Abstract
High density lipoprotein (HDL) is the major atheroprotective particle in plasma. Recent studies demonstrate that myeloperoxidase (MPO) binds to HDL in vivo, selectively targeting apolipoprotein A1 (apoA1) of HDL for oxidative modification and concurrent loss in cholesterol efflux and lecithin cholesterol acyl transferase activating activities, generating a “dysfunctional HDL” particle. We now show that (patho)physiologically relevant levels of MPO-catalyzed oxidation result in loss of non-cholesterol efflux activities of HDL including anti-apoptotic and anti-inflammatory functions. One mechanism responsible is shown to involve the loss of modified HDL binding to the HDL receptor, scavenger receptor B1, and concurrent acquisition of saturable and specific binding to a novel unknown receptor independent of scavenger receptors CD36 and SR-A1. HDL modification by MPO is further shown to confer pro-inflammatory gain of function activities as monitored by NF-κB activation and surface vascular cell adhesion molecule levels on aortic endothelial cells exposed to MPO-oxidized HDL. The loss of non-cholesterol efflux activities and the gain of pro-inflammatory functions requires modification of the entire particle and can be recapitulated by oxidation of reconstituted HDL particles comprised of apoA1 and nonoxidizable phosphatidylcholine species. Multiple site-directed mutagenesis studies of apoA1 suggest that the pro-inflammatory activity of MPO-modified HDL does not involve methionine, tyrosine, or tryptophan, oxidant-sensitive residues previously mapped as sites of apoA1 oxidation within human atheroma. Thus, MPO-catalyzed oxidation of HDL results not only in the loss of classic atheroprotective reverse cholesterol transport activities of the lipoprotein but also both the loss of non-cholesterol efflux related activities and the gain of pro-inflammatory functions.
Introduction
High density lipoprotein (HDL)3 is a complex mixture of cholesterol carrying lipoprotein particles built upon a predominantly apolipoprotein A1 (apoA1) backbone. HDL is currently thought to function primarily in mediating reverse cholesterol transport (RCT), the net transport of cholesterol from peripheral tissues to the liver for ultimate elimination into the intestinal lumen as biliary cholesterol for excretion in feces (1). RCT involves multiple biochemical processes, including both lipid poor apoA1 and HDL serving as acceptors of cholesterol efflux from peripheral cholesterol loaded cells, maturation of HDL from a nascent relatively cholesterol poor particle into a cholesterol-laden spherical form through interaction with lecithin cholesterol acyl transferase (LCAT), and delivery of cholesterol to liver and steroidogenic tissues through the HDL receptor, scavenger receptor B1 (SR-B1) (2).
Although the RCT related functions of apoA1 and HDL are thought to primarily account for both the atheroprotective activity and the strong inverse association of HDL cholesterol and apoA1 levels and cardiovascular risks, other non-cholesterol efflux-related activities have also been identified and thus potentially contribute to the protective functions of HDL (3). For example, early seminal studies by Chisolm and co-workers (4) showed anti-inflammatory properties of HDL where the cytotoxicity of oxidized low density lipoprotein for vascular endothelial cells and smooth muscle cells in culture could be prevented by HDL. Subsequent studies by Fogelman and co-workers (5, 6) have considerably extended upon these findings, including early demonstration that the anti-inflammatory function of HDL may become pro-inflammatory during the acute phase response, such as during acute influenza A infection. HDL shows anti-inflammatory activities when incubated with cultured vascular endothelial cells activated by cytokines (7), and bolus infusion of HDL promotes anti-inflammatory effects in vivo, such as in a porcine model of acute inflammation (8). More recent studies have demonstrated a critical role for SR-B1 binding of HDL in mediating many anti-inflammatory and anti-apoptotic activities of HDL via initiation of a cascade of downstream signaling pathways involving activation of both Akt and MAPKs and eventual endothelial nitric-oxide synthase (eNOS) activation (9, 10).
Since the initial findings of Van Lenten et al. (5), a growing body of data support the notion that both acute phase responses and chronic inflammatory conditions, including cardiovascular disease (CVD), can render HDL “dysfunctional” or “pro-inflammatory,” lacking in biological activities important in RCT (11, 12). HDL isolated from patients with CVD or chronic inflammatory disorders are less effective at preventing low density lipoprotein-induced monocyte migration in vitro (13). Recent studies have extended such observations to direct in vivo measures of RCT, demonstrating that inflammation impairs reverse cholesterol transport in murine models of endotoxemia (14).
One potential mechanism that may contribute to impairment in HDL function during inflammation and CVD is oxidative modification of the particle by myeloperoxidase (MPO). Zheng et al. (15, 16) first discovered that MPO, a leukocyte-derived heme protein implicated in atherosclerosis, binds to HDL via a specific binding domain on apoA1, promoting selective targeting of the lipoprotein in human plasma and atherosclerotic plaque for oxidative modification and a resultant loss of cholesterol efflux function. Independent studies have confirmed these observations (17, 18) as well as shown that site-specific oxidative modification of apoA1 within nascent HDL may inhibit the ability of the particle to activate LCAT, a critical process in HDL particle maturation and presumably the overall RCT pathway (19, 20).
Thus far, the functional consequences of MPO-mediated oxidative modification of HDL have only been linked to inhibition in the classic atheroprotective functions of the particle, namely inhibition in cholesterol efflux activity and LCAT activation. The impact of MPO-catalyzed oxidation of HDL on alternative processes critical to RCT such as SR-B1 interaction and non-cholesterol efflux-related activities of the particle remains unknown. Herein we show that apoA1 oxidation by the MPO/H2O2/Cl− system at levels comparable with those observed within apoA1 recovered from human atheroma results in total ablation of HDL-mediated anti-apoptotic and anti-inflammatory activities through a mechanism involving loss of SR-B1 binding. We further show that MPO-dependent modification of HDL confers pro-inflammatory activities to the intact particle but not individual components of HDL, resulting in vascular endothelial cell activation as monitored by both NF-κB activation and vascular cell adhesion molecule (VCAM-1) up-regulation and enhanced surface protein expression. Pathophysiological levels of MPO-catalyzed oxidation of HDL confer acquisition of saturable and specific binding activity to an unknown receptor(s) distinct from classic scavenger receptors (SR-B1, CD36, and SR-A1) on multiple primary and immortalized endothelial cells.
EXPERIMENTAL PROCEDURES
General Procedures
MPO was purified from detergent extracts of human leukocytes by lectin affinity and gel filtration chromatography, as described previously (21). HDL (1.063 < d < 1.21) was isolated by sequential ultracentrifugation from human plasma as described previously (22). ApoA1 was isolated from the plasma of healthy donors using established methods (23, 24). Recombinant human apoA1 was generated in an Escherichia coli expression system and isolated by sequential column chromatographies as described (19). Reconstituted nascent HDL (rHDL) was prepared using a sodium cholate dialysis method (25), and mass spectrometry confirmed a particle with a molar ratio similar to the starting composition of 100:10:1, POPC:cholesterol: apoA1, and <0.1% residual cholate as determined by stable isotope dilution mass spectrometry. rHDL particle size was characterized by native gel electrophoresis and dual beam light scattering, confirming a 96 Å particle size. HDL was iodinated by the method of Bolton and Hunter (26) to a specific activity of 80 dpm/ng protein and subsequently modified by the MPO/H2O2/Cl− system as outlined below. Protein content was determined by a Lowry assay (27). Biological materials isolated from the blood of healthy donors (e.g. MPO, HDL, apoA1) were performed under protocols approved by the Cleveland Clinic Institutional Review Board, and all of the participants gave written informed consent.
MPO Modification of HDL and apoA1
MPO-mediated modification of HDL and apoA1 was carried out as described previously at a mol ratio of 10 mol of H2O2/mol of apoA1 (16). Protein bound chlorotyrosine (ClTyr) was quantified by stable isotope dilution HPLC with on-line electrospray ionization tandem mass spectrometry using an API 4000 triple quadrupole mass spectrometer (Applied Biosystems, Foster, CA) interfaced with a Cohesive HPLC (Franklin, MA) with Ionics redesigned source as an upgrade (21).
