Abstract
ECRG2 is a novel gene that shows sequence similarity to KAZAL-type serine protease inhibitor. We have previously demonstrated that ECRG2 inhibits migration/invasion of lung cancer PG cells. However, the mechanism by which ECRG2 performs these activities is a compelling question. Urokinase-type plasmin activator (uPA) binding to uPAR induces migration/invasion through multiple interactors including integrins. In this study, we found that ECRG2 binds specifically to the kringle domain of uPA. Moreover, we demonstrated that ECRG2 forms a complex with uPA·uPAR, that such a complex modifies the dynamical association of uPAR with β1 integrins, and that disruption inhibits Src/MAP (mitogen-activated protein) kinase pathway, resulting in suppression of cell migration/invasion in an in vitro Matrigel migration/invasion assay. Conversely, depletion of ECRG2 markedly enhanced the association of uPAR with β1 integrins, elevated basal Src/MAP kinase activation, and stimulated HT1080, MDA-MB-231, and MCF-7 cell migration/invasion. Together, our results provide evidence that ECRG2 is involved in the regulation of migration/invasion through uPA/uPAR/β1 integrins/Src/MAP kinase pathway and may represent a novel therapeutic target for cancer.
Introduction
Tumor cells must invade through the adjacent basement membrane into surrounding tissues and then migrate to and invade the vasculature to metastasize to distant sites (1). The processes of tumor cell migration and invasion involve a dynamic interaction between the tumor cells and the extracellular matrix and are regulated by multiple cytokines and growth factors, integrins, cell-cell adhesion molecules/communication, matrix-degrading enzymes, and loss of activity of degradative enzyme inhibitors (2). The esophageal cancer-related gene 2 (ECRG2) is a novel gene that is highly expressed in adult esophageal mucosa, brain, thyroid, mouth epithelia, fetal skin, and thymus and poorly expressed in the fetal esophagus, brain, lung, heart, stomach, liver, spleen, colon, kidney, testis, and muscle tissue. Reverse transcription-PCR and Northern blot results showed that the ECRG2 gene was expressed in normal esophagus, liver, colon, and lung tissues but was down-regulated in the adjacent and cancerous tissues, especially with low frequency in esophageal cancer (3). The ECRG2 gene contains a characteristic secondary structure of a KAZAL-type conserved domain and is a novel member of the KAZAL-type-related serine protease inhibitor family (4). The serine protease inhibitor (serpin) superfamily includes inhibitors of a number of serine proteases with roles in a variety of cellular process, including cell migration and adhesion, preventing tumor metastasis (5). Some serpins have biological activities independent of protease inhibition. For example, PAI-1 is a specific inhibitor and regulator of the serine proteases urokinase-type and tissue-type plasminogen activator, modulating cell adhesion and migration (6). Other serpins lack intrinsic inhibitory activity. Examples of this are ovalbumin, thyroid-binding globulin (SERPINA6), angiotensinogen (SERPINA8), and pigment epithelium-derived factor (SERPINF1), which has neurotrophic and antiangiogenic activity (7, 8). Maspin (SERPINB5) is thought to be another non-inhibitory serpin and inhibits cell migration in the absence of detectable protease inhibitory activity (9). In our previous experiments, the ECRG2 gene was shown to reduce the migration/invasion of PG cancer cells and suppress metastases in nude mice (10). In addition, we showed that there is a direct interaction between ECRG2 and the urokinase-type plasminogen activator (uPA)2 (10). Thus, our previous studies provide evidences that the ECRG2 gene plays an important role in the prevention of tumor cell migration and invasion, possibly through the uPA. However, little is known about how ECRG2 regulates the cellular response to uPA binding.
The uPA·uPA receptor (uPAR) system has been shown to play a critical role in the regulation of cancer cell migration, extracellular matrix invasion, and metastasis (11). uPA binds with high affinity to a cell surface uPAR (12). uPAR is a heavily glycosylated glycosylphosphatidylinositol-anchored protein formed by three cysteine-rich LY6-like extracellular domains (LU domains D1, D2, and D3) (13). There are three basic steps involved in migration/invasion and intracellular signaling. (a) uPA·uPAR promotes extracellular proteolysis by regulating plasminogen activation; (b) uPA·uPAR regulates cell-extracellular matrix interactions as an adhesion receptor for vitronectin and through its capacity to modulate integrin function; and (c) uPA·uPAR regulates cell migration as a signal transduction molecule and by its intrinsic chemotactic activity (14). One important mechanism through which uPAR directs these actions is by complexing with other membrane proteins (e.g. integrins) for signal transduction (15, 16). It has also become clear that uPAR complexes can transduce intracellular signals (17). Several groups have reported that the binding of uPA to uPAR stimulates intracellular signaling, and much of this signaling is consistent with an integrin-mediated pathway (18). Integrins are known to activate the PI3K, Src, and extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway, and recent studies have shown that the higher activation of FAK is associated with increased cell motility and cytoskeletal changes (19–21).
In this study, we have investigated the mechanism by which ECRG2 regulates the fibrosarcoma HT1080, breast cancer MDA-MB-231, and MCF-7 cell migration/invasion. We demonstrate that the direct binding of ECRG2 to uPA present in the uPA·uPAR complex disrupts the association of uPAR with β1 integrins, leading to reduced activation of the Src/MAP kinase pathway, resulting in abatement of uPA signaling through the uPAR·β1 integrins complex. In contrast, depletion of ECRG2 enhanced association of uPAR·β1 integrins, increased Src/MAP kinase activity, and promoted cell migration/invasion. Thus, our results reveal an important role of the ECRG2 gene in suppression of cancer cell migration/invasion and shed new light on how ECRG2 participates in the regulation of cancer cell migration/invasion.
EXPERIMENTAL PROCEDURES
Reagents
Monoclonal anti-ECRG2 antibody was produced by our laboratory (22, 23). Human uPA and uPAR monoclonal antibodies were purchased from American Diagnostica (Greenwich, CT). Monoclonal antibodies to integrin α3 (P1B5), α5β1 (HA5), αvβ3 (LM609), and polyclonal anti-β1 (AB1937) integrin were obtained from Chemicon International (Temecula, CA). The monoclonal antibodies to FAK kinase and phospho-FAK were obtained from Transduction Laboratories (Lexington, KY). Polyclonal anti-phospho-ERK, anti-phospho-Src, and total Src antibodies were purchased from Cell Signaling Technology (Beverly, MA). Rabbit PI3K and p-PI3K antibody were purchased from Abcam (Cambridge, MA). The phospho-ERK inhibitor PD98059 and PI3K inhibitor wortmannin were purchased from Sigma-Aldrich. Inhibitor to Src family tyrosine kinases PP2 was from Calbiochem. Peptide α325 (PRHRHMGAVFLLSQEAG) was synthesized by Quality Controlled Biochemicals (Framingham, MA).
