Abstract
Desmocollin (Dsc) 1–3 and desmoglein (Dsg) 1–4, transmembrane proteins of the cadherin family, form the adhesive core of desmosomes. Here we provide evidence that Dsc3 homo- and heterophilic trans-interaction is crucial for epidermal integrity. Single molecule atomic force microscopy (AFM) revealed homophilic trans-interaction of Dsc3. Dsc3 displayed heterophilic interaction with Dsg1 but not with Dsg3. A monoclonal antibody targeted against the extracellular domain reduced homophilic and heterophilic binding as measured by AFM, caused intraepidermal blistering in a model of human skin, and a loss of intercellular adhesion in cultured keratinocytes. Because autoantibodies against Dsg1 are associated with skin blistering in pemphigus, we characterized the role of Dsc3 binding for pemphigus pathogenesis. In contrast to AFM experiments, laser tweezer trapping revealed that pemphigus autoantibodies reduced binding of Dsc3-coated beads to the keratinocyte cell surface. These data indicate that loss of heterophilic Dsc3/Dsg1 binding may contribute to pemphigus skin blistering.
Desmogleins (Dsg)2 and desmocollins (Dsc) are members of the Ca2+-dependent cadherin family of adhesion molecules that extend with their outer domains into the extracellular core of desmosomes. Desmosomal cadherins include four Dsg (Dsg1–4) and three Dsc3 isoforms (Dsc1–3) (1, 2). Desmosomal cadherins share a common domain organization with five N-terminally located extracellular subdomains (EC1–5). The membrane-distal EC1 domain is thought to contain the adhesive interface necessary for trans-interaction as could be concluded from structural analysis and blocking studies using peptides and antibodies (3–5). By establishing trans- and cis-interacting adhesive complexes, desmosomal cadherins participate in providing mechanical strength to stratified epithelia (6). In human epidermis Dsg1 and Dsc1 expression decreases from the outermost granular layer toward deeper layers, whereas Dsg3 and Dsc3 are primarily found in the basal layer and display an inverse expression gradient (7, 8). In contrast to classical cadherins present in adherens junctions that primarily undergo homophilic trans-interaction, desmosomal cadherins are generally believed to mediate both homo- and heterophilic binding (9). Recently, an important role of Dsc3 for integrity of murine epidermis was demonstrated in animals with conditional epidermal Dsc3 deficiency that suffered from severe intraepidermal blister formation (10) comparable with the phenotype of the autoimmune bullous skin disease pemphigus vulgaris (PV) (11). PV is associated with antibodies (Abs) against Dsg3, in part combined with Abs targeting Dsg1, whereas Dsg1 Abs alone are associated with pemphigus foliaceus (PF). However, PV and PF sera usually do not contain autoantibodies targeting Dsc3 (12). In view of the apparently important role of Dsc3 in epidermal adhesion, we addressed whether Dsg1 and Dsg3 might heterophilically interact with Dsc3 and whether Abs in pemphigus might interfere with such type of interaction.
MATERIALS AND METHODS
Cell Culture, Test Reagents, and shRNA
As described previously (13), the spontaneously immortalized keratinocyte cell line HaCaT was grown in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) containing 1.8 mm Ca2+ supplemented with 10% fetal calf serum (Biochrom, Berlin, Germany), 50 units/ml penicillin-G, and 50 μg of streptomycin. Confluent monolayers were used throughout all experiments. Monoclonal Abs against the extracellular domains of Dsc3 (clone U-114, Progen, Heidelberg, Germany), Dsg1 (clone p124, Progen), and VE-cadherin (clone 11D4.1 (14)) were applied in a 1:20 dilution. Dsc3 mAb is directed against a membrane-proximal region of the extracellular anchor domain, whereas Dsg1 mAb was raised against a peptide derived from the EC2 to EC4 domains. Monoclonal mouse PV antibody AK23, which recognizes an epitope within amino acids 1–160 of human Dsg3 EC1 domain, was purchased from Biozol (Eching, Germany) and used at 75 μg/ml for experiments. SB202190 (Calbiochem) was preincubated for 1 h at 30 μm. The human TRIPZ lentiviral inducible shRNAmir construct V2THS_151242 (BioCat, Heidelberg, Germany) was transfected into HaCaT using TurboFect (Fermentas, St. Leon-Rot, Germany), and a stable-transfected cell line (Dsc3dim) was obtained by selection with 2 μg/ml puromycin (Sigma). Expression of shRNAmir was induced by 0.1 μg/ml doxycycline (Sigma) for 48 h, and Dsc3 expression was determined by immunostaining and Western blot analysis.
Electrophoresis and Western Blotting
Immunodetection of Dsc3 in Dsc3dim HaCaT was performed according to standard procedures as described earlier (15). Quality and purity of recombinant proteins were determined by the same approach after every production cycle. Dsc3 mAb (clone U-114, Progen), Dsg1 mAb (clone p124, Progen), and a polyclonal Dsg3 Ab (clone H-145, Santa Cruz Biotechnology, Heidelberg, Germany) were applied as primary antibodies in a 1:500 dilution.
Purification of Pemphigus-IgG
In this study, sera from two patients with PV and from one patient with PF were used. The diagnosis was based on the typical clinical appearance, intercellular deposits of IgG, and C3 in the epidermis by direct immunofluorescence microscopy of a perilesional biopsy and detection of circulating Dsg Abs by enzyme-linked immunosorbent assay (MBL, Nagoya, Japan; see Table 1). PV-IgG 1 contained only Abs against Dsg3, whereas PV-IgG 2 was positive for Dsg1 and Dsg3 Abs. PF-IgG contained Dsg1 but no Dsg3 Abs. For detection of possible Dsc3 Abs in pemphigus IgG fractions, we applied enzyme-linked immunosorbent assay measurements and immunoblotting analysis as described elsewhere in detail (16). All three sera were negative for Dsc3 by enzyme-linked immunosorbent assay (Table 1) and in Western blots (data not shown). A control serum was obtained from a healthy donor not suffering from any skin disease (control IgG). Purification of whole IgG fractions was performed using protein A-agarose affinity chromatography as described earlier (17). The amount of IgG used in each experiment was adjusted to 200–500 μg/ml.
TABLE 1.
Antibody profile of IgG in patients
| ELISAa score | Dsg3 | Dsg1 | Dsc3 |
|---|---|---|---|
| units/ml | units/ml | units/ml | |
| PV-IgG 1 | 6586 | − | − |
| PV-IgG 2 | 787 | 656 | − |
| PF-IgG | − | 97 | − |
a ELISA is enzyme-linked immunosorbent assay.
