Abstract
Human interleukin-24 (IL-24) is unique among the IL-10 superfamily as there is considerable evidence that it possesses multiple anti-cancer properties, including direct tumor cell cytotoxicity, helper T cell (TH1) immune stimulation, and anti-angiogenic activities. The primary sequence of human IL-24 differs from homologous cytokines, because it possesses three consensus N-linked glycosylation sites and the potential for a single disulfide bond. To address the significance of these modifications in human IL-24, we analyzed the relationship between post-translational modifications and the cytokine activity of the human IL-24 protein. In contrast to related interleukins, we identified a relationship between net glycosylation, protein solubility, and cytokine activity. In addition, abrogation of the two cysteine residues by mutagenesis dramatically altered the ability of IL-24 to secrete from host cells and resulted in the concomitant loss of IL-24 activity. We conclude that, unlike other IL-10 family members, human IL-24 must be glycosylated to maintain solubility and bioavailability. Further, a single, unique disulfide bond is required for secretion and activity. These structure-function relationships show that, although IL-24 is a member of the IL-19 subfamily of IL-10-like cytokines by sequence similarity, its surface properties and its distinctive disulfide arrangement make it unique. These observations could explain the novel biological activities measured of this cytokine. Understanding the structural basis of IL-24 activity will be important in the interpretation of the function of this cytokine and in the development of scale-up strategies for biophysical and clinical applications.
Melanoma differentiation-associated gene 7 was identified as a novel tumor suppressor gene in human melanoma cells (1). Because of its physical location within the IL-104 gene cluster, its homology with the IL-10 protein and its cytokine activity, melanoma differentiation-associated gene 7 was reclassified as interleukin-24 (IL-24) (2). Gene delivery of IL-24 using plasmid or adenoviral vectors has demonstrated that the product of the human IL-24 gene exhibits powerful tumor specific pro-apoptotic, growth inhibitory, and anti-angiogenic activities (3). In primary human endothelial cells, IL-24 interacts with a unique set of cell surface receptors, IL-22R1/IL-20R2 (4, 5), resulting in potent anti-angiogenic activity (3). In tumor cells that express these receptors, IL-24 induces apoptosis; however, no cytotoxicity is observed in normal cells that also express the IL-24 receptors (6). Given these properties, IL-24 is now being considered as a promising new bio-therapeutic agent in the treatment of various cancers (7, 8).
IL-24 has been categorized as a member of the IL-19 subfamily of IL-10-like cytokines (9). This subfamily includes IL-19, IL-20, IL-22, and IL-24. The three-dimensional structures of two of the four IL-19 subfamily members, IL-19 and IL-22, have been determined to high resolution (10–12). Like IL-10, both IL-19 and IL-22 are composed of a bundle of 6 α-helices; however, unlike IL-10, these cytokines are active as monomers. Each of these cytokines possesses a set of highly conserved disulfide bonds, which are characteristic of each cytokine. Presumably, these disulfide bridges stabilize the α-helical structure for efficient receptor engagement in the extracellular environment (13). IL-24 is the lone exception, because it is missing two complementary cysteine residues that form consensus disulfide bonds in the other members of this family.
In many of the known IL-10-like cytokines (IL-10, IL-20, IL-22, and others), glycosylation is not required for activity. Either the proteins are not glycosylated, as is the case for IL-10 and IL-20, or the cytokine is glycosylated, but the glycan can be removed either enzymatically or by mutagenesis without compromising activity, as is the case for IL-22. Refolding IL-22 from bacterially expressed protein has no effect on the cytokine activity or the overall three-dimensional structure (11, 14). Although similar claims have been reported for IL-24 (15–17), these analyses relied on very small amounts of protein, so it was difficult to accurately associate protein structure with function. It is also clear that IL-24 is not as robust as other related cytokines, because several groups have shown varied responses of this cytokine in different biological assays (18, 19). One possibility to explain the discrepancy between IL-24 and the other IL-19-like cytokines is that the IL-24 protein possesses unique structural features that contribute both to its distinctive biology and to the characteristic properties of the protein.
In this study, we utilized cytokine activity assays in tandem with genomic and biophysical analyses to assess the role of post-translational modifications in IL-24. We found that, unlike the other members of the IL-19 subfamily, human IL-24 requires at least two contiguous glycosylated sites for efficient secretion and activity. These neighboring glycan groups likely mask a non-polar region on the surface of IL-24 located near the putative receptor binding site on helix B. Further, the IL-24 protein possesses a novel pairing of disulfide bonds that has not been identified in related interleukins. Understanding the structural characteristics of human IL-24 will be essential for its development as a therapeutic cytokine.
