Abstract
Studies of the organic anion transporters (Oats) have focused mainly on their interactions with organic anionic substrates. However, as suggested when Oat1 was originally identified as NKT (Lopez-Nieto, C. E., You, G., Bush, K. T., Barros, E. J., Beier, D. R., and Nigam, S. K. (1997) J. Biol. Chem. 272, 6471–6478), since the Oats share close homology with organic cation transporters (Octs), it is possible that Oats interact with cations as well. We now show that mouse Oat1 (mOat1) and mOat3 and, to a lesser degree, mOat6 bind a number of “prototypical” Oct substrates, including 1-methyl-4-phenylpyridinium. In addition to oocyte expression assays, we have tested binding of organic cations to Oat1 and Oat3 in ex vivo assays by analyzing interactions in kidney organ cultures deficient in Oat1 and Oat3. We also demonstrate that mOat3 transports organic cations such as 1-methyl-4-phenylpyridinium and cimetidine. A pharmacophore based on the binding affinities of the tested organic cations for Oat3 was generated. Using this pharmacophore, we screened a chemical library and were able to identify novel cationic compounds that bound to Oat1 and Oat3. These compounds bound Oat3 with an affinity higher than the highest affinity compounds in the original set of prototypical Oct substrates. Thus, whereas Oat1, Oat3, and Oat6 appear to function largely in organic anion transport, they also bind and transport some organic cations. These findings could be of clinical significance, since drugs and metabolites that under normal physiological conditions do not bind to the Oats may undergo changes in charge and become Oat substrates during pathologic conditions wherein significant variations in body fluid pH occur.
Introduction
The proximal tubule is the main site of reabsorption and elimination of a wide range of charged organic molecules, encompassing both xenobiotics and endogenous metabolites. The transport of these compounds across the basolateral or apical surfaces of the proximal tubular cell is mediated in large part by organic anion and cation transporters (Oats2 and Octs) (2). The prototypical Oat, Oat1, initially identified as novel kidney transporter NKT (1), and Oat3 form part of a rate-limiting tertiary transport system on the basolateral side of the kidney, where they couple organic anion entry with dicarboxylate exit, driven by concentration gradients produced by the sodium dicarboxylate cotransporter (NaDC3) and Na+/K+ ATPase (3, 4). Recently, a closely related transporter, Oat6, was identified in mouse olfactory mucosa and was shown to be involved in the transport of odorant organic anions (5, 6). Oct1 and -2, located on the basolateral side of the proximal tubule (7–9), mediate the bidirectional transport of cations by facilitative diffusion (10).
The Oats and Octs are closely related, both being members of the SLC22 family of solute transporters, and share close structural and sequence homology. Both Oats and Octs possess 12 transmembrane domains and a long hydrophilic extracellular loop between transmembrane domains 1 and 2. Based on this, when Oat1 was originally identified as NKT (1), it was suggested that the transporter might bind organic cations as well as anions. In studies over the past decade, the broad substrate specificity of Oats and Octs and the structural homologies of newer family members (11) have continued to raise the possibility that overlapping substrate specificity may be present and that the Oats can also interact with cations. However, despite a few early renal physiological reports on interaction of some cations with the probenecid-sensitive PAH transport system (12–15), molecular studies until now have focused on the binding and transport of organic anions (mainly pharmaceutical drugs) by the Oats.
We initially examined the kinetics of binding of organic cations to Oats by assessing inhibition of transport in Xenopus oocyte expression systems. We noted that a number of cationic compounds manifested significant binding affinity for Oat1 and -3 and, to a lesser degree, for Oat6 in the oocyte binding assays. To determine whether this was also true at the level of whole tissues, we examined the interaction of cationic compounds with transport of fluorescent organic anion substrates in cultures of kidney organ derived from Oat knock-out mice (16, 17). Organ culture represents a living organ system where the Oat is in interaction with the cellular membrane and other transporters. Results from the organ culture inhibition assay were consistent with those from the oocyte assays. We also used radiolabeled organic cations to directly measure transport by Oat1, -3, and -6 and found that cimetidine and 1-methyl-4-phenylpyridinium (1-MPP) were transported by Oat3. Using the binding affinity data generated from these assays, we were able to generate a pharmacophore common to both Oat1 and -3. The pharmacophore consists of a positive ionizable feature, a hydrophobic region and two hydrogen bond acceptors. This pharmacophore was then used to screen a chemical library of cationic compounds, and novel cationic compounds that interacted with Oat1 and 3 were verified in vitro. Thus, our findings demonstrate novel binding interactions between organic cations and Oats using in vitro and ex vivo assays, which were further validated by pharmacophore modeling. Because pathological conditions can result in altered body fluid pH (and thus in altered ionization of charged drugs, toxins, and metabolites) and because of the importance of Oats in regulating the distribution of organic ions between blood, cerebrospinal fluid, and urine, these findings could well be particularly important in disease states, such as metabolic alkalosis, diabetic ketoacidosis, and lactic acidosis.