Recombinant Dsc3-Fc, Dsg1-Fc, and Dsg3-Fc
Human Dsc3a cDNA was a kind gift from Takashi Hashimoto (Department of Dermatology, Kurume University School of Medicine, Kurume, Japan). The complete extracellular Dsc3 domain, including the endoplasmic reticulum signal sequence, was amplified using 5′-ggatcagctagccaccatggccgccgctgggccccggcgct-3′ and 5′-ccgctcgagttttccaagtattactcctgtactc-3′ (introduced NheI and XhoI restriction sites are underlined) primers and cloned in-frame with the cDNA encoding the Fc fragment of human IgG 1 (including the hinge region and Ig domains CH2 and CH3) using NheI/XhoI-digested plasmid pEGFP-Cad11-Fc (18). Chinese hamster ovarian cells were transfected with pEGFP-Dsc3-Fc using Effectene transfection reagent (Qiagen, Hilden, Germany) following the manufacturer's protocol and cultured in DMEM in 140-mm Petri dishes. Purification of secreted Dsc3-Fc from supernatant was performed using protein A affinity chromatography as described earlier (17). Expression and purification of Dsg1-Fc and Dsg3-Fc followed a similar procedure and were explained in detail elsewhere (15). Purity was determined by Coomassie Brilliant Blue staining of 7.5% SDS-PAGE and Western blot detection.
Conformational Epitope Modeling
Sequence comparisons with sequences of cadherins with known structures identified C-cadherin as the closest homologue for the EC1-EC2 pair of Dsc3 and Dsg1. Thus a three-dimensional model was built for Dsc3 and Dsg1 using the structure of the C-cadherin ectodomain (Protein Data Bank entry 1L3W) as a template. Amino acid sequences of human Dsc3 and Dsg1 EC1-EC2 pair were aligned to that of Xenopus laevis C-cadherin, and residues that differed between Dsc3 (or Dsg1) and C-cadherin EC1-EC2 were exchanged to finally present the amino acid sequence of human Dsg1 (or human Dsc3) using the software tool ProteinDesign in Quanta2008 (Accelrys Inc, San Diego). Insertions and deletions between Dsc3/Dsg1 and C-cadherin were located only in loop regions within the EC2 domain (i.e. the β2β3-, β3β4-, and β6β7-loop) and thus did not affect the cadherin fold or secondary structure arrangement. Because no insertions or deletions were found between the N-terminal EC1 domain of our Dsc3/Dsg1 models and the C-cadherin template, no large structural deviations were observed after refinement. Steric clashes between side chains were removed by short molecular dynamics simulations in vacuo excluding electrostatic energy terms. Because of the compliance of the amino acid sequence in Dsc3 and Dsg1 with the requirements for the strand swapping trans-interaction model (5) and the presence of several structures supporting this model, it was used for homology model building and structure analysis.
AFM Measurements
Characterization of homo- and heterophilic trans-interaction properties of Dsc3, Dsg1, and Dsg3 was performed with a Bioscope AFM driven by a Nanoscope III controller (Digital Instruments Inc.). Principle and setup have been extensively described earlier (18). Recombinant proteins were covalently linked to the tip of a Si3N4 AFM cantilever (Veeco Instruments, Santa Barbara, CA) and freshly cleaved mica (SPI Supplies, Unterfoehring, Germany) by flexible polyethyleneglycol spacers. This heterobifunctional linker contained an amino-reactive cross-linker group (N-hydroxysuccinimide ester) at one end to bind to amino groups of ethanolamine-functionalized tips and mica. The other end consisted of a benzaldehyde function for coupling to lysine residues of the recombinant protein. Functionalizing procedures were performed essentially as described (19), with the concentration of the linker adjusted to 6.6 mg/ml and the protein to 0.2 mg/ml. Binding frequency measurements were performed in HBSS buffer containing 1.3 mm Ca2+, and force distance cycles were recorded at 1 Hz vertical scan rate and 300 nm z-amplitude yielding a tip velocity of 600 nm/s. At least four different tip/mica combinations from a minimum of two different coating procedures were assayed for each condition with typically >2000 force distance cycles obtained. The resulting data were evaluated by Matlab-based software analysis (version, 7.0.1, Mathworks Inc., Natick, MA). For determination of τ0, AFM tip velocity was varied between 300 and 5500 nm/s.
Laser Tweezer
Laser tweezer trapping of microbeads coated with desmosomal cadherins was carried out as outlined previously (17). Recombinant Dsc3-Fc (10 mg/ml) was covalently linked to protein A-coated beads (Dynal Tech, Oslo, Norway). HaCaTs were grown for 8 days on glass coverslips. 10 μl of bead stock solution was added in 200 μl of DMEM. After settling for 30 min, 100 beads were measured, and the number of beads that could not be displaced by a 1064-nm Nd:Yag laser beam (adjusted to 42 milliwatt in focal plane) was taken as control value. After addition of mAbs or pemphigus-IgG for 30 min, beads were again exposed to the laser beam, and number of bound beads was compared with controls. For some experiments, PV-IgG 2 was incubated overnight at 4 °C with Dsg1-Fc-coated beads to remove Dsg1 Abs present in this IgG fraction. Beads were spun down, and supernatant was used in laser tweezer experiments.
Dispase-based Dissociation Assay
The technique was carried out essentially as described before (20). HaCaT cells were grown to confluency for 8 days in 12-well plates. After incubation with mAbs or pemphigus-IgG in DMEM for 24 h, cells were washed twice with HBSS and incubated for 30 min with 250 μl of Bacillus polymyxa-derived dispase II in HBSS (>2.4 units/ml; Sigma) to release monolayers from well bottoms. After exchange of dispase solution to 500 μl of HBSS, the monolayer was pipetted up and down five times with a 1-ml pipette at constant speed. The resulting fragments per well were counted under a binocular microscope and compared with control values from untreated wells.
Ex Vivo Model of Human Skin
As described previously in detail (21), a patch of epidermis was obtained from the upper thigh of two recently deceased humans who donated their bodies to the Institute of Anatomy and Cell Biology for scientific and teaching purposes. Viability of epidermis was ensured by color change after incubation with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma), and skin specimens were processed as described earlier in detail. Three samples per cadaver and experimental conditions were completely processed and checked for blister formation after hematoxylin and eosin staining according to standard procedures. Some sections were subjected to immunostaining as described earlier. Monoclonal Dsc3 (clone U-114, Progen), Dsg1 (clone p124, Progen), or rabbit polyclonal Dsg3 Abs (clone H-145, Santa Cruz Biotechnology) were used at a 1:100 dilution. Antibody-stained sections were examined and imaged with a confocal laser scanning microscope (LSM510) equipped with a 40× NA 1.3 oil plan NeoFluar objective (both Carl Zeiss Microimaging, Goettingen, Germany).