EXPERIMENTAL PROCEDURES
Sequence Alignment
IL-24 sequences were identified by tblastn searches of the primary nucleotide sequences at NCBI using the Homo sapiens amino acid sequence as the probe. Scientific names, common names, and data base accessions for translated gene sequences that correlate with the putative secreted IL-24 proteins are as follows: H. sapiens (human) IL-24, AC098935.2; Pan troglodytes (chimp), AACZ02012631.1; Pongo abelii (orangutan) ABGA01259979.1; Macaca mulatta (rhesus monkey), AC194488.2; Microcebus murinus (gray mouse lemur), ABDC01084535.1; Tupaia belangeri (northern tree shrew), AAPY01334263.1; Cavia porcellus (Guinea pig), AAKN02017883.1; Dipodomys ordii (kangaroo rat), ABRO01173591.1; Mus musculus (mouse), AC165322.12; Rattus norvegicus (Norway rat), AAHX01075042.1; Spermophilus tridecemlineatus (ground squirrel), AAQQ01504599.1, AAQQ01504600.1, and AAQQ01786339.1; Canis familiaris (dog), AAEX02025893.1; Felis catus (domestic cat), ACBE01499449.1; Bos taurus (cow), AAFC03056217.1; Tursiops truncatus (dolphin), ABRN01323706.1; Myotis lucifugus (brown bat), AAPE01426984.1 and AAPE01426909.1; Equus caballus (horse), AAWR02036827.1; Loxodonta africana (African elephant), AAGU02213483.1; Monodelphis domestica (short-tailed opossum (20)), AAFR03023107.1; H. sapiens IL-20, AAH74948; H. sapiens IL-19, Protein Data Bank (pdb) code 1N1F; H. sapiens IL-22, pdb code 1M4R; H. sapiens IL-10, pdb code 1ILK; and cytomegalovirus IL-10, pdb code 1LQS_A. Sequences were aligned using salign in Modeler (21).
Homology Model of IL-24
We used Modeler (21) to construct and refine a homology model of human IL-24 based on the known structures of IL-19 and IL-22. As the pairing of disulfide bonds is unique in IL-24, the software was explicitly instructed to construct the model including a single disulfide bond between Cys-59 and Cys-106 (Fig. 1). Structural representations were created using PyMOL (22).
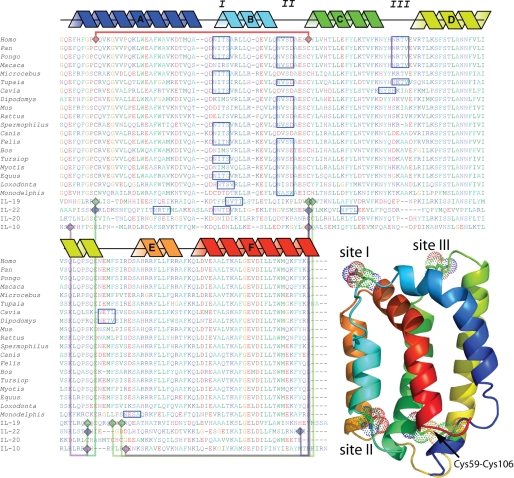
FIGURE 1.
Zoo alignment of the available IL-24 sequences. Blue boxes correspond to consensus N-linked glycosylation sites that were selected above a 50% threshold glycosylation potential by NetNGlyc (R. Gupta, E. Jung, and S. Brunak, NetNGlyc server). Purple (IL-10), blue (IL-22), green (IL-19), and red lines (IL-24) connect cysteine residues in known disulfide bonds. Schematic helices correspond to known secondary structure from the IL-19/IL-22 template. The three-dimensional model corresponds to the homology model of human IL-24 (residues 51–206). Helices are colored blue (N terminus) through red (C terminus). I, II, and III correspond to consensus N-linked glycosylation sites in the human IL-24 sequence.
Bacterial Expression, Refolding, and Analysis
A cDNA encoding residues 52–206 of human IL-24 was subcloned into pET28A using the NcoI and BamHI restriction sites and overexpressed as a non-His tagged, insoluble protein. The bacterial cells were lysed and centrifuged at 5,000 rpm in an SS-34 rotor at 4 °C. The resulting pellet was dissolved in 2 m Urea and re-centrifuged at 5,000 rpm at 4 °C. This step was repeated three times. The final pellet was dissolved in 6 m Urea, 10 mm Tris, pH 8.0, and refolded overnight at 4 °C by dilution to 50 μg/ml into 2 mm reduced glutathione:0.2 mm oxidized glutathione with 0.5% decyl-maltoside. Misfolded, aggregated protein was removed by centrifugation at 10,000 rpm for 20 min at 4 °C. Protein purity was assayed by SDS-PAGE gel electrophoresis and stained with Coomassie Blue (data not shown) and/or Western blot analysis using both polyclonal and monoclonal IL-24 specific antibodies. Protein was concentrated to 3 mm IL-24 using an Amicon pressure cell.
CD Analysis
All CD measurements were carried out at room temperature on an Aviv 215 spectropolarimeter using a quartz cell with a path length of 1 mm. Samples were scanned from 195 nm to 260 nm in 1 nm steps in 5 mm Tris, pH 8.0. For CD analysis, the IL-24 was diluted from 3 mm to 50 μm in 5 mm Tris, pH 8.0. The spectrum is an average of 10 scans. Background corrections for variance within the buffer were made in all spectra.
Cell Culture
The H1299 lung cancer cell line was obtained from the America Type Culture Collection (ATCC, Manassas, VA) and was maintained in Dulbecco's modified Eagle's medium (HyClone, Inc., Logan, UT) supplemented with 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine, and HEPES buffer (Invitrogen). The cells were routinely screened to verify lack of mycoplasma contamination and were used in the log phase of growth. Monoclonal anti-IL-24 antibody was prepared as described previously (23). Rabbit phospho-STAT3 (Tyr-705) was purchased from Cell Signaling Technology Inc. (Beverly, MA), β-actin monoclonal antibody was purchased from Oncogene Research Products (San Diego, CA). H1299 cells were transfected with 4 μg of expression plasmid DNAs using Lipofectamine 2000 (Invitrogen) for 18–48 h. Stable cell lines were obtained by cotransfection with psv2neo and selection in G418 (800 ng/ml) for 10 days.
Secretion Assay
Supernatants were harvested from the culture of transfected cells and centrifuged at 14,000 rpm for 10 min to measure only soluble, non-aggregated IL-24 protein. Supernatant was saved for Western blot analysis or used to treat tumor cells.