EXPERIMENTAL PROCEDURES
Radiolabeled substrates [3H]verapamil (specific activity 80 Ci/mmol), [3H]nicotine (specific activity 85 Ci/mmol), [3H]cimetidine (specific activity 20 Ci/mmol), [3H]quinidine (specific activity 20 Ci/mmol), [3H]procainamide (specific activity 55 mCi/mmol), and [3H]1-methyl-4-phenylpyridinium (specific activity 85 Ci/mmol) were obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO). The radiolabeled tracers [3H]p-aminohippurate (PAH) (specific activity 4.22 Ci/mmol) and [3H]estrone 3-sulfate (57.3 Ci/mmol) were purchased from PerkinElmer Life Sciences. Cations and fluorescent dyes (5-carboxyfluorescein (5CF), 6-carboxyfluorescein (6CF), and fluorescein) were obtained from Sigma, and matches to the pharmacophore were ordered from Maybridge (Cornwall, UK).
Oocyte Isolation/Microinjection
Xenopus oocyte assays were performed as previously described (5, 18). Briefly, using the mMessage mMachine in vitro transcription kit (Ambion, Austin, TX), capped RNA was synthesized from linearized plasmid DNA for mOat1 (Image clone ID 4163278), mOat3 (Image clone ID 4239544), and mOat6 (Image clone ID 6309674). Oocytes were defolliculated and maintained in Barth's buffer growth medium. Stage IV-V oocytes were selected and injected with cRNA solution (1.0 μg/μl, 23 nl/oocyte) using the Nanoliter 2000 nanoinjector (World Precision Instruments, Sarasota, FL). Transport assays were performed 2 days after injection.
Binding and Transport Assays
Oocytes were divided into experimental groups of 16–20 and placed in a 24-well plate with 1 ml of Barth's buffer containing a fluorescent anion tracer with a final concentration of 30 μm 6-carboxyfluorescein for mOat1-injected oocytes, 50 μm 5CF (mOat3), or 30 μm fluorescein (mOat6), as well as various concentrations of unlabeled organic cations. After 1 h of incubation at 25 °C, the oocytes were washed five times at 4 °C with Barth's buffer, and each experimental group was divided into four groups of 4–5 oocytes. The oocytes were lysed, the lysate was centrifuged, and the fluorescence of the supernatant was measured by a fluorometer (PolarStar plate reader, BMG Labtechnologies, Durham, NC). To correct for transport due to diffusion or to endogenously expressed transporters in the oocytes, the uptake of fluorescent tracer was measured in uninjected oocytes, and this value was subtracted from the uptake into Oat-injected oocytes in all experimental samples.
Transport assays using radiolabeled cations were performed in a similar manner. Two days after injection with cRNA, oocytes were incubated in 1 ml of Barth's buffer containing ∼1 μCi of the cation being assayed. For experiments measuring affinity of the cations for mOat3, the oocytes were incubated in 1 ml of Barth's buffer, together with 1 μCi of the cation being tested and different concentrations of unlabeled cation. After 1 h of incubation at 25 °C, the oocytes were washed five times with ice-cold Barth's buffer and subsequently lysed. Radioactivity was measured by scintillation counting (Rackbeta, Beckman-Coulter, Fullerton, CA).
Calculations and Statistics
Ki of an inhibitor cation was determined by measuring tracer uptake in the presence of 3–4 inhibitor concentrations in 10-fold increments. Each data point is the average of four measurements. Inhibition data were curve-fitted using nonlinear regression in Prism software 5.0 (GraphPad Inc., San Diego, CA) to calculate the Ki ± S.E. as before (16). Km of cations was determined by measuring uptake in the presence of 3–4 cation concentrations, followed by curve-fitting using nonlinear regression in Prism software, version 5.0. Vmax, the maximum transporter mediated uptake rate, was calculated as Vmax = CL × (S + Km), where S was the substrate concentration of the radiolabeled tracer in the medium, and clearance (CL; the amount of radiolabeled substrate cleared from the incubation medium) was calculated as Vtransport/S. S.E. for Vmax was measured as S.E.Vmax = (S.E.CL2 × (S + K)2 + S.E.K2 × CL2)½.
Kidney Organ Culture
Oat1 and Oat3 knock-out kidney organ cultures were preformed as described previously (18, 19). Briefly, embryonic day 13.5 murine kidneys were grown on polycarbonate transwell filters with Dulbecco's modified Eagle's medium/F-12 (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Mediatech) and 1% penicillin/streptomycin (Invitrogen) at 37 °C for 5 days. All animal protocols conformed to National Institutes of Health guidelines for acceptable animal use.
Kidney Organ Culture Uptake Assay and Confocal Microscopy
After 5 days of culture, the cultured kidneys were washed once in phosphate-buffered saline and then incubated (on the filter) for 1 h at 25 °C in phosphate-buffered saline (containing 0.1 mm CaCl2 and 1 mm MgCl2) with 1 μm 6CF and with or without tracer uptake inhibitors (2 mm PAH, estrone 3-sulfate, or organic cations) (17). After the incubation, organ cultures were washed three times in 4 °C phosphate-buffered saline, cut from the transwell filter, and mounted on a glass slide with Fluormount (Southern Biotech, Birmingham, AL). Samples were kept on ice until imaged. Confocal images were taken with the Nikon D-eclipse C1 confocal microscope (A.G. Heinze, Lake Forest, CA). Images were taken using the same settings for intensity comparisons.