Statistics
Data were processed with SigmaStat (Systat Software GmbH, Erkrath, Germany). Data values were compared using unpaired Student's t test. Nonparametric Mann-Whitney rank sum test was used for non-Gaussian variables. Statistical significance was assumed for p < 0.05. Error bars in graphs represent S.E.
RESULTS
Dsc3 Homophilic and Heterophilic Binding
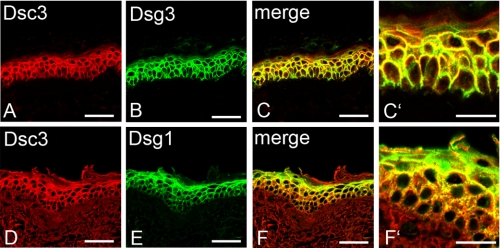
First, we studied the distribution of Dsc3, Dsg1, and Dsg3 in human epidermis. In cryosections of adult human skin, Dsc3 staining was present throughout the basal and the spinous layer of the epidermis with weak signals in the granular layer (Fig. 1). Dsg3 displayed a distribution similar to Dsc3 (Fig. 1, A–C′), whereas Dsg1 was expressed in all suprabasal layers of the epidermis but with most abundance in the granular layer (d–F′). In general, these data are consistent with previous studies on the distribution of Dsc3, Dsg1, and Dsg3 in human epidermis (7, 8). Thus, because of extensive overlap of Dsc3 distribution with expression patterns of both Dsg1 and Dsg3, we applied single molecule AFM to characterize Dsc3 homo- and heterophilic adhesion.
FIGURE 1.
Immunostaining of desmosomal cadherins in human epidermis. Dsc3 is expressed throughout basal, spinous, and lower granular layer similar to Dsg3 (A–C and C′). Dsg1 predominantly localizes in the granular layer but is still present in the spinous layer (E). Thus, Dsg1 staining extensively overlaps with Dsc3 staining throughout the epidermis (D, F, and F′). Bars, 50 μm in A–F and 20 μm in C′ and F′.
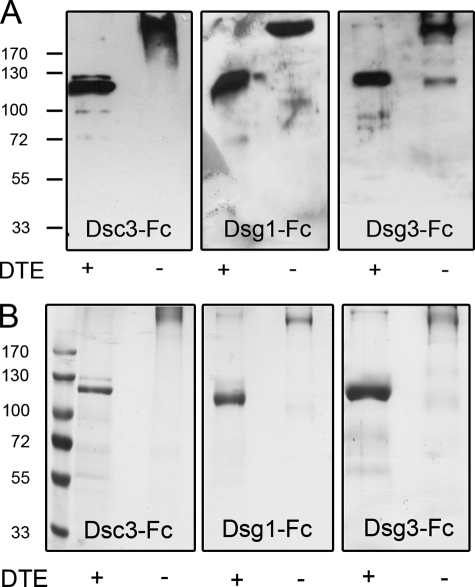
We functionalized AFM tips and mica with recombinantly generated fusion proteins of human Dsc3a, Dsg1, and Dsg3. These proteins consisted of two full-length extracellular domains of each protein coupled to the Fc part of human IgG 1 containing the hinge, C2, and C3 region. The proteins migrate at ∼250 kDa under nonreducing conditions in SDS-PAGE and could be converted to monomers of half the size by reduction of disulfide bonds with dithioerythritol (Fig. 2, A and B). Recently, it was demonstrated for Dsg1 and Dsg3 that pathogenic pemphigus antibodies preferentially recognize the mature form of these proteins (22, 23). High amounts of pro-protein after purification, which seem to be common to various extents when utilizing the baculovirus expression system, might mask effects of pathogenic pemphigus antibodies. Pro-proteins and mature Dsg and Dsc molecules can be distinguished by SDS-PAGE with the mature form being smaller because of proteolytic cleavage of the pro-protein in the Golgi complex. According to Fig. 2, the eukaryotic expression system used for this study does not seem to result in the production of significant amounts of pro-protein as only for Dsc3 a minor additional band appeared slightly above the main band.
FIGURE 2.
A, immunodetection of purified recombinant proteins with specific antibodies. B, Coomassie Blue staining of purified proteins. Proteins, consisting of two full-length extracellular domains of either Dsc3, Dsg1, or Dsg3 fused to the Fc part of human IgG 1, migrate around 250 kDa. Addition of dithioerythritol (DTE) to sample buffer served to reduce disulfide bonds, resulting in monomeric proteins of half the original size.
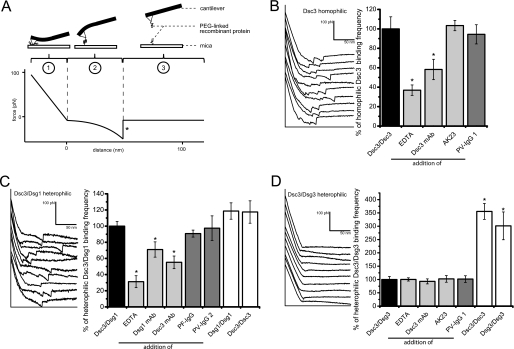
In AFM experiments we previously obtained evidence for homophilic trans-interaction of both Dsg1 and Dsg3 (3, 15, 17, 24). The deflection of a flexible cantilever attached to the AFM head is monitored during its approach and retrace to and from a fixed mica plate and recorded as force distance curves (Fig. 3A). AFM cantilevers and mica plates coated with entire extracellular domains of cadherins via short polyethyleneglycol linkers are cyclically brought into contact leading to retraction of the cantilever (Fig. 3A, ①). In case of a binding event the adhesion force between trans-interacting cadherins causes a downward bend of the cantilever in the following retrace movement during which the force acting on the cantilever will characteristically increase as indicated by a nonlinear slope of the retrace curve (Fig. 3A, ②). When the pulling force acting via the cantilever exceeds the adhesive force between the interacting cadherins, bonds will undergo disrupture, and the cantilever abruptly jumps back into its neutral position (Fig. 3A, marked by an asterisk in the retrace curve). The height of jump within the force-distance curve represents the unbinding force of the cadherin bonds. During the remaining retrace range, the cantilever stays in its neutral position (Fig. 3A, ③). The number of unbinding events recorded in a given series of force distance cycles was set to 100%. After addition of EDTA or specific Abs for 30 min, another set of force-distance cycles was recorded, and the number of unbinding events was compared with control value. The left panel of Fig. 3B shows typical retrace curves when recombinant Dsc3 was attached to plate and mica, i.e. homophilic binding was measured. The average number of unbinding events per force distance cycle (binding frequency) under this control condition was 0.41 ± 0.03. Addition of EDTA to buffer solution for 30 min to remove Ca2+ decreased binding activity to 36.9 ± 5.4% compared with control values (Fig. 3B, right panel). Specific inhibition with a mouse monoclonal Dsc3 Ab directed against the extracellular domain reduced activity to 58.2 ± 10.2% after 30 min. In antibody control experiments we used AK23, a monoclonal Dsg3 antibody (mAb) derived from a pemphigus mouse model (4), as well as PV-IgG 1 containing autoantibodies against Dsg3. Both Abs had no significant effect on Dsc3 trans-interaction (103.4 ± 5.3 and 94.4 ± 9.9%, respectively). These data provide strong evidence for homophilic binding activity of Dsc3.
