Abstract
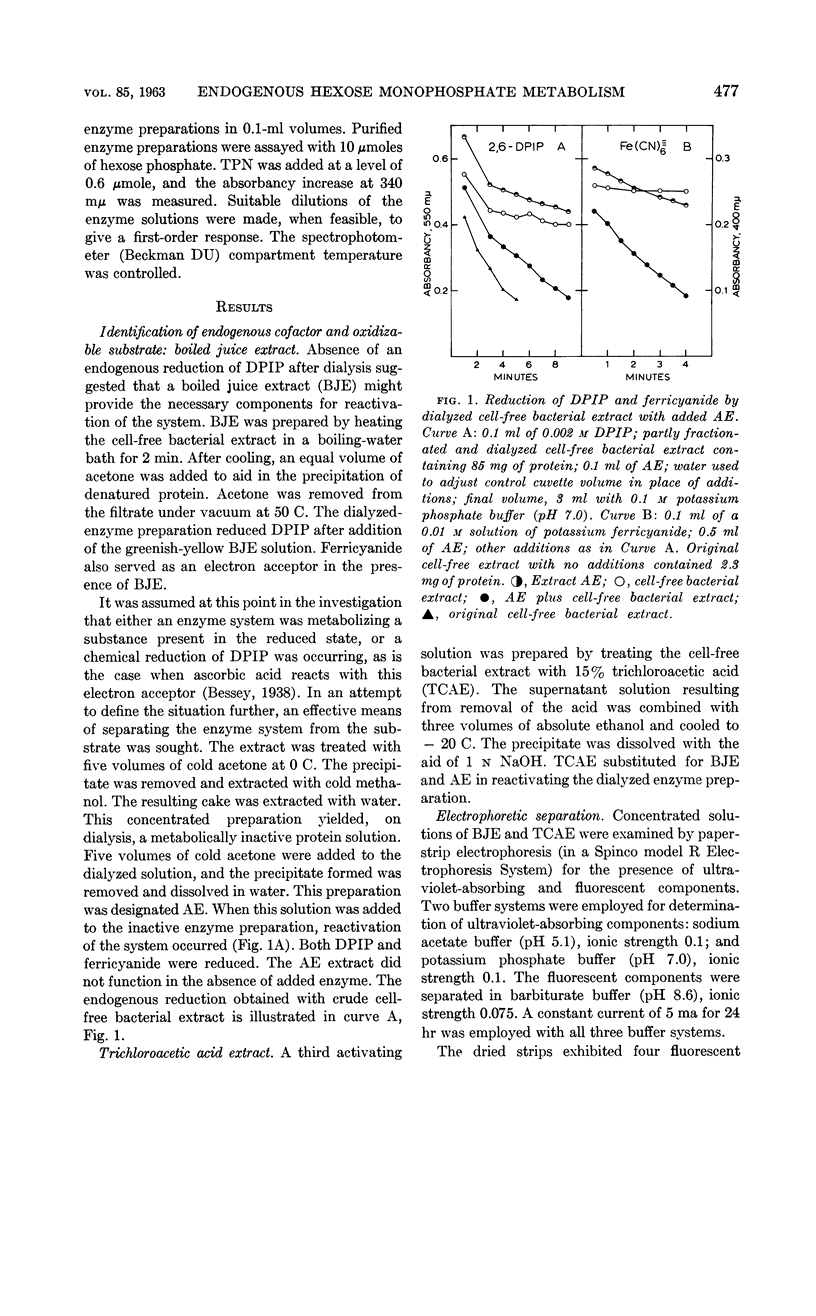
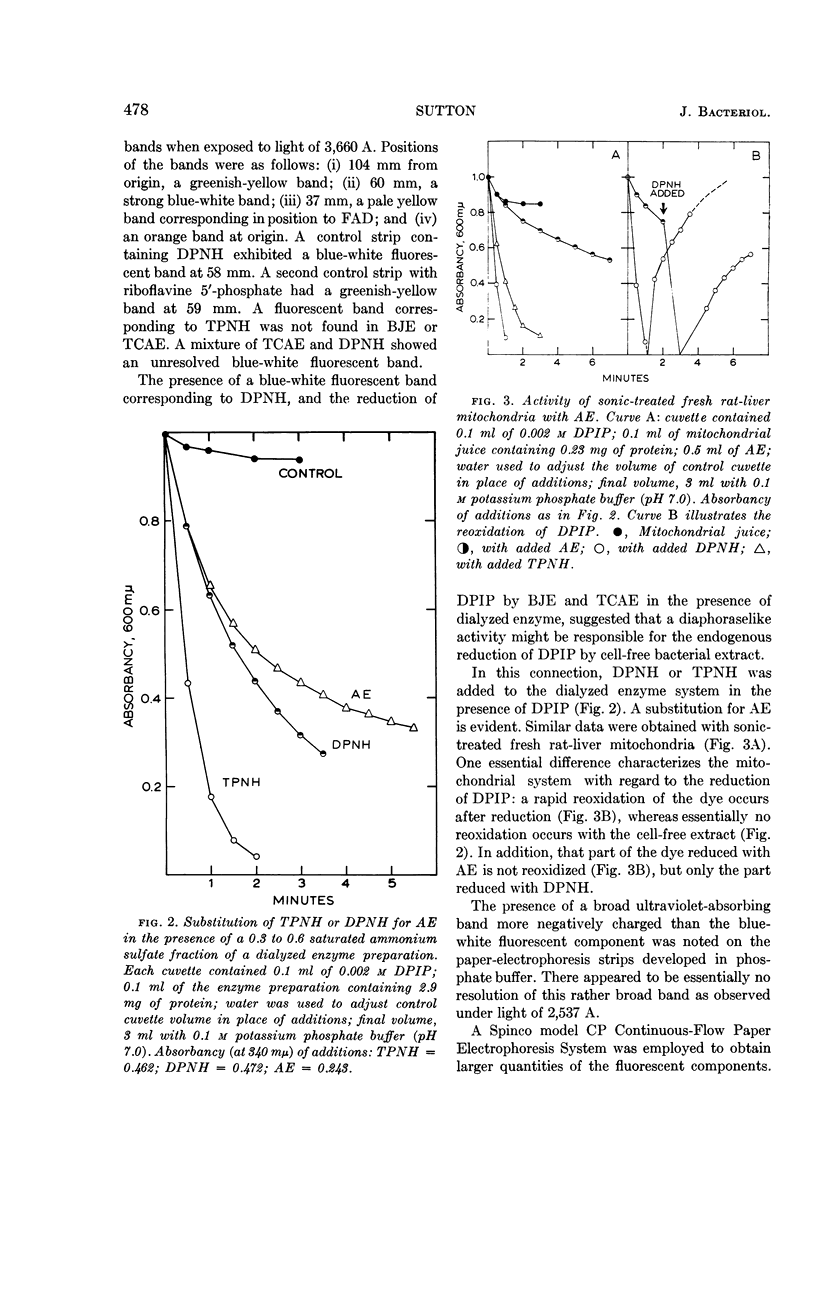
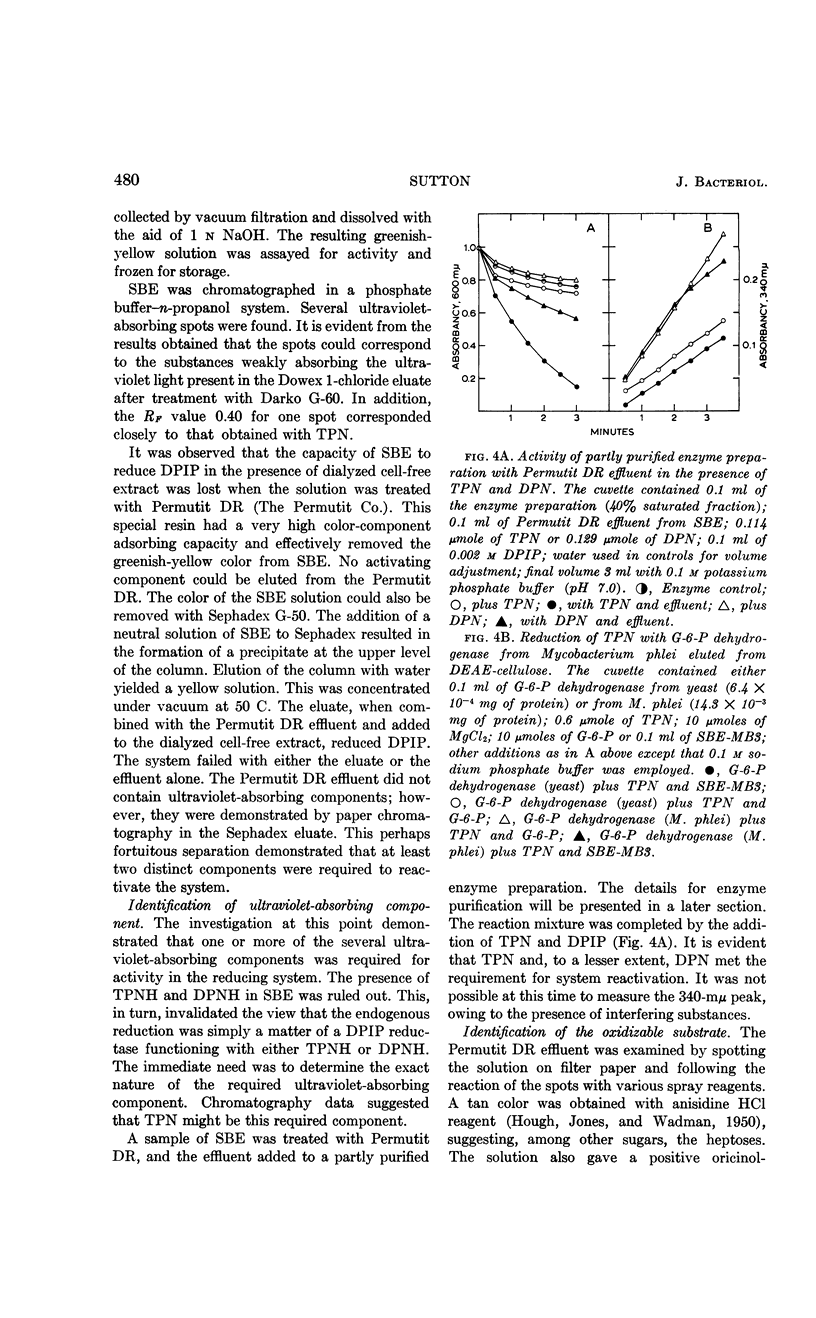
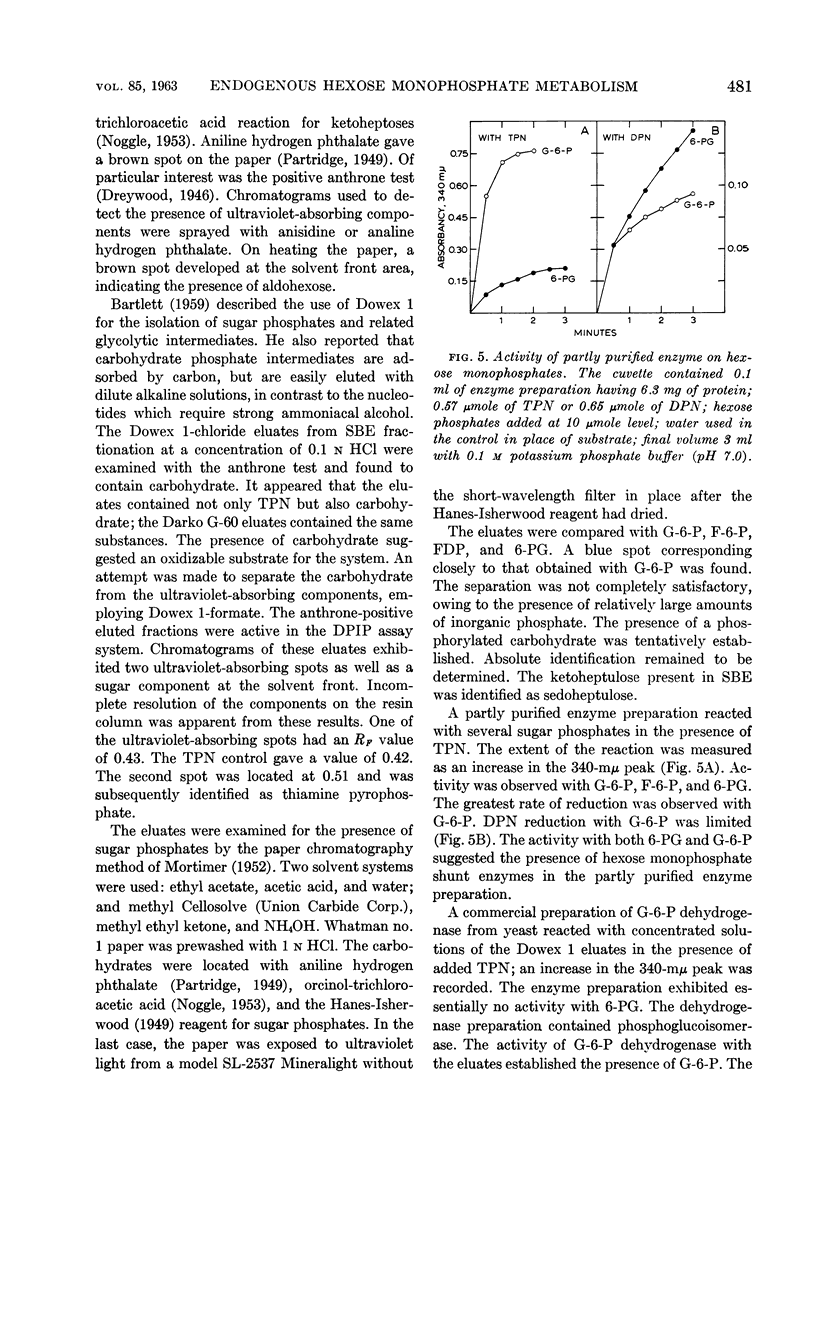
Sutton, W. B. (The Lilly Research Laboratories, Indianapolis, Ind.). Relationship of the hexose monophosphate shunt to the endogenous metabolism of cell-free extracts of Mycobacterium phlei. J. Bacteriol. 85:476–484. 1963.—The endogenous reduction of 2,6-dichlorophenol-indophenol (DPIP) by cell-free extracts of Mycobacterium phlei has been linked to the presence of glucose-6-phosphate (G-6-P) dehydrogenase functioning in connection with a reduced triphosphopyridine nucleotide (TPN)-DPIP reductase. The necessary substrate and coenzyme, i.e., G-6-P and TPN, are contained in the cell-free bacterial extract. The only required addition to activate the system is a suitable electron acceptor. The accumulation of G-6-P and the presence of 6-phosphogluconic dehydrogenase in the cell-free extract suggest that the hexose monophosphate shunt mechanism is impaired by sonic treatment of M. phlei.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BARTLETT G. R. Methods for the isolation of glycolytic intermediated by column chromatography with ion exchange resins. J Biol Chem. 1959 Mar;234(3):459–465. [PubMed] [Google Scholar]
- COLOWICK S. P., KAPLAN N. O., CIOTTI M. M. The reaction of pyridine nucleotide with cyanide and its analytical use. J Biol Chem. 1951 Aug;191(2):447–459. [PubMed] [Google Scholar]
- EDSON N. L. The intermediary metabolism of the mycobacteria. Bacteriol Rev. 1951 Sep;15(3):147–182. doi: 10.1128/br.15.3.147-182.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson N. L., Hunter G. J. Respiration and nutritional requirements of certain members of the genus Mycobacterium. Biochem J. 1943;37(5):563–571. doi: 10.1042/bj0370563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN D. S. Enzyme systems in the mycobacteria. A review. Bibl Tuberc. 1961;16:1–44. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- LANGAN T. A., Jr, KAPLAN N. O., SHUSTER L. Formation of the nicotinic acid analogue of diphosphopyridine nucleotide after nicotinamide administration. J Biol Chem. 1959 Aug;234(8):2161–2168. [PubMed] [Google Scholar]
- NOGGLE G. R. Paper chromatography of some ketoheptoses. Arch Biochem Biophys. 1953 Mar;43(1):238–239. doi: 10.1016/0003-9861(53)90106-4. [DOI] [PubMed] [Google Scholar]
- SEBRELL W. H., Jr Some problems in food toxicology. Fed Proc. 1960 Sep;19(Suppl 4):31–32. [PubMed] [Google Scholar]
- SUTTON W. B. Isolation and properties of a lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1954 Sep;210(1):309–320. [PubMed] [Google Scholar]
- SUTTON W. B. Mechanism of action and crystallization of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1957 May;226(1):395–405. [PubMed] [Google Scholar]
- SUTTON W. B. Sulfhydryl and prosthetic groups of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1955 Oct;216(2):749–761. [PubMed] [Google Scholar]
- VOLK W. A., MYRVEK Q. N. Intermediary metabolism of glucose by mycobacterium smegmatis. Am Rev Tuberc. 1956 Apr;73(4):589–592. doi: 10.1164/artpd.1956.73.4.589. [DOI] [PubMed] [Google Scholar]
- WEBER M. M., SWARTZ M. N. Pyridine nucleotide coenzymes of Mycobacterium phlei. Arch Biochem Biophys. 1960 Feb;86:233–237. doi: 10.1016/0003-9861(60)90411-2. [DOI] [PubMed] [Google Scholar]