Mice and Isolation of Mouse Peritoneal Macrophages
All animal studies were approved by the Institutional Animal Care and Utilization Committee of Cleveland Clinic. All of the mice used were on a C57Bl6/J background (>10 generations) and maintained on normal chow. CD36 knock-out (KO), SR-A1 KO, and CD36/SR-A1 double knock out (DKO) mice were generously provided by Dr. Maria Febbraio (Cleveland Clinic). Mice peritoneal macrophages (MPMs) were elicited by thioglycollate injection as previously described (28). MPMs were cultured in RPMI with 10% fetal bovine serum overnight, and nonadherent cells were removed by washing.
Functional Characterization of MPO-modified HDL
Cholesterol efflux (total) and LCAT activity were determined as previously described (19, 29). Human umbilical vein endothelial cells (HUVEC) were serum-deprived for 6 h with simultaneous incubation with 500 μg of protein/ml of HDL or 500 μg of protein/ml oxidized HDL. Where indicated, individual components (lipids extracted by the Bligh-Dyer method (30), delipidated apoA1 or POPC small unilamellar vesicles) were added. After 6 h, apoptosis was measured with an annexin V-fluorescein isothiocyanate apoptosis detection kit (BD Pharmingen, Franklin Lakes, NJ) or APO-BRDU kit (BD Biosciences). Flow cytometry of labeled cells was performed on a FACScan. An alternate endothelial cell type, bovine aortic endothelial cells (BAEC), and an alternate apoptogenic trigger, UV irradiation (254 nm, 12.4 watts, for 10 min), were used where indicated. Surface VCAM-1 protein levels were determined in HUVEC (TNF-α-induced VCAM-1) and BAEC (no TNF-α addition). The cells were incubated with 500 μg of protein/ml of HDL, 500 μg of protein/ml of oxidized HDL or, where indicated, individual protein and lipid components for 6 h. After three washes with phosphate-buffered saline, the cells were fixed in 4% paraformaldehyde on ice for 30 min. Surface VCAM-1 protein was determined using anti-VCAM-1 primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) with sheep anti-mouse horseradish peroxidase (GE Healthcare) as a secondary antibody, detection by SureBlue tetramethylbenzidine peroxidase substrate (KPL, Gaithersburg, MD) and measuring absorbance at 450 nm on a 96-well plate reader (Spectramax 384 Plus; Molecular Devices, Sunnyvale, CA) after the addition of 1 m HCl to stop the reaction. Caspase-3 activity in HUVEC was determined as described previously (9). Conversion of tritium-labeled arginine (GE Healthcare) to citrulline was used as a measure of eNOS activity. HUVEC were treated with the indicated protein concentrations of HDL or oxHDL for 10 min, and the media were collected and spun down to pellet debris. Arginine and citrulline separation was achieved by HPLC, and the [3H]citrulline peak was quantified by liquid scintillation counting. BAEC were treated with HDL or oxidized HDL for 6 h, and NF-κB activation in whole cell extracts was determined by electrophoretic mobility shift assay (EMSA) with supershift detected using antibody specific to p65 subunit of NF-κB (31). IκB kinase (IKK) activity was determined by immunoprecipitation of the IKK complex using IKKγ antibody, followed by performance of a kinase assay using recombinant GST-IκB-α(1–54) and [32P]ATP as substrates, as described previously (32). As an additional specificity control, recombinant mutant GST-IκB-α where serines 32 and 36 were mutated to alanine was generated and used as substrate.
SR-B1 Specific Binding Assay of HDL and MPO-oxidized HDL
Binding to SR-B1 was assessed as described previously (33). HEK 293T cells transfected with SR-B1 or pCGCG vector (34) were incubated with radiolabeled HDL or oxidized HDL for 1.5 h at 4 °C. Binding to wild type and knock-out MPMs was performed as described previously (35). Specific binding was calculated as total binding minus binding in the presence of 30-fold excess of unlabeled HDL (nonspecific binding). Competition binding experiments with 125I-labeled oxidized HDL isolated from plasma and oxidized rHDL synthesized with the pan Trp → Phe mutant apoA1 were performed at 30-fold molar excess of the unlabeled ligands.
Statistical Analysis
All of the experiments were performed at least three times. The results are reported as the means ± S.E. of at least triplicate determinations. Student's two-tailed t test was used for statistical analyses, and p < 0.05 was considered significant. Nonlinear regression was used to fit binding curves with GraphPad Prism 5.0 software.
RESULTS
HDL Oxidative Modification by (Patho)physiologically Relevant Levels of the MPO/H2O2/Cl− System Inhibits the Anti-apoptotic Activity of the Lipoprotein
In initial studies, we sought to test the hypothesis that HDL oxidation by (patho)physiologically relevant levels of the MPO/H2O2/Cl− system resulted in a loss of anti-apoptotic activity concurrent with loss of classic cholesterol efflux and LCAT activation properties of the particle. To do so we first exposed isolated human HDL to the MPO oxidant system and confirmed that exposure to the complete MPO/H2O2/Cl− system resulted in the loss of HDL-dependent cholesterol efflux activity when the modified lipoprotein was incubated with cholesterol-loaded macrophages (Fig. 1A), as well as inhibition of LCAT-catalyzed cholesteryl ester formation (Fig. 1B). MPO is the only known enzyme in mammals capable of generating chlorinating oxidants, enabling quantification of protein-bound ClTyr levels to serve as a specific “dosimeter” of exposure to MPO-mediated oxidation in vivo (36). The specificity of ClTyr as a post-translational modification of proteins formed by MPO-generated chlorinating oxidants has been confirmed in multiple models of inflammation using MPO knock-out mice (21, 37), with human MPO transgenic mice (38), and in studies employing neutrophils from MPO-deficient human donors (39). We therefore quantified ClTyr levels in the MPO-oxidized HDL preparations and confirmed that the degree of oxidation produced in vitro (Fig. 1C) is comparable with levels previously observed in apoA1 recovered from human atherosclerotic aortic tissues, where ClTyr levels as high as 25 mmol of ClTyr/mol of Tyr have been observed (15). Functional impairment in HDL-mediated cholesterol efflux and LCAT activity and parallel increases in HDL apoA1 ClTyr content showed an absolute requirement for the presence of each of the components of the oxidation system (MPO, H2O2, and Cl−) because eliminating any one of the components inhibited ClTyr production and functional impairment in the classic cholesterol transporting functions of HDL (Fig. 1). For all subsequent functional characterization studies described below for HDL exposed to MPO-catalyzed oxidation, the oxHDL preparations used were first similarly characterized by mass spectrometry to confirm that the degree of oxidation was pathophysiologically relevant (as indicated by levels of ClTyr within range observed for apoA1 recovered from human arterial tissues) and to be accompanied by significant impairment in cholesterol efflux and LCAT activating activities.
FIGURE 1.
Oxidation of HDL by the MPO/H2O2/Cl− system has functional consequences for classic atheroprotective activities of HDL. A, RAW macrophages were loaded with [3H]cholesterol and incubated for 6 h with 100 μg of protein/ml of HDL, HDL oxidized by the complete MPO/H2O2/Cl− system (oxHDL), or HDL exposed to the indicated components of the complete MPO system. The percentage cholesterol efflux was determined as described under “Experimental Procedures.” B, demonstration that MPO-catalyzed oxidation of reconstituted nascent HDL inhibits LCAT activating activity. C, the content of protein bound chlorotyrosine on HDL exposed to the MPO/H2O2/Cl− system (or the indicated components) was determined by stable isotope dilution liquid chromatography-tandem mass spectrometry as described under “Experimental Procedures.” The arrow indicates the upper range of chlorotyrosine content reported in apoA1 recovered from human atherosclerotic plaque (15). The results represent the means of triplicate determinations of a representative experiment performed at least three times.