FIGURE 3.
A, principle of AFM force measurements. B, example force curves obtained when homophilic Dsc3 interaction was measured (left panel) showing typical unbinding events. Homophilic Dsc3 trans-interaction frequency was attenuated by EDTA to remove Ca2+ and Dsc3 mAb targeting the extracellular domain (right panel), indicating specific binding events. C, example curves with numerous unbinding events when trans-interaction of Dsc3 and Dsg1 was evaluated (left panel). Binding frequency was attenuated by treatment with Dsc3 mAb, Dsg1 mAb, or EDTA (right panel). PF-IgG 1 and PV-IgG 2 had no effect. D, example force curves without specific unbinding events when trans-interaction of Dsc3 and Dsg3 was probed (left panel). The low binding frequency of Dsc3/Dsg3 trans-interaction was not reduced by Dsc3 mAb, AK23, or PV-IgG 1 and 2, indicating lack of specific binding events. n = 4–5 for every condition.
Next, we tested a possible interaction of Dsc3 with Dsg1. The force distance cycles were run with Dsc3 attached to the tip and Dsg1 to the mica or vice versa with the setup switched every test run. Binding frequency for heterophilic binding of Dsc3 and Dsg1 was 0.29 ± 0.01. The left panel in Fig. 3C shows typical retrace curves obtained with this experimental setup. Addition of EDTA reduced binding to 31.2 ± 7.4% of controls (Fig. 3C, right panel). Similarly, specific inhibition by mAbs targeting the extracellular domain of Dsc3 and Dsg1 significantly reduced binding frequency to 55.2 ± 7.8 and 71.0 ± 9.4%, respectively. In contrast, both PF-IgG and PV-IgG 2, although containing Dsg1 autoantibodies and used under conditions where they blocked keratinocyte cohesion as shown in cell dissociation assays (Fig. 5), did not significantly impair Dsc3/Dsg1 trans-interaction (90.7 ± 4.3 and 97.5 ± 15.2%, respectively). This is similar to our previous studies where PF-IgG and PV-IgG were found not to directly interfere with Dsg1 homophilic binding (15, 17). We then replaced either tip or plate by ones coated with the respective other cadherin to measure homophilic adhesion of Dsc3 as well as Dsg1. No significant differences compared with heterophilic Dsc3/Dsg1 binding (117.5 ± 13.9% for homophilic Dsc3 and 118.6 ± 10.1% for homophilic Dsg1) could be detected. Taken together, these data clearly demonstrate heterophilic trans-interaction of Dsc3 and Dsg1.
FIGURE 5.
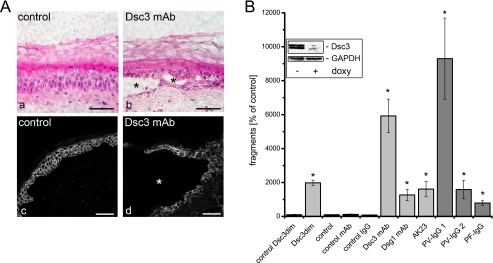
A, effects of blocking Dsc3 function in human epidermis. Incubation with DMEM did not affect morphology of human epidermis (panel a, hematoxylin and eosin staining) or linear Dsc3 distribution (panel c, immunostaining). In contrast, treatment with Dsc3 mAb for 24 h induced suprabasal cleavage (*, panels b and d) and fragmentation of Dsc3-staining (panel d). Bar, 50 μm. B, keratinocyte dissociation assays performed using HaCaT monolayers. Knockdown of Dsc3 with shRNA (induced by doxycycline (doxy), inset) and blocking of function with specific mAb increased the number of fragments compared with controls. Also, Dsg1 mAb and AK23 but not VE-cadherin (control) mAb induced fragment formation as well as PV-IgG 1 (Dsg3 Abs only), PV-IgG 2 (Dsg1/3 Abs), and PF-IgG (Dsg1 Abs only). n = 9. *, p < 0.05 compared with the control.
Interestingly, binding frequency of heterophilic Dsc3 interaction with Dsg3 was rather low (0.13 ± 0.01) compared with conditions described above. Representative retrace curves are shown in the left panel of Fig. 3D. To eliminate the possibility of a failed coating procedure, experiments were typically carried out as follows. First, homophilic binding of Dsc3 was measured. Second, the tip was then exchanged by a tip coated with Dsg3, and heterophilic binding on the same Dsc3-coated mica was evaluated. Finally, Dsc3 mica was replaced by mica coated with Dsg3 to measure homophilic Dsg3 binding. Under these conditions, binding frequency of both homophilic Dsc3 and homophilic Dsg3 trans-interaction increased to 355.7 ± 30.0 and 301.5 ± 52.2% of values obtained for heterophilic binding. Neither EDTA (100.3 ± 6.2%) nor Dsc3 mAb, AK23, or PV-IgG 1 significantly reduced the low frequency of heterophilic Dsc3/Dsg3 binding (92.9 ± 9.5, 102.3 ± 12.5, and 101.5 ± 12.5%, Fig. 3D, right panel). These data strongly indicate that Dsc3 does not significantly interact with Dsg3.
In a further series of experiments we varied cantilever retrace velocity and recorded resulting unbinding forces to determine the lifetime of a single bond. Unbinding force maxima displayed a logarithmic increase when pulling velocity was gradually raised from 300 to 5500 nm/s. Heterophilic Dsc3/Dsg1 interaction forces increased from 27 to 58 pN, homophilic Dsc3 forces from 24 to 66 pN, and homophilic Dsg3 forces from 24 to 61 pN. Thus, homophilic and heterophilic unbinding forces were very similar at given retrace velocities. However, fitting Bell's equation (25) to the unbinding forces yielded a lifetime of a single bond at zero force of τ0 ≈ 0.55 s for heterophilic Dsc3/Dsg1, which was about 2-fold higher compared with homophilic Dsc3 and Dsg3 interaction (≈0.24 and ≈0.31 s, respectively).