Generation of Inducible ECRG2-Tet-on, ECRG2-Tet-on-3′UTR-si, ECRG2-Tet-on-3′UTR-si-uPA-KD, and ECRG2-Tet-on-3′UTR-si-β1-KD Cells
HT1080 cells, a fibrosarcoma cell line that is commonly used in migration/invasion experiments, were purchased from the American Type Culture Collection. Breast cancer cell lines MDA-MB-231 and MCF-7 were kind gifts from Dr. D. X. Lin (Cancer Institute, Peking Union Medical College and Chinese Academy of Medical Sciences). All cells were grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 10 mm Hepes, 2 mm l-glutamine, 1 mm minimal essential medium, and sodium pyruvate. We used a doxycycline-inducible expression system to obtain the conditional expression of ECRG2 in HT1080, MDA-MB-231, and MCF-7 cells (ECRG2-Tet-on) as previously described (23). As shown in supplemental Fig. 1A, the stable ECRG2 knockdown cells were generated by infection of the ECRG2-Tet-on cells or Tet-On empty vector control cells with pSUPER.Retro.puro-ECRG2–3′UTR small interfering RNA (siRNA) (ECRG2-Tet-on-3′UTR-si) or the pSUPER.Retro.puro-LacZ siRNA retrovirus (Tet-On empty and shRNA control) as described before (23). Colonies showing resistance to puromycin (5 μg/ml) were clonally isolated, and an immunoblotting assay was used to screen for the expression or suppression of ECRG2 protein.
The pSUPER.retro.puro-uPA or pSUPER.retro.puro-β1 plasmids were constructed by cloning human uPA complimentary sequence from +346 to +367 (5′-agcttgagagccctgctggcgcgccatatataatggcgcgccagcagggctctca-3′) (13, 24) or siRNAs for β1 integrin (Stealth Select RNA interference; oligonucleotide IDs HSS105559, HSS105560, and HSS105561, purchased from Invitrogen) into pSUPER.retro.puro expressing vector, respectively. The retroviruses were produced as described previously (23). ECRG2-Tet-on-3′UTR-si cells were infected with either pSUPER.retro.puro-uPA (ECRG2 Tet-on-3′UTR-si-uPA-KD) or pSUPER.retro.puro-β1 (ECRG2-Tet-on-3′UTR-si-β1-KD) or pSUPER.retro.puro-LacZ siRNA retrovirus (Scramble control). All clones were cultured in complete Dulbecco's modified Eagle's medium containing 3 μg/ml puromycin.
Transwell Invasion and Migration Assays
An in vitro invasion assay was performed by using 24-well Transwell units with polycarbonate filters (pore size 8 μm) coated on the upper side with Matrigel (BD Biosciences). The lower part of the Transwell unit was filled with 10% fetal bovine serum medium. 1 × 104 cells in 500 μl of medium for each group were placed in the upper part of the Transwell unit and allowed to invade for 24 h. After incubation, non-invaded cells on the upper part of the membrane were removed with a cotton swab. Invaded cells on the bottom surface of the membrane were counted under a microscope. Results expressed as the mean ± S.D. from triplicates samples are representative of at least three experiments. A migration assay was performed by similar procedures except that the polycarbonate filters were not coated with Matrigel. Some cell migration experiments were done in the presence of MAP kinase inhibitor (PD98059), Src kinase inhibitor (PP2), PI3K kinase inhibitor (wortmannin), FAK kinase inhibitor (TAE226), or peptide α325(100 μm). The same volume of DMSO was used as control.
Immunoprecipitation and Immunoblotting
Cells (5 × 106) were lysed on ice for 30 min in 1.5 ml of radioimmune precipitation buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% deoxycholate, 0.1% SDS, 1% Triton X-100, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, and 10 μg/ml leupeptin) or Triton lysis buffer (50 mm HEPES, pH 7.5, 150 mm NaCl, 1% Triton X-100) supplemented with protease inhibitors. After preclearing with protein A-agarose, lysates were incubated with anti-ECRG2 antibody, anti-uPA antibody, anti-uPAR antibody or with normal IgG (control) at 4 °C overnight. The beads were pelleted, washed extensively with immunoprecipitation buffer, boiled in SDS sample buffer, fractionated by SDS-PAGE, and analyzed by uPA, uPAR, α3β1, α5β1, or αvβ3 antibody.
Preparation of Cell Lysates and Immunoblotting Analysis
Adherent cells were washed in ice-cold phosphate-buffered saline, scraped, and resuspended into cell lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EGTA, 0.5% Nonidet P-40) containing protease and phosphatase inhibitors (1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 25 mm NaF, 1 mm sodium vanadate) and then centrifuged at 14,000 rpm for 15 min at 4 °C to pellet insoluble cell debris. Protein concentrations of supernatants were determined using the DC protein assay (Bio-Rad). Equal amounts of protein were resolved by SDS-PAGE, electrophoretically transferred to polyvinylidene difluoride membranes (Millipore Corporation), and subjected to immunoblot analysis using the following primary antibodies: anti-ECRG2 monoclonal antibody (4E8) or p-ERK/ERK, p-Src/Src, p-PI3K/PI3K, or p-FAK/FAK antibodies. Secondary antibodies, Alkapanel phosphatase-conjugated Affinipure goat anti-mouse IgG or anti-rabbit IgG-Alkapanel phosphatase antibody (Sigma), were incubated for 1 h at room temperature and then processed for chemiluminescence detection using the CDP-Star reagent (Roche Applied Science).
Statistical Analyses
In general, all experiments were performed three times. Statistical analyses were done using the InStat 3.01 software. The significance of differences between treatment groups and control groups was determined using the Student's t test. Significance was determined with 95% confidence. Quantitative results are presented as the average of the three experiments, each performed in triplicate, ±S.D.
RESULTS
Identification of ECRG2-binding Sequence in uPA
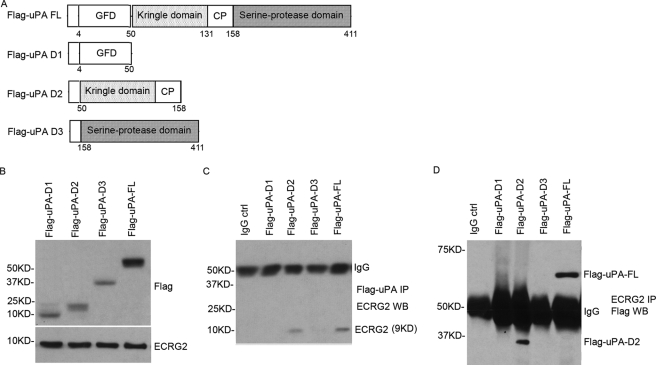
Previous observations have demonstrated that ECRG2 physically interacts with uPA (10). uPA consists of two disulfide bridge-linked polypeptide chains: the N-terminal polypeptide containing a uPA growth factor-like domain (Fig. 1A, GFD), a kringle domain, and a linked or connecting peptide region and a large C-terminal, serine protease polypeptide (21). In an effort to characterize the binding domain of ECRG2 in uPA, several uPA variants were generated as shown in Fig. 1A. FLAG-tagged full-length human uPA (FLAG-uPA-FL) and three variants carrying the uPAR-binding region growth factor-like domain (amino acids 1–50), the kringle region (amino acids 50–158), and serine protease region (158–411) were transfected into HT1080-ECRG2-Tet-On cells and cultured in the presence of doxycycline for 24 h. Cell lysates were subjected to Western blotting for the expression of ECRG2 and different FLAG-uPA deletion mutants (Fig. 1B). Immunoprecipitations were performed using anti-FLAG antibody. As shown in Fig. 1C, ECRG2 was observed in the FLAG-uPA-D2 deletion mutant and full-length uPA expression cells. No ECRG2 was seen in FLAG-uPA-D1 and D3 deletion mutant expression cells, although equivalent amounts of ECRG2 were expressed. In parallel experiments, FLAG-uPA-D2 and FLAG-uPA-FL were specifically detected in the ECRG2 immunoprecipitates (Fig. 1D). Similar results were observed in breast cancer cell lines MDA-MB-231 and MCF-7 (supplemental Fig. 2). These data suggest that the kringle and CP regions (50–158) are required for the association of uPA with ECRG2 in vivo.
FIGURE 1.
Identification of ECRG2-binding sequence in uPA. A, schematic representation of the human urokinase structure shows the N-terminal growth factor-like domain (GFD, residues 1–49), the kringle domain (residues 50–131), the CP region (residues 132–158), and the catalytic domain (residues 159–411). FLAG-tagged variant deletion mutants of human uPA are shown. B, FLAG-uPA full-length and variant deletion mutants were transiently transfected into HT1080 ECRG2-Tet-On cells. Cells were incubated in the presence of doxycycline (0.1 μg/ml) for 24 h and subjected to Western blotting. The expression of ECRG2 and FLAG-uPA deletion mutants is shown. C, cells were subjected to immunoprecipitation using anti-FLAG antibody or IgG (control (ctrl)). Immunoprecipitates (IP) were then analyzed by Western blotting (WB) with anti-ECRG2 antibody. D, a parallel experiment was performed using immunoprecipitation with ECRG2 antibody and Western blotting with FLAG antibody.
ECRG2 Does Not Impair the uPA·uPAR Complex Formation
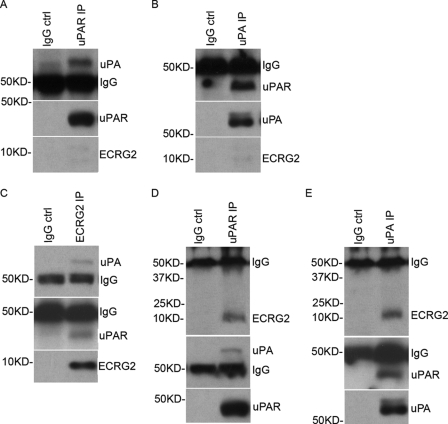
To explore whether ECRG2, which physically associates with uPA as described above, affects the direct binding of uPA to uPAR, ECRG2-Tet-on-3′UTR-si cells were cultured in the absence or presence of doxycycline for 24 h and subjected to immunoprecipitation. As expected, uPA·uPAR forms a complex in absence of doxycycline in ECRG2-Tet-on-3′UTR-si cells (Fig. 2, A and B). We then examined the binding of uPA to uPAR in the presence of doxycycline in ECRG2-Tet-on-3′UTR-si cells. Cell lysates were immunoprecipitated with anti-ECRG2 antibody, and the resultant ECRG2-immunoprecipitate was probed for uPA and uPAR (Fig. 2C). Similar experiments were performed using either immunoprecipitation with anti-uPAR antibody and Western blotting with anti-ECRG2 and anti-uPA antibodies (Fig. 2D) or immunoprecipitation with uPA antibody and Western blotting with anti-ECRG2 and anti-uPAR antibodies (Fig. 2E). uPA and uPAR were detected in ECRG2 immunoprecipitate (Fig. 2C). Similar patterns were obtained from uPAR immunoprecipitation (Fig. 2D) and uPA immunoprecipitation (Fig. 2E) experiments. We obtained very similar results in MDA-MB-231 and MCF-7 cells (supplemental Fig. 3), indicating that ECRG2 does not disrupt the association of uPA with uPAR and forms an ECRG2·uPA·uPAR complex in culture cells.
FIGURE 2.
The binding of ECRG2 to uPA forms an ECRG2·uPA·uPAR complex. A, cell lysates from ECRG2-Tet-on-3′UTR-si cells in the absence of doxycycline were subjected to immunoprecipitation (IP) with anti-uPAR antibody or IgG (control (ctrl)) and Western blotting for analysis for uPA. B, cell lysates from ECRG2-Tet-on-3′UTR-si cells in the absence of doxycycline were subjected to immunoprecipitation with anti-uPA antibody or IgG (control) and Western blotting for analysis for uPAR. C, cell lysates from ECRG2-Tet-on-3′UTR-si cells in the presence of doxycycline were subjected to immunoprecipitation with anti-ECRG2 antibody or IgG (control) and Western blotting for analysis of uPA and uPAR. D, cell lysates from ECRG2-Tet-on-3′UTR-si cells in the presence of doxycycline were subjected to immunoprecipitation with anti-uPAR antibody or IgG (control) and Western blotting for analysis of ECRG2 and uPA. E, cell lysates from ECRG2-Tet-on-3′UTR-si cells in the presence of doxycycline were subjected to immunoprecipitation with anti-uPA antibody or IgG (control) and Western blotting for analysis of ECRG2 and uPAR.