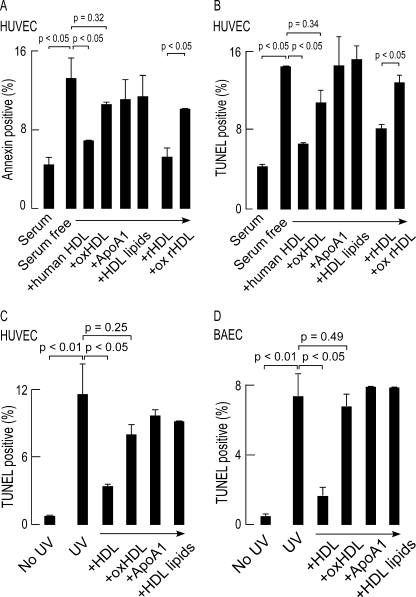
HUVECs undergo apoptosis upon serum deprivation, and previous studies have shown that isolated human HDL, but not low density lipoprotein, inhibits serum starvation-induced endothelial cell apoptosis (40). In cells that were serum-deprived for 6 h, the level of apoptosis as measured by both annexin staining (Fig. 2A) and TUNEL (Fig. 2B) increased nearly 3-fold. As expected, simultaneous incubation with 500 μg of protein/ml of isolated HDL markedly reduced the level of endothelial cell apoptosis. In contrast, the same concentration of MPO-oxHDL was ineffective at preventing HUVEC apoptosis (Fig. 2, A and B). Further, anti-apoptotic activity required the presence of the intact native particle because co-incubation of comparable levels of isolated human plasma-derived apoA1 and liposomes generated from HDL lipids failed to promote anti-apoptotic effect (Fig. 2).
FIGURE 2.
HDL protects HUVEC and BAEC from multiple apoptogenic triggers, whereas MPO-oxidized HDL fails to do so. A, HUVEC were placed in serum-free medium along with the indicated treatments for 6 h. B, apoptosis was quantified by either annexin positive staining by flow cytometry or TUNEL staining. C and D, HUVEC and BAEC were exposed to 254-nm UV irradiation for 10 min followed by incubation with the indicated treatments for 6 h. Apoptosis was quantified by TUNEL staining. The results represent the means of triplicate determinations of a representative experiment performed at least three times.
Because plasma HDL is a heterogeneous group of lipoproteins (41) with varying lipid composition, we next sought to identify the components of the lipoprotein involved in the anti-apoptotic activity. rHDL was prepared using human apoA1 (isolated from plasma) as the sole protein and the relatively oxidant-resistant lipid POPC as the sole phospholipid. Exposure of serum-starved HUVEC to 500 μg of protein/ml of rHDL recapitulated the anti-apoptotic activity observed with isolated human plasma derived HDL, demonstrating that an intact particle comprised of only apoA1 and a relatively nonoxidizable phosphatidylcholine molecular species was all that is needed for facilitating the anti-apoptotic activity of HDL (Fig. 2). Interestingly, oxidation of rHDL with MPO resulted in the loss of the anti-apoptotic effect (Fig. 2, A and B). Similar behavior for rHDL versus oxidized rHDL and the loss of macrophage cholesterol efflux activity and LCAT activation were also observed (data not shown). The similar anti-apoptotic activity of plasma HDL and rHDL but not with isolated apoA1 or HDL lipid liposomes, highlights the importance of the structural integrity of HDL for its anti-apoptotic activity, as well as the likelihood that oxidation of the protein (apoA1) and not the lipid component of rHDL is responsible for ablating the anti-apoptotic effect.
To further explore the generality of these observations, the anti-apoptotic activities of isolated human HDL versus MPO-generated oxHDL were examined using multiple distinct endothelial cells (HUVECs, BAECs, and human aortic endothelial cells) to alternative apoptogenic triggers. Ultraviolet light exposure is a classic apoptogenic trigger. When each of the endothelial cell lines were irradiated with 254-nm UV light, a nearly 10-fold increase in apoptosis was observed, which was markedly attenuated by concomitant addition of 500 μg of protein/ml of isolated human HDL. In contrast, neither MPO-generated oxHDL nor the individual components of native HDL (isolated lipid poor apoA1 or liposomes produced from HDL extracted lipids) reduced the rate of apoptosis significantly (Fig. 2, C and D; data for only HUVECs and BAECs shown).
HDL Modified by MPO Loses Its Capacity to Inhibit Caspase Activation and to Activate Endothelial Nitric-oxide Synthase Activity
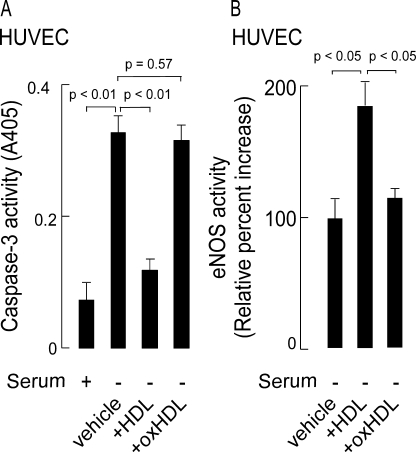
One of the mechanisms that accounts for the anti-apoptotic effect of HDL is its ability to inhibit caspase-3 activation, the terminal caspase that mediates proteolytic cleavage of key cellular proteins (9). Because pathophysiologically relevant levels of MPO-catalyzed oxidation of HDL causes the lipoprotein to lose its anti-apoptotic activity, we decided to measure the ability of native versus MPO-oxidized HDL to inhibit caspase-3 activation. Under serum starvation conditions, HDL inhibited caspase-3 activation, whereas HDL modified by the complete MPO oxidation system failed to inhibit caspase-3 activity (Fig. 3A). Previous investigations have also shown that the mechanism whereby HDL exerts anti-apoptotic activity originates with HDL binding to its receptor, SR-B1, followed by activation of signaling pathways involving the survival kinases Akt and MAPK, which phosphorylate and activate eNOS (10). Activation of eNOS results in simultaneous production of nitric oxide, which is protective against apoptosis, and the co-product citrulline. We therefore next examined the ability of native HDL versus oxHDL to activate eNOS. [3H]Citrulline production from tracer levels of [3H]arginine was used as a means of gauging the activity level of eNOS following exposure of HUVEC to HDL versus HDL exposed to the MPO oxidant system. As expected, HDL treatment of HUVEC lead to activation of eNOS as indicated by the increased conversion of arginine to citrulline (Fig. 3B). In contrast, endothelial cell exposure to oxHDL failed to induce eNOS activation (Fig. 3B). Inhibition of both caspase-3 and eNOS activation by oxHDL was only seen upon incubation with HDL exposed to the complete MPO/H2O2/Cl− system because omission of any individual component permitted HDL to retain its anti-apoptotic activity.
FIGURE 3.
Exposure of HDL to the MPO oxidant system inhibits the anti-apoptotic activity of the particle as monitored by loss of capacity to both inhibit caspase-3 activity and induce eNOS activity. A, serum starvation of HUVEC for 24 h increases endothelial cell caspase-3 activity. The anti-apoptotic activity of HDL as monitored by inhibition in caspase-3 activation. The anti-apoptotic activity of HDL exposed to the complete MPO/H2O2/Cl− system is also shown. B, the capacity of HDL or HDL previously exposed to the MPO/H2O2/Cl− system to activate endothelial cell eNOS was determined by monitoring [3H]citrulline formation from [3H]arginine as described under “Experimental Procedures.” The results represent the means of triplicate determinations of a representative experiment performed at least three times.