Conformational Epitope Modeling of Homophilic and Heterophilic Dsc3 Trans-interaction
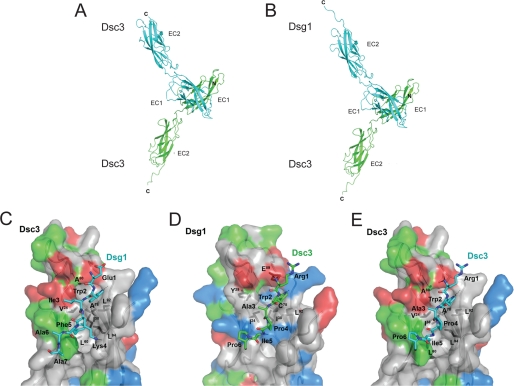
Because sequence comparisons identified C-cadherin as the closest homologue for the EC1-EC2 pair of Dsc3 and Dsg1, a three-dimensional model was built for Dsc3 and Dsg1 using the structure of the C-cadherin ectodomain (Protein Data Bank entry 1L3W) as a template. The strand-swapping model with the N-terminal conserved Trp residue swapped between the two molecules, which has been proposed (5) as a possible general interaction model for classical but also desmosomal cadherins, was used to model homo- or heterophilic interactions between Dsc3 and/or Dsg1 (Fig. 4).
FIGURE 4.
Modeling of the homo- and heterophilic trans-interaction of Dsc3/Dsc3 and Dsc3/Dsg1. Trans-interaction between two Dsc3 molecules (green and cyan) (A) and between Dsc3 (green) and Dsg1 (cyan) (B) is shown in ribbon representation. C, details of the heterophilic Dsc3-Dsg1 trans-interaction with Dsc3 shown as surface representation with the surface colored in red for negatively charged and in blue for positively charged amino acids and in green for polar residues. The N terminus of Dsg1 is shown in stick representation, and putative hydrogen bonds are shown as stippled lines. D, same as C but with Dsg1 shown as surface and the N terminus of Dsc3 as stick representation. E, same as C but for the interaction of homophilic Dsc3 trans-interaction. Residues of the interacting N terminus are labeled using the three-letter amino acid code, whereas residues of trans-interacting Dsc3 or Dsg1 molecules are indicated using the one-letter code.
Because the strand-swapping model is based on a “symmetrical” interaction with a (pseudo)2-fold axis located between the N termini of both EC1 domains of the homo- and heterophilic trans-interactions, the same secondary structure elements of both molecules are involved. Briefly, the N terminus of one cadherin domain (Dsc3, Arg-1 to Ala-5; Dsg1, Glu-1 to Phe-5) “intercalates” between the β2β3-loop (Dsc3, Gln-23 to Ala-28; Dsg1, Lys-23 to Cys-28) and the C-terminal end of the β6β7-loop (Dsc3, Ala-88 to Leu-92; Dsg1, Leu-88 to Leu-92) of the cadherin domain of the second interacting molecule. Hydrophobic forces dominate the interaction with the conserved Trp residue being surrounded by a cluster of hydrophobic residues, namely Val-24, Ala-78, and Leu-92 of Dsc3 (identical in Dsg1). In addition there is a hydrogen bond between the indole proton of Trp-2 and the main chain carbonyl of Leu-90 in the trans-interacting Dsc3 (also conserved in the interaction with Dsg1). There is a second hydrophobic interaction cluster, which is, however, different in the homo- and heterophilic interaction. In the homophilic Dsc3-Dsc3 interaction, the adjacent four residues of Trp-2 are all hydrophobic (Ala-3 to Pro-6) and pack intramolecularly against a hydrophobic surface patch in the β-strand 7 (Leu-92 to Pro-95). The side chain of the fifth residue in the N terminus, Ile-5 in Dsc3, is involved in a weak intermolecular interaction packing against Gln-22, Gln-23, and Val-24. In the heterophilic interaction, Ile-5 of Dsc3 is replaced by the larger Phe-5 in Dsg1. Together with the exchange of Pro-4 of Dsc3 to a Lys in Dsg1, the side chain of Phe-5 is located further into a hydrophobic cleft formed by Val-24, Leu-60, and Leu-94 of the trans-interacting Dsc3. These data suggest that heterophilic Dsc3/Dsg1 trans-interaction might be slightly stronger compared with homophilic Dsc3 binding.
Role of Dsc3 for Integrity of Human Epidermis and Cohesion of Cultured Keratinocytes
Because the Dsc3 mAb used in AFM experiments has proven to effectively block Dsc3 trans-interaction, we sought to use this approach to disturb Dsc3 function in the human skin model used in previous studies (13, 21). Incubation of small skin pieces with culture medium for 24 h did not affect the morphology of the epidermis as revealed by hematoxylin and eosin staining (Fig. 5A, panel a). In contrast, treatment with Dsc3 mAb for 24 h resulted in intraepidermal blister formation (Fig. 5A, panel b) with the cleavage plane typically located in suprabasal or spinous layers. Immunostaining for Dsc3 revealed that blisters were always located in areas with strong Dsc3 expression (Fig. 5A, panel d). Dsc3 staining changed markedly from a linear distribution under control conditions (Fig. 5A, panel c) to a fragmented pattern after incubation with Dsc3 mAb (Fig. 5A, panel d). These data show that Dsc3-mediated binding is also crucial for cohesion of human epidermis.
We next applied dispase-based dissociation assays on HaCaT keratinocyte monolayers to test their ability to withstand mechanical stress under conditions of impaired Dsc3-mediated adhesion (Fig. 5B). In controls, typically 1–8 fragments were counted. For conditional reduction of Dsc3 expression in HaCaT cells, we used an shRNA approach (Fig. 5B). 48 h following induction of Dsc3 knockdown by doxycycline, fragment numbers increased to 1080 ± 175% of noninduced controls. Similarly, incubation of normal HaCaT cells with Dsc3 mAb for 24 h resulted in strongly increased fragment numbers (5930 ± 990%), whereas a control mAb (directed against VE-cadherin) and control IgG from a healthy volunteer had no effect. Dsg1 mAb and AK23 increased fragments to 1260 ± 330 and 1610 ± 450% of controls, respectively. We next tested the potency of different pemphigus-IgG to weaken intercellular adhesion. Application of PV-IgG 1 for 24 h resulted in strong increase in fragment numbers (9300 ± 2390%), whereas PV-IgG 2 was less effective (1590 ± 540% fragments). Application of PF-IgG increased fragments to 790 ± 135%. Thus, conditional knockdown of Dsc3 via shRNA and treatment with Dsc3 mAb were similarly effective to impair intercellular adhesion, supporting the idea of a pivotal function of Dsc3 for cohesion of human keratinocytes.