Immunoblotting Assay
Immunoblotting using various antibodies and standard procedures were performed as described previously (24). Proteins were visualized using enhanced chemiluminescence (Amersham Biosciences) after incubation with primary or secondary antibodies. Activation of STAT3 was determined by immunofluorescence assay using a phospho-STAT3-specific antibody (24). Pictures were taken using a fluorescence microscope 1–2 h after staining.
Cell Killing Assay
Cell killing was determined by the trypan blue exclusion assay (25). At designated times after treatment with IL-24 (wild type and mutants), cells were harvested by trypsinization, and a small aliquot was suspended in a 1:10 volume with 0.1% trypan blue (Invitrogen). Total cell numbers, and the number of dead cells was counted using a hemocytometer under light microscopy. Assays were performed three times.
Immunofluorescent Cellular Localization
H1299 lung cancer cells (5 × 104 cells/well) were grown on chamber slides to 70% confluence and then transfected with the wild-type or mutant IL-24 cDNA or treated with phosphate-buffered saline as a negative control. Seventy-two hours later, the cells were washed with phosphate-buffered saline and fixed with 4% paraformaldehyde/phosphate-buffered saline for confocal microscopic analysis. Cells were blocked with 1% normal goat serum for 1 h and then incubated overnight at a dilution of 1:100 with the primary mouse monoclonal IL-24 antibody. The slides were then washed to remove primary antibody, rinsed with phosphate-buffered saline, and placed in a prewarmed staining solution containing ER-TrackerTM red dyes or MitoTracker Deep Red 633 (Molecular Probes) for ∼15–20 min at 37 °C. The slides were then washed and incubated with a fluorescein isothiocyanate- or rhodamine-conjugated secondary antibody (Invitrogen) for 1 h. Next, the slides were mounted with ProLong Gold Antifade reagent containing 4′,6-diamidino-2-phenylindole (Invitrogen) and analyzed under an Olympus FluoView FV500 laser confocal microscope (Olympus America, Melville, NY) after adjustment for background staining.
RESULTS
Conservation of N-Glycosylation Sequons
We compared the IL-24 protein sequences from 19 mammalian genomes to reveal characteristic glycosylation trends that have been maintained in this cytokine throughout evolution (25). Human IL-24 possesses three consensus sites for N-linked glycosylation (Fig. 1). Here, each site is labeled (using the human IL-24 amino acid numbering) as site I (Asn-85), site II (Asn-99), and site III (Asn-126) (Fig. 1). Interestingly, the relatively heavy glycosylation pattern characteristic of the IL-24 in higher primates is not conserved across all species. Site I is represented in 12 of 19 of the available sequences, while site II is represented in 16 of 19 of the sequences; site III is found in only 5 of 19 of the sequences. Furthermore, 8 of 19 of the IL-24 proteins examined in this analysis possess only one consensus glycosylation site. In most cases, the protein sequences with a single glycosylation sequon are coincident with site II, suggesting that this is a unique characteristic of IL-24. Of the other members of the IL-19-like subfamily, IL-20 lacks any post-translational N-linked glycosylation signals, IL-19 possesses two consensus sites, but only one is utilized (10), and IL-22 has three consensus N-linked glycosylation sites (Fig. 1). Only IL-24 site I is common to all the glycosylated family members.
Homology Model of Human IL-24
The crystal structures of the related cytokines, IL-19 and IL-22, have been solved to high resolution (10–12). Considering only the range of amino acids from the secreted form of these cytokines, IL-19 is ∼37% similar to IL-24, whereas IL-22 is ∼40% similar. Using these two three-dimensional structures and the primary sequence similarity to IL-24, we computed a model of human IL-24 (Fig. 1) using Modeler (21, 26). The resulting model (human IL-24 residues 51–206) consists of 6 α-helices (indicated as A–F in Fig. 1), with a single disulfide bond joining helices A and C (Fig. 1). Although the quality of this homology model requires further experimental consideration, it does predict that Lys-77 and Asp-194 can form a highly conserved salt bridge joining helices A and F. The function of this salt bridge in the IL-19 subfamily is not well understood; however, it is highly characteristic of this class of α-helical interleukins (e.g. IL-10, IL-19, IL-20, and IL-22) (27). Our homology model of IL-24 also predicts that two of the N-linked glycosylation sites (sites I and III) localize to loops at the “top” of the molecule, whereas an additional site (site II) localizes to a loop at the base of helix B.
Bacterial Expression and Refolding
The cDNA corresponding to the secreted form of human IL-24 (residues 52–206) was cloned into multiple bacterial expression systems. We attempted to produce soluble protein as glutathione S-transferase, maltose-binding protein, and His-tagged fusion proteins; however, only very small amounts of soluble protein could be obtained. In each case, once the fusion partner was separated from IL-24 by protease cleavage, the IL-24 became insoluble. A screen for refolding conditions using the untagged IL-24 identified a detergent-based protocol that yielded human IL-24 with very high solubility (>50 mg/ml). However, upon lowering the detergent concentration by dialysis or dilution, the IL-24 protein quantitatively precipitated. IL-24 produced in Escherichia coli migrated to ∼20 kDa on PAGE gels, which is similar in size to the unglycosylated mammalian IL-24 (∼20 kDa) (Fig. 3). The protein was also immunoreactive with human IL-24 monoclonal antibody. When diluted into culture media, the biological activity of the bacterially expressed, detergent-refolded IL-24 demonstrated no significant cell killing; however, it was difficult to control for the presence bacterial endotoxins and excess detergent in cell assays (data not shown). Other groups have confirmed that bacterially expressed IL-24 does not show significant cytokine activity (18). On the other hand, the glycosylated human IL-24 produced in mammalian cells showed specific tumor cell killing (6, 24). The cell killing activity of the fully glycosylated protein was inhibited using specific anti-IL-24 antibodies (6, 24).
