Abstract
DNA double-stranded breaks (DSBs) are lethal if not repaired and are highly mutagenic if misrepaired. Nonhomologous end joining (NHEJ) is one of the major DSB repair pathways and can rejoin the DSB ends either precisely or with mistakes. Recent evidence suggests the existence of two NHEJ subpathways: conservative NHEJ (C-NHEJ), which does not require microhomology and can join ends precisely; and deletional NHEJ (D-NHEJ), which utilizes microhomology to join the ends with small deletions. Little is known about how these NHEJ subpathways are regulated. Mre11 has been implicated in DNA damage response, thus we investigated whether Mre11 function also extended to NHEJ. We utilized an intrachromosomal NHEJ substrate in which DSBs are generated by the I-SceI to address this question. The cohesive ends are fully complementary and were either repaired by C-NHEJ or D-NHEJ with similar efficiency. We found that disruption of Mre11 by RNA interference in human cells led to a 10-fold decrease in the frequency of D-NHEJ compared with cells with functional Mre11. Interestingly, C-NHEJ was not affected by Mre11 status. Expression of wild type but not exonuclease-defective Mre11 mutants was able to rescue D-NHEJ in Mre11-deficient cells. Further mutational analysis suggested that additional mechanisms associated with methylation of Mre11 at the C-terminal glycine–arginine-rich domain contributed to the promotion of D-NHEJ by Mre11. This study provides new insights into the mechanisms by which Mre11 affects the accuracy of DSB end joining specifically through control of the D-NHEJ subpathway, thus illustrating the complexity of the Mre11 role in maintaining genomic stability.
DNA double-stranded breaks (DSBs)3 can be produced in physiological and genotoxic processes. Improper repair or failure to repair DSBs can lead to gene deletions, duplications, translocations, and missegregation of large chromosome fragments, which may result in gene dosage imbalance, cancer development, or cell death (1–3). Historically, two distinct pathways have been described which ensure that DSBs are repaired: nonhomologous end joining (NHEJ) and homologous recombination (HR). During HR, the damaged chromosome interacts via synapsis with an undamaged DNA molecule with which it shares extensive sequence homology, usually its sister chromatid (4, 5). HR is most active in the late S and G2 phases of the cell cycle. In contrast, NHEJ is active throughout the cell cycle and requires little or no DNA homology during repair; thus, it is traditionally considered an error-prone repair pathway (6, 7). However, accumulating evidence from recent studies suggests that there exists an error-free NHEJ subpathway (8, 9).
Two types of end-joining reactions can be defined operationally. The first one, which may be called conservative NHEJ (C-NHEJ), is characterized by the precise joining of short, overhanging, complementary ends. Proteins including Ku70/Ku80 and XRCC4 (10–12) are associated with this highly efficient pathway, whