Abstract
This study was initiated to induce experimental autoimmune anterior uveitis (EAAU) in Lewis rats by melanin-associated antigen (MAA; 22-kDa fragment of type I collagen α2 chain) derived from rat iris and ciliary body (CB), to localize MAA within the eye, and to investigate the possible mechanism of MAA generation in vivo. The EAAU model replicates idiopathic human anterior uveitis. Lewis rats sensitized to rat MAA developed anterior uveitis, and EAAU induced by rat MAA can be adoptively transferred to naive syngenic rats by MAA-primed T cells. Animals immunized with rat MAA developed cellular immunity to the antigen. MAA was detected only in the iris and CB of the eye. Iris and CB were the major source of matrix metalloproteinase-1 (MMP-1) in the naive eye, and ocular expression of MMP-1 was up-regulated, whereas expression of tissue inhibitor of metalloproteinase 1 decreased before the onset of EAAU. These results demonstrated that EAAU can be induced by autologous MAA. Uveitogenic antigen is present only in the iris and CB of the eye, and the imbalance between MMP-1 and tissue inhibitor of metalloproteinase 1 may play a role in the generation of MAA in vivo. Collectively, the evidence presented here suggests that MAA is an autoantigen in EAAU. These observations may extend to idiopathic human anterior uveitis and facilitate the development of antigen-specific therapy.
Introduction
Uveitis is broadly defined as the inflammation of the uvea (iris, ciliary body, and choroid). It is responsible for over 2.8% of blindness in the United States with a higher disease rate for the older population (1–3). Anterior uveitis (AU)2 of unknown etiology is the most common form of intraocular inflammation in humans. In a nonreferral clinic, ∼52% of patients may present with idiopathic AU (1–3). It has been considered an autoimmune disease and presents with the inflammation of the iris and/or ciliary body (CB) with no involvement of retina (1–3). The recurrent nature of idiopathic AU results in visual complications that lead to the permanent loss of vision (1–3). Experimental autoimmune anterior uveitis (EAAU) is an autoimmune ocular inflammatory disease that serves as a model of idiopathic human AU (4–12). In this experimental model, inbred Lewis rats are subcutaneously immunized in the foot pad with melanin-associated antigen (MAA) isolated from bovine iris and CB, and EAAU is induced in these animals by an antigen-specific CD4+ T cell response to MAA (5–12). EAAU is characterized histologically by a lymphocytic infiltration in the iris and CB (4–12), and antigen-specific CD4+ T cells can adoptively transfer disease into naive syngenic recipients (7, 8, 12). EAAU cannot be induced by the adoptive transfer of primed CD8+ T cells or immune sera (6–8). MAA has been purified to homogeneity in our laboratory, and our published results have shown that a 22-kDa fragment of type I collagen α2 chain (CI-α2 (22 kDa)) derived from bovine iris and CB contains the antigenic determinant(s) necessary to induce EAAU in Lewis rats (8).
We have previously reported that in animals immunized with bovine MAA, no inflammation was observed in the retina as well as in other parts of the eye, including cornea and choroid (5–10). Other organs, such as pineal gland, liver, and kidney, were not affected in these animals (5–10). We have further demonstrated that MAA was only found in the autoantigenic form in the iris and CB because the antigen isolated from bovine skin, bovine Achilles tendon, bovine conjunctiva, bovine gut, and rat tail was not uveitogenic (8). However, to our knowledge, induction of EAAU by rat MAA, the precise expression/localization of this uveitogenic antigen, and the possible mechanism(s) of MAA generation in vivo have not been investigated. We initiated this study to investigate if EAAU can be induced by MAA (CI-α2 (22 kDa)) purified from rat iris and CB and to analyze the expression/localization of MAA within the rat eye. Our study was also designed to investigate the possible role of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in the generation of MAA in the anterior segment of the eye. MMPs are a family of zinc-containing endoproteases that degrade matrix proteins, and TIMPs are endogenous (natural) inhibitors of MMPs (13). The results reported here provide evidence that MAA is the target autoantigen in EAAU, and the imbalance between the expression of MMP-1 and TIMP-1 may play a role in the generation of MAA in vivo and in the pathogenesis of EAAU.
EXPERIMENTAL PROCEDURES
Animals
Pathogen-free male Lewis rats (5–6 weeks old) were obtained from Harlan Sprague-Dawley (Indianapolis, IN). This study was approved by the Institutional Animal Care and Use Committee, University of Arkansas for Medical Sciences (Little Rock, AR).
Preparation of Melanin-associated Antigen
Melanin-associated antigen, CI-α2 (22 kDa), was separately isolated from bovine iris and CB as well as the iris and CB of naive Lewis rats as described previously by us (8). Fifty bovine and 500 rat eyes were used. Briefly, bovine iris and CB and rat iris and CB were homogenized and extracted with 0.5 m acetic acid at 45 °C for 48 h separately. The α2 chain of type I collagen was purified separately from rat and bovine iris and CB using the method described before (8). Purified α2 chain was treated with proteolytic enzyme, Staphylococcus aureus V8 protease (Sigma), and the 22-kDa antigen was purified using preparative SDS-PAGE and preparative isoelectric focusing (8). The NH2-terminal sequence of purified rat protein was determined as described previously (8).
Induction and Evaluation of EAAU
Lewis rats were immunized with 50 μl of stable emulsion containing 50 μg of protein (bovine or rat MAA) emulsified (1:1) in complete Freund's adjuvant (Sigma) in the hind foot pad (5–12). Animals were graded for the clinical signs of EAAU as described previously by us (5). Eyes were harvested at various time points for histological analysis to assess the course and severity of inflammation (5). The intensity of uveitis was histologically scored in a masked fashion on an arbitrary scale of 0–4 as follows: 0, normal; 1, dilated iris vessels plus thickened iris stroma exudates in the anterior chamber with protein or a few scattered inflammatory cells or both; 2, moderate infiltration of inflammatory cells in the stroma of the iris or CB or both and a moderate number of inflammatory cells within the anterior chamber; 3, heavy infiltration of inflammatory cells within the iris stroma and the CB and heavy infiltration of inflammatory cells within the anterior chamber; 4, heavy exudation of cells with dense protein aggregation in the anterior chamber and inflammatory cell deposits on the corneal endothelium.
Adoptive Transfer of EAAU
Lewis rats were divided into two groups (n = 5 animals/group). Animals in group 1 were immunized with rat MAA, and the animals in group 2 were immunized with bovine MAA. Popliteal lymph nodes were harvested separately from donor rats in each group at day 14 postimmunization (7, 8). A single-cell suspension of popliteal lymph node cells (LNCs) was made in Dulbecco's modified minimum essential medium, and LNCs (20 × 106) harvested from animals in group 1 and group 2 were cultured separately with the rat and bovine antigen (20 μg/ml), respectively, for 3 days. After this, T cells were purified by using Cellect immunocolumns (Cytovax Biotechnologies, Inc.) and were separately injected into naive Lewis rats via the tail vein. These experiments were repeated three times with similar results.
T Cell Proliferation Assay
At day 14 postimmunization, popliteal lymph nodes were harvested from Lewis rats immunized with rat antigen, and a single-cell suspension was prepared (8). LNCs (2 × 105/well) were stimulated with rat antigen (20 μg/ml) in 96-well flat bottom plates (BD Biosciences). LNC obtained from Lewis rats immunized with bovine antigen and cultured in vitro with bovine antigen served as the positive control. Negative control consisted of cells cultured without antigen. Plates were cultured for 72 h at 37 °C and incubated with [3H]thymidine (1 μCi/well) (GE Healthcare) for an additional 18 h. The stimulation index (the ratio between the cpm of a culture in the presence of the antigen and the proliferation of the same cells in the absence of the antigen) was determined, and a value of 3.0 and above was considered positive. These experiments were repeated three times with similar results.
Preparation of Polyclonal Antibodies
Purified bovine MAA (200 ng) mixed with complete Freund's adjuvant (Sigma) was injected into New Zealand White rabbits (Charles River Laboratories, Wilmington, MA) every 10 days. Three days after the sixth injection, blood was withdrawn, serum was collected, and the specificity of the antibody was determined by Western blot analysis.
Western Blot Analysis
Cornea, retina, iris and CB, and choroid and retinal pigment epithelium (RPE) harvested from 30 eyes of naive Lewis rats were pooled separately to detect the expression of MAA. Iris and CB were collected in one tube; similarly, RPE and choroid were collected together in another tube. In a separate set of experiments, intraocular tissue was prepared from the eyes harvested from naive Lewis rats as well as Lewis rats sacrificed at different time points during EAAU. Intraocular tissues, which consisted of uvea, retina, lens, aqueous humor, and vitreous, were prepared using a previously described method (14) and were used to detect protein levels of β-actin, TIMP-1, TIMP-2, and TIMP-3. Tissues prepared as described above were homogenized in 500 μl of ice-cold PBS containing protease inhibitors and 1% Nonidet P-40. The homogenate was centrifuged at 14,000 × g at 4 °C for 15 min, and the supernatant was subjected to SDS-PAGE. After SDS-PAGE on a 14% linear slab gel, separated proteins were transferred to polyvinylidene difluoride membranes using a Trans-Blot semidry electrophoretic transfer cell (Bio-Rad). Western blot analysis (14) was performed using polyclonal antibodies against bovine MAA (1:1000 dilution), TIMP-1 (1:500 dilution, R&D Systems, Minneapolis, MN), TIMP-2, and TIMP-3 (Abbiotech, San Diego, CA). Blots were also incubated with the monoclonal β-actin (mouse IgG1, Sigma) housekeeping gene. Control blots were reacted with equivalent concentrations of normal rabbit serum or isotype-matched nonspecific control antibody. After washing and incubation with horseradish peroxidase-conjugated appropriate secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), blots were developed using the ECL Plus Western blotting detection system (GE Healthcare). Quantification of proteins was accomplished by analyzing the intensity of the bands using Quantity One 4.2.0 (Bio-Rad). These experiments were repeated three times with similar results.
Immunohistochemistry
For frozen sections, eyes harvested from naive Lewis rats were placed in optimal cutting temperature compound (Electron Microscopy Sciences, Hatfield, PA) and were snap frozen. Eyes were sectioned by cryostat, and the tissue (5 μm thick) was air-dried overnight (18 h), fixed in cold acetone for 10 min, and rehydrated in phosphate-buffered saline (pH 7.2). Sections were stained for MAA using polyclonal antibody against bovine MAA (1:200 dilution) and an immunoperoxidase staining kit from Vector Laboratories (Burlingame, CA) as described before (6). Slides were then washed in tap water, counterstained for 10 min with Mayer's hematoxylin, washed thoroughly in cold tap water, and coverslipped with an aqueous mounting medium for viewing by light microscopy. Control stains were performed with normal rabbit serum at a concentration similar to that of the primary antibody. Additional controls consisted of staining by omission of the primary and secondary antibody. This experiment was repeated three times with similar results.
Flow Cytometry
Cornea, iris, CB, choroid, RPE, and retina harvested from 18 eyes of naive Lewis rats were pooled separately. Iris and CB were collected in one tube; similarly, RPE, choroid, and retina were collected together in another tube. Total cells isolated from these tissues as well as from the whole rat eyes were used in flow cytometry for MMP-1, MMP-8, and MMP-13. In a parallel experiment, total cells isolated from the whole eyes of Lewis rats sacrificed at different time points (n = 3 rats/time point) during EAAU were used in flow cytometry for MMP-1.
A single-cell suspension was prepared by mashing the above mentioned tissues with frosted slides, followed by filtration through the cell strainer. Cells were incubated with anti-rat CD32 antibody (BD Biosciences) for 15 min at 4 °C. This was followed by the intracellular stain using the BD Cytoperm/Cytofix kit (BD Biosciences) according to the manufacturer's instructions. The cells were stained using primary antibodies for MMP-1 (Lifespan Biosciences, Seattle, WA), MMP-8, and MMP-13 (Santa Cruz Biotechnology, Inc.), followed by appropriate fluorescein isothiocyanate or phosphatidylethanolamine secondary antibodies. The stained cells were analyzed using a BD FACSCalibur (BD Biosciences). These experiments were repeated three times with similar results.
Semiquantitative RT-PCR
Cornea, iris, CB, choroid, RPE, and retina harvested from 18 eyes of naive Lewis rats were pooled separately. Iris and CB were collected in one tube; similarly, RPE, choroid, and retina were collected together in another tube. Total RNA extracted from these tissues was used to detect mRNA levels of β-actin, MMP-1, MMP-8, and MMP-13. In a separate set of experiments, Lewis rats were sacrificed at different time points (n = 3 rats/time point) during EAAU, and intraocular tissues, which consisted of uvea, retina, lens, aqueous humor, and vitreous, were prepared using a previously described method (14). Total RNA extracted from pooled intraocular tissues was used to detect the mRNA levels of β-actin, MMP-1, TIMP-1, TIMP-2, and TIMP-3. Total RNA was prepared using Qiagen total RNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer's specifications. Semiquantitative RT-PCR was performed using the reagents purchased from Bio-Rad. The oligonucleotide primers were synthesized at Integrated DNA Technologies (Coralville, IA). The primer sequences as well as the predicted sizes of amplified cDNA were as follows: β-actin, 5′-GCGCTCGTCGTCGACAACGG-3′ (forward) and 5′-GTGTGGTGCCAAATCTTCTCC-3′ (reverse) (335 bp); MMP-1, 5′-TTGTTGCTGCCCATGAGCTT-3′ (forward) and 5′-ACTTTGTCGCCAATTCCAGG-3′ (reverse) (639 bp); MMP-8, 5′-AGTGCCCGACTCTGGTGATTTCTT-3′ (forward) and 5′-AGGTGCTGGGTTCTCTGTAAGCAT-3′ (reverse) (464 bp); MMP-13, 5′-AGGATCACCTGATTCTTGGGTGCT-3′ (forward) and 5′-AGGAGCATGAAAGGGTGGTCTCAA-3′ (reverse) (782 bp); TIMP- 1, 5′-GCTAGAGCAGATACCACGATGGCG-3′ (forward) and 5′-TGCAAGGGATGGCTGAACAGGG-3′ (reverse) (499 bp), TIMP-2, 5′-GTGAGCGAGAAGGAGGTGGATTCC-3′ (forward) and 5′-CTTGATGCAGGCAAAGAACTTGGC-3′ (reverse) (438 bp);, TIMP-3, 5′-CGTGCACATGCTCGCCCAGC-3′ (forward) and 5′-GGCCCTTGCGCTGGGACAG-3′ (reverse) (335 bp).
Four different cycles, 25, 28, 30, and 35 were used for PCR, and all four cycles gave similar results. All reactions were normalized for β-actin expression. The negative controls consisted of the omission of RNA template or reverse transcriptase from the reaction mixture. PCR products were analyzed on a 2% agarose gel and quantitated by densitometry using Quantity One 4.2.0 (Bio-Rad). These experiments were repeated three times with similar results.
Quantitative Real-time RT-PCR
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). Quantitative real-time RT-PCR was performed using iQTM SYBR® Green Supermix and the iQTM5 quantitative real-time RT-PCR unit (both purchased from Bio-Rad). The primers were designed and ordered from Integrated DNA Technologies, using the criteria provided in the Bio-Rad users' manual. Primer sequences, including the predicted sizes of amplified cDNA, are as follows: β-actin (103 bp), 5′-AACCCTAAGGCCAACCGTGAAA-3′ (forward) and 5′-AGGCATACAGGGACAACACA-3′ (reverse); MMP-1 (133 bp), 5′-TTGCTTCTCTTGGCTACCAGCTCA- 3′ (forward) and 5′-TAGCTTGGACGTCTTCACCCAAGT-3′ (reverse); TIMP-1 (124 bp), 5′-AGCTTCCTGGTTCCCTGGCATAAT-3′ (forward) and 5′-CAGTTTGCAAGGGATGGCTGAACA-3′ (reverse); MMP-8 (101 bp), 5′-CGTGGCTGCTCATGAATTTGGACA-3′ (forward) and 5′-AGGTGCTGGGTTCTCTGTAAGCAT-3′ (reverse); MMP-13 (82 bp), 5′-TCTTTGGCTTAGAGGTGACTGGCA-3′ (forward) and 5′- ACATCAGGCACTCCACATCTTGGT-3′ (reverse). Pilot quantitative real-time RT-PCR experiments were performed to determine the optimal condition for each primer. All quantitative real-time RT-PCR experiments were performed in duplicate. The primer specificity of the amplification product was confirmed by melting curve analysis of the reaction products using SYBR Green as well as by visualization on ethidium bromide-stained agarose (1.5%) gels. The housekeeping gene β-actin was used as an internal control, and gene-specific mRNA expression was normalized against β-actin expression. iQTM5 optical system software version 2.0 (Bio-Rad) was used to analyze quantitative real-time RT-PCR data and derive threshold cycle (CT) values according to the manufacturer's instructions. The DDCT method was used to transform CT values into relative quantities with S.D. values. The same software was used to calculate the normalized expression of the gene of interest, using β-actin as a reference gene, and the results were expressed as normalized -fold expression.
Cleavage of Rat Type I Collagen by MMP-1
Human MMP-1 (Millipore, Billerica, CA) was activated with trypsin for 10 min at 25 °C, and the trypsin was inactivated with a 10-fold excess of soybean trypsin inhibitor. Rat type I collagen was incubated with activated human MMP-1 for 26 h at 25 °C, and the reaction was stopped by the addition of EDTA to a final concentration of 50 mm. Digestion products were resolved on 12% SDS-PAGE, and the gel was stained with Coomassie Blue to assess the cleavage of rat type I collagen by activated MMP-1.
Statistical Analysis
The data are expressed as the mean ± S.D. Data were analyzed and compared using the Student's t test, and differences were considered statistically significant with p < 0.05.
RESULTS
Induction of EAAU by rat MAA (CI-α2 (22 kDa))
In this study, the α2 chain of type I collagen was purified from the iris and the CB harvested from the eyes of naive Lewis rats. Purified α2 chain of type I collagen was then digested with V8 protease, and the 22-kDa fragment of type I collagen α2 chain (CI-α2 (22 kDa) MAA) was purified as previously described by us (8). The NH2-terminal composition of the purified rat antigen is given in Table 1. Amino acid sequence alignment revealed 97.3% homology with the 22-kDa fragment of type I collagen α2 chain isolated from bovine iris and CB (Table 1). Further analysis of the primary structure of rat type I collagen α2 chain (Protein Data Bank code 1y0f) demonstrated the localization of MAA in rat type I collagen α2 chain (Fig. 1A). This structural analysis was performed using the UCSF Chimera software for molecular modeling (15).
TABLE 1.
Comparison of amino terminal amino acid sequences of rat and bovine MAA (CI-α2 (22 kDa))
Standard one letter amino acid symbols are used, and the difference in amino acid residue is indicated by boldface type.
| Protein | Amino acid number | Amino acid sequence |
|---|---|---|
| Rat MAA | 1 | IGPAGPPGPPGLRGNPGSRGLPGADGVAGVMGPAGSR |
| Bovine MAA | 1 | IGPAGPPGPPGLRGNPGSRGLPGADGRAGVMGPAGSR |
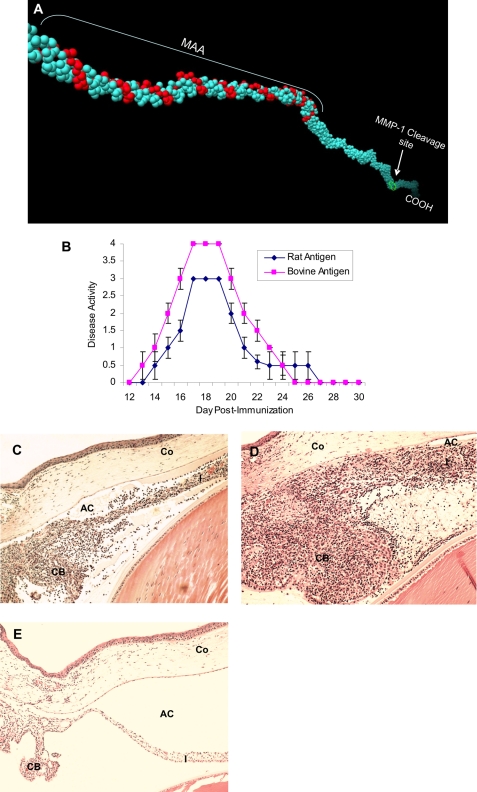
FIGURE 1.
Rat MAA (CI-α2 (22 kDa)) and EAAU. A, localization of MAA (CI-α2 (22 kDa)) and MMP-1 cleavage site on rat type I collagen α2 chain (Protein Data Bank code 1y0f) using UCSF Chimera software for molecular modeling. B, clinical course of EAAU induced by rat and bovine antigen. Data are presented as mean disease activity ± S.D. Histopathologic changes in the eyes of Lewis rats immunized with rat (C) and bovine (D) antigen. Note the heavy infiltration of inflammatory cells within the iris (I), the ciliary body (CB), and the anterior chamber (AC) at the peak of EAAU (day 19 postimmunization); however, cornea (Co) was not affected. E, EAAU did not develop in animals injected with adjuvant alone. Objective magnification was ×10.
Next we examined the ability of rat CI-α2 (22 kDa) to induce EAAU in Lewis rats. Animals were divided into two groups, and animals in group 1 (n = 20 animals) were immunized with bovine antigen (22-kDa protein), whereas animals in group 2 (n = 10 animals) were immunized with 22-kDa rat antigen. In agreement with our previous report (8), immunization with pure bovine antigen (22-kDa protein) induced severe uveitis in both eyes of all 20 Lewis rats in group 1 (Table 2 and Fig. 1, B and D). Interestingly, the disease induced in Lewis rats by rat antigen was similar to the EAAU induced in Lewis rats by bovine antigen (Table 2). All 10 animals immunized with pure 22-kDa protein isolated from rat iris and CB developed EAAU in both eyes (Table 2 and Fig. 1, B and C). However, the clinical and histopathologic examination revealed that the pathogenic antigen isolated from rat iris and CB (Fig. 1, B and C) was less uveitogenic than bovine antigen (Fig. 1, B–D). The cornea (Fig. 1, C and D), retina, and choroid (data not shown) were not affected in these animals. EAAU did not develop in control animals injected with adjuvant alone (Table 2 and Fig. 1E). Intact α2 chain of type I collagen purified from rat iris and ciliary body (emulsified in complete Freund's adjuvant) did not induce EAAU (Table 2). None of the animals immunized with purified rat or bovine MAA (CI-α2 (22 kDa)) manifested clinical signs of arthritis (data not shown).
TABLE 2.
Pathogenicity of MAA (CI-α2 (22kDa)) isolated from rat iris and ciliary body
| Antigen | Antigen dose | Adjuvant | EAAUa |
Day of onsetb | ||
|---|---|---|---|---|---|---|
| Incidence | Moderate | Severe | ||||
| μg | ||||||
| Rat MAA (CI-α2 (22 kDa)) | 50 | CFAc | 20/20 | 20/20 | 0 | 14 ± 0.5d |
| Bovine MAA (CI-α2 (22 kDa)) | 50 | CFA | 40/40 | 0 | 40/40 | 13 ± 1.0d |
| 0 | CFA | 0/20 | 0 | 0 | ||
| Intact α2 chain (rat iris/CB) | 50 | CFA | 0/20 | 0 | 0 | |
a The incidence of EAAU is given as positive/total eyes following clinical examination.
b Mean ± S.D. Severity of inflammation upon histopathologic examination was grouped as mild (1+), moderate (2+ to 3+), or severe (4+).
c CFA, complete Freund's adjuvant.
dp < 0.05.
Adoptive Transfer of Rat MAA (CI-α2 (22 kDa))-induced EAAU
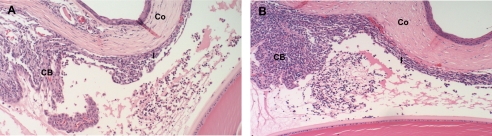
Adoptive transfer experiments with in vitro primed antigen-specific T cells were used to test the ability of rat MAA to transfer EAAU to naive syngenic rats. A single-cell suspension of popliteal LNCs harvested from Lewis rats that were immunized with rat antigen was cultured with the rat antigen for 3 days. In parallel, a single-cell suspension of popliteal LNCs harvested from Lewis rats immunized with bovine antigen was cultured with bovine antigen and served as the positive control. Our data show that 10 million T cells isolated from the draining lymph nodes of Lewis rats immunized with purified rat antigen transferred EAAU to naive syngenic rats (Table 3 and Fig. 2). The disease started at day 9 after cell transfer and remained active for 4 days. However, the disease activity of EAAU in these animals was less severe (Fig. 2A) than EAAU observed in the animals receiving the same number of T cells from donor rats immunized with bovine antigen (Fig. 2B), and these differences were statistically significant (Table 3). Thus, by adoptive transfer experiments, we demonstrated that EAAU induced by rat MAA is also a T cell-mediated autoimmune disease.
TABLE 3.
Adoptive transfer of EAAU induced by rat MAA (CI-α2 (22 kDa))
| Donor immunization | Cells transferred to recipients |
EAAU in recipientsa |
Day of onsetb | |||
|---|---|---|---|---|---|---|
| Stimulus in culture | Number of cells (×106) | Cell population | Incidence | Score | ||
| Rat MAA | Rat MAA | 10 | T cells | 18/18 | Moderate | 9 ± 0.5c |
| Bovine MAA | Bovine MAA | 10 | T cells | 18/18 | Severe | 7 ± 1.0c |
a The incidence of EAAU is given as positive/total eyes following clinical examination. Cells were transferred intravenously via the tail vein.
b Mean ± S.D. Severity of inflammation on histopathologic examination was grouped as mild (1+), moderate (2+ to 3+), or severe (4+).
cp < 0.05.
FIGURE 2.
Adoptive transfer of EAAU by rat MAA (CI-α2 (22 kDa)). EAAU was induced after adoptive transfer of rat MAA-primed T cells (A). Recipient animals were sacrificed at the peak of adoptive transfer EAAU. B, histopathologic changes in the eyes of recipient Lewis rats sacrificed at the peak of adoptive transfer EAAU induced by transfer of bovine MAA-primed T cells. I, iris; CB, ciliary body; CO, cornea. Objective magnification was ×10.
Immunogenecity of Rat MAA (CI-α2 (22 kDa))
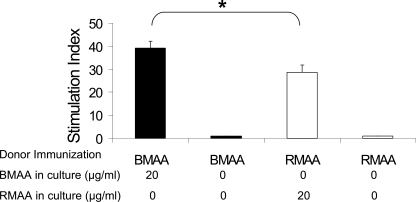
We have previously reported that EAAU induced by bovine MAA could not be adoptively transferred by immune sera (7). Furthermore, animals immunized with bovine MAA developed antibodies to this antigen, but there was no correlation between humoral immune response and disease activity in EAAU (8). Therefore, in the current study, cellular response to rat MAA was investigated using an in vitro lymphocyte proliferation assay. Popliteal lymph nodes were harvested from Lewis rats that were immunized with rat MAA or bovine MAA, and a single-cell suspension of LNCs was cultured separately for 72 h. LNCs from rat MAA-immunized animals were cultured in the presence or absence of rat MAA. Similarly, the LNCs from bovine MAA-immunized animals were cultured in the presence or absence of bovine MAA. Our data presented in Fig. 3 demonstrated that LNCs from rats challenged with rat antigen elicited a proliferative lymphocyte response (stimulation index = 28.6 ± 3.2). However, this proliferative response (Fig. 3) was significantly (p < 0.05) less than the proliferative response observed when LNCs harvested from Lewis rats immunized with bovine antigen were treated in vitro with the bovine antigen (stimulation index = 39.2 ± 2.9). LNCs from animals sensitized with rat MAA or bovine MAA failed to proliferate in vitro in the absence of the antigen (Fig. 3).
FIGURE 3.
Proliferation of lymph node cells in vitro. Data from three independent experiments are shown as a bar graph. Results are expressed as mean stimulation index ± S.D. BMAA, bovine CI-α2 (22 kDa); RMAA, rat CI-α2 (22 kDa). *, p < 0.05.
Localization of MAA (CI-α2 (22 kDa)) in the Rat Eye
Because in animals with EAAU the inflammation is observed only in the iris and CB of the eye (5–12), we next asked if MAA was only present within the iris and CB or if it is expressed in other parts of the eye also. Extraction of MAA from Lewis rat eye is time-consuming and cost-intensive. Five hundred rat versus 50 bovine eyes were required for similar recovery (∼1.0 mg) of the purified protein (MAA). Therefore, polyclonal antibodies used for MAA localization studies were raised against bovine antigen only.
Western Blot Analysis
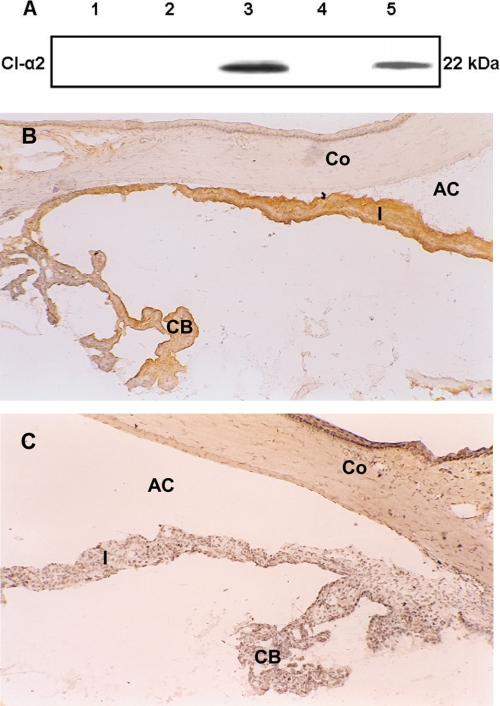
A representative immunoblot for MAA localization (Fig. 4A) demonstrates that polyclonal antibody against MAA specifically identified MAA in the iris and CB harvested from the eyes of naive Lewis rats as a single protein band with an apparent molecular mass of 22 kDa (lane 3). In contrast, MAA was not detected in the cornea (lane 1), retina (lane 2), and choroid and RPE (lane 4). Pure bovine MAA was used as the positive control (Fig. 4A, lane 5). Control blots stained with normal rabbit serum did not show any reactivity (data not shown).
FIGURE 4.
Localization of MAA [CI-α2 (22 kDa)) in the eye. A, Western blot analysis using polyclonal antibody against bovine MAA (CI-α2 (22 kDa)) to detect MAA in different rat ocular tissues. Samples were analyzed on 14% SDS-PAGE. Total protein (40 μg) from cornea (lane 1), retina (lane 2), iris and CB (lane 3), and RPE and choroid (lane 4) were used. Purified bovine MAA (CI-α2 (22 kDa)) loaded (5 μg) in lane 5 was used as the positive control. B and C, MAA (CI-α2 (22 kDa)) in the rat eye (frozen sections). Naive rat eye was examined immunohistochemically for MAA using polyclonal antibody against bovine MAA. B, MAA is constitutively expressed on the iris (I) and ciliary body (CB); MAA was not detected on the cornea (Co). No staining was observed in the control section stained with normal rabbit serum (C). The data shown are representative of three separate experiments. AC, anterior chamber. Objective magnification was ×10.
Immunohistochemical Analysis
Immunohistochemistry using frozen sections of the eye harvested from naive Lewis rats was performed to confirm that MAA (CI-α2 (22 kDa)) is present within the iris and CB of the rat eye. Staining was observed on the iris and CB when anti-MAA antibody was used (Fig. 4B). MAA was not detected on the cornea (Fig. 4B), lens, retina, RPE, and choroid (data not shown). No staining was detected in the sections stained with an equivalent dilution of normal rabbit serum (Fig. 4C). Taken together, these results established that MAA (CI-α2 (22 kDa)) was only present and constitutively expressed in the iris and CB of the eyes of naive Lewis rats.
Mechanism of MAA Generation in Vivo
To investigate how the ocular microenvironment possibly may mediate the generation and/or exposure of MAA in vivo, we analyzed the local expression of MMPs and TIMPs in the eyes of naive Lewis rats as well as in the eyes during EAAU.
MMP-1, MMP-8, and MMP-13 in the Eyes of Naive Lewis Rats
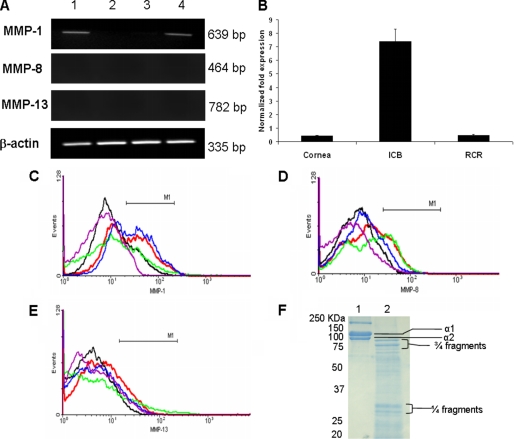
Because MAA is a 22-kDa fragment of type I collagen α2 chain, ocular expression of MMP-1 (collagenase 1), MMP-8 (collagenase 2), and MMP-13 (collagenase 3) was investigated at the mRNA level using semiquantitative RT-PCR and quantitative real-time RT-PCR and at the protein level by flow cytometry. RT-PCR analysis detected a band of 639 bp, representing MMP-1 transcripts in the iris and CB (Fig. 5A, lane 1) and in the whole rat eye (Fig. 5A, lane 4). MMP-1 mRNA was barely detected in the cornea (Fig. 5A, lane 2) and RPE-retina-choroid (Fig. 5A, lane 3) of the naive rat eye. The quantitative real-time RT-PCR analysis further confirmed the higher expression of MMP-1 mRNA in iris and CB of naive rat eye compared with extremely low levels of MMP-1 in cornea and RPE-retina-choroid (Fig. 5B). We were not able to detect MMP-8 and MMP-13 transcripts in these samples by quantitative real-time RT-PCR. A similar pattern was observed for MMP-1 protein (present intracellularly), as determined by flow cytometric analysis (Fig. 5C). Interestingly, MMP-8 and MMP-13 transcripts (Fig. 5A) and protein (Fig. 5, D and E) could not be detected in these samples. Taken together, our results established that MMP-1 is constitutively expressed in the iris and CB of the naive rat eye.
FIGURE 5.
Distribution of MMP-1, MMP-8, and MMP-13 in the normal rat eye. A, representative RT-PCR (using 25 cycles) profile of MMP-1, MMP-8, and MMP-13 mRNA in the iris and the CB (lane 1), cornea (lane 2), RPE-choroid-retina (lane 3), and intraocular tissue (lane 4). A single band of 335 bp for β-actin demonstrated an equal amount of PCR product in each lane. MMP-1 transcripts (639 bp) were detected in the iris and the CB (lane 1) and intraocular tissue (lane 4). MMP-1 mRNA was barely detected in cornea (lane 2) and RPE-choroid-retina (lane 3). In contrast, MMP-8 and MMP-13 mRNA were not detected in these samples. B, representative quantitative real-time RT-PCR profile of MMP-1 mRNA in the cornea, iris-ciliary body (ICB), and RPE-choroid-retina (RCR). MMP-1 was barely detected in cornea and RPE-choroid-retina (RCR). C, flow cytometric analysis showing MMP-1+-positive cells in the whole eye (blue) and in the iris and the CB (red). Few MMP-1+ cells were detected in the cornea (green) and RPE-choroid-retina (black). MMP-8+ (D) and MMP-13+ (E) cells were not detected in these samples. Purple color in C–E, negative control (without primary antibody). The data shown are representative of three different experiments. F, Coomassie-stained SDS-PAGE (12%) showing the cleavage of rat type I collagen by activated MMP-1. Treatment of rat type I collagen with MMP-1 in vitro generated several collagen peptides of different sizes (lane 2). Type I collagen incubated with the reaction mixture but without MMP-1 was loaded in lane 1.
Since the MMP-1 cleavage site is present on the α2 chain of rat type I collagen (Fig. 1A), we investigated if rat type I collagen can be cleaved by activated MMP-1 in vitro. Our data presented in Fig. 5F demonstrate that MMP-1 cleaved α1 and α2 chains of rat type I collagen efficiently into classical three-quarters and one-quarter fragments along with several other collagen peptides of different sizes (lane 2). It is of interest to note that some of these collagen peptides were present between 20 and 25 kDa (Fig. 5F, lane 2) and that the molecular mass of MAA is 22 kDa. No cleavage of α1 and α2 chains of rat type I collagen was observed in the absence of activated MMP-1 (Fig. 5F, lane 1).
Ocular Expression of MMP-1 during EAAU
Because only MMP-1 could be detected in the iris and CB of the normal rat eye, we investigated the expression profile of MMP-1 in the eyes of Lewis rats during the course of EAAU. Lewis rats immunized with bovine MAA were sacrificed at days 11, 14, 19, 23, and 30 postimmunization, and the expression of MMP-1 transcripts and protein was assessed by semiquantitative RT-PCR and flow cytometric analysis, respectively.
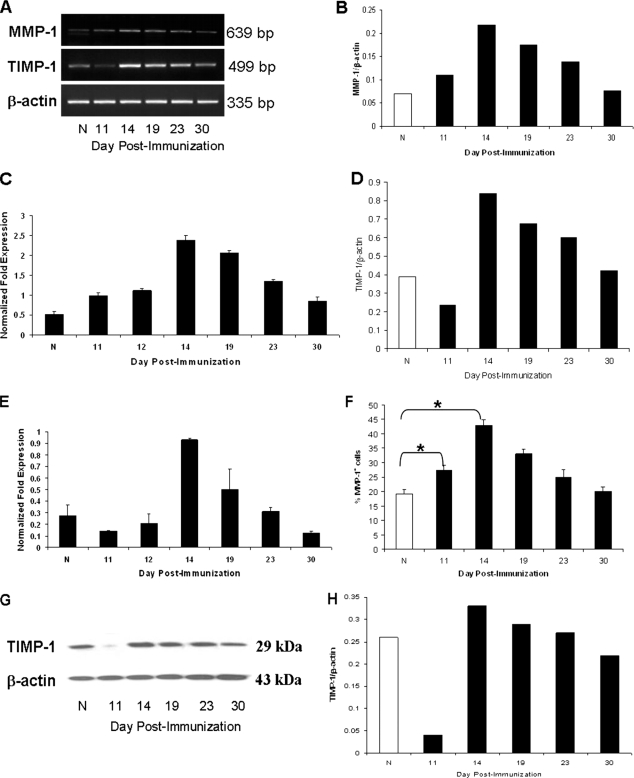
In MAA-sensitized animals, a substantial increase in MMP-1 mRNA was observed at day 11 postimmunization (before the onset of EAAU) compared with naive animals (Fig. 6, A and B). MMP-1 transcripts were further up-regulated at day 14 (the onset of EAAU) but declined at day 19 (the peak of EAAU). As shown in Fig. 6, A and B, the expression of MMP-1 transcripts further decreased during the resolution (day 23) and after the resolution of EAAU (day 30). These results were further confirmed by including day 12 postimmunization as an additional day and using quantitative real-time RT-PCR. Similar to day 11, low levels of MMP-1 transcripts were detected at day 12 also (Fig. 6C). The pattern of MMP-1 mRNA expression during EAAU detected by quantitative real-time RT-PCR (Fig. 6C) was similar to that observed with semiquantitative RT-PCR (Fig. 6, A and B).
FIGURE 6.
Expression of MMP-1 and TIMP-1 during EAAU. A, RT-PCR products for MMP-1 (639 bp) and TIMP1 (499 bp) in the rat eye at different time points during EAAU. A strong band at 335 bp for β-actin indicated an equal amount of RNA in each lane. B, densitometric analysis of PCR products of MMP-1. The intensity of PCR products was quantitated using an image analyzer, and the relative intensity was expressed as the ratio of the intensity of MMP-1 transcripts to the intensity of β-actin transcripts. C, quantitative real-time RT-PCR profile of MMP-1 transcripts in the rat eye during EAAU. D, densitometric analysis of PCR products of TIMP-1. The intensity of PCR products was quantitated using an image analyzer, and the relative intensity was expressed as the ratio of the intensity of TIMP-1 transcripts to the intensity of β-actin transcripts. E, quantitative real-time RT-PCR profile of TIMP-1 during EAAU. F, flow cytometric analysis of MMP-1+ cells in the eyes of Lewis rats during the course of EAAU. The percentage of MMP-1+ cells during EAAU was determined by flow cytometry, and cumulative data are shown as a bar graph. Semiquantitative Western blot (G) and densitometric (H) analysis of TIMP-1 protein in MAA-sensitized animals is shown. The results of densitometric analysis are expressed as the ratio of the intensity of the TIMP-1 protein to the intensity of β-actin protein bands (H). N, naive animals. The data shown are representative of three separate experiments. *, p < 0.05.
A similar pattern for MMP-1+ cells in the eye was observed by flow cytometric analysis during the course of EAAU (Fig. 6F). MMP-1+ cells increased significantly on days 11 and 14 postimmunization in the eyes of rats with EAAU compared with naive rats (p < 0.05; Fig. 6F). The levels of MMP-1-expressing cells declined on days 19–30 (Fig. 6F).
Ocular Expression of TIMPs during EAAU
Compared with naive rats, a dramatic decrease in TIMP-1 mRNA (Fig. 6, A and D) and protein (Fig. 6, G and H) was observed in the eyes of Lewis rats immunized with bovine MAA before the onset of EAAU (day 11 postimmunization). Increased expression of TIMP-1 (both mRNA and protein) was observed on day 14 (the onset of EAAU), followed by decreased levels of TIMP-1 on days 19, 23, and 30 (Fig. 6, A, D, E, G, and H) in these animals. In contrast, both mRNA and protein levels of TIMP-2 and TIMP-3 did not change during the course of EAAU and were similar to those observed in the eyes of naive Lewis rats (data not shown). Since TIMP-1 mRNA expression decreased drastically at day 11, we confirmed these results by performing quantitative real-time RT-PCR for TIMP-1 transcripts at different time points, including an additional time point of day 12 postimmunization. Our results revealed that, similar to day 11, TIMP-1 mRNA expression was down-regulated at day 12 postimmunization also (Fig. 6E).
DISCUSSION
EAAU is an organ-specific inflammatory disease that affects the iris and the CB of the eye and serves as an animal model of human idiopathic anterior uveitis (4–12). In the present study, we have purified a 22-kDa fragment of type I collagen α2 chain (CI-α2 (22 kDa)) from the iris and CB of Lewis rats and have demonstrated that this protein can induce EAAU in Lewis rats. We have determined that the uveitogenic antigen is solely localized to the iris and the CB of the rat eye and have addressed a possible mechanism of autoantigen generation in vivo. Our previous work has shown that the pathogenic antigen in EAAU is a 22-kDa fragment of type I collagen α2 chain associated with bovine uveal melanin (8) and referred it to as MAA (5–12). Melanin pigment is synthesized within the melanocytes and deposited on a proteinaceous matrix structure, called melanosomes (16). Biologic melanin contains several matrix proteins, which are bound to the chromophoric backbone during polymer formation (16, 17). Although the Lewis rat is an amelanotic (i.e. no melanin is present) animal, the melanocytes with premelanosomes and matrix proteins are present within the eyes of this animal (18). Results from the present study demonstrated that the uveitogenic antigen purified from Lewis rat iris and CB is also a 22-kDa fragment of type I collagen α2 chain. Therefore, in order to be consistent with our previous publications (5–12), EAAU-causing antigen (CI-α2 (22 kDa)) purified from Lewis rat eye is also referred as MAA in the current paper.
Our results have shown that the clinical and histopathologic patterns of EAAU induced by rat MAA are similar to those induced by bovine MAA. However, the severity of disease with rat antigen was less than that observed with the bovine antigen when EAAU was induced by active immunization or by adoptive transfer of antigen-specific T cells. Furthermore, lymph node cells from rat MAA-sensitized Lewis rats demonstrated a proliferative response that was less than the proliferative response observed with lymph node cells harvested from bovine MAA-sensitized animals. These differences in the pathogenecity may arise from the differences in the primary structures (i.e. amino acids) as well as the glycosylation patterns of rat and bovine MAA. The present study revealed a 97.3% sequence homology at the amino acid level between the amino-terminal regions of purified rat and bovine MAA. Additionally, we have previously reported that bovine MAA (CI-α2 (22 kDa)) was pathogenic only if bound carbohydrates were intact and carbohydrate moieties bound to the antigen were crucial to the pathogenecity (8). Type I collagen contains covalently attached carbohydrate units; the nature and the number of carbohydrate units per collagen molecule varies among different species (19, 20). Previous studies using an EAU model have shown that heterologous antigen is more potent than the homologous antigen, with a higher level of T cell responsiveness to the heterologous antigens (21, 22).
The data presented in the current paper provide a direct correlation between the site of inflammation in EAAU and constitutive expression of MAA in Lewis rat eye. Results from our study demonstrated that the 22-kDa MAA was specifically present in the iris and CB of the naive Lewis rat eye. Since MAA is endogenously expressed only on the iris and CB of the rat eye, its recognition by T cells is most likely responsible for inflammation of these ocular tissues during EAAU.
Type I collagen is a major component of the anterior uveal tract (i.e. the iris and CB) (23). We have previously reported that intact α1 and α2 chains of bovine iris- and CB-derived type I collagen are not uveitogenic (8). Interestingly, our published data (8) and the results presented in this paper clearly demonstrate that Lewis rats developed EAAU only if the α2 chain of type I collagen (purified from bovine and rat iris and CB) underwent proteolysis. These observations suggest that the proteolytic degradation of the α2 chain led to the exposure of an antigenic epitope(s) that is necessary to induce uveitis in Lewis rats. To explore this possibility, we focused our attention on defining an ocular microenvironment in which MAA may be generated and/or exposed in vivo and hypothesized that tissue-specific MMPs may mediate the fragmentation of type I collagen present in the iris and CB of the eye. Generation of an autoantigen by MMPs has been reported in the literature (24, 25).
We specifically analyzed the expression of MMP-1 (collagenase 1), MMP-8 (collagenase 2), and MMP-13 (collagenase 3) in the naive rat eyes as well as in the eyes during EAAU. Our results clearly demonstrated that iris and CB were the major source of MMP-1 in the naive rat eye. It has been reported that in the human eye, MMP-1 is present in significantly higher amounts in iris and CB compared with other ocular tissues and may play an important role in the normal turnover of matrix proteins (26).
We were unable to detect MMP-8 and MMP-13 in the rat eye. Together, these results suggested that MMP-1 constitutively expressed in the iris and CB of the naive rat eye may participate in the generation and/or exposure of MAA, which may otherwise be masked in the triple helix of type I collagen. It is known that MMP-1 exhibits specificity for type I collagen. MMP-1 first unwinds the helical collagen molecule before preferentially binding to and cleaving the α2 chain of type I collagen (27). Importantly, our present and past (8) studies demonstrated that MAA is a 22-kDa fragment of type I collagen α2 chain, and molecular modeling using the UCSF Chimera software (15) revealed the presence of MMP-1 cleavage on the α2 chain of type I collagen. Additionally, in the present study, we observed that MMP-1 can cleave rat type I collagen in vitro, into classical three-quarters and one-quarter fragments along with several collagen peptides of different sizes, including collagen fragments between 20 and 25 kDa. These results are in agreement with those reported in the literature (28–30).
The presence of MMP-1 in the iris and CB may have pathologic significance in EAAU. Up-regulation of MMP-1 simultaneous with decreased expression of its natural inhibitor, TIMP-1, before the onset of EAAU as observed in our study will lead to accelerated generation of MAA in vivo. This may be needed for the propagation of the autoimmune response that drives EAAU. On the other hand, up-regulation of TIMP-1 at the onset of autoimmune uveitis may have a protective effect in EAAU because it will counteract the activity of up-regulated MMP-1 and prevent further generation of MAA. Increased levels of MMP-1 and TIMP-1 observed at the onset of EAAU may be in part due to tumor necrosis factor-α. Our published study (12) has shown that the levels of tumor necrosis factor-α increased at the onset of EAAU. Inflammatory cytokines, such as tumor necrosis factor-α, have been reported to augment the expression of MMPs and TIMPs (31, 32). Our results indicating that the levels of MMP-1 were up-regulated before the onset of EAAU when no inflammatory cells could be detected in the eye (5–12) and decreased moderately at the peak of EAAU when there is a massive infiltration of the inflammatory cells in the anterior segment of the eye (5–12) suggest that the expression of MMP-1 originated mainly from the iris and CB of the eye. However, invading inflammatory cells will also have some contribution toward MMP-1 expression observed during EAAU, because MMP-1 is expressed on a wide variety of cell types, including T cells (12).
In conclusion, we report three novel observations in this paper. First, EAAU can be induced by a fragment of rat type I collagen; second, the pathogenic antigen is present only in the iris and CB of the eye; third, the generation and/or exposure of MAA (CI-α2 (22 kDa)) by MMP-1 is a possible mechanism of autoantigen generation in vivo. Furthermore, our results suggest that regulation of MMP-1 by TIMP-1 may play an important role in the pathogenesis of autoimmune uveitis. A tight balance has been reported to exist between the levels of MMPs and TIMPs under normal conditions (13), and alteration of the balance between MMPs and TIMPs has been implicated in the pathogenesis of various diseases (33–35). Many autoantigens are not generated from rare molecules but from common and abundant proteins. In organ-specific autoimmune diseases, autoreactivity against ubiquitous antigen develops, but the disease is restricted to a particular organ, presumably due to greater accessibility and/or amount of the autoantigen in the target organ (36–38). Type I collagen is the most abundant protein in mammals (39).
Collectively, the findings reported in the current manuscript establish an etiologic role for MAA (CI-α2 (22 kDa)) in EAAU with broader implications to human idiopathic AU. It is possible that idiopathic human AU may also be the result of an autoimmune response to fragment(s) of type I collagen confined to the anterior segment of the eye. Human AU has been historically characterized as a collagen disease (40). Idiopathic human AU and its complications lead to permanent loss of vision, and due to unknown etiology, the disease can only be treated symptomatically. Precise identification of MAA (CI-α2 (22 kDa)) as the autoantigen and understanding of the possible mechanism by which uveitogenic antigen is generated in vivo are crucial to elucidate the etiopathogenesis of idiopathic AU and may lead to the development of effective and safe antigen specific therapies. Currently, nonspecific therapies, such as steroid and immunosuppressive agents, are used to treat patients with idiopathic AU, and these treatment modalities are associated with serious side effects (1–3).
Acknowledgments
We thank Ruslana G. Tytarenko for help with histologic studies and Andrea Harris for help with the use of the flow cytometer. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (15) (supported by National Institutes of Health Grant P41 RR-01081).
This work was supported, in whole or in part, by National Institutes of Health Grants EY016205 and EY 014623. This work was also supported by the Pat and Willard Walker Eye Research Center, the Jones Eye Institute and Arkansas Master Tobacco Settlement, and the Arkansas Biosciences Institute, University of Arkansas for Medical Sciences (Little Rock, AR).
- AU
- anterior uveitis
- CB
- ciliary body
- EAAU
- experimental autoimmune anterior uveitis
- MAA
- melanin-associated antigen
- MMP
- matrix metalloproteinase
- TIMP
- tissue inhibitor of metalloproteinases
- LNC
- lymph node cell
- RPE
- retinal pigment epithelium
- CI-α2 (22 kDa)
- 22-kDa fragment of type I collagen α2 chain
- RT-PCR
- reverse transcription-PCR.
REFERENCES
- 1.Gritz D. C., Wong I. G. (2004) Ophthalmology 111, 491–500 [DOI] [PubMed] [Google Scholar]
- 2.Bora N. S., Kaplan H. J. (2007) Chem. Immunol. Allergy 92, 213–220 [DOI] [PubMed] [Google Scholar]
- 3.Bloch-Michel E., Nussenblatt R. B. (1987) Am. J. Ophthalmol. 103, 234–235 [DOI] [PubMed] [Google Scholar]
- 4.Broekhuyse R. M., Kuhlmann E. D., Winkens H. J., Van Vugt A. H. (1991) Exp. Eye Res. 52, 465–474 [DOI] [PubMed] [Google Scholar]
- 5.Bora N. S., Kim M. C., Kabeer N. H., Simpson S. C., Tandhasetti M. T., Cirrito T. P., Kaplan A. D., Kaplan H. J. (1995) Invest. Ophthalmol. Vis. Sci. 36, 1056–1066 [PubMed] [Google Scholar]
- 6.Kim M. C., Kabeer N. H., Tandhasetti M. T., Kaplan H. J., Bora N. S. (1995) Curr. Eye Res. 14, 703–710 [DOI] [PubMed] [Google Scholar]
- 7.Bora N. S., Woon M. D., Tandhasetti M. T., Cirrito T. P., Kaplan H. J. (1997) Invest. Ophthalmol. Vis. Sci. 38, 2171–2175 [PubMed] [Google Scholar]
- 8.Bora N. S., Sohn J. H., Kang S. G., Cruz J. M., Nishihori H., Suk H. J., Wang Y., Kaplan H. J., Bora P. S. (2004) J. Immunol. 172, 7086–7094 [DOI] [PubMed] [Google Scholar]
- 9.Jha P., Sohn J. H., Xu Q., Nishihori H., Wang Y., Nishihori S., Manickam B., Kaplan H. J., Bora P. S., Bora N. S. (2006) Invest. Ophthalmol. Vis. Sci. 47, 1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jha P., Sohn J. H., Xu Q., Wang Y., Kaplan H. J., Bora P. S., Bora N. S. (2006) J. Immunol. 176, 7221–7231 [DOI] [PubMed] [Google Scholar]
- 11.Jha P., Matta B., Lyzogubov V., Tytarenko R., Bora P. S., Bora N. S. (2007) Invest. Ophthalmol. Vis. Sci. 48, 5091–5100 [DOI] [PubMed] [Google Scholar]
- 12.Matta B., Jha P., Bora P. S., Bora N. S. (2008) Am. J. Pathol. 173, 1440–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visse R., Nagase H. (2003) Circ. Res. 92, 827–839 [DOI] [PubMed] [Google Scholar]
- 14.Sohn J. H., Kaplan H. J., Suk H. J., Bora P. S., Bora N. S. (2000) Invest. Ophthalmol. Vis. Sci. 41, 3492–3502 [PMC free article] [PubMed] [Google Scholar]
- 15.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 16.Nordlund J. J., Abdel-Malek Z. A., Boissy R. E., Rheins L. A. (1989) J. Invest. Dermatol. 92, 53S–60S [DOI] [PubMed] [Google Scholar]
- 17.Chedekel M. R., Ahene A. B., Zeise L. (1992) Pigment Cell Res. 5, 240–246 [DOI] [PubMed] [Google Scholar]
- 18.Searle A. (1968) Comparative Genetics of Coat Color in Mammals, Logos Academic Press, London [Google Scholar]
- 19.Kivirikko K., Myllyla R., Pihlajaniemi T. (1992) in Hydroxylation of Proline and Lysine Residues in Collagens and Other Animal and Plant Proteins (Harding J. J., Crabbe M. J. C., eds) pp. 1–51, CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 20.Kielty C., Hopkinson I., Grant M. E. (1993) Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects (Royce P., Steinmann P. M. eds) pp. 103–147, Wiley-Liss, New York [Google Scholar]
- 21.Abe T., Satoh N., Nakajima A., Koizumi T., Tamada M., Sakuragi S. (1997) Exp. Eye Res. 65, 703–710 [DOI] [PubMed] [Google Scholar]
- 22.Fling S. P., Donoso L. A., Gregerson D. S. (1991) J. Immunol. 147, 483–489 [PubMed] [Google Scholar]
- 23.Marshall G. E., Konstas A. G., Lee W. R. (1993) Br. J. Ophthalmol. 77, 515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo A., Lee G. C., Terai K., Takami K., Hickey W. F., McGeer E. G., McGeer P. L. (1997) Am. J. Pathol. 150, 1253–1266 [PMC free article] [PubMed] [Google Scholar]
- 25.Van den Steen P. E., Proost P., Grillet B., Brand D. D., Kang A. H., Van Damme J., Opdenakker G. (2002) FASEB J. 16, 379–389 [DOI] [PubMed] [Google Scholar]
- 26.Gaton D. D., Sagara T., Lindsey J. D., Weinreb R. N. (1999) Invest. Ophthalmol. Vis. Sci. 40, 363–369 [PubMed] [Google Scholar]
- 27.Chung L., Dinakarpandian D., Yoshida N., Lauer-Fields J. L., Fields G. B., Visse R., Nagase H. (2004) EMBO J. 23, 3020–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krane S. M., Byrne M. H., Lemaître V., Henriet P., Jeffrey J. J., Witter J. P., Liu X., Wu H., Jaenisch R., Eeckhout Y. (1996) J. Biol. Chem. 271, 28509–28515 [DOI] [PubMed] [Google Scholar]
- 29.Fields G. B., Van Wart H. E., Birkedal-Hansen H. (1987) J. Biol. Chem. 262, 6221–6226 [PubMed] [Google Scholar]
- 30.Ottl J., Gabriel D., Murphy G., Knäuper V., Tominaga Y., Nagase H., Kröger M., Tschesche H., Bode W., Moroder L. (2000) Chem. Biol. 7, 119–132 [DOI] [PubMed] [Google Scholar]
- 31.MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. (1990) J. Biol. Chem. 265, 17238–17245 [PubMed] [Google Scholar]
- 32.Leonardi A., Cortivo R., Fregona I., Plebani M., Secchi A. G., Abatangelo G. (2003) Invest. Ophthalmol. Vis. Sci. 44, 183–189 [DOI] [PubMed] [Google Scholar]
- 33.Burger D., Rezzonico R., Li J. M., Modoux C., Pierce R. A., Welgus H. G., Dayer J. M. (1998) Arthritis Rheum. 41, 1748–1759 [DOI] [PubMed] [Google Scholar]
- 34.Akiyama K., Shikata K., Sugimoto H., Matsuda M., Shikata Y., Fujimoto N., Obata K., Matsui H., Makino H. (1997) Res. Commun. Mol. Pathol. Pharmacol. 95, 115–128 [PubMed] [Google Scholar]
- 35.Higashikata T., Yamagishi M., Higashi T., Nagata I., Iihara K., Miyamoto S., Ishibashi-Ueda H., Nagaya N., Iwase T., Tomoike H., Sakamoto A. (2006) Atherosclerosis 185, 165–172 [DOI] [PubMed] [Google Scholar]
- 36.Davidson A., Diamond B. (2001) N. Engl. J. Med. 345, 340–350 [DOI] [PubMed] [Google Scholar]
- 37.Wu C. T., Gershwin M. E., Davis P. A. (2005) Ann. N.Y. Acad. Sci. 1050, 134–145 [DOI] [PubMed] [Google Scholar]
- 38.Atassi M. Z., Casali P. (2008) Autoimmunity 41, 123–132 [DOI] [PubMed] [Google Scholar]
- 39.Prockop D. J., Kivirikko K. I. (1995) Annu. Rev. Biochem. 64, 403–434 [DOI] [PubMed] [Google Scholar]
- 40.Woods A. C. (1962) Uveitis Associated with Collagen Disease, Wilkins and Wilkins, Baltimore [Google Scholar]