MPO-dependent Oxidation of HDL Inhibits the Anti-inflammatory Properties of the Particle as Monitored by Inhibition in TNF-α-induced VCAM-1 Protein Expression in Endothelial Cells
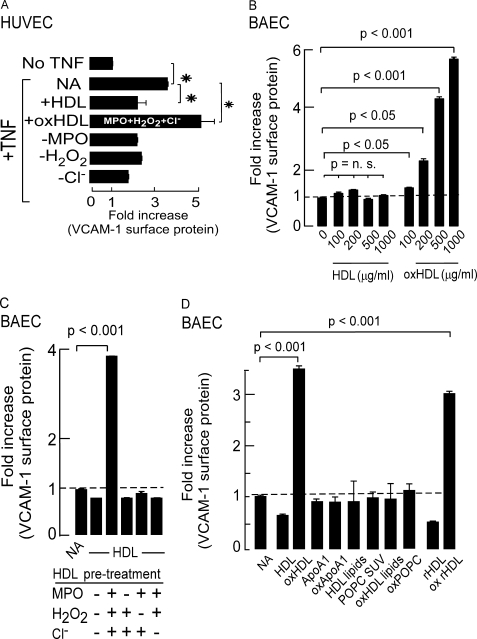
VCAM-1 is involved in the development of atherosclerosis by mediating the migration and extravasation of leukocytes across the vascular endothelium (42, 43). HDL is reported to inhibit VCAM-1 expression in endothelial cells (4) in an SR-B1-specific manner (44). We hypothesized that MPO-dependent oxidative modification of HDL may inhibit this anti-inflammatory and atheroprotective activity of HDL. When activated with TNF-α, HUVEC surface VCAM-1 protein levels increased 3-fold (Fig. 4A). HDL pretreatment (500 μg of protein/ml) resulted in a significant decrease in TNF-α-stimulated surface VCAM-1 protein (Fig. 4A), attesting to the anti-inflammatory actions of the lipoprotein. Remarkably, incubation of endothelial cells with MPO-oxidized HDL did not inhibit, but rather, further enhanced TNF-α-induced VCAM-1 protein expression in HUVEC, with the surface VCAM-1 level increasing nearly 5-fold. The marked increase in TNF-α-induced VCAM-1 protein expression promoted by co-incubation of HDL modified by MPO required the complete MPO oxidant system because elimination of any one of the components during the oxidation reaction (MPO, H2O2, and Cl−) lead to failure to confer pro-inflammatory activity to the particle (Fig. 4A).
FIGURE 4.
HDL oxidized by physiologically relevant levels MPO-generated oxidants inhibits the anti-inflammatory activity of the particle in HUVEC and promotes VCAM-1 protein expression in BAEC independent of TNF-α. A, HUVEC surface VCAM-1 protein expression was quantified by enzyme-linked immunosorbent assay in the absence and presence of TNFα as described under “Experimental Procedures.” In parallel, the impact of concomitant incubation with 500 μg of protein/ml of HDL, HDL previously exposed to the complete MPO/H2O2/Cl− system (oxHDL), or HDL incubated with the indicated components of the MPO/H2O2/Cl− system, on HUVEC surface VCAM-1 protein levels was determined by enzyme-linked immunosorbent assay as described under “Experimental Procedures.” *, p < 0.05. B, BAEC were incubated with the indicated concentrations of isolated human HDL or HDL exposed to the complete MPO/H2O2/Cl− system (oxHDL), and VCAM-1 surface protein levels were determined as described under “Experimental Procedures.” C, BAEC were incubated with 500 μg of protein/ml of HDL previously exposed to the indicated components of the MPO oxidation system and surface VCAM-1 protein levels were determined by enzyme-linked immunosorbent assay as described under “Experimental Procedures.” D, BAEC were incubated for 6 h with 500 μg of protein/ml of HDL, oxHDL, isolated human apoA1, apoA1 previously exposed to the complete MPO/H2O2/Cl− system (oxApoA1), lipid extract of HDL, small unilamellar vesicles (SUV) comprised of POPC, lipid extract of oxHDL, POPC small unilamellar vesicles exposed to the complete MPO/H2O2/Cl− system (oxPOPC), reconstituted nascent HDL (rHDL), or rHDL exposed to the complete MPO/H2O2/Cl− system (ox rHDL), and then cell surface VCAM-1 protein levels in BAEC were determined as described under “Experimental Procedures.” NA represents “no addition.” All of the results represent the means of triplicate determinations of a representative experiment performed at least three times.
Exposure of HDL to the MPO/H2O2/Cl− System Confers a Pro-inflammatory Gain of Function Activity to the Modified Particle
The increased VCAM-1 surface expression noted on endothelial cells exposed to oxHDL beyond levels observed with TNF-α activation suggested that oxHDL may activate endothelial cells independent of TNF-α stimulation. To test this hypothesis, BAECs were cultured in medium supplemented with increasing concentrations of either HDL or MPO-generated oxHDL in the absence of cytokine agonists, and surface levels of VCAM-1 protein were determined (for reference, normal plasma levels of apoA1 are 1200–1600 μg of protein/ml). Exposure to oxHDL induced over a 4-fold increase in endothelial cell surface VCAM-1 protein within 6 h, whereas exposure to native HDL failed to increase surface VCAM-1 protein (Fig. 4B). oxHDL-induced increase in surface VCAM-1 protein was dose-dependent (Fig. 4B) and required HDL modification by the complete oxidation system to confer the pro-inflammatory activity to the lipoprotein (Fig. 4C). Similar to the cholesterol efflux, LCAT activating, anti-apoptotic, and anti-inflammatory activities of HDL, the newly acquired pro-inflammatory activity of oxHDL was only observed with a structurally intact lipoprotein particle and could be recapitulated with reconstituted HDL particles generated with nonoxidizable phospholipid species but not oxidation of either lipid poor apoA1 or HDL lipids (Fig. 4D).
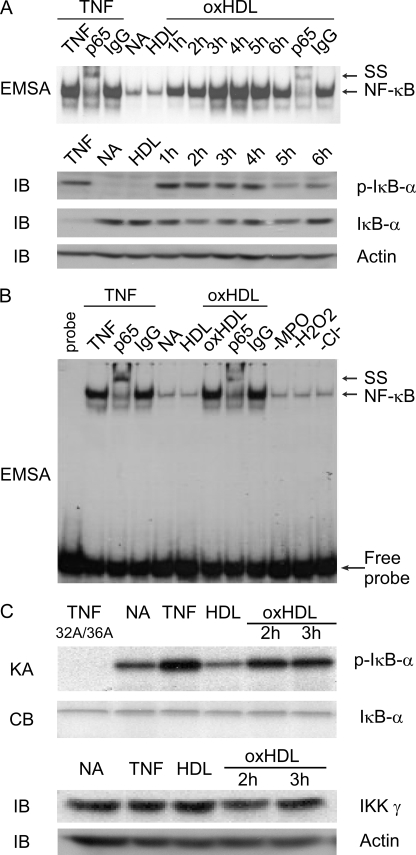
MPO-modified HDL Activates Endothelial Cell NF-κB in BAEC and Induces the Phosphorylation of IκBα
VCAM-1 expression is strongly influenced by the transcription factor NF-κB (45). Previous studies have demonstrated that HDL can inhibit TNF-α-induced activation of NF-κB (46). We therefore next tested the hypothesis that exposure of BAECs to oxHDL induces NF-κB activation. Although incubation of BAEC with HDL did not activate NF-κB, treatment with MPO-oxidized HDL induced NF-κB activation in a time-dependent manner (from between 1 and 6 h of oxidized HDL exposure), as determined by EMSA (Fig. 5A). Also, immunoblot analysis revealed phosphorylation of IκBα on IκB conserved serine residues 32 and 36, a hallmark of IKK activity, which is immediately upstream of NF-κB activation (47) (Fig. 5A). To verify that the DNA-protein complex was indeed NF-κB, an antibody specific to the p65 subunit of NF-κB was used and demonstrated a “supershift,” whereas an irrelevant control IgG antibody did not (Fig. 5A). MPO-generated oxHDL-induced NF-κB activation required the complete oxidation system; when MPO, H2O2, or Cl− was eliminated during the oxidation reaction, the HDL failed to be oxidized and did not activate NF-κB (Fig. 5B). IKK antibody-specific immuno-pulldown coupled kinase assays using IκBα as a specific substrate for IKK demonstrated that oxidized HDL treatment of BAEC activates the IKK complex (Fig. 5C).
FIGURE 5.
MPO-oxidized HDL induces bovine aortic endothelial cell NF-κB activation, IKK activation, and phosphorylation of IκBα. A, BAEC were incubated with TNFα for 30 min (first through third lanes), media only (NA), HDL for 3 h, or oxHDL for the indicated times. EMSA for NF-κB activation were then performed in whole cell lysates as described under “Experimental Procedures.” Where indicated, the lysates were also incubated with anti-NF-κB p65 or isotype control IgG and supershift (SS) of the NF-κB complex monitored. Parallel immunoblots (IB) were generated using phosphoserine 32- and 36-specific IκB-α antibody (p-IκB-α), demonstrating phosphorylation of IκB-α on serines 32 and 36 in oxHDL-treated cells. Immunoblot with specific antibody to IκB-α is also shown, along with a immunoblot of lysates probed with anti-β-actin to demonstrate equal loading in each lane. B, EMSA analysis of BAEC lysates as in A except that the cells were exposed to HDL modified by the complete MPO/H2O2/Cl− system (oxHDL) or the complete oxidant system minus the indicated components (i.e. −MPO, −H2O2, or −Cl−). Note that BAEC NF-κB activation is only observed by exposure to HDL previously incubated with the complete MPO/H2O2/Cl− system because eliminating any one of the components of the oxidation system produces a HDL particle that fails to activate endothelial cell NF-κB. C, BAEC were incubated with TNFα (30 min) as positive control, media alone (NA) as negative control, or either HDL (3 h) or HDL previously exposed to the complete MPO/H2O2/Cl− system (oxHDL, 2 or 3 h). IKK activity was then determined in BAEC lysates using IKK-specific immuno-pulldown coupled kinase assay (KA). IKK complex was immunoprecipitated with antibody to IKKγ, and kinase activity using recombinant GST-IκBα(1–54) and [32P]ATP as substrate was performed as described under “Experimental Procedures.” Note that IκBα is phosphorylated in response to stimulation by TNFα and oxHDL but not HDL. Specificity of the kinase reaction was confirmed by demonstrating failure of the site-specific mutant GST-IκB-α(1–54) (32A/36A) to be phosphorylated in TNFα-stimulated extracts. Parallel immunoblots using antibodies specific to either IKKγ or β-actin are also shown. Equivalent levels of GST-IκB-α substrate addition to the IKK complexes are shown by Coomassie Blue (CB) staining. NA refers to “no addition.”
Mechanism of Loss of Anti-apoptotic and Anti-inflammatory Activities of oxHDL Involves Loss of Binding to the HDL Receptor, SR-B1
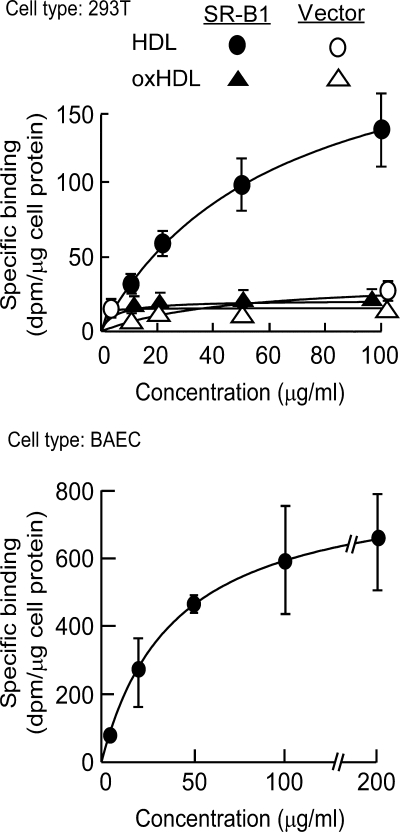
SR-B1 is the physiologic HDL receptor (48). Previous studies have shown that HDL binding to SR-B1 is a prerequisite step in the signaling cascade that leads to phosphorylation and activation of eNOS, inhibition of caspase-3, and subsequent anti-apoptotic activity of HDL (9, 10). SR-B1 binding has also been shown to mediate the anti-inflammatory activities of the particle (49). We hypothesized that a possible mechanism accounting for the loss of anti-apoptotic and anti-inflammatory activities of MPO generated oxHDL could be the loss of binding to SR-B1, thus accounting for why the modified particle no longer could turn on the survival pathway that protects endothelial cells from apoptosis or activate eNOS. Consistent with this notion, binding studies in 293T cells transfected with SR-B1 showed that HDL binds to SR-B1 in a saturable and specific manner, whereas HDL modified by (patho)physiologic levels of MPO-catalyzed oxidation can no longer bind to SR-B1, even at high concentrations (Fig. 6, top panel).
FIGURE 6.
MPO-oxidized HDL fails to bind to the physiologic HDL receptor SR-B1 and gains binding to an alternate receptor on endothelial cells. Top, specific binding of HDL and HDL previously oxidized by exposure to the complete MPO/H2O2/Cl− system (oxHDL) were determined on 293T human embryonic kidney cells transiently transfected with either human SR-B1 or vector as described under “Experimental Procedures.” Bottom, specific binding of HDL previously modified by the complete MPO/H2O2/Cl− system determined using BAECs as described under “Experimental Procedures.” The results represent the means of triplicate determinations of a representative experiment performed at least three times.
Oxidized HDL Acquires Saturable and Specific Binding Activity to Endothelial Cells via a Receptor(s) Independent of the Scavenger Receptors CD-36 and SR-A1
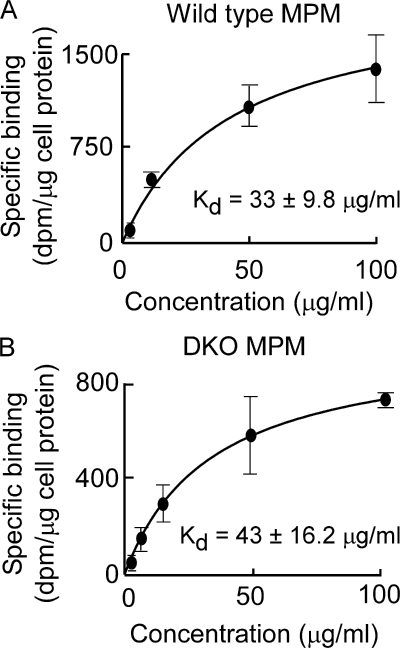
Although the loss of SR-B1 binding could account for the loss of anti-apoptotic and anti-inflammatory activities of oxidized HDL, it does not satisfactorily explain the gain of pro-inflammatory function observed with MPO-modified HDL. Interestingly, despite the loss of SR-B1 binding activity, oxHDL demonstrated saturable and specific binding to endothelial cells, consistent with recognition by an alternative receptor(s) (Fig. 6, bottom panel). We hypothesized that the scavenger receptors CD36 and SR-A1, pattern recognition receptors with broad ligand specificity, may facilitate the observed specific binding of oxHDL to endothelial cells. This was a particularly attractive hypothesis because these scavenger receptors have been linked to both recognition of modified lipoproteins and the pathogenesis of atherosclerosis (50, 51). However, binding studies with MPO-generated oxHDL and mouse peritoneal macrophages excluded a potential role for either CD36 or SR-A1 as a receptor for the modified particle because the absence of each receptor individually (Kd for binding of oxHDL to CD36 knock out (KO) macrophages was 42 ± 13.6 μg/ml, and Kd for binding of oxHDL to SR-A1 KO macrophages was 30 ± 8.2 μg/ml), as well as in combination in cells recovered from the double knock-out failed to alter the observed saturable and specific binding of oxHDL to cells (Fig. 7).
FIGURE 7.
The scavenger receptors CD36 and SR-A1 do not recognize HDL modified by the MPO/H2O2/Cl− system. Specific binding of HDL previously modified by the complete MPO/H2O2/Cl− system (oxHDL) to the indicated MPMs was determined as described under “Experimental Procedures.” Note that oxHDL binds equally well to wild type MPMs (A) and DKO MPMs (B). The results represent the means of triplicate determinations of a representative experiment performed at least three times.
Methionine, Tyrosine, and Tryptophan Residues Identified as Targets for MPO-catalyzed Oxidation of HDL in Vivo Do Not Appear to Participate in oxHDL Binding to Endothelial Cells
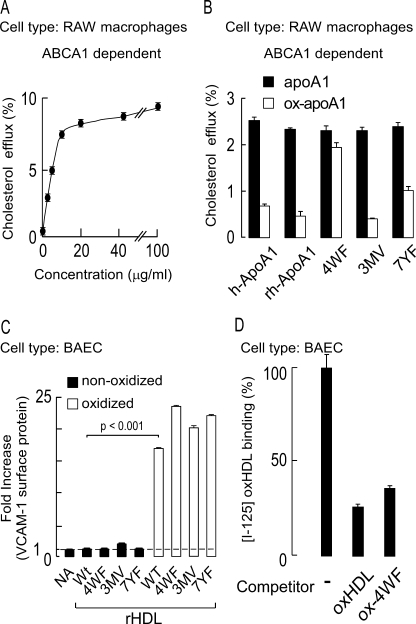
In a final series of studies, we sought to identify potential residues on apoA1 within nascent HDL that may participate in the acquisition of saturable and specific binding to endothelial cells upon exposure to MPO-generated halogenating oxidants. MPO-generated halogenating oxidants can potentially modify any amino acid residue via amide bonds and susceptible groups on side chains, including Cys, Met, Trp, His, Lys, Arg, and Gln. Multiple site-specific oxidative modifications to apoA1 that occur within human atherosclerotic plaque have been extensively mapped through proteomics studies (16, 52). Although the inventory of residues targeted for oxidation in vivo is by no means complete, we hypothesized that one or more of the residues already identified as targets of oxidation through in vitro and in vivo studies may participate in the acquisition of saturable and specific binding upon HDL oxidation. To test this hypothesis, we examined the binding characteristics of rHDL generated with distinct site-directed mutant forms of apoA1. In addition to recombinant wild type human apoA1 as control, three mutants were prepared for study: one in which all four tryptophan residues (Trp-8, -50, -72, and -108) are converted to phenylalanine (4WF); a second in which all three methionine residues (Met-86, -112, and -148) are converted to valine (3MV); and third in which all seven tyrosine residues (Tyr-18, -29, -100, -115, -166, -192, and -236) are converted to phenylalanine (7YF). Cholesterol efflux studies with equivalent levels of wild type apoA1 versus each of these individual mutant forms of apoA1 (the amount of lipid free apoA1 used, 5 μg/ml, was chosen based on the concentration curve in Fig. 8A) showed that the lipid free protein possessed comparable cholesterol efflux activity (Fig. 8B). Upon exposure to the MPO/H2O2/Cl− system, the pan Trp → Phe mutant demonstrated resistance to oxidative inactivation of cholesterol efflux activity as previously reported (49), whereas each of the alternative mutant forms showed sensitivity similar to that of wild type for MPO-dependent oxidative functional inactivation (Fig. 8B), consistent with prior published observations (29). As expected, both wild type and all rHDL forms containing the site-directed mutant forms of apoA1 did not show VCAM-1 activation upon incubation with endothelial cells in the nonoxidized form. Interestingly (unfortunately), upon MPO-catalyzed oxidation, all of the rHDL generated with mutant apoA1 behaved similarly to wild type rHDL, promoting endothelial cell surface expression of VCAM (Fig. 8C). In a final series of studies we sought to confirm that VCAM up-regulation induced by binding oxHDL versus oxidized mutant forms of rHDL occurred via the same potential receptor. Competition binding studies were therefore performed within BAEC. Binding of [125I]oxHDL was substantially inhibited in the presence of a 30-fold molar excess of nonlabeled oxidized pan Trp → Phe mutant rHDL (Fig. 8D), indicating that binding of the two oxidized lipoproteins occurs via the same receptor(s). Collectively, these studies suggest that methionines, tyrosines, and tryptophans, residues identified as targets for MPO-catalyzed oxidation in vivo, do not participate in the acquisition of pro-inflammatory gain of function phenotype conferred to HDL following exposure to (patho)physiological levels of the MPO/H2O2/Cl− system.
FIGURE 8.
ApoA1 tyrosine, tryptophan, and methionine residues do not appear to be involved in endothelial activation by MPO-oxidized HDL. A, dose-response curve of isolated human apoA1-mediated cholesterol efflux activity (ABCA1-dependent) from cholesterol-laden RAW macrophages. B, ABCA1-mediated cholesterol efflux activity of various apoA1 in the absence versus presence of MPO-catalyzed oxidation was examined in RAW macrophages at subsaturating levels of protein (5 μg/ml). ApoA1 forms used included isolated human apoA1 (h-ApoA1), recombinant human apoA1 (rh-ApoA1), and the indicated site-directed mutant forms of recombinant human apoA1. 4WF represents recombinant human apoA1 in which the endogenous tryptophans at residues 8, 50, 72, and 108 were converted to phenylalanine. 3MV represents recombinant human apoA1 in which endogenous methionines at residues 86, 112, and 148 were converted to valine. 7YF represents recombinant human apoA1 in which endogenous tyrosines at residues 18, 29, 100, 115, 166, 192, and 236 were converted to phenylalanine. Note that oxidation by the complete MPO system substantially inhibits ABCA1-mediated cholesterol efflux from all apoA1 forms examined except for the oxidant-resistant 4WF mutant. C, recombinant HDL (rHDL) were generated using each of the recombinant human apoA1 forms indicated in B. The capacity of the indicated rHDL to promote BAEC activation in native form versus following oxidation by the MPO/H2O2/Cl− system was then determined by quantifying endothelial cell VCAM-1 surface protein levels. NA refers to “no addition.” Wt refers to rHDL generated with the wild type human sequence for apoA1. D, competition binding data demonstrating that excess MPO-oxidized rHDL generated with the 4WF apoA1 mutant significantly inhibits binding of oxHDL to BAECs. The results represent the means of triplicate determinations of a representative experiment performed at least three times.
DISCUSSION
Taken together, the present studies show that the biological consequence of MPO-catalyzed oxidation of HDL extends well beyond the classic RCT-related activities of cholesterol efflux and LCAT activation. New insights shown into how MPO catalyzed oxidation of HDL affects the non-cholesterol efflux activities of the lipoprotein include: (i) the discovery that oxidation of HDL by MPO to pathophysiologically relevant levels results in the loss of binding to the HDL receptor SR-B1; (ii) the discovery that loss of oxHDL binding to SR-B1 ultimately results in loss of ability of oxHDL to activate eNOS and inhibit caspase-3 and is the mechanism contributing to the loss of anti-apoptotic activity of the lipoprotein; (iii) the discovery that pathophysiologically relevant levels of MPO catalyzed oxidation of HDL generate a particle that not only loses its anti-inflammatory activity but also gains a pro-inflammatory function as monitored by endothelial cell VCAM-1 protein up-regulation and NF-κB activation via activation of the IKK complex; (iv) the demonstration that the mechanism underlying the gain of function pro-inflammatory activity of oxHDL is the acquisition of saturable and specific binding to an as yet unrecognized receptor(s) that is distinct from the scavenger receptors CD36 and SR-A1; and (v) the demonstration that the pro-inflammatory activity of oxHDL is mediated by apoA1 residues that are distinct from those involved in the loss of cholesterol efflux and the loss of LCAT binding and activation activity.