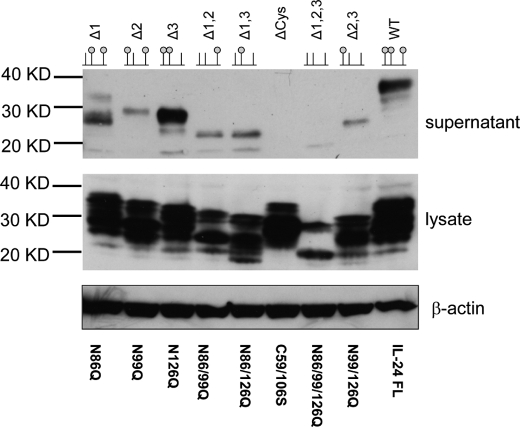
FIGURE 3.
Western blot of SDS gel showing soluble secreted human IL-24 (supernatant) and insoluble, intracellular IL-24 (lysate). The top row shows the identity of the mutation and a schematic lollipop representation of the mutation. The small green balls correspond to the occupancy of a glycan moiety. In almost every case, the lysate shows immune-reactive protein at various stages of processing. β-Actin was used as a positive control for sample loading.
Secondary Structure Analysis
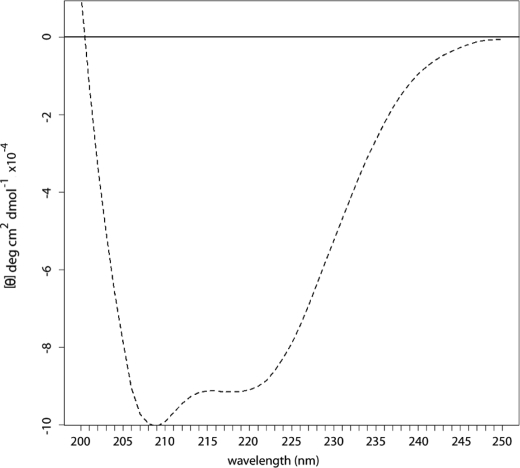
To test whether the detergent-soluble IL-24 possessed measurable secondary structure, we analyzed our re-folded protein by CD. This analysis of refolded human IL-24 confirmed that the cytokine is predominantly α-helical (Fig. 2). Ellman's reagent (28) confirmed that two Cys residues were reactive in our detergent-refolded samples (data not shown), indicating that the disulfide bridge was not formed in the bacterial protein. This is consistent with the known secondary structure of related cytokines. In the case of recombinant human IL-10, reduction of the two disulfide bridges by the addition of 1 mm dithiothreitol resulted in a loss of biological activity, but had only a relatively minor effect on the overall secondary structure of the protein (29).
FIGURE 2.
CD spectra of detergent re-folded human IL-24.
Efficient Secretion of IL-24 Requires N-Linked Glycosylation
To test the overall role of N-linked glycosylation in human IL-24, we eliminated all of the glycosylation sites in human IL-24 by mutagenesis (N86Q, N99Q, and N126Q; labeled as Δ1,2,3 in Fig. 3). This construct was transfected into H1299 cells, and the IL-24 was screened for solubility and tumor killing activity. We found that little or no mutated IL-24 was secreted from the H1299 cells (Fig. 3, lane Δ1,2,3, supernatant). When we analyzed the intracellular levels of IL-24, we found that it was also markedly diminished compared with the wild-type controls (Fig. 3, lane Δ1,2,3, lysate). This is likely due to rapid ubiquitination and subsequent intracellular degradation of IL-24 (30).
All N-Linked Glycosylation Sequons in IL-24 Are Post-translationally Modified
Although there are three consensus sites for N-linked glycosylation in the human IL-24 sequence, the actual extent of post-translational modification has not been described in this cytokine. To test whether each individual sequon is available for post-translational modification, we mutated all combinations of pairs of sequons, thereby restricting glycosylation to single sites, and tested for secretion and activity. These constructs were labeled according to the sites deleted by mutagenesis (Δ1,2 = ΔN86/99Q; Δ1,3 = ΔN86/126Q; and Δ2,3 = ΔN99/126Q). The resulting secreted protein products ran on SDS-gels at sizes corresponding to singly glycosylated IL-24 (Fig. 3, lanes Δ1,2, Δ1,3, and Δ2,3). Each of the three double mutants was secreted from H1299 cells at similar levels but significantly less than wild-type levels. Because of the marked reduction in secreted IL-24 levels, we can conclude that a single glycosylation event is not sufficient to mediate wild-type secretion levels in human IL-24. This result also demonstrates that each of the three sequons is amenable to post-translational modification, and the mature secreted cytokine utilizes all three sites.
Combinatorial Deletion of Glycosylation Sequons in Human IL-24
To test the contribution of pairs of glycosylation sites, we mutated individual sequons (Fig. 3, lanes Δ1, Δ2, and Δ3). The predominant band in the Δ1 construct corresponds in mass to IL-24 glycosylated at only sites II and III (Fig. 3, Δ1). Levels of expression for this mutant are comparable to the wild-type secreted protein. The secreted amounts of the Δ3 construct in the media were reproducibly higher relative to the wild-type IL-24; however, immunoreactive lower molecular weight bands corresponding to the single glycosylated species and unglycosylated IL-24 were also present. Surprisingly, the Δ2 mutation displayed a marked reduction in secreted levels in the media. Either the Δ2 site is structurally unique compared to the other two sites or two contiguous glycosylation events must cooperate to overcome unfavorable aggregation properties of the protein surface coincident with helix B.