Quantitative Image Analysis
Image analysis was performed with ImageJ software (National Institutes of Health, Bethesda, MD) using pixel intensity measurements. Images were manually outlined twice to measure intensity, and the average value obtained was taken as the total intensity of a kidney. Four representative background areas were traced in interstitial spaces. Average background measurements were then subtracted from the total intensity. Significance values were determined using the t test, and all values represent triplicate kidney samples.
Computational Modeling
The consensus pharmacophore model was constructed on the basis of the cationic compounds that had high binding affinity for Oat3 (cimetidine, procainamide, metoclopramide, and verapamil), using the program Catalyst (Accelrys, 2007). The following steps were conducted according to the HipHop Catalyst strategy: (a) generation of conformational models for each of the compounds, (b) estimation of chemical features within each of the compounds, and (c) eventual creation of the closest superimposition of geometrical position of common features in three-dimensional space for the best fitting conformation of the compounds (20, 21). Conformations of the compounds were generated by the program within the limits of the energy differences from the initial conformation. It is known that in many cases, the conformation of a bound compound is not a global minimum conformation. We used the limit of 10 kcal/mol. The program superimposes up to 10 features (including hydrogen bond donor, hydrogen bond acceptor, hydrophobic, positive and negative ionizable features, etc.) for a set of compounds. These features are then included in the common feature hypotheses (pharmacophores). These hypotheses are tested by submitting a set of the structures of the compounds that have different activities to the program. Their fit to the hypothesis (pharmacophore) should correspond to their activities (i.e. compounds with better fits to the pharmacophore have higher activities, and those with worse fits have lower activities). Using such criteria, we selected the best hypothesis (pharmacophore), which was then used to screen a chemical data base from Maybridge. Compounds with a good fit were tested for binding to mOat1 and mOat3.
RESULTS
Interaction of Organic Cations with mOat1, mOat3, and mOat6
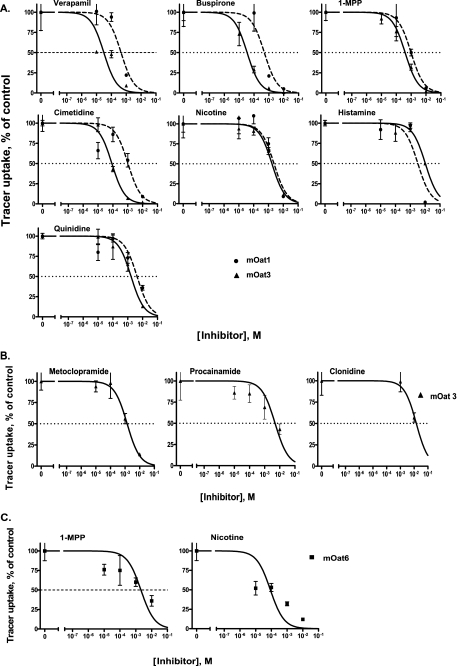
A set of 14 organic cations that had been previously demonstrated to interact with the Octs (22) and that were considered to be prototypical Oct substrates were selected to assess their interactions with mOat1, mOat3, and mOat6; these were tetraethylammonium, 1-MPP, buspirone, cimetidine, nicotine, histamine, quinidine, verapamil, metoclopramide, procainamide, clonidine, guanidine, metformin, and dopamine. The inhibition of fluorescent tracer uptake by the cations was used to determine Ki (affinity) values. It has previously been shown that, of several fluorescent tracers tested, 6CF, 5CF, and fluorescein had the highest Vmax values for mOat1, mOat3, and mOat6, respectively (17); they were therefore suitable tracers for these transporters. Based on these findings, we used 6CF, 5CF, and fluorescein as probes to determine the affinities of the aforementioned cations for mOat1, mOat3, and mOat6, respectively. Seven cations demonstrated binding to mOat1, whereas 10 of the 14 showed binding to mOat3 (Table 1). The seven cations that interacted with mOat1 were a subset of those that interacted with mOat3. Of these, although histamine manifested greater affinity for mOat1 than for mOat3, buspirone, 1-MPP, cimetidine, nicotine, quinidine, and verapamil manifested greater affinity for mOat3 than for mOat1 (Fig. 1A).
TABLE 1.