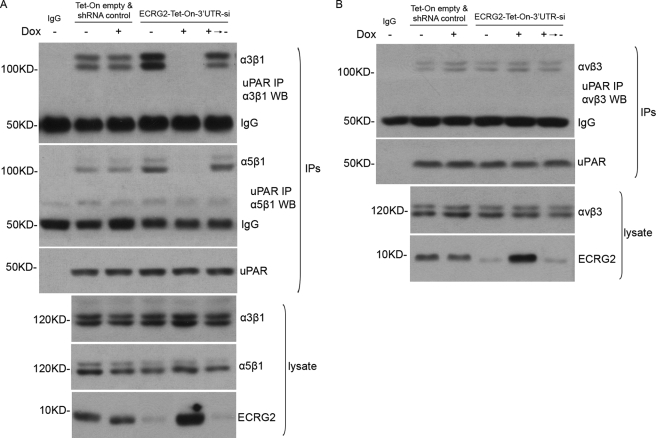
uPAR Fails to Complex with α3β1 and α5β1 Integrins in ECRG2 Expression Cells
Having demonstrated that ECRG2 binds to uPA present in the uPA·uPAR complex on the cell surface, we further tested whether ECRG2 was likely to affect the direct interaction of uPAR with integrins as they are well characterized transmembrane adaptor proteins for uPAR (2). Tet-On empty and shRNA control or ECRG2-Tet-on-3′UTR-si cells were incubated in the presence or absence of doxycycline for 24 h and subjected to immunoprecipitation and Western blotting analysis. As expected, uPAR-α3β1 and uPAR-α5β1 interactions were verified by immunoblotting analysis after immunoprecipitation with an anti-uPAR antibody in Tet-On empty and shRNA control cells in the presence or absence of doxycycline. Depletion of ECRG2 strongly enhanced the interaction of both α3β1 and α5β1 with uPAR. Surprisingly, the uPAR-α3β1 and uPAR-α5β1 interactions were completely blocked in ECRG2-Tet-on-3′UTR-si cells in the presence of doxycycline, whereas removing doxycycline restored the associations again, indicating that ECRG2 impairs uPAR·α3β1 and uPAR·α5β1 complex formation (Fig. 3A). In the case of αvβ3, ECRG2 does not affect the association of uPAR with αvβ3 (Fig. 3B). In these experiments, both the transmembrane adaptors (integrins) and uPAR were comparably expressed as judged by immunoblotting analysis of total cell extracts. Similar results were obtained in MDA-MB-231 and MCF-7 cells (supplemental Fig. 4). Together, these results suggest that ECRG2 could regulate the capacity of uPAR to complex with its transmembrane adaptors, β1 integrins. The formation of the ECRG2·uPA·uPAR complex impairs the binding ability of uPAR to α3β1 and α5β1 integrins but not αvβ5 integrin.
FIGURE 3.
Effect of ECRG2 on complex formation between uPAR and integrins (α3β1, α5β1, αvβ3). HT1080 Tet-On empty and shRNA control or ECRG2-Tet-On-3′UTR-si cells were grown in the presence or absence of doxycycline (Dox, 0.1 μg/ml) for 24 h. Some ECRG2-Tet-On-3′UTR-si cells were incubated in the medium with doxycycline for 24 h and then changed to fresh medium for another 48 h. Cells were incubated with uPA (10 nm) for 1 h before being collected. uPAR was immunoprecipitated from total cell extracts using an anti-uPAR antibody. The uPAR immunoprecipitates were blotted for α3β1 and α5β1 (A) or αvβ3 (B).
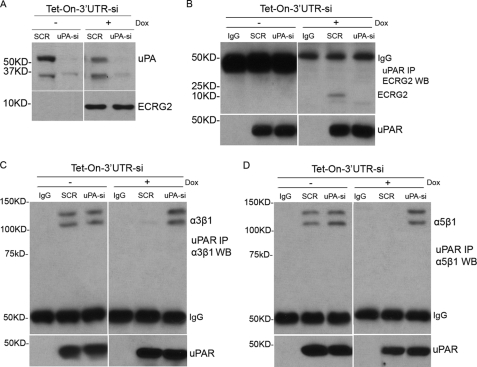
ECRG2 Regulates the Association of uPAR with β1 Integrins through uPA
The above data indicate that binding of ECRG2 to uPA present in the uPA·uPAR complex on the cell surface alters uPAR conformation and changes its matrix ligand binding properties. To probe this idea further, ECRG2-Tet-on-3′UTR-si cells were infected with pSUPER.retro.puro-uPA containing a small siRNA targeting uPA previously shown to suppress uPA mRNA (ECRG2-Tet-on-3′UTR-si-uPA-KD) (24) or pSUPER.retro.puro-LacZ (scramble control) and incubated in the presence or absence of doxycycline. The suppression or expression of uPA and ECRG2 was verified 24 h later by Western blotting (Fig. 4A). Cell lysates were immunoprecipitated by anti-uPAR antibody and Western blotting by anti-ECRG2, anti-α3β1, or anti-α5β1 antibody, respectively. Consistent with our data described above, ECRG2 was detected in uPAR immunoprecipitates in scramble control cells (Fig. 4B, right panel, second lane). However, the association of ECRG2 with uPAR was completely blocked in uPA depletion cells (Fig. 4B, right panel, third lane), confirming that ECRG2 associated with uPAR through uPA. Moreover, further analyses of interaction between uPAR and integrins show that ECRG2 expression compromises the interaction of uPAR with α3β1 (Fig. 4C, right panel, second lane) and α5β1 (Fig. 4D, right panel, second lane) in scramble control cells, consistent with the results described above. However, α3β1 (Fig. 4C, right panel, third lane) and α5β1 (Fig. 4D, right panel, third lane) were found in uPAR immunoprecipitates in uPA-si cells even when ECRG2 was expressed. These results confirm that uPAR forms complexes with β1 integrins when ECRG2 was depleted, and these associations were completely blocked when ECRG2 was expressed in the presence of uPA. However, the inhibition function of ECRG2 on interaction of uPAR with β1 integrins was compromised when uPA was depleted. Together, these data confirm our finding that ECRG2, through its direct interaction with uPA, disrupts the binding ability of uPAR to β1 integrins.
FIGURE 4.
ECRG2 disrupts the interaction of uPAR with integrins through uPA. A, ECRG2-Tet-on-3′UTR-si cells were transfected with either an siRNA previously shown to suppress uPA mRNA or a scramble control siRNA and incubated in the presence or absence of doxycycline for 24 h. Suppression of uPA expression and induction of ECRG2 expression were verified by Western blotting. B–D, cells were then immunoprecipitated (IP) with anti-uPAR antibody or IgG (control (ctrl)) and subjected to Western blotting (WB) using anti-ECRG2 (B), anti-α3β1 (C), or anti-α5β1 (D) antibodies. The efficiencies of immunoprecipitations were also shown by Western blotting with anti-uPAR antibody.