An intriguing finding in the present studies is the acquisition of saturable and specific binding of MPO-generated oxHDL to an alternative as of yet unrecognized receptor on endothelial cells. Studies with cells from CD-36 KO, SR-A1 KO, and DKO mice unambiguously show that the classic scavenger receptors CD-36 and SR-A1 do not participate in binding because the apparent binding affinity of the modified HDL did not change upon exposure to cells from the genetically engineered strains. Moreover, the ability to recapitulate specific and saturable binding to endothelial cells, NF-κB activation, and up-regulation in VCAM expression, using oxidized reconstituted HDL comprised of only human apoA1 and the relatively nonoxidizable phospholipid POPC, but not oxidized individual components of the particle, is consistent with recognition of a new structural motif generated on the apolipoprotein following oxidant exposure. Unfortunately, attempts to identify the precise modified residue(s) on apoA1 that facilitate the saturable and specific binding to endothelial cells have thus far proven inconclusive. It was somewhat surprising that many residues previously identified as targets for oxidation on apoA1 in vitro and in vivo by MPO are not apparently involved in binding. However, there are numerous alternative oxidant-sensitive groups within apoA1 that are no doubt targets for HOCl-mediated oxidation whose products may mediate binding to the alternate receptor. For example, the amine moiety of lysine (apoA1 has 21 lysines) and the N terminus are highly reactive with hypochlorous acid forming chloramines, secondary reactive chlorinating species that can further react with adjacent susceptible groups or decompose into multiple alternative species. In prior studies we have shown that the Nϵ-chloramine formed by HOCl-dependent oxidation of Nϵ-amine groups on lysine of apoA1 can form 2-aminoadipic acid (29). Moreover, Nϵ-dichloramine formation is also a relatively facile reaction, although the final decomposition product(s) formed are less clear. In model dipeptide systems where halogenation of the N termini formed Nα-dichloramines, nitriles were one of the observed products (54). Alternatively, chlorination of the N termini of target proteins has also been reported to generate multiple deamination derivatives (54). Even the amide bond itself within a polypeptide is a potential target for halogenation by HOCl, forming chloramides. Further studies are needed to define the identity of the receptor recognizing oxHDL and to better understand at the structural level the features responsible on oxHDL that promote binding to the endothelial cell receptor(s) and subsequent NF-κB activation with a resultant gain of pro-inflammatory activity.
In summary, the present studies identify further mechanisms whereby MPO-mediated oxidative reactions may contribute to the pathogenesis of CVD. MPO levels have been shown in multiple studies to track with incident risks of CVD events in subjects (53, 55–57). The present studies may help to explain prior reports of pro-inflammatory HDL in the acute phase setting, as well as during chronic inflammation such as in CVD. MPO is the most abundant protein within neutrophils and monocytes and is classically used as a quantitative index of acute inflammation and leukocyte activation. Two novel targets for pharmacologic inhibition are suggested by the present studies. The first is MPO itself, to inhibit formation of dysfunctional HDL forms. The second and equally intriguing possibility is to inhibit MPO-generated oxHDL binding to the as of yet unrecognized receptor. Finally, the present studies lend further support to the idea that it is both the quality and the quantity of HDL that is important for understanding its overall biological functions in CVD.
Acknowledgments
We thank Cathy Shemo and Sage O'Bryant for expert help with the flow cytometry experiments and Xiaoming Fu for expert help with mass spectrometry based measures of chlorotyrosine content in HDL. We also thank Dr. Maria Febbraio for generously providing mice for the peritoneal macrophage binding studies and Dr. Paul DiCorleto for generously providing HUVEC.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 HL076491-055328, P01 HL077107-050004, P01 HL087018-02001. This work was also supported by Case Western Reserve University/Cleveland Clinic Clinical and Translational Science Award 1KL2RR024990.
- HDL
- high density lipoprotein
- MPO
- myeloperoxidase
- apo
- apolipoprotein
- VCAM
- vascular cell adhesion molecule
- RCT
- reverse cholesterol transport
- LCAT
- lecithin cholesterol acyl transferase
- SR-B1
- scavenger receptor B1
- MAPK
- mitogen-activated protein kinase
- eNOS
- endothelial nitric-oxide synthase
- CVD
- cardiovascular disease
- rHDL
- reconstituted nascent HDL
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- ClTyr
- chlorotyrosine
- HPLC
- high pressure liquid chromatography
- MPM
- mouse peritoneal macrophage
- KO
- knock-out
- DKO
- double knock-out
- HUVEC
- human umbilical vein endothelial cell(s)
- BAEC
- bovine aortic endothelial cell(s)
- TNF
- tumor necrosis factor
- oxHDL
- oxidized HDL
- EMSA
- electrophoretic mobility shift assay
- IKK
- IκB kinase
- GST
- glutathione S-transferase.
REFERENCES
- 1.Fielding C. J., Fielding P. E. (1995) J. Lipid Res. 36, 211–228 [PubMed] [Google Scholar]
- 2.Tall A. R. (1998) Eur. Heart. J. 19, (Suppl. A) A31–A35 [PubMed] [Google Scholar]
- 3.Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., Fogelman A. M. (2004) Circ. Res. 95, 764–772 [DOI] [PubMed] [Google Scholar]
- 4.Hessler J. R., Robertson A. L., Jr., Chisolm G. M., 3rd (1979) Atherosclerosis 32, 213–229 [DOI] [PubMed] [Google Scholar]
- 5.Van Lenten B. J., Hama S. Y., de Beer F. C., Stafforini D. M., McIntyre T. M., Prescott S. M., La Du B. N., Fogelman A. M., Navab M. (1995) J. Clin. Invest. 96, 2758–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Lenten B. J., Wagner A. C., Nayak D. P., Hama S., Navab M., Fogelman A. M. (2001) Circulation 103, 2283–2288 [DOI] [PubMed] [Google Scholar]
- 7.Baker P. W., Rye K. A., Gamble J. R., Vadas M. A., Barter P. J. (1999) J. Lipid Res. 40, 345–353 [PubMed] [Google Scholar]
- 8.Cockerill G. W., Huehns T. Y., Weerasinghe A., Stocker C., Lerch P. G., Miller N. E., Haskard D. O. (2001) Circulation 103, 108–112 [DOI] [PubMed] [Google Scholar]
- 9.Li X. A., Guo L., Dressman J. L., Asmis R., Smart E. J. (2005) J. Biol. Chem. 280, 19087–19096 [DOI] [PubMed] [Google Scholar]
- 10.Mineo C., Yuhanna I. S., Quon M. J., Shaul P. W. (2003) J. Biol. Chem. 278, 9142–9149 [DOI] [PubMed] [Google Scholar]
- 11.Nicholls S. J., Zheng L., Hazen S. L. (2005) Trends Cardiovasc. Med. 15, 212–219 [DOI] [PubMed] [Google Scholar]
- 12.Navab M., Reddy S. T., Van Lenten B. J., Anantharamaiah G. M., Fogelman A. M. (2009) J. Lipid Res. 50, (suppl.) S145–S149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., Fogelman A. M. (2003) Circulation 108, 2751–2756 [DOI] [PubMed] [Google Scholar]