Binding affinities of the tested organic cations and selected organic anions for mOat1, mOat3, and mOat6
|
Ki (mean ± S.E.) |
|||
|---|---|---|---|
| mOat1 | mOat3 | mOat6 | |
| μm | |||
| Organic cation | |||
| Verapamil | 447 ± 116 | 31 ± 15 | NAa |
| Buspirone | 494 ± 195 | 34 ± 4 | NA |
| 1-MPP | 990 ± 67 | 391 ± 50 | 2,096 ± 967 |
| Cimetidine | 1,038 ± 445 | 85 ± 11 | NA |
| Nicotine | 2,365 ± 646 | 1,713 ± 219 | 86 ± 42 |
| Histamine | 3,214 ± 1,424 | 10,230 ± 3,776 | NA |
| Quinidine | 4,251 ± 1,382 | 1,675 ± 214 | NA |
| Metoclopramide | NA | 1,376 ± 150 | NA |
| Procainamide | NA | 4,873 ± 1,736 | NA |
| Clonidine | NA | 14,360 ± 7,694 | NA |
| Organic anion | |||
| p-Aminohippurate | 8 ± 2b | 92 ± 14c | 446 ± 98d |
| Estrone 3-sulfate | 203 ± 10d | 8 ± 2b | 58 ± 10d |
| Methotrexate | 901 ± 108d | 303 ± 142c | 597 ± 79d |
| N-Acetylaspartate | 840 ± 260d | NTe | 27,800 ± 9,900d |
| Cidofovir | 25 ± 7f | NA | NA |
| Fluorescein | 3 ± 0.1f | 35 ± 2f | 93 ± 6f |
| 5-Carboxyfluorescein | 523 ± 80f | 373 ± 28f | 3581 ± 582f |
| 6-Carboxyfluorescein | 57 ± 3f | 11 ± 0.1f | 3162 ± 241f |
FIGURE 1.
Interaction of organic cations with mOat1, mOat3, and mOat6. A, seven organic cations inhibited uptake of fluorescent tracers (6CF and 5CF; see “Experimental Procedures”) in both mOat1 and mOat3-expressing Xenopus oocytes. Each data point represents the mean ± S.E. values of four groups of 4–5 oocytes each. B, three cations that did not inhibit Oat1 inhibited 5CF uptake in Oat3. C, only two cations inhibited fluorescein uptake in Oat6-expressing oocytes.
Three cations, metoclopramide, procainamide, and clonidine, showed binding to mOat3 but not to mOat1 (Fig. 1B). Only two cations, 1-MPP and nicotine, demonstrated binding to mOat6 (Fig. 1C). Of these, 1-MPP manifested lower affinity for mOat6 (Ki = 2,096 ± 967 μm) than for mOat1 (Ki = 990 ± 67 μm) and mOat3 (Ki = 391 ± 50 μm), whereas nicotine showed higher affinity for mOat6 (Ki = 86 ± 42 μm).
The Ki values of some standard anions previously measured in our laboratory were included in Table 1 for comparison with the Ki values of the cations tested. Although the Ki values of the cations were found to be in a similar range as that of the standard anions, these concentrations are higher than are typically found in vivo (23), and therefore the interactions of these compounds with the Oats in vivo remains uncertain.
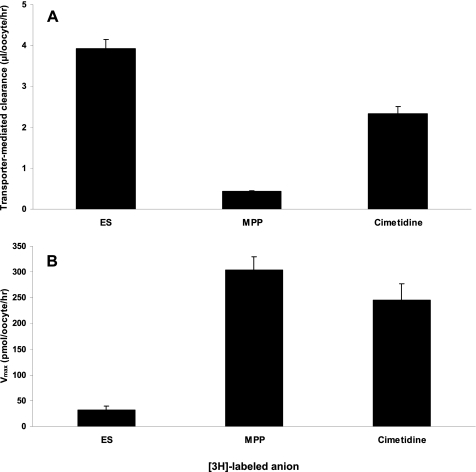
Transport via mOat1, mOat3, and mOat6 was directly assessed by measuring uptake of those compounds for which radiolabeled versions were commercially available: [3H]nicotine, [3H]cimetidine, [3H]procainamide, [3H]verapamil, [3H]quinidine, and [3H]1-MPP. Of these, cimetidine and 1-MPP were transported by mOat3 (Fig. 2), whereas significant transport by mOat1 or mOat6 was not detected. We proceeded to measure the affinities (Km) of these two cations for mOat3 and found the Km values to be 105 ± 28 and 693 ± 208 μm for cimetidine and 1-MPP, respectively. These values were similar to the inhibition constants (Ki) obtained for these compounds (Table 1).
FIGURE 2.
Transport of organic cations by mOat3. A, transporter-mediated clearance of 3H-labeled estrone 3-sulfate (ES), cimetidine, and 1-MPP was calculated as the clearance in injected oocytes minus the clearance of uninjected oocytes. The measurements were performed in a single experiment using the same group of oocytes. Estrone 3-sulfate, the prototypical substrate for Oat3, was used as a positive control. B, Vmax, the maximum uptake rate, of the 3H-labeled compounds was calculated from the clearance (see “Experimental Procedures”).