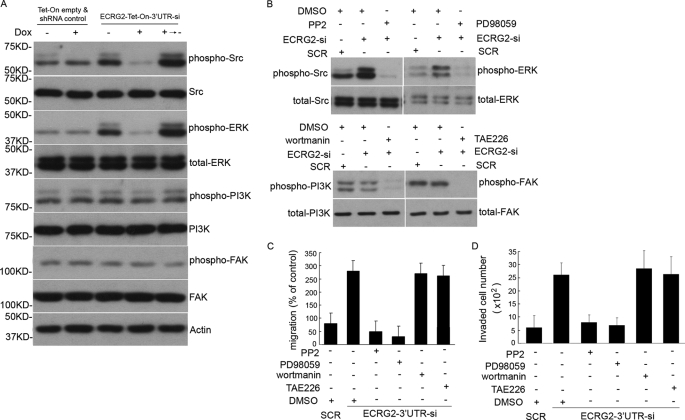
Src/MAP Kinase, but Not FAK and PI3K, Is Involved in ECRG2-regulated, uPA-dependent Cell Migration/Invasion
uPA·uPAR regulates cell migration as a signal transduction molecule and by its intrinsic chemotactic activity (13–15). To explore a molecular pathway by which ECRG2 influences migration and invasion through disrupting the physical association between uPAR and β1 integrins, HT1080 Tet-On empty vector and shRNA control cells or ECRG2-Tet-On-3′UTR-si cells were cultured in the presence or absence of doxycycline for 24 h. Cells were collected and subjected to Western blotting to probe the normal and phosphorylation forms of second messenger pathways known to be activated by integrin signaling (Src, MAP kinase, PI3K, and FAK) (19–21). As shown in Fig. 5A, total Src expression remained unchanged, whereas the level of p-Src increased in the absence of doxycycline and decreased in the presence of doxycycline in ECRG2-Tet-On-3′UTR-si cells when compared with that of controls. Similar results were obtained for the expression of ERK and p-ERK. In the case of PI3K and FAK, total and phosphorylation forms of PI3K and FAK showed similar levels with or without ECRG2 expression, indicating that the ECRG2·uPA·uPAR complex retards the phosphorylation of Src and MAP kinase but not PI3K and FAK. To further confirm this, ECRG2 depletion cells were exposed to various inhibitors of kinases or the same volume of DMSO for 6 h before being subjected to the cell migration assay. Fig. 5B confirmed that all the inhibitors effectively inhibited the kinase activity. As shown in Fig. 5C, cells exposed to inhibitors of PI3K (wortmannin, 100 nm) or FAK inhibitor (TAE226, 100 nm) maintained a similar migration with ECRG2 depletion cells, indicating that their activity did not account for the enhanced migration caused by ECRG2 depletion. In contrast, a specific inhibitor of Src family kinases (PP2, 1 μm) and a specific inhibitor of MAP kinase (PD98059, 10 μm) were found to reverse the enhanced migration of ECRG2 depletion cells, indicating that Src/MAP kinase was involved in cell migration regulated by ECRG2. Similar patterns were observed in cell invasion assays, as shown in Fig. 5D. We observed similar results in MDA-MB-231 and MCF-7 cells (supplemental Fig. 5).
FIGURE 5.
Involvement of Src/MAP kinases in ECRG2-regulated, uPA-induced cell migration. A, ECRG2-Tet-On-3′UTR-si and Tet-On empty and shRNA control cells were cultured in the presence or absence of doxycycline (Dox) for 24 h and treated with uPA (10 nm) for 1 h before being collected, and then total cell lysates were prepared, fractionated by SDS-PAGE, and subjected to Western blotting for normal and phosphorylated forms of Src, ERK, PI3K, and FAK. Actin level served as a loading control. B, ECRG2–3′UTR-si cells were incubated with Src kinase inhibitor PP2, MAP kinase inhibitor PD98059, PI3K kinase inhibitor wortmannin, or FAK kinase inhibitor TAE226 in the medium contained uPA (10 nm), respectively. The same volume of DMSO was used as control. The inhibitory activity of inhibitors was verified by Western blotting. C, ECRG2–3′UTR-si cells and scrambled (SCR) control (Tet-on empty and shRNA control) cells were induced to migrate in the presence of inhibitors or same volume of DMSO. Random cell migration of non-pretreated cells is referred to as 100% of migration. Results are representative of three independent experiments. D, a Transwell invasion assay was done in ECRG2-3′UTR-si cells and SCR control (Tet-on empty and shRNA control) cells in the presence of inhibitors or the same volume of DMSO. An equal volume (0.5 ml/chamber) of cell suspensions (2 × 104 cells/ml) was seeded in Matrigel invasion chambers and incubated at 37 °C for 48 h. The number of cells that invaded through the Matrigel was quantified by counting cells from 10 microscopic fields. Columns, means of 20 high power fields from two independent experiments; error bars, S.D.
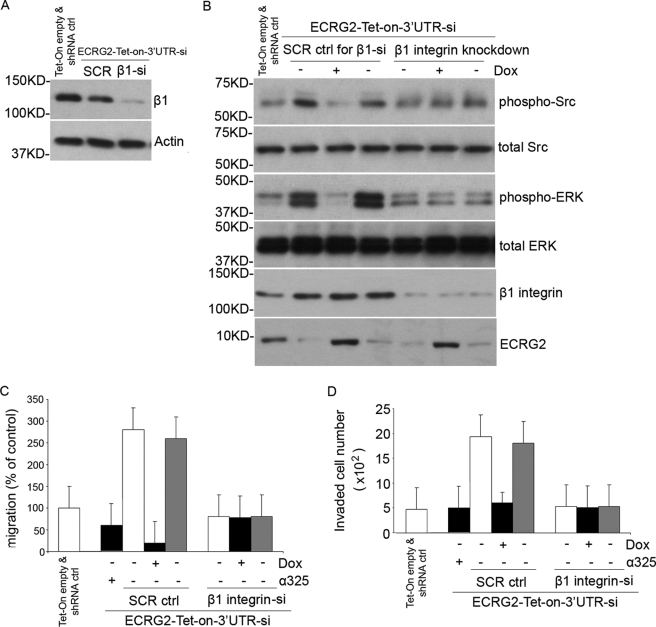
ECRG2 Regulates uPA-dependent Cell Migration/Invasion through β1 Integrin/Src/MAP Kinase Pathway
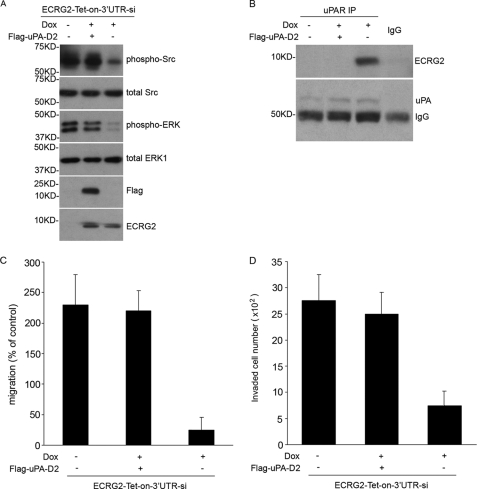
Our results indicate that the signal response to ECRG2 in cell migration/invasion is achieved through the α3β1 or α5β1 integrin pathway, and Src/MAP kinase is involved in ECRG2-regulated cell migration/invasion. We hypothesize that ECRG2 regulates cell migration/invasion mainly through the uPA/uPAR/α3β1 or α5β1 integrins/Src/MAP kinase pathway. To test this possibility, we depleted β1 integrin in ECRG2-Tet-on-3′UTR-si cells by using the siRNA retrovirus infection approach (ECRG2-Tet-on-3′UTR-si-β1-KD). Cells were cultured in the presence or absence of doxycycline for 24 h, and the depletion of β1 integrin was confirmed by Western blotting analysis (Fig. 6A). As shown in Fig. 6B, ECRG2 depletion significantly increased, whereas overexpression decreased the phosphorylation forms of Src and MAP kinase in scramble control cells. However, the