- 14.McGillicuddy F. C., de la Llera Moya M., Hinkle C. C., Joshi M. R., Chiquoine E. H., Billheimer J. T., Rothblat G. H., Reilly M. P. (2009) Circulation 119, 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng L., Nukuna B., Brennan M. L., Sun M., Goormastic M., Settle M., Schmitt D., Fu X., Thomson L., Fox P. L., Ischiropoulos H., Smith J. D., Kinter M., Hazen S. L. (2004) J. Clin. Invest. 114, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng L., Settle M., Brubaker G., Schmitt D., Hazen S. L., Smith J. D., Kinter M. (2005) J. Biol. Chem. 280, 38–47 [DOI] [PubMed] [Google Scholar]
- 17.Bergt C., Pennathur S., Fu X., Byun J., O'Brien K., McDonald T. O., Singh P., Anantharamaiah G. M., Chait A., Brunzell J., Geary R. L., Oram J. F., Heinecke J. W. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13032–13037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao B., Oda M. N., Bergt C., Fu X., Green P. S., Brot N., Oram J. F., Heinecke J. W. (2006) J. Biol. Chem. 281, 9001–9004 [DOI] [PubMed] [Google Scholar]
- 19.Wu Z., Wagner M. A., Zheng L., Parks J. S., Shy J. M., 3rd, Smith J. D., Gogonea V., Hazen S. L. (2007) Nat. Struct. Mol. Biol. 14, 861–868 [DOI] [PubMed] [Google Scholar]
- 20.Shao B., Cavigiolio G., Brot N., Oda M. N., Heinecke J. W. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12224–12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan M. L., Wu W., Fu X., Shen Z., Song W., Frost H., Vadseth C., Narine L., Lenkiewicz E., Borchers M. T., Lusis A. J., Lee J. J., Lee N. A., Abu-Soud H. M., Ischiropoulos H., Hazen S. L. (2002) J. Biol. Chem. 277, 17415–17427 [DOI] [PubMed] [Google Scholar]
- 22.Paromov V. M., Morton R. E. (2003) J. Biol. Chem. 278, 40859–40866 [DOI] [PubMed] [Google Scholar]
- 23.Osborne J. C. J. (1968) Methods Enzymol. 128A, 213–222 [DOI] [PubMed] [Google Scholar]
- 24.Rye K. A., Garrety K. H., Barter P. J. (1992) J. Lipid Res. 33, 215–224 [PubMed] [Google Scholar]
- 25.Matz C. E., Jonas A. (1982) J. Biol. Chem. 257, 4535–4540 [PubMed] [Google Scholar]
- 26.Bolton A. E., Hunter W. M. (1973) Biochem. J. 133, 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry O. H., Rosbrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 28.Park Y. M., Febbraio M., Silverstein R. L. (2009) J. Clin. Invest. 119, 136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng D. Q., Wu Z., Brubaker G., Zheng L., Settle M., Gross E., Kinter M., Hazen S. L., Smith J. D. (2005) J. Biol. Chem. 280, 33775–33784 [DOI] [PubMed] [Google Scholar]
- 30.Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 31.Bauer J. A., Lupica J. A., Schmidt H., Morrison B. H., Haney R. M., Masci R. K., Lee R. M., Didonato J. A., Lindner D. J. (2007) PLoS ONE 2, e1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiDonato J. A., Hayakawa M., Rothwarf D. M., Zandi E., Karin M. (1997) Nature 388, 548–554 [DOI] [PubMed] [Google Scholar]
- 33.Gu X., Trigatti B., Xu S., Acton S., Babitt J., Krieger M. (1998) J. Biol. Chem. 273, 26338–26348 [DOI] [PubMed] [Google Scholar]
- 34.Greenberg M. E., Sun M., Zhang R., Febbraio M., Silverstein R., Hazen S. L. (2006) J. Exp. Med. 203, 2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Gugiu B., Fox P. L., Hoff H. F., Salomon R. G., Hazen S. L. (2002) J. Biol. Chem. 277, 38503–38516 [DOI] [PubMed] [Google Scholar]
- 36.Hazen S. L., Heinecke J. W. (1997) J. Clin. Invest. 99, 2075–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Askari A. T., Brennan M. L., Zhou X., Drinko J., Morehead A., Thomas J. D., Topol E. J., Hazen S. L., Penn M. S. (2003) J. Exp. Med. 197, 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z., Nicholls S. J., Rodriguez E. R., Kummu O., Hörkkö S., Barnard J., Reynolds W. F., Topol E. J., DiDonato J. A., Hazen S. L. (2007) Nat. Med. 13, 1176–1184 [DOI] [PubMed] [Google Scholar]
- 39.Rosen H., Crowley J. R., Heinecke J. W. (2002) J. Biol. Chem. 277, 30463–30468 [DOI] [PubMed] [Google Scholar]
- 40.Nofer J. R., Levkau B., Wolinska I., Junker R., Fobker M., von Eckardstein A., Seedorf U., Assmann G. (2001) J. Biol. Chem. 276, 34480–34485 [DOI] [PubMed] [Google Scholar]
- 41.Blanche P. J., Gong E. L., Forte T. M., Nichols A. V. (1981) Biochim. Biophys. Acta 665, 408–419 [DOI] [PubMed] [Google Scholar]
- 42.Cybulsky M. I., Gimbrone M. A., Jr. (1991) Science 251, 788–791 [DOI] [PubMed] [Google Scholar]
- 43.Nakashima Y., Raines E. W., Plump A. S., Breslow J. L., Ross R. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 842–851 [DOI] [PubMed] [Google Scholar]
- 44.Kimura T., Mogi C., Tomura H., Kuwabara A., Im D. S., Sato K., Kurose H., Murakami M., Okajima F. (2008) J. Immunol. 181, 7332–7340 [DOI] [PubMed] [Google Scholar]
- 45.Iademarco M. F., McQuillan J. J., Rosen G. D., Dean D. C. (1992) J. Biol. Chem. 267, 16323–16329 [PubMed] [Google Scholar]
- 46.Cockerill G. W., Rye K. A., Gamble J. R., Vadas M. A., Barter P. J. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 1987–1994 [DOI] [PubMed] [Google Scholar]
- 47.DiDonato J., Mercurio F., Rosette C., Wu-Li J., Suyang H., Ghosh S., Karin M. (1996) Mol. Cell. Biol. 16, 1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acton S., Rigotti A., Landschulz K. T., Xu S., Hobbs H. H., Krieger M. (1996) Science 271, 518–520 [DOI] [PubMed] [Google Scholar]
- 49.Tölle M., Pawlak A., Schuchardt M., Kawamura A., Tietge U. J., Lorkowski S., Keul P., Assmann G., Chun J., Levkau B., van der Giet M., Nofer J. R. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1542–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Febbraio M., Hajjar D. P., Silverstein R. L. (2001) J. Clin. Invest. 108, 785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Winther M. P., van Dijk K. W., Havekes L. M., Hofker M. H. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 290–297 [DOI] [PubMed] [Google Scholar]
- 52.Peng D. Q., Brubaker G., Wu Z., Zheng L., Willard B., Kinter M., Hazen S. L., Smith J. D. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 2063–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang W. H., Tong W., Troughton R. W., Martin M. G., Shrestha K., Borowski A., Jasper S., Hazen S. L., Klein A. L. (2007) J. Am. Coll. Cardiol. 49, 2364–2370 [DOI] [PubMed] [Google Scholar]
- 54.Stelmaszyńska T., Zgliczynski J. M. (1978) Eur. J. Biochem. 92, 301–308 [DOI] [PubMed] [Google Scholar]
- 55.Brennan M. L., Penn M. S., Van Lente F., Nambi V., Shishehbor M. H., Aviles R. J., Goormastic M., Pepoy M. L., McErlean E. S., Topol E. J., Nissen S. E., Hazen S. L. (2003) N. Engl. J. Med. 349, 1595–1604 [DOI] [PubMed] [Google Scholar]
- 56.Baldus S., Heeschen C., Meinertz T., Zeiher A. M., Eiserich J. P., Münzel T., Simoons M. L., Hamm C. W. (2003) Circulation 108, 1440–1445 [DOI] [PubMed] [Google Scholar]
- 57.Meuwese M. C., Stroes E. S., Hazen S. L., van Miert J. N., Kuivenhoven J. A., Schaub R. G., Wareham N. J., Luben R., Kastelein J. J., Khaw K. T., Boekholdt S. M. (2007) J. Am. Coll. Cardiol. 50, 159–165 [DOI] [PubMed] [Google Scholar]