Computational Modeling
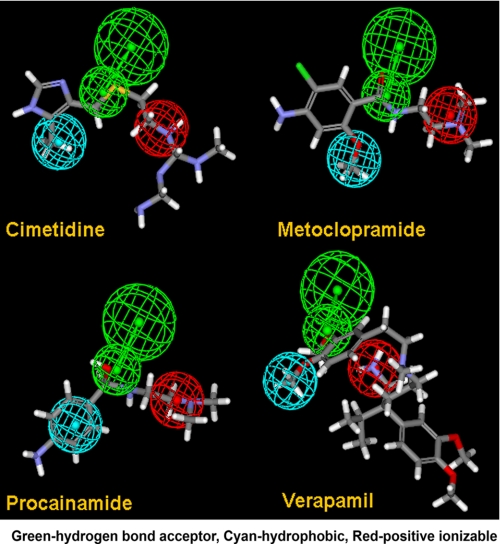
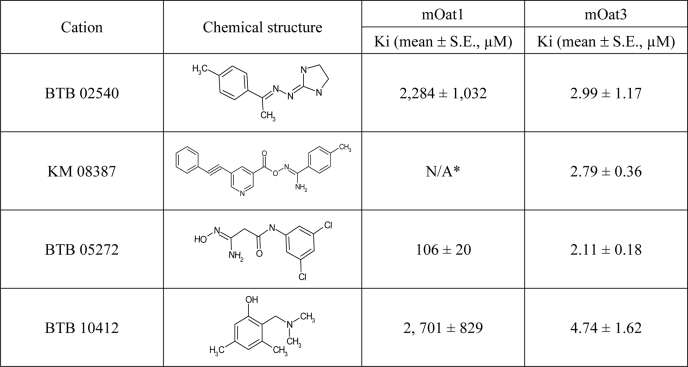
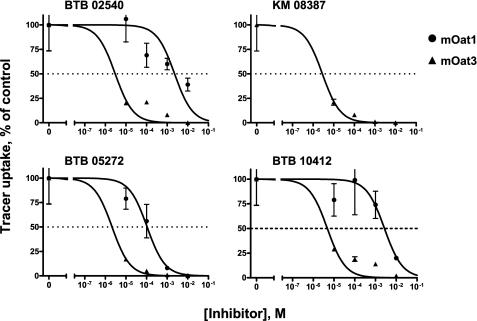
Analysis of the structures of the tested compounds led to the derivation of a four-feature pharmacophore comprising two hydrogen bond acceptors, a hydrophobic feature and a positive ionizable feature (Fig. 3), which was used to screen a structural data base (see “Experimental Procedures”). Seven cationic compounds with a good fit to the pharmacophore were tested for binding to mOat1 and mOat3. Four compounds were found to bind to mOat3, and three were found to bind to mOat1 (Table 2 and Fig. 4). Consistent with the data for the 14 prototypical organic cations, the affinities were highest for the compounds that bound Oat3; in fact, they were better than the best in the original cation set by an order of magnitude (e.g. compare Ki for verapamil of 31 μm in Table 1 with Ki of BTB 05272 of 2 μm in Table 2). These findings support the notion that the pharmacophore features make important contributions to organic cation binding to the Oats.
FIGURE 3.
Oat3 pharmacophore. A four-feature pharmacophore model of the interaction of organic cations with Oat3 is shown superimposed on the structures of four of the tested compounds. Pharmacophore features are color-coded as follows. Red, positive ionizable; green, hydrogen bond acceptor; cyan, hydrophobic. Compounds are color-coded as follows. Red, oxygen; blue, nitrogen; gray, carbon; white, hydrogen.
TABLE 2.
Binding affinities of cations from the Maybridge library for mOat1 and mOat3
* Did not show affinity for Oat1.
FIGURE 4.
Compounds matching the pharmacophore interact with Oat1 and Oat3. Four cationic compounds that were identified from the Maybridge chemical library as pharmacophore matches strongly inhibited Oat3-mediated transport in Xenopus oocyte assays, whereas three of these compounds inhibited Oat1-mediated transport. Oat3 showed a higher affinity for these compounds than did Oat1.