phosphorylation forms of these kinases remained more or less unchanged in β1 integrin-depleted cells, indicating that ECRG2 loses the ability to regulate Src/MAP kinase activity when β1 integrin was depleted. To evaluate the effect of β1 integrin depletion on ECRG2-regulated cell migration, Transwell migration assays were performed. We used a synthetic peptide α325, a 17-mer derived from the α3 integrin sequence shown to block uPAR-α3β1 and uPAR-α5β1 interaction (2), as a positive control. Scramble control or ECRG2-Tet-on-3′UTR-si-β1-KD cells were analyzed for their ability to migrate. As expected, cells showed a substantially higher basal migration when ECRG2 was depleted in scramble control cells, and the addition of α325 strongly suppressed the migration. No migration was observed in the presence of doxycycline in scramble control cells. However, the cell migration remained unchanged no matter what with or without ECRG2 expression in β1-depleted cells (Fig. 6C), indicating that β1 integrin is necessary for ECRG2-regulated cell migration. Similar results were observed in a cell invasion assay as shown in Fig. 6D. Thus, the complex of ECRG2·uPA·uPAR suppresses cell migration/invasion by abolishing the interaction of uPAR with α3β1 and α5β1 integrins and subsequently inhibiting the downstream Src/MAP kinase pathway. To further confirm this, we overexpressed FLAG-uPA D2 to disrupt the effect of ECRG2 on the uPA·uPAR·integrin complex and then examined the p-Src and p-ERK levels and cell migration/invasion. As shown in Fig. 7, the expression of ECRG2 inhibited the p-Src and p-ERK levels as described above (third lane). However, overexpression of FLAG-uPA D2, which disrupted the ECRG2·uPA·uPAR complex (Fig. 7B), rescued the inhibitions of ECRG2 on activity of Src and ERK (Fig. 7A, second lane), thus promoting cell migration (Fig. 7C) and cell invasion (Fig. 7D). Similar results were observed in MDA-MB-231 and MCF-7 cells (supplemental Fig. 6).
FIGURE 6.
β1 integrin is necessary for ECRG2-dependent promotion or suppression of cell migration. A, ECRG2-Tet-on-3′UTR-si cells were infected with β1-integrin siRNA retrovirus (ECRG2-Tet-On-3′UTR-si-β1-KD) or scramble control siRNA (SCR control). After 48 h, cells were subjected to Western blotting for suppression of β1-integrin. Actin was used as loading control. shRNA ctrl, shRNA control. B, ECRG2-Tet-On-3′UTR-si-β1-KD cells or SCR control cells were incubated with or without doxycycline (Dox) for 24 h and subjected to Western blotting for phosphorylation and total forms of Src and ERK. The expression of ECRG2 and depletion of β1-integrin were also shown. C, 48 h after infection, SCR control (SCR ctrl) (Tet-on empty and shRNA control) cells or ECRG2-Tet-On-3′UTR-si-β1-KD cells were seeded in Matrigel migration chambers cultured with or without doxycycline. α325, a positive control, was added to ECRG2-Tet-On-3′UTR-si cells in the absence of doxycycline for 20 min at 37 °C. Migrated cells were counted. Data are expressed as mean ± S.D. from three independent experiments, each in triplicate. Random cell migration of non-pretreated cells is referred to as 100% of migration. D, a Transwell invasion assay was done in SCR control (Tet-on empty and shRNA control) cells or ECRG2-Tet-On-3′UTR-si-β1-KD cells in the presence or absence of doxycycline. α325, a positive control, was added to ECRG2-Tet-On-3′UTR-si cells in the absence of doxycycline for 20 min at 37 °C. Equal volume (0.5 ml/chamber) of cell suspensions (2 × 104 cells/ml) was seeded in Matrigel invasion chambers and incubated at 37 °C for 48 h. The number of cells invaded through the Matrigel was quantified by counting cells from 10 microscopic fields. Columns, means of 20 high power fields from two independent experiments; error bars, S.D.
FIGURE 7.
ECRG2 regulates cell migration through uPA/uPAR/integrin/Src/ERK pathway. ECRG2-Tet-on-3′UTR-si cells were transiently transfected with or without FLAG-uPA-D2 plasmid and incubated in the absence or presence of doxycycline (Dox) for 24 h. A, cell lysates were subjected to Western blotting analysis for phospho-Src and -ERK. B, cell lysates were subjected to immunoprecipitation (IP) with anti-uPAR antibody and Western blotting for ECRG2 and uPA. C, cells were subjected to the in vitro migration assay as described above. D, cells were subjected to the in vitro Transwell invasion assay as described above.
DISCUSSION
Tumor cell invasion and migration are critical steps in tumor progression and metastasis, and the uPA·uPAR system has been shown to mediate these processes (1–2). Previously, our group reported that ECRG2 inhibits invasiveness and metastasis of the highly metastatic lung cancer cell line PG, possibly via suppression of uPA/plasmin activity (10). In this study, we report evidence that ECRG2 directly binds to uPA and that such binding modifies uPAR association with its transmembrane adaptor β1 integrins, regulating Src/MAP kinase pathway and contributing to cell invasion and migration.