IL-24 Possesses a Single Unique Disulfide Bond
Each of the IL-10-like cytokines whose x-ray structure has been solved possesses multiple sets of disulfide bonds (Fig. 1). The cysteine residues that contribute to these disulfide bonds are highly conserved; therefore, one can usually predict the presence of a homologous disulfide bridge in other family members. IL-24 lacks two of these cysteine residues, making alignment-based prediction of disulfide pairs problematic. Although these two cysteine residues are present in the homologous cytokines, they have never been observed forming a bond with each other. This leaves two possible scenarios for the disulfide connectivity of IL-24. In the first case, the cytokine is monomeric like IL-19 and IL-22. This would force a novel arrangement of disulfide bonds between helices A and C and would likely result in a novel structure for the cytokine (27). In the second case, IL-24 could be a dimer like IL-10 and use this disulfide to inter-connect the two molecules. However, the disulfide-linked IL-10 dimer has only been observed in the cytomegalovirus IL-10 (31), so this possibility is unlikely. To evaluate the role of disulfide bonds in human IL-24, we mutated the two cysteine residues in IL-24 to Ser, thereby removing any potential for disulfide formation. The C59S/C106S protein is expressed at high levels inside the cells but is very poorly secreted (Fig. 3, lane ΔCys). We conclude that IL-24 requires a single disulfide bond for productive folding and secretion of the molecule.
In our genomic analysis, each of the 19 IL-24 protein sequences shares the same pair of cysteine residues; therefore, the lone disulfide bond must be a unique characteristic of IL-24. The only exception is Monodelphis IL-24. This sequence possesses a total of four cysteine residues: the two cysteine residues in common with the other IL-24 sequences, in addition to two unique positions. Interestingly, one site is present within the first glycosylation sequon, whereas an additional cysteine residue is shared with the other IL-10-like proteins.
Receptor-mediated Killing and Receptor Activation Are Disulfide- and Glycosylation-dependent
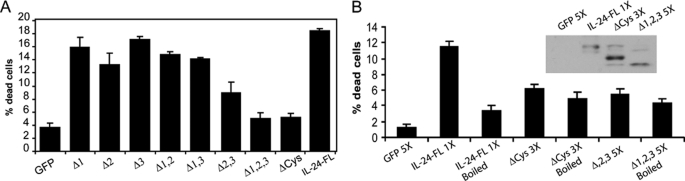
We have previously shown that IL-24 protein can kill melanoma and breast tumor cells in a paracrine manner via engagement of the IL-22/IL-20 receptors (24, 32). To assay the secreted protein for killing activity, we harvested the supernatant from cells transfected with wild-type or mutant IL-24 and applied them to IL-24 receptor-positive melanoma cells (MeWo). As expected, wild-type IL-24 full-length induced significant cell death in the tumor cells (Fig. 4A). The Δ1 or Δ3 mutations killed tumor cells at a comparable level to the wild-type protein, but Δ2 mutant showed reduced killing (Fig. 4A; p < 0.05). Loss of disulfide bonding, ΔCys, or mutation of three glycosylation residues, Δ1,2,3, reduced killing levels comparable to the green fluorescent protein control protein. This is probably the result of low concentrations secreted protein and not due to specific alteration in the cytokine-receptor complex. As measured in Fig. 3, cells transfected with genes corresponding to ΔCys or Δ1,2,3 secrete much less IL-24 compared with the other mutants. To compensate for the reduced protein levels, supernatant from the ΔCys or Δ1,2,3 cell lines was concentrated and applied to tumor cells (Fig. 4B). Only the wild-type IL-24 protein resulted in cell death. Supernatant from ΔCys or Δ1,2,3 was severely impaired in induction of cell death. Heat inactivation blocked activity of wild-type IL-24, but had no effect on the mutants (Fig. 4B).
FIGURE 4.
A, IL-24 killing of receptor-positive tumor cells. Supernatants from cells transiently transfected with indicated plasmids were applied to IL-24 receptor-positive MeWo tumor cells. The number of dead cells was counted against total number of cells and expressed as percent dead cells. B, IL-24 mutant proteins were concentrated (X3) to achieve protein amounts equivalent to wild-type IL24 protein (inset); the proteins were either boiled or not boiled prior to adding to MeWo cells. Specific killing activity was examined in cell proliferation assay.
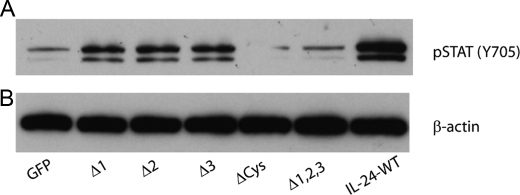
To evaluate receptor interaction by IL-24 mutants, target cells were treated with culture supernatants containing wild-type IL-24 or mutant IL-24 protein, and receptor engagement was evaluated using activation of STAT3 as a marker (Fig. 5). This qualitative assay reports on the downstream effects of cytokine-receptor interactions and is therefore less sensitive to absolute quantity of cytokine in the assay. ΔCys or Δ1,2,3 mutants failed to activate STAT3, whereas loss of individual glycosylation sites did not significantly affect STAT3 activation (Fig. 5). These results indicate that ΔCys or Δ1,2,3 mutants are poorly secreted and prone to aggregation and, hence, have lost the ability to engage receptors and initiate apoptosis.