Cation Interaction with Kidney Organ Cultures
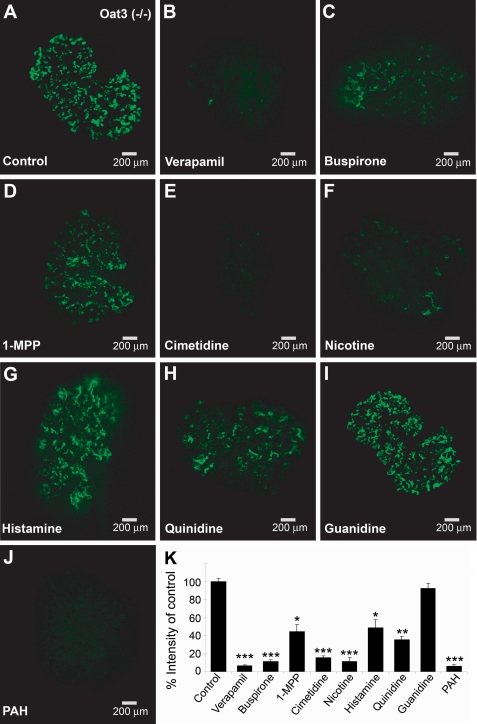
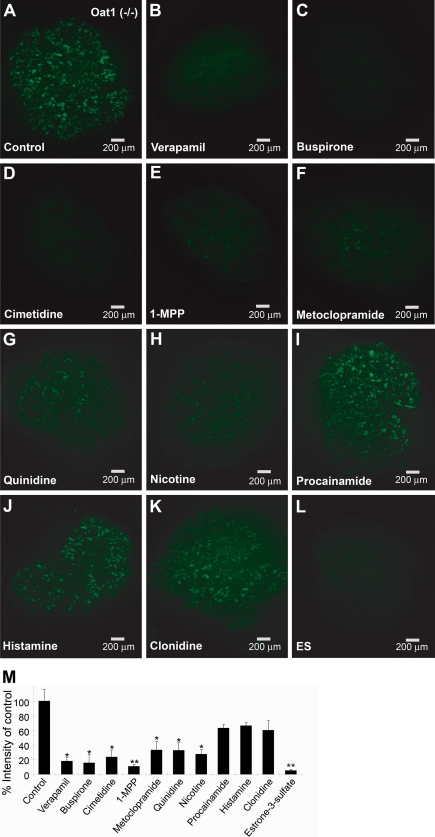
Although the oocyte expression system has been extremely useful in studies of organic anion transporters, it is not whole living mammalian tissue. Because of the complex nature of the Oat tertiary transport system and issues related to cytoskeletal associations and cell polarity in renal tubules, it is important to confirm these findings in the more complex setting of live mammalian tissues. The kidney organ culture is an ex vivo model that can be used to study the activity of transporters through the use of fluorescent tracers. It has previously been shown that 6CF can be used to track translocation through mOat1 and mOat3 in the organ culture model (17). Moreover, Oat1 and Oat3 knock-out tissue can be used to isolate the specific contributions of Oat3 and Oat1-mediated uptake, respectively, in whole live tissue. Accordingly, we determined the interaction of the aforementioned cations with organ cultures from mOat1 and mOat3 knockouts (Figs. 5 and 6). By quantifying fluorescent tracer uptake in organ cultures and determining the extent of inhibition of uptake by the cations, we were able to determine inhibitory potency. The results closely correlated with the data from the Xenopus oocyte assays, with the exception of 1-MPP. In Oat3−/− organ cultures (where tracer transport is due to Oat1), coincubation with 2 mm verapamil, buspirone, cimetidine, and nicotine resulted in inhibition of 80–90% of tracer uptake. At this concentration, 1-MPP, histamine, and quinidine showed 50–65% inhibition; guanidine, which did not inhibit tracer uptake in the mOat1-expressing oocytes, also did not show significant inhibition of tracer uptake in Oat3−/− organ cultures; and PAH, the prototypical Oat1 substrate, showed greater than 90% inhibition of tracer uptake (Fig. 5). Conversely, in Oat1−/− tissue (where tracer transport is due to Oat3), 2 mm verapamil, buspirone, cimetidine, and 1-MPP showed greater than 75% inhibition of tracer uptake, whereas metoclopramide, quinidine, and nicotine showed more than 60% inhibition; procainamide, histamine, and clonidine showed weaker inhibition, at around 40%; and estrone sulfate, a well known substrate of Oat3, showed the strongest inhibition, at 95% (Fig. 6). Again, these results correlated well with the oocyte transport studies shown in Table 1 and Fig. 1.
FIGURE 5.
Inhibition of fluorescent tracer uptake in Oat3 knock-out kidney cultures by cations. Organ cultures from Oat3 knockouts were incubated with a 2 mm concentration of the indicated compounds and 1 μm 6CF. Fluorescence was quantified, and results are presented as a percentage of control tracer uptake. J, PAH, the prototypical substrate for Oat1, was used as a positive control and showed the greatest degree of inhibition. I, guanidine, which did not show inhibition in the Xenopus oocyte assays, was the negative control. A–J, all images represent triplicate kidney cultures from the same experiment. K, values in the graph represent mean ± S.E. *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
FIGURE 6.
Inhibition of tracer uptake in Oat1 knock-out kidney culture. Organ cultures from Oat1 knockouts were coincubated with tracer (6CF; 1 μm) and the indicated compounds (2 mm), and data were analyzed as described in the legend to Fig. 5. L, estrone 3-sulfate (ES), the prototypical substrate for Oat3, served as the positive control. A–L, all images represent triplicate kidney cultures from the same experiment. M, values in the graph represent mean ± S.E. percentage of control. *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
DISCUSSION
In this study, we analyzed the interaction of a set of prototypical Oct substrates with mOat1, mOat3, and mOat6 at multiple levels, including transporter-expressing Xenopus oocytes, kidney culture, and computational modeling, followed by in vitro testing of predictions. Somewhat unexpectedly, Oat1 and -3 shared affinity for most of these cations, which are classically viewed as Oct substrates. Oat3 generally appeared to demonstrate greater affinity and broader specificity for the tested cations. Oat6 did not appear to have the same degree or range of affinity for these compounds as did the other two transporters, although its relatively high affinity for nicotine is notable. These results support the view that although Oat1 and -3 share close structural homology and often overlapping substrate specificities, there are distinct differences in their binding sites (16, 17). Furthermore, Oat6, although a close phylogenetic relation of Oat1, has been observed to have charge and hydrophobicity preferences distinct from those of Oat1 (16).