Dsc3-mediated Binding in Cultured Keratinocytes Is Impaired by Pemphigus-IgG
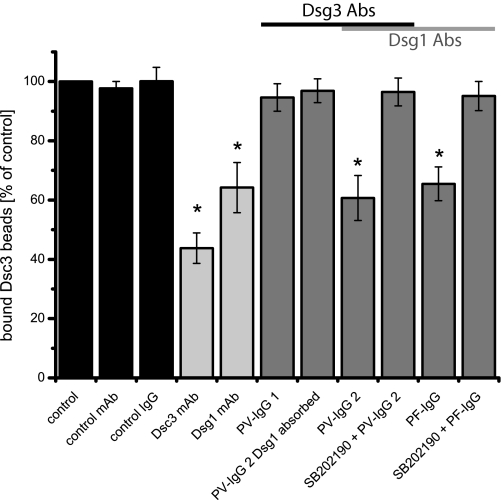
Because challenge of keratinocyte cohesion by dissociation assays interferes with the concerted action of all intercellular adhesion molecules, we next used a laser tweezer approach to determine whether pemphigus autoantibodies might affect Dsc3-mediated binding. Because laser trapping is based on binding of Dsc3-coated microbeads to desmosomal cadherins located on the keratinocyte cell surface, this approach does not only allow us to detect direct autoantibody-mediated interference but also to observe changes in cadherin binding mediated by cellular signaling pathways (3, 15, 17). Settling of cadherin-coated microbeads for 30 min on HaCaT cells induced formation of cell-to-bead contacts as described previously for Dsg1-coated beads (17). In our study, typically about 70% of settled Dsc3-coated beads could not be displaced by a laser beam and were thus considered tightly bound (control). After incubation with various antibody fractions for 30 min, the percentage of laser-resistant bound beads was determined again and compared with control values. Addition of Dsc3 mAb as well as of Dsg1 mAb reduced bead binding to 45.9 ± 5.0 and 64.2 ± 8.5% of controls (Fig. 6). However, PV-IgG 1 containing Abs against Dsg3 but not Dsg1 had no effect (94.6 ± 5.4%). Accordingly, PV-IgG 2 with absorbed Dsg1 Abs also did not interfere with Dsc3-mediated adhesion (96.9 ± 4.0%). In contrast to data obtained by AFM measurements, addition of unabsorbed PV-IgG 2 and PF-IgG both containing Dsg1 Abs reduced bead binding to 51.2 ± 6.5 and 65.5 ± 5.7% of controls, respectively. Because inhibition of p38MAPK has proven to effectively block pemphigus-IgG-mediated effects in vitro and in vivo, we applied the p38 MAPK inhibitor SB202190 to elucidate whether intracellular signaling processes participate in pemphigus-IgG-induced loss of Dsc3-mediated adhesion. Preincubation with 30 μm SB202190 for 1 h blocked the effects of PV-IgG 2 and PF-IgG on Dsc3-mediated binding (96.5 ± 4.7 and 95.1 ± 4.9% of controls, respectively). Taken together, these data indicate that pemphigus-IgG containing autoantibodies against Dsg1 interfere with Dsc3 adhesion.
FIGURE 6.
Laser tweezer experiments performed using HaCaT cells. Incubation with Dsc3 and Dsg1 mAbs as well as with PV-IgG 2 and PF-IgG for 30 min reduced binding of Dsc3-coated beads compared with controls. PV-IgG 1 (Dsg3 Abs only) or PV-IgG 2 with absorbed Dsg1 Abs had no effect. Reduction of bead binding could be prevented by inhibition of p38 MAPK with SB202190. n = 6. *, p < 0.05 compared with the control.
DISCUSSION
Our data provide evidence for a crucial role of Dsc3 in human epidermis. Blocking of Dsc3 function using a monoclonal antibody led to the formation of intraepidermal blisters. Moreover, treatment of human keratinocyte monolayers with the same Dsc3 mAb was similarly effective in reducing intercellular adhesive strength as sh-RNA-mediated knockdown of Dsc3. Because distribution of Dsc, Dsg1, and Dsg3 broadly overlapped in the skin sections examined, we investigated whether the two desmogleins heterophilically interact with Dsc3 on a single molecule level. Both homophilic Dsc3 binding as well as heterophilic trans-interaction of Dsc3 with Dsg1 were detected. In contrast, no evidence for specific trans-interaction of Dsc3 with Dsg3 was obtained. AFM revealed no significant differences in the unbinding forces of homophilic Dsc3 trans-interaction compared with homophilic Dsg3 or heterophilic Dsc3/Dsg1 interaction on a single molecule level. Because intraepidermal blistering and keratinocyte dissociation in response to the Dsc3 mAb closely resembled the effects of IgG fractions from pemphigus patients, we studied the effects of pemphigus IgG containing autoantibodies against Dsg3 and Dsg1 on Dsc3 binding to the surface of human keratinocytes using the laser tweezer technique. We found that Dsc3 bead binding was reduced by both PV-IgG and PF-IgG when containing autoantibodies against Dsg1. This effect was abolished by inhibition of p38 MAPK, which is known to be critical for pemphigus pathogenesis. Taken together, the data presented support the hypothesis that Dsc3 homo- and heterophilic binding is required for maintenance of keratinocyte cohesion and that interference with Dsc3 binding may contribute to skin blistering in pemphigus.
Dsc3 Plays a Critical Role in Keratinocyte Cohesion
In the ex vivo model of human skin splitting, the monoclonal antibody targeting the extracellular domain of Dsc3 induced intraepidermal blister formation similar to pemphigus autoantibodies in previous studies (13, 21). Dsc3 mAb treatment and shRNA-mediated knockdown of Dsc3 weakened adhesion in dissociation assays of cultured keratinocytes. These results support data obtained from a conditional Dsc3 knock-out mouse model (10). Epidermal deficiency for Dsc3 caused severe intraepidermal blister formation, which was even more pronounced than blistering in mice deficient for Dsg3 (26). Also, keratinocytes derived from this Dsc3 knock-out mouse model displayed reduced adhesion (10). On first glance, the more severe phenotype of Dsc3-deficient compared with Dsg3-deficient mice is surprising, especially because Dsg3 binding is impaired in the autoimmune disease pemphigus. However, according to our AFM measurements, Dsc3 undergoes both homo- and heterophilic interaction, whereas Dsg3 has only been shown to trans-interact homophilically (15, 29). Therefore, it can be speculated that Dsc3 is essential for epidermal integrity because it is able to undergo heterophilic binding to Dsg 1 in addition to homophilic trans-interaction. To address this important question, we characterized the molecular binding properties of homo- and heterophilic Dsc3 adhesion.
