., Lott F., Sun N., Welcher A. A., Dong C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11695–11700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussell T., Spender L. C., Georgiou A., O'Garra A., Openshaw P. J. (1996) J. Gen. Virol. 77, 2447–2455 [DOI] [PubMed] [Google Scholar]
- 20.Dong C., Juedes A. E., Temann U. A., Shresta S., Allison J. P., Ruddle N. H., Flavell R. A. (2001) Nature 409, 97–101 [DOI] [PubMed] [Google Scholar]
- 21.Fischer A. M., Katayama C. D., Pagès G., Pouysségur J., Hedrick S. M. (2005) Immunity 23, 431–443 [DOI] [PubMed] [Google Scholar]
- 22.Rao A., Luo C., Hogan P. G. (1997) Annu. Rev. Immunol. 15, 707–747 [DOI] [PubMed] [Google Scholar]
- 23.Yoshida H., Nishina H., Takimoto H., Marengère L. E., Wakeham A. C., Bouchard D., Kong Y. Y., Ohteki T., Shahinian A., Bachmann M., Ohashi P. S., Penninger J. M., Crabtree G. R., Mak T. W. (1998) Immunity 8, 115–124 [DOI] [PubMed] [Google Scholar]
- 24.Chow C. W., Dong C., Flavell R. A., Davis R. J. (2000) Mol. Cell. Biol. 20, 5227–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranger A. M., Hodge M. R., Gravallese E. M., Oukka M., Davidson L., Alt F. W., de la Brousse F. C., Hoey T., Grusby M., Glimcher L. H. (1998) Immunity 8, 125–134 [DOI] [PubMed] [Google Scholar]
- 26.Eichelberger M., Allan W., Zijlstra M., Jaenisch R., Doherty P. C. (1991) J. Exp. Med. 174, 875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruder D., Srikiatkhachorn A., Enelow R. I. (2006) Viral Immunol. 19, 147–155 [DOI] [PubMed] [Google Scholar]
- 28.Wiley J. A., Hogan R. J., Woodland D. L., Harmsen A. G. (2001) J. Immunol. 167, 3293–3299 [DOI] [PubMed] [Google Scholar]
- 29.Belz G. T., Xie W., Altman J. D., Doherty P. C. (2000) J. Virol. 74, 3486–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langrish C. L., Chen Y., Blumenschein W. M., Mattson J., Basham B., Sedgwick J. D., McClanahan T., Kastelein R. A., Cua D. J. (2005) J. Exp. Med. 201, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H., Li Z., Yang X. O., Chang S. H., Nurieva R., Wang Y. H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. (2005) Nat. Immunol. 6, 1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung Y., Chang S. H., Martinez G. J., Yang X. O., Nurieva R., Kang H. S., Ma L., Watowich S. S., Jetten A. M., Tian Q., Dong C. (2009) Immunity 30, 576–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willoughby E. A., Perkins G. R., Collins M. K., Whitmarsh A. J. (2003) J. Biol. Chem. 278, 10731–10736 [DOI] [PubMed] [Google Scholar]
- 34.Kolch W. (2005) Nat. Rev. Mol. Cell Biol. 6, 827–837 [DOI] [PubMed] [Google Scholar]
- 35.Dong C., Yang D. D., Tournier C., Whitmarsh A. J., Xu J., Davis R. J., Flavell R. A. (2000) Nature 405, 91–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.