The inhibition of Oat-mediated transport by the organic cations in our study using microinjected oocytes and cultured knock-out tissue is consistent with older physiological studies (prior to the molecular cloning of the Oats), showing inhibition of PAH transport by several cations in the renal proximal tubule (12, 15, 24). These cations were found to inhibit both the organic cation (N1-methylnicotinamide) transport system and the organic anion (PAH) transport system. It was postulated that hydrophobicity and the presence of electronegative groups, such as Cl−, Br−, or NO2−, determined inhibition of PAH transport by these substrates. We, however, found no correlation between hydrophobicity or increasing electron-attracting properties of the cations and their inhibitory potencies (data not shown).
The kidney organ culture represents a model wherein the interaction of substrates with Oats in a live organ system can be observed. It has been shown that certain fluorescent tracers, such as 6CF, are transported by Oat1 and Oat3 (17). This property was used in Oat1 and Oat3 knock-out tissue to determine the interaction of the cations with Oat1 and Oat3 individually in a whole organ system. Since the fluorescence was quantifiable, we were able to determine the inhibition profiles of the cations tested, and these results were similar to those obtained from the Xenopus oocyte assays, with the exception of 1-MPP. The organ culture is a more complex system than the oocyte system, and thus it is possible that other factors may affect binding in the organ culture. (Table 1 and Figs. 1, 5, and 6).
The pharmacophore generated for Oat3 provides additional insight into the physicochemical characteristics that determine interaction with the binding site of the transporter. The features of the pharmacophore included two hydrogen bond acceptors, which is consistent with previous quantitative structure-activity relationship analysis of the binding determinants of mOat3, which was based on inhibition profiles of antivirals (17) and which had also shown that the presence of hydrogen bond acceptors was an important factor in binding to mOat3.
The use of computational analysis to characterize binding determinants for the Oats has implications for drug design and prediction of substrate interactions. We used the Oat3 pharmacophore to screen a collection of cations, and, through in vitro testing of matches to the pharmacophore, were able to identify novel cations that bound Oat3 and Oat1. The affinities for Oat3 of these compounds were 10 times greater than those of the original set of prototypical Oct substrates, supporting the validity of the pharmacophore model. In the clinical setting, multidrug regimens are common, and competitive excretion of several drugs through Oat-mediated mechanisms can lead to accumulation of some of these drugs in the body potentially leading to toxicity. In such cases, a pharmacophore could be used to identify those drugs that are excreted through the Oats and therefore at risk for accumulation in the body and causing toxicity. Our results also suggest that competition between organic anionic and cationic drugs and metabolites could be clinically important, especially for Oat3-mediated transport. Furthermore, since changes in body fluid pH are common in pathological states (e.g. diabetic ketoacidosis, lactic acidosis, and respiratory and metabolic alkalosis) and because Oats mediate the distribution of drugs, metabolites, and toxins between blood, CSF, and urine, it seems plausible that, as their charge changes, drugs and metabolites that do not bind Oats under normal physiological conditions could become Oat substrates.
When NKT, later termed Oat1, was first identified, it was suggested that it might interact with organic anions or cations (1). Our data indicate that, despite their current nomenclature, Oat1 and Oat3 interact with organic cations to a surprising degree. However, among the cations that bound to the Oats, only cimetidine and, to a lesser degree, 1-MPP were actually transported. This supports previous observations that substrate binding to the Oats and translocation across the membrane are two distinct processes (16), with the former being dependent on the physicochemical characteristics of the substrate and the latter on the molecular features of the substrate, which lead to transporter conformational changes. Finally, it seems worth considering in more detail whether other SLC22 transporters, including recently identified family members (11), might have the ability to bind and/or transport cations as well as anions.
Acknowledgments
We gratefully acknowledge the technical assistance provided by Shammy Closson, manuscript assistance by Megan Bettilyon, and the helpful discussions with Wei Wu, Kevin Bush, and Megha Nagle.
This work was supported, in whole or in part, by National Institutes of Health Grants AI057695, DK079784, and NS062156 (to S. K. N.) and DK064839 and DK075486. This work was also supported by a Coplon award (to S. A. E.).
- Oat
- organic anion transporter
- mOat
- mouse Oat
- Oct
- organic cation transporter
- PAH
- p-aminohippurate
- 1-MPP
- 1-methyl-4-phenylpyridinium
- 5CF
- 5-carboxyfluorescein
- 6CF
- 6-carboxyfluorescein.