Heterophilic Trans-interaction Does Not Appear to be a General Principle for Desmosomal Cadherins
Using AFM measurements, we found evidence for homophilic Dsc3 interaction and heterophilic Dsc3/Dsg1 binding but not for specific Dsc3/Dsg3 trans-interaction. This observation is remarkable because in previous studies Ca2+-dependent heterophilic binding of Dsc2 and Dsg2 was demonstrated by BIAcore measurements using recombinant EC1-EC2 constructs (27). Similarly, for Dsc2 and Dsg1, heterophilic binding was shown by cell aggregation assays using cells expressing either Dsc2 or Dsg1 (28). In view of these data, heterophilic trans-interaction of desmosomal cadherins does not appear to be a general principle. This is underscored by our observation that heterophilic interaction of Dsg1 and Dsg3 does not occur on a single molecule level (15). Thus, heterophilic interaction of desmosomal cadherins likely depends on the isoforms involved, whereas similar to classical cadherins homophilic trans-interaction seems to be more of a general rule. So far, homophilic interaction of desmosomal cadherins has been demonstrated for Dsg1–3, Dsc2, and Dsc3 (15, 17, 27, 29). Further studies are required to evaluate which isoforms of desmosomal cadherins do heterophilically interact and to elucidate the mechanisms underlying trans-interaction of specific isoforms.
Our modeling data suggest that the interaction mechanism of homophilic Dsc3 binding slightly differs from interaction of Dsc3 with Dsg1. The stronger hydrophobic interaction and the additional intermolecular hydrogen bond between the side chain amino group of Lys-4 of Dsg1 and the main chain amide of Ile-5 of Dsc3 suggest that heterophilic trans-interaction of Dsc3 and Dsg1 might even be slightly stronger than the homophilic Dsc3 interaction. However, adhesion force measurements did not reveal significant differences between homophilic Dsg3, homophilic Dsc3, and heterophilic Dsc3/Dsg1 interaction. By varying the retrace velocity of the AFM tip, we determined the lifetime of single unstressed bonds for these interaction pairs and found the values to be within the range of other desmosomal and nondesmosomal cadherins such as Dsg1 and VE-, E-, N-, and LI-cadherin (24, 30–34). However, among desmosomal cadherins studied so far by AFM, the lifetime of heterophilic trans-interaction was about 2-fold longer compared with homophilic binding, which might reflect the possibly stronger heterophilic binding indicated by conformational epitope modeling. It is intriguing to speculate that such differences in the molecular binding mechanism may help to explain why heterophilic adhesion of desmosomal cadherins appears to be important for epidermal integrity.
Role of Dsc3 Binding in Pemphigus
Our data show that pemphigus IgG reduces the adhesion of Dsc3-coated beads to the surface of human keratinocytes and thus interferes with Dsc3-mediated binding. However, these effects were only detectable when Dsg1 antibody-containing IgG fractions were used. These experiments indicate that primarily Dsg1 autoantibodies inhibit Dsc3-mediated binding, possibly by targeting heterophilic Dsc3/Dsg1 binding on the keratinocyte cell surface as can be assumed from AFM measurements. It is not surprising that Dsc3 and Dsg1 mAbs reduced bead binding because they were also effective at directly interfering with heterophilic Dsc3/Dsg1 trans-interaction on a single molecule level. In contrast, PV-IgG 2 and PF-IgG both reduced Dsc3 bead binding but were ineffective at directly inhibiting Dsg1 trans-interaction in AFM studies. However, application of the p38 MAPK inhibitor SB202190, which is known to effectively block PV- and PF-IgG-mediated effects both in vitro and in vivo (35–38), abolished the interference with Dsc3 bead binding, indicating that heterophilic Dsc3/Dsg1 interaction may be reduced by autoantibody-triggered cellular signaling events. Similar findings were obtained in earlier studies where PF-IgG reduced binding of Dsg3-coated beads to the keratinocyte cell surface but was not effective at blocking trans-interaction in a cell-free system (13, 15).
Because PV-IgG 1 (Dsg3 Abs only) and PV-IgG 2 with absorbed Dsg1 Abs in contrast to PF-IgG and PV-IgG 2 (both containing Dsg1 Abs) did not reduce binding of Dsc3-coated beads, one may speculate whether this effect may be linked to epidermal involvement in PV and PF. Because mucosal dominant PV is characterized by autoantibodies against Dsg3 only, whereas epidermal involvement in PV is usually associated with a combination of Dsg1 and Dsg3 Abs, it is at least possible that autoantibody-induced loss of heterophilic Dsc3/Dsg1 interaction contributes to skin blistering in pemphigus. As outlined above, this may be relevant because our data suggest that the molecular binding properties of heterophilic Dsc3/Dsg1 interaction may result in stronger adhesion compared with homophilic trans-interaction of other types of desmosomal cadherins. Moreover, because circulating autoantibodies targeting Dsc3 have been described in patients with atypical pemphigus (12, 39), interference with Dsc3 binding may be of pathogenic relevance.
Collectively, our data do the following: 1) provide evidence for a pivotal role of Dsc3-mediated adhesion for the integrity of the human epidermis; 2) identify Dsc3 and Dsg1 but not Dsg3 as trans-interaction partners of Dsc3; and 3) for the first time indicate a possible implication of Dsc3 in pemphigus pathogenesis.
Acknowledgments
We thank Lisa Bergauer, Tetjana Frantzeskakis, and Seraj Khan for excellent technical assistance. Human Dsc3a cDNA was a kind gift of Takashi Hashimoto (Department of Dermatology, Kurume University School of Medicine, Kurume, Japan).
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB487 (TP B5 and B2).
- Dsg
- desmoglein(s)
- Dsc
- desmocollin
- AFM
- atomic force microscopy
- PV
- pemphigus vulgaris
- PF
- pemphigus foliaceus
- Ab
- antibody
- mAb
- monoclonal antibody
- DMEM
- Dulbecco's modified Eagle's medium
- HBSS
- Hanks' balanced saline solution
- pN
- piconewton
- shRNA
- short hairpin RNA.