To evaluate the mechanism by which ECRG2 regulates cell invasion and migration, we initially identified the binding domain of ECRG2 in uPA. Our studies using co-immunoprecipitation showed that ECRG2 directly binds to the Kringle domain of uPA (Fig. 1 and supplemental Fig. 2). The putative binding site for uPA protein in ECRG2 was mapped by the heteronuclear NMR method recently (25), whereas the significance of ECRG2 binding to the Kringle and CP domain of uPA remains to be established in the future. A growing body of evidence has suggested that the uPA·uPAR system promotes tumor metastasis by several different mechanisms and not just solely by breaking down the extracellular matrix (26). The uPAR is linked to cellular migration through its capacity to promote pericellular proteolysis, regulate integrin function, and mediate cell signaling in response to urokinase (uPA) binding (27). To further examine the possibility of mechanisms, other than extracellular matrix degradation, of ECRG2 in cell invasion and migration, we next tested whether ECRG2 affects the uPAR function through binding to uPA. Immunoprecipitation experiments show that ECRG2 does not impair the association of uPA with uPAR and forms a triple complex: ECRG2·uPA·uPAR (Fig. 2 and supplemental Fig. 3). The uPAR is a glycosylphosphatidylinositol-anchored protein that lacks a transmembrane domain. At least three types of transmembrane protein have now been identified as mediators of uPAR, and they are involved in signaling in response to uPA: integrins, G-protein-coupled receptors, and caveolin. Several different lines of evidence indicate that uPAR can dynamically and functionally interact with multiple integrins including β1, β2, β3, and β5 integrins and control their activation, which contributes to migration and invasion (28). Among β1 integrins, uPAR directly associates with α3β1 via a surface loop within the β-propeller (29). uPAR is also able to associate with α5β1 and to regulate α5β1-mediated cell migration (30). Other integrins such as αvβ3 were also involved in the association with uPAR (2, 21). The interaction of integrins to uPAR is not constitutive, but it is dynamically regulated by conformational changes in integrin extracellular domains, by intracellular signal transduction pathways, and by the interaction of integrins with neighboring membrane-associated proteins, including integrin-associated protein, caveolin, and uPAR (16, 31). Our present studies have focused on the regulation of integrin function by uPAR. Surprisingly, the α3β1 and α5β1 integrins were completely lost in uPAR immunoprecipitates when ECRG2 was overexpressed. In contrast, the protein levels of α3β1 and α5β1 integrins in uPAR immunoprecipitate were significantly increased in ECRG2 depletion cells (Fig. 3 and supplemental Fig. 4), indicating that the binding of ECRG2 to uPA disrupts the association of the uPA·uPAR complex with α3β1 and α5β1 integrins. The block function of uPAR-α3β1 or uPAR-α5β1 interaction disappeared no matter whether ECRG2 was depleted or overexpressed when uPA was knocked down using the siRNA approach (Fig. 4), confirming that ECRG2 regulates the association of uPAR with integrins through uPA. The binding of uPA to uPAR induces conformational changes in uPAR, which promotes its interaction with a variety of integrins (25). The active molecular complex seems to be a trimeric uPA·uPAR·integrin complex. Our results indicate that ECRG2 modifies uPAR through binding to the uPA·uPAR complex, and such modification blocks the dynamical association of uPAR with α3β1 and α5β1 integrins, inactivates the trimeric uPA·uPAR·integrins complex, and thus inhibits uPA-stimulated migration.
The uPAR-integrin interaction has been shown to transduce intracellular signals and plays critical roles in cell invasion and migration. Integrins are known to activate the Src, ERK/MAPK, FAK, and PI3K pathways (13). Our results demonstrated that depletion of ECRG2 causes the high activation of p-Src and p-ERK, whereas overexpression of ECRG2 leads to dephosphorylation of Src and ERK. An in vitro migration/invasion assay showed that depletion of ECRG2 markedly increased the cell migration/invasion when compared with control cells. However, cell migration/invasion was not increased in the presence of Src inhibitor (PP2) or MAP kinase inhibitor (PD98059) in ECRG2 depletion cells (Fig. 5 and supplemental Fig. 5), indicating that Src and MAP kinases are a downstream pathway stimulated by the uPAR·integrin complex response to the binding of ECRG2 to uPA and are required for ECRG2 to regulate cell migration/invasion. Based on our results, we hypothesize that ECRG2 regulates cell migration/invasion through uPAR/α3β1 or α5β1 integrin/Src/MAP kinase pathway. To test this possibility, β1 integrin was depleted using the siRNA approach. Similar patterns of p-Src and p-ERK were observed in scramble control cells. However, the phospho-kinases remained more or less changed with or without ECRG2 expression in β1 integrin depletion cells (Fig. 6B). Moreover, the depletion of ECRG2 markedly increases, whereas the expression of ECRG2 markedly inhibits cell migration/invasion in scramble control cells, whereas no significant change of cell migration/invasion was observed whether ECRG2 was depleted or overexpressed in β1 integrin depletion cells (Fig. 6, C and D). Furthermore, disrupting the effect of ECRG2 on uPA·uPAR·integrin complexes by overexpression FLAG-uPA D2 reverted the inhibition of ECRG2 on Src/ERk pathway, promoting cell migration and invasion (Fig. 7 and supplemental Fig. 6). Our results suggest that the interaction of ECRG2 with uPA·uPAR·β1 integrin complexes is required for ECRG2 to regulate the Src/MAP kinase pathway and cell migration/invasion.
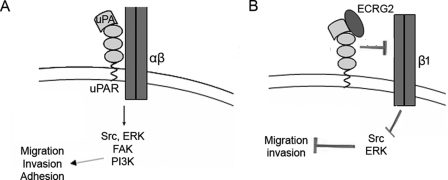
We obtained very similar results for MDA-MB-231 and MCF-7 breast cancer cells (supplemental Figs. 2–6). Taken together, we propose a model in which ECRG2 regulates cell migration and invasion (Fig. 8). The binding of ECRG2 to the uPA·uPAR complex blocks the interaction of uPAR with α3β1 or α5β1 integrin and results in inactivation of integrin intracellular signaling components (Src/MAP kinase). High levels of uPA and uPAR often correlate with poor prognosis of cancer patients. Therefore, the specific inhibition of uPA·uPAR with small molecule active site inhibitors is one strategy to decrease the invasive and metastatic activity of tumor cells. Our data show that ECRG2 can regulate cell migration and invasion through several mechanisms. Understanding the detail of this model will provide more direct and efficient tools for the control of uPA·uPAR-mediated migration in tumor metastasis.
FIGURE 8.
Schematic representation showing the signaling and functional consequences from uPA-uPAR-integrin interactions and model of the plausible signaling events regulated by ECRG2. A, at the leading edge of migrating cells, the uPAR binds inactive urokinase (pro-uPA), which is then converted to active uPA. uPAR lacks a cytosolic domain but transmits intracellular signals through its association with transmembrane integrins. uPA-bound uPAR frequently interacts with α3β1 or α5β1 integrin, causing its activation. This leads to integrin-dependent recruitment of Src, FAK, or PI3K, which results in activation of MAPK signaling. B, model of ECRG2 regulating cell migration and invasion. The binding of ECRG2 to the uPA·uPAR complex blocks the interaction of uPAR with α3β1 or α5β1 integrin and results in integrin inactivation, disassembly of the uPA·uPAR·integrin complex, inactivation of its intracellular signaling components, and reduced Src/MAP kinase activation.