FIGURE 5.
STAT3 assay. Cell lysates prepared from wild-type and mutant IL-24-treated MeWo cells were analyzed for phosphorylated (p) STAT3 (Ser-705) and total STAT3 at 30 min after treatment by Western blotting. STAT3 activation, as indicated by increased pSTAT3, was observed in cells treated with wild-type IL-24 protein and in cells treated with single mutants (1, 2, or 3). STAT3 activation was reduced in cells treated with ΔCys and Δ1,2,3 and comparable to control cells (GFP). β-Actin was used as loading control.
Subcellular Localization of Mutant IL-24
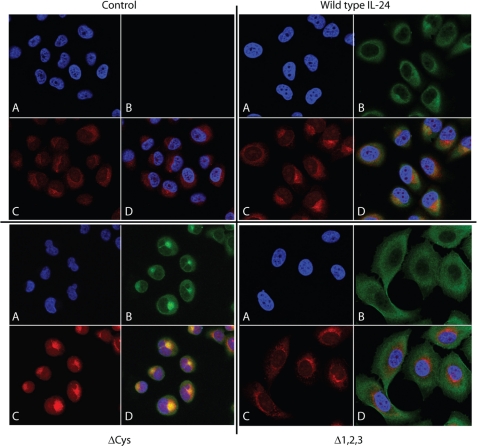
To determine if deletion in glycosylation affect IL-24 subcellular localization, stable cell lines expressing mutant or wild-type IL-24 were evaluated by confocal microscopy using monoclonal antibodies specific for IL-24. Wild-type IL-24 is primarily localized in the endoplasmic reticulum (ER), and IL-24 staining (green) is co-incident with staining of the ER (red) (Fig. 6). In contrast, cells expressing ΔCys or Δ1,2,3 mutant show dramatically altered subcellular localization of mutant IL-24. The ΔCys mutant IL-24 appears to be retained in the ER, and little protein is found in the cytoplasm. In contrast, the Δ1,2,3 mutant appears to be released from the ER, widely distributed throughout the cell, but is poorly secreted.
FIGURE 6.
Subcellular localization of the mutant IL-24 proteins in H1299 cells compared with known molecular markers. In each block of four panels, A is 4′,6-diamidino-2-phenylindole staining (blue); B uses IL-24 antibody (green), C is ER staining (red), and D is the overlay of all three panels.
DISCUSSION
In this study, we have identified that N-glycosylation at site II in IL-24 is unique and is required for efficient secretion and activity. Further, IL-24 possesses a unique disulfide bridge that is not found in other related cytokines. Our results also show that all three sites are accessible to the glycosylation machinery of the cell, but not all sites are equally advantageous to secretion and folding. This is in contrast to most other IL-19-like cytokines as glycosylation is not necessary for secretion or activity.
Our homology model of human IL-24 predicts that site I is located on an exposed loop between helices A and B. In our secretion assays, the elimination of this site from human IL-24 (Δ1) produces a soluble protein that is ∼10 kDa smaller than wild-type, but larger than the singly glycosylated forms of the protein. This molecular mass is consistent with the two remaining glycosyl moieties on the secreted cytokine. The Δ1 protein retains near wild-type activity in the cell killing assays. This mutant is also able to phosphorylate STAT3, an indicator of receptor engagement. Similar results were observed with the Δ3 mutant. Interestingly, the elimination of site III resulted in IL-24 secretion levels higher than that of wild-type protein, and it did not show any significant functional consequences with cytokine activity. This observation held across numerous repeat experiments (n > 6 independent experiments).
The Δ2 mutant shows a completely different result. In our homology model, site II is located between helices B and C (Fig. 1). This site is unique to most of the IL-24 homologs (with the exception of the Canis, Cavia, and Microcebus sequences); however, in each of these exceptions, a cryptic N-linked glycosylation sequon may still be present, but the essential asparagine residue was replaced by a conservative aspartic acid. When we assayed the Δ2 mutation in human IL-24 for secretion, we noticed that there was markedly less secreted protein than with the other two single mutants. Although there is evidence on the Western blot for soluble protein, levels are reduced compared with either wild-type, Δ1, or Δ3 mutant IL-24. Therefore, we conclude that only the mutants where the glycosyl modifications were contiguous to site II did the secreted expression approach wild-type levels. For example, Δ1 and Δ3 both expressed at levels comparable to wild-type; however, Δ2 did not. This suggests a degree of synergy between glycosylation sites.