REFERENCES
- 1.Lopez-Nieto C. E., You G., Bush K. T., Barros E. J., Beier D. R., Nigam S. K. (1997) J. Biol. Chem. 272, 6471–6478 [DOI] [PubMed] [Google Scholar]
- 2.Ahn S. Y., Nigam S. K. (2009) Mol. Pharmacol. 76, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweet D. H., Chan L. M., Walden R., Yang X. P., Miller D. S., Pritchard J. B. (2003) Am. J. Physiol. Renal Physiol. 284, F763–F769 [DOI] [PubMed] [Google Scholar]
- 4.Sweet D. H., Wolff N. A., Pritchard J. B. (1997) J. Biol. Chem. 272, 30088–30095 [DOI] [PubMed] [Google Scholar]
- 5.Kaler G., Truong D. M., Sweeney D. E., Logan D. W., Nagle M., Wu W., Eraly S. A., Nigam S. K. (2006) Biochem. Biophys. Res. Commun. 351, 872–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monte J. C., Nagle M. A., Eraly S. A., Nigam S. K. (2004) Biochem. Biophys. Res. Commun. 323, 429–436 [DOI] [PubMed] [Google Scholar]
- 7.Karbach U., Kricke J., Meyer-Wentrup F., Gorboulev V., Volk C., Loffing-Cueni D., Kaissling B., Bachmann S., Koepsell H. (2000) Am. J. Physiol. Renal Physiol 279, F679–F687 [DOI] [PubMed] [Google Scholar]
- 8.Motohashi H., Sakurai Y., Saito H., Masuda S., Urakami Y., Goto M., Fukatsu A., Ogawa O., Inui K. (2002) J. Am. Soc. Nephrol. 13, 866–874 [DOI] [PubMed] [Google Scholar]
- 9.Sugawara-Yokoo M., Urakami Y., Koyama H., Fujikura K., Masuda S., Saito H., Naruse T., Inui K., Takata K. (2000) Histochem. Cell Biol. 114, 175–180 [DOI] [PubMed] [Google Scholar]
- 10.Koepsell H., Schmitt B. M., Gorboulev V. (2003) Rev. Physiol. Biochem. Pharmacol. 150, 36–90 [DOI] [PubMed] [Google Scholar]
- 11.Wu W., Baker M. E., Eraly S. A., Bush K. T., Nigam S. K. (2009) Physiol. Genomics 38, 116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzler W. H., Brokl O. H. (1984) Am. J. Physiol. 246, F188–F200 [DOI] [PubMed] [Google Scholar]
- 13.Jensen R. E., Berndt W. O. (1988) J. Pharmacol. Exp. Ther 244, 543–549 [PubMed] [Google Scholar]
- 14.Sokol P. P., Huiatt K. R., Holohan P. D., Ross C. R. (1989) J. Pharmacol. Exp. Ther. 251, 937–942 [PubMed] [Google Scholar]
- 15.Ullrich K. J., Rumrich G., David C., Fritzsch G. (1993) Pflugers Arch. 425, 280–299 [DOI] [PubMed] [Google Scholar]
- 16.Kaler G., Truong D. M., Khandelwal A., Nagle M., Eraly S. A., Swaan P. W., Nigam S. K. (2007) J. Biol. Chem. 282, 23841–23853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truong D. M., Kaler G., Khandelwal A., Swaan P. W., Nigam S. K. (2008) J. Biol. Chem. 283, 8654–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eraly S. A., Vallon V., Vaughn D. A., Gangoiti J. A., Richter K., Nagle M., Monte J. C., Rieg T., Truong D. M., Long J. M., Barshop B. A., Kaler G., Nigam S. K. (2006) J. Biol. Chem. 281, 5072–5083 [DOI] [PubMed] [Google Scholar]
- 19.Sweet D. H., Miller D. S., Pritchard J. B., Fujiwara Y., Beier D. R., Nigam S. K. (2002) J. Biol. Chem. 277, 26934–26943 [DOI] [PubMed] [Google Scholar]
- 20.Barnum D., Greene J., Smellie A., Sprague P. (1996) J. Chem. Inf. Comput. Sci. 36, 563–571 [DOI] [PubMed] [Google Scholar]
- 21.Clement O. O., Mehl A. T. (2000) in Pharmacophore Perception, Development, and Use in Drug Design (Guner O. F. ed) pp. 69–83, International University Line, La Jolla, CA [Google Scholar]
- 22.Wright S. H. (2005) Toxicol. Appl. Pharmacol. 204, 309–319 [DOI] [PubMed] [Google Scholar]
- 23.Russell M. A., Jarvis M., Iyer R., Feyerabend C. (1980) Br. Med. J. 280, 972–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullrich K. J., Rumrich G., David C., Fritzsch G. (1993) Pflugers Arch. 425, 300–312 [DOI] [PubMed] [Google Scholar]
- 25.Vallon V., Rieg T., Ahn S. Y., Wu W., Eraly S. A., Nigam S. K. (2008) Am. J. Physiol. Renal Physiol. 294, F867–F873 [DOI] [PubMed] [Google Scholar]
- 26.Vallon V., Eraly S. A., Wikoff W. R., Rieg T., Kaler G., Truong D. M., Ahn S. Y., Mahapatra N. R., Mahata S. K., Gangoiti J. A., Wu W., Barshop B. A., Siuzdak G., Nigam S. K. (2008) J. Am. Soc. Nephrol. 19, 1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]