REFERENCES
- 1.Waschke J. (2008) Histochem. Cell Biol. 130, 21–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kottke M. D., Delva E., Kowalczyk A. P. (2006) J. Cell Sci. 119, 797–806 [DOI] [PubMed] [Google Scholar]
- 3.Heupel W. M., Müller T., Efthymiadis A., Schmidt E., Drenckhahn D., Waschke J. (2009) J. Biol. Chem. 284, 8589–8595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsunoda K., Ota T., Aoki M., Yamada T., Nagai T., Nakagawa T., Koyasu S., Nishikawa T., Amagai M. (2003) J. Immunol. 170, 2170–2178 [DOI] [PubMed] [Google Scholar]
- 5.Posy S., Shapiro L., Honig B. (2008) J. Mol. Biol. 378, 954–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Getsios S., Huen A. C., Green K. J. (2004) Nat. Rev. Mol. Cell Biol. 5, 271–281 [DOI] [PubMed] [Google Scholar]
- 7.Nuber U. A., Schäfer S., Stehr S., Rackwitz H. R., Franke W. W. (1996) Eur. J. Cell Biol. 71, 1–13 [PubMed] [Google Scholar]
- 8.Mahoney M. G., Hu Y., Brennan D., Bazzi H., Christiano A. M., Wahl J. K., 3rd (2006) Exp. Dermatol. 15, 101–109 [DOI] [PubMed] [Google Scholar]
- 9.Garrod D., Chidgey M. (2008) Biochim. Biophys. Acta 1778, 572–587 [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Den Z., Koch P. J. (2008) J. Cell Sci. 121, 2844–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley J. R., Amagai M. (2006) N. Engl. J. Med. 355, 1800–1810 [DOI] [PubMed] [Google Scholar]
- 12.Hisamatsu Y., Amagai M., Garrod D. R., Kanzaki T., Hashimoto T. (2004) Br. J. Dermatol. 151, 73–83 [DOI] [PubMed] [Google Scholar]
- 13.Spindler V., Drenckhahn D., Zillikens D., Waschke J. (2007) Am. J. Pathol. 171, 906–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotsch U., Borges E., Bosse R., Böggemeyer E., Simon M., Mossmann H., Vestweber D. (1997) J. Cell Sci. 110, 583–588 [DOI] [PubMed] [Google Scholar]
- 15.Heupel W. M., Zillikens D., Drenckhahn D., Waschke J. (2008) J. Immunol. 181, 1825–1834 [DOI] [PubMed] [Google Scholar]
- 16.Müller R., Heber B., Hashimoto T., Messer G., Müllegger R., Niedermeier A., Hertl M. (2009) Clin. Exp. Dermatol., in press [DOI] [PubMed] [Google Scholar]
- 17.Waschke J., Bruggeman P., Baumgartner W., Zillikens D., Drenckhahn D. (2005) J. Clin. Invest. 115, 3157–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heupel W. M., Baumgartner W., Laymann B., Drenckhahn D., Golenhofen N. (2008) Mol. Cell. Neurosci. 37, 548–558 [DOI] [PubMed] [Google Scholar]
- 19.Ebner A., Wildling L., Kamruzzahan A. S., Rankl C., Wruss J., Hahn C. D., Holzl M., Zhu R., Kienberger F., Blaas D., Hinterdorfer P., Gruber H. J. (2007) Bioconjugate Chem. 18, 1176–1184 [DOI] [PubMed] [Google Scholar]
- 20.Schmidt E., Gutberlet J., Siegmund D., Berg D., Wajant H., Waschke J. (2009) Am. J. Physiol. Cell Physiol. 296, C162–C172 [DOI] [PubMed] [Google Scholar]
- 21.Waschke J., Spindler V., Bruggeman P., Zillikens D., Schmidt G., Drenckhahn D. (2006) J. Cell Biol. 175, 721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma P. M., Choi E. J., Kuroda K., Hachiya T., Ishii K., Payne A. S. (2009) J. Invest. Dermatol. 129, 2309–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokouchi M., Saleh M. A., Kuroda K., Hachiya T., Stanley J. R., Amagai M., Ishii K. (2009) J. Invest. Dermatol. 129, 2156–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waschke J., Menendez-Castro C., Bruggeman P., Koob R., Amagai M., Gruber H. J., Drenckhahn D., Baumgartner W. (2007) J. Membr. Biol. 216, 83–92 [DOI] [PubMed] [Google Scholar]
- 25.Bell G. I. (1978) Science 200, 618–627 [DOI] [PubMed] [Google Scholar]
- 26.Koch P. J., Mahoney M. G., Ishikawa H., Pulkkinen L., Uitto J., Shultz L., Murphy G. F., Whitaker-Menezes D., Stanley J. R. (1997) J. Cell Biol. 137, 1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syed S. E., Trinnaman B., Martin S., Major S., Hutchinson J., Magee A. I. (2002) Biochem. J. 362, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcozzi C., Burdett I. D., Buxton R. S., Magee A. I. (1998) J. Cell Sci. 111, 495–509 [DOI] [PubMed] [Google Scholar]
- 29.Amagai M., Kàrpàti S., Klaus-Kovtun V., Udey M. C., Stanley J. R. (1994) J. Invest. Dermatol. 103, 609–615 [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner W., Hinterdorfer P., Ness W., Raab A., Vestweber D., Schindler H., Drenckhahn D. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 4005–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgartner W., Golenhofen N., Grundhöfer N., Wiegand J., Drenckhahn D. (2003) J. Neurosci. 23, 11008–11014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgartner W., Wendeler M. W., Weth A., Koob R., Drenckhahn D., Gessner R. (2008) J. Mol. Biol. 378, 44–54 [DOI] [PubMed] [Google Scholar]
- 33.Perret E., Benoliel A. M., Nassoy P., Pierres A., Delmas V., Thiery J. P., Bongrand P., Feracci H. (2002) EMBO J. 21, 2537–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendeler M. W., Drenckhahn D., Gessner R., Baumgartner W. (2007) J. Mol. Biol. 370, 220–230 [DOI] [PubMed] [Google Scholar]
- 35.Berkowitz P., Chua M., Liu Z., Diaz L. A., Rubenstein D. S. (2008) Am. J. Pathol. 173, 1628–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkowitz P., Diaz L. A., Hall R. P., Rubenstein D. S. (2008) J. Invest. Dermatol. 128, 738–740 [DOI] [PubMed] [Google Scholar]
- 37.Berkowitz P., Hu P., Liu Z., Diaz L. A., Enghild J. J., Chua M. P., Rubenstein D. S. (2005) J. Biol. Chem. 280, 23778–23784 [DOI] [PubMed] [Google Scholar]
- 38.Berkowitz P., Hu P., Warren S., Liu Z., Diaz L. A., Rubenstein D. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12855–12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolling M. C., Mekkes J. R., Goldschmidt W. F., van Noesel C. J., Jonkman M. F., Pas H. H. (2007) Br. J. Dermatol. 157, 168–173 [DOI] [PubMed] [Google Scholar]