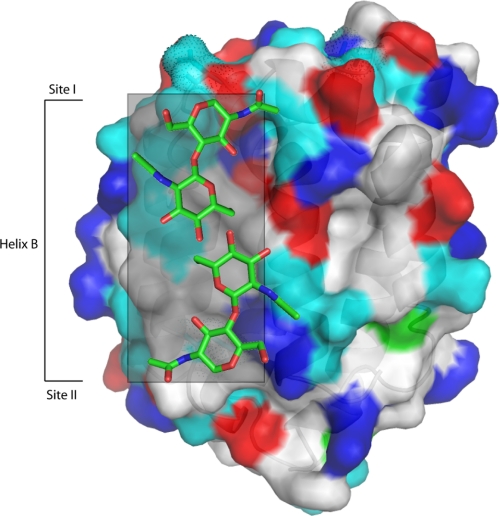
A similar synergistic relationship among glycosylation sites has also been observed in other proteins. In viral hemagglutinin, for example, N-linked glycosylation sequons were systematically deleted by mutagenesis and tested for function (33, 34). Similar to our results with IL-24, they concluded that specific sugar modifications in hemagglutinin act together to maintain a hydrophilic surface to promote trimer formation and to discourage non-productive aggregation. A similar conclusion was also reached with recombinant interferon-β (IFN-β) (35). In the crystal structure of IFNβ-1a (Avonex®), N-linked glycan modification screened the hydrophobic surface of the IFN-β from aggregation by forming hydrogen bonds between the glycan moiety and the peptide backbone of the cytokine. Removal of the glycan by expression in bacteria, IFNβ-1b (Betaseron®), exposed this uncharged surface to solvent and accounts for the propensity of the non-glycosylated protein to aggregate. This engineered version of interferon-β is 10 times less potent in in vitro assays than the fully glycosylated version. This is presumably due to reduced bioavailability due to an increased potential to aggregate. If our homology model of IL-24 is accurate, the requirement for site-specific glycosylation in human IL-24 could indicate that IL-24 shares an apolar surface similar to that of IFN-β (Fig. 7). The location of the glycosyl group on the folded molecule could shield hydrophobic and polar-uncharged amino acids on the surface of human IL-24 from aggregation as it did with IFNβ-1a.
FIGURE 7.
Surface rendering of human IL-24 with two of the sugars. The gray box corresponds to the region around helix B thought to harbor the hydrophobic (white surface) and polar, non-charged (cyan surface) residues that could mediate aggregation. Two fucose molecules cover a predicted hydrophobic region.
Conserved Disulfide Patterns in the IL-10 Superfamily
One characteristic shared by the entire IL-10 superfamily is the conserved pairing of disulfide-bonded residues that apparently stabilize the secreted cytokine (36). Of the known crystal structures, human IL-10 (pdb code: 1ILK) possesses two disulfide bonds connecting helix A to helix D, and helix C to the linker region between helices D and E. IL-19 and IL-22 have three pairs of disulfide-bonded residues that link similar regions of the molecule. IL-24, on the other hand, possesses only two Cys residues. From the primary sequence evidence alone, one can conclude that either IL-24 has one unique disulfide bond or that the molecule has no disulfide bonds. Because IL-24 is a secreted cytokine and it shares significant similarity to other IL-10-like proteins, it is unlikely that it maintains free cysteine residues. Given that the refolded protein possesses the expected α-helical signature without a disulfide bond, and because the protein does not secrete without the two conserved cysteine residues, we can conclude that IL-24 must form a unique disulfide bond between Cys-52 and Cys-126. Loss of this disulfide bond causes the protein to lose activity and to be retained in the ER. This may reflect the loss of folding or structural integrity conferred by disulfide bonds. In contrast, the non-glycosylated IL-24 in the Δ1,2,3 construct appears to be released from the ER but remains in the cytosol without being secreted.
In conclusion, using a combination of genomic, molecular modeling, and cell-based secretion methods, we have mapped several critical structural features in human IL-24. Our data show that, in the absence of any N-linked glycosylation, as is the case for bacterially expressed protein, the cytokine lacks significant biological activity or bioavailability. Our data also indicate that human IL-24 requires at least two contiguous N-linked glycosylation events and the formation of a single disulfide bridge to maintain solubility. Either the lack of the disulfide bonds or the removal of the N-linked glycan groups on human IL-24 will lead to aggregation and a general lack of bioactivity. To fully explore the potential for human IL-24 as a biotherapeutic agent, we must fully understand the requirements of the molecule for proper folding and solubility in mammalian systems.
This work was supported by a grant from the Melanoma Research Foundation and a University of Texas M. D. Anderson SPORE in Melanoma developmental award (CA093459).
- IL-10
- interleukin-10
- STAT3
- signal transducers and activators of transcription 3
- ER
- endoplasmic reticulum
- IFN
- interferon.
REFERENCES
- 1.Jiang H., Lin J. J., Su Z. Z., Goldstein N. I., Fisher P. B. (1995) Oncogene 11, 2477–2486 [PubMed] [Google Scholar]
- 2.Caudell E. G., Mumm J. B., Poindexter N., Ekmekcioglu S., Mhashilkar A. M., Yang X. H., Retter M. W., Hill P., Chada S., Grimm E. A. (2002) J. Immunol. 168, 6041–6046 [DOI] [PubMed] [Google Scholar]
- 3.Ramesh R., Mhashilkar A. M., Tanaka F., Saito Y., Branch C. D., Sieger K., Mumm J. B., Stewart A. L., Boquoi A., Dumoutier L., Grimm E. A., Renauld J. C., Kotenko S., Chada S., Boquio A. (2003) Cancer Res. 63, 5105–5113 [PubMed] [Google Scholar]