Acknowledgment
We thank the Medical Experiment Center of Shanxi Medical University for Microscopy analysis.
This work was supported by National Nature Science Foundation of China Grants 30971518 and 30500588 and by the Program for the Top Young Academic Leaders of Higher Learning Institutions of Shanxi (to Y. C.).
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
- uPA
- urokinase-type plasminogen activator
- uPAR
- urokinase-type plasminogen activator receptor
- ERK
- extracellular-signal-regulated kinase
- MAP
- mitogen-activated protein
- MAPK
- MAP kinase
- FAK
- focal adhesion kinase
- PI3K
- phosphatidylinositol 3-kinase
- DMSO
- dimethyl sulfoxide
- PP2
- protein phosphatase 2A
- siRNA
- small interfering RNA
- shRNA
- short hairpin RNA
- UTR
- untranslated region
- P
- phosphorylated
- SCR
- scrambled.
REFERENCES
- 1.Friedl P., Wolf K. (2003) Nat. Rev. Cancer. 3, 362–374 [DOI] [PubMed] [Google Scholar]
- 2.Tang C. H., Wei Y. (2008) Cell Mol. Life Sci. 65, 1916–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su T., Liu H., Lu S. H. (1998) Zhonghua Zhong Liu Za Zhi. 20, 254–257 [PubMed] [Google Scholar]
- 4.Cui Y., Wang J., Zhang X., Lang R., Bi M., Guo L., Lu S. H. (2003) Biochem. Biophys. Res. Commun. 302, 904–915 [DOI] [PubMed] [Google Scholar]
- 5.Smith S. L., Watson S. G., Ratschiller D., Gugger M., Betticher D. C., Heighway J. (2003) Oncogene 22, 8677–8687 [DOI] [PubMed] [Google Scholar]
- 6.Stefansson S., Lawrence D. A. (1996) Nature 383, 441–443 [DOI] [PubMed] [Google Scholar]
- 7.Silverman G. A., Bird P. I., Carrell R. W., Church F. C., Coughlin P. B., Gettins P. G., Irving J. A., Lomas D. A., Luke C. J., Moyer R. W., Pemberton P. A., Remold-O'Donnell E., Salvesen G. S., Travis J., Whisstock J. C. (2001) J. Biol. Chem. 276, 33293–33296 [DOI] [PubMed] [Google Scholar]
- 8.Dawson D. W., Volpert O. V., Gillis P., Crawford S. E., Xu H., Benedict W., Bouck N. P. (1999) Science 285, 245–248 [DOI] [PubMed] [Google Scholar]
- 9.Bass R., Fernández A. M., Ellis V. (2002) J. Biol. Chem. 277, 46845–46848 [DOI] [PubMed] [Google Scholar]
- 10.Huang G., Hu Z., Li M., Cui Y., Li Y., Guo L., Jiang W., Lu S. H. (2007) Carcinogenesis 28, 2274–2281 [DOI] [PubMed] [Google Scholar]
- 11.Blasi F., Carmeliet P. (2002) Nat. Rev. Mol. Cell Biol. 3, 932–943 [DOI] [PubMed] [Google Scholar]
- 12.Rao J. S. (2003) Nat. Rev. Cancer 3, 489–501 [DOI] [PubMed] [Google Scholar]
- 13.Ahmed N., Oliva K., Wang Y., Quinn M., Rice G. (2003) Brit. J. Cancer 89, 374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman H. A., Wei Y. (2001) Thromb. Haemost. 86, 124–129 [PubMed] [Google Scholar]
- 15.Ossowski L., Aguirre-Ghiso J. A. (2000) Curr. Opin. Cell Biol. 12, 613–620 [DOI] [PubMed] [Google Scholar]
- 16.Sturge J., Wienke D., East L., Jones G. E., Isacke C. M. (2003) J. Cell Biol. 162, 789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidenius N., Blasi F. (2003) Cancer Metastasis Rev. 22, 205–222 [DOI] [PubMed] [Google Scholar]
- 18.Sitrin R. G., Johnson D. R., Pan P. M., Harsh D. M., Huang J., Petty H. R., Blackwood R. A. (2004) Am. J. Respir. Cell Mol. Biol. 30, 233–241 [DOI] [PubMed] [Google Scholar]
- 19.Aguirre-Ghiso J. A., Estrada Y., Liu D., Ossowski L. (2003) Cancer Res. 63, 1684–1695 [PubMed] [Google Scholar]
- 20.Aguirre Ghiso J. A., Kovalski K., Ossowski L. (1999) J. Cell Biol. 147, 89–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franco P., Vocca I., Carriero M. V., Alfano D., Cito L., Longanesi-Cattani I., Grieco P., Ossowski L., Stoppelli M. P. (2006) J. Cell Sci. 119, 3424–3434 [DOI] [PubMed] [Google Scholar]
- 22.Huang G., Wang D., Guo L., Zhao N., Li Y., Lu S. H. (2005) Hybridoma 24, 86–91 [DOI] [PubMed] [Google Scholar]
- 23.Cheng X., Shen Z., Yang J., Lu S. H., Cui Y. (2008) J. Biol. Chem. 283, 5888–5898 [DOI] [PubMed] [Google Scholar]
- 24.Gondi C. S., Kandhukuri N., Dinh D. H., Gujrati M., Rao J. S. (2007) Int. J. Oncol. 31, 19–27 [PMC free article] [PubMed] [Google Scholar]
- 25.Geng Y., Feng Y., Xie T., Dai Y., Wang J., Lu S. H. (2008) Arch. Biochem. Biophys. 479, 153–157 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M., Kobayashi H., Kanayama N., Saga Y., Suzuki M., Lin C. Y., Dickson R. B., Terao T. (2004) J. Biol. Chem. 279, 14899–14908 [DOI] [PubMed] [Google Scholar]
- 27.Czekay R. P., Aertgeerts K., Curriden S. A., Loskutoff D. J. (2003) J. Cell Biol. 160, 781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer T. W., Liu W., Fan F., Camp E. R., Yang A., Somcio R. J., Bucana C. D., Callahan J., Parry G. C., Evans D. B., Boyd D. D., Mazar A. P., Ellis L. M. (2005) Cancer Res. 65, 7775–7781 [DOI] [PubMed] [Google Scholar]
- 29.Wei Y., Eble J. A., Wang Z., Kreidberg J. A., Chapman H. A. (2001) Mol. Biol. Cell 12, 2975–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y., Czekay R. P., Robillard L., Kugler M. C., Zhang F., Kim K. K., Xiong J. P., Humphries M. J., Chapman H. A. (2005) J. Cell Biol. 168, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Y., Yang X., Liu Q., Wilkins J. A., Chapman H. A. (1999) J. Cell Biol. 144, 1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]