- 4.Wang M., Tan Z., Zhang R., Kotenko S. V., Liang P. (2002) J. Biol. Chem. 277, 7341–7347 [DOI] [PubMed] [Google Scholar]
- 5.Dumoutier L., Leemans C., Lejeune D., Kotenko S. V., Renauld J. C. (2001) J. Immunol. 167, 3545–3549 [DOI] [PubMed] [Google Scholar]
- 6.Zheng M., Bocangel D., Doneske B., Mhashilkar A., Ramesh R., Hunt K. K., Ekmekcioglu S., Sutton R. B., Poindexter N., Grimm E. A., Chada S. (2007) Cancer Immunol. Immunother. 56, 205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong A. W., Nemunaitis J., Su D., Zhang Y., Cunningham C., Senzer N., Netto G., Rich D., Mhashilkar A., Parker K., Coffee K., Ramesh R., Ekmekcioglu S., Grimm E. A., van Wart Hood J., Merritt J., Chada S. (2005) Mol. Ther. 11, 160–172 [DOI] [PubMed] [Google Scholar]
- 8.Inoue S., Shanker M., Miyahara R., Gopalan B., Patel S., Oida Y., Branch C. D., Munshi A., Meyn R. E., Andreeff M., Tanaka F., Mhashilkar A. M., Chada S., Ramesh R. (2006) Curr. Gene Ther. 6, 73–91 [DOI] [PubMed] [Google Scholar]
- 9.Zdanov A. (2006) Vitam. Horm. 74, 61–76 [DOI] [PubMed] [Google Scholar]
- 10.Chang C., Magracheva E., Kozlov S., Fong S., Tobin G., Kotenko S., Wlodawer A., Zdanov A. (2003) J. Biol. Chem. 278, 3308–3313 [DOI] [PubMed] [Google Scholar]
- 11.Nagem R. A., Colau D., Dumoutier L., Renauld J. C., Ogata C., Polikarpov I. (2002) Structure 10, 1051–1062 [DOI] [PubMed] [Google Scholar]
- 12.Xu T., Logsdon N. J., Walter M. R. (2005) Acta Crystallogr. D. Biol. Crystallogr. 61, 942–950 [DOI] [PubMed] [Google Scholar]
- 13.Thornton J. M. (1981) J. Mol. Biol. 151, 261–287 [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira Neto M., Ferreira J. R., Jr., Colau D., Fischer H., Nascimento A. S., Craievich A. F., Dumoutier L., Renauld J. C., Polikarpov I. (2008) Biophys. J. 94, 1754–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauane M., Gupta P., Lebedeva I. V., Su Z. Z., Sarkar D., Randolph A., Valerie K., Gopalkrishnan R. V., Fisher P. B. (2006) Cancer Res. 66, 11869–11877 [DOI] [PubMed] [Google Scholar]
- 16.Mumm J. B., Ekmekcioglu S., Poindexter N. J., Chada S., Grimm E. A. (2006) J. Interferon Cytokine Res. 26, 877–886 [DOI] [PubMed] [Google Scholar]
- 17.Sauane M., Gopalkrishnan R. V., Choo H. T., Gupta P., Lebedeva I. V., Yacoub A., Dent P., Fisher P. B. (2004) Oncogene 23, 7679–7690 [DOI] [PubMed] [Google Scholar]
- 18.Kreis S., Philippidou D., Margue C., Rolvering C., Haan C., Dumoutier L., Renauld J. C., Behrmann I. (2007) PLoS ONE 2, e1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreis S., Philippidou D., Margue C., Behrmann I. (2008) J. Cell. Mol. Med. 12, 2505–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong E. S., Young L. J., Papenfuss A. T., Belov K. (2006) Immunome Res. 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sali A., Blundell T. L. (1993) J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 22.DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA [Google Scholar]
- 23.Poindexter N. J., Walch E. T., Chada S., Grimm E. A. (2005) J. Leukocyte Biol. 78, 745–752 [DOI] [PubMed] [Google Scholar]
- 24.Chada S., Mhashilkar A. M., Ramesh R., Mumm J. B., Sutton R. B., Bocangel D., Zheng M., Grimm E. A., Ekmekcioglu S. (2004) Mol. Ther. 10, 1085–1095 [DOI] [PubMed] [Google Scholar]
- 25.Beintema J. J. (1986) J. Mol. Evol. 24, 118–120 [DOI] [PubMed] [Google Scholar]
- 26.Martí-Renom M. A., Stuart A. C., Fiser A., Sánchez R., Melo F., Sali A. (2000) Annu. Rev. Biophys. Biomol. Struct. 29, 291–325 [DOI] [PubMed] [Google Scholar]
- 27.Zdanov A. (2004) Curr. Pharm. Des. 10, 3873–3884 [DOI] [PubMed] [Google Scholar]
- 28.Ellman G. L. (1959) Arch. Biochem. Biophys. 82, 70–77 [DOI] [PubMed] [Google Scholar]
- 29.Windsor W. T., Syto R., Tsarbopoulos A., Zhang R., Durkin J., Baldwin S., Paliwal S., Mui P. W., Pramanik B., Trotta P. P., et al. (1993) Biochemistry 32, 8807–8815 [DOI] [PubMed] [Google Scholar]
- 30.Gopalan B., Shanker M., Scott A., Branch C. D., Chada S., Ramesh R. (2008) Cancer Gene Ther. 15, 1–8 [DOI] [PubMed] [Google Scholar]
- 31.Jones B. C., Logsdon N. J., Josephson K., Cook J., Barry P. A., Walter M. R. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9404–9409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chada S., Bocangel D., Ramesh R., Grimm E. A., Mumm J. B., Mhashilkar A. M., Zheng M. (2005) Mol. Ther. 11, 724–733 [DOI] [PubMed] [Google Scholar]
- 33.Roberts P. C., Garten W., Klenk H. D. (1993) J. Virol. 67, 3048–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng D. T., Hiebert S. W., Lamb R. A. (1990) Mol. Cell. Biol. 10, 1989–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Runkel L., Meier W., Pepinsky R. B., Karpusas M., Whitty A., Kimball K., Brickelmaier M., Muldowney C., Jones W., Goelz S. E. (1998) Pharm. Res. 15, 641–649 [DOI] [PubMed] [Google Scholar]
- 36.Commins S., Steinke J. W., Borish L. (2008) J. Allergy Clin. Immunol. 121, 1108–1111 [DOI] [PubMed] [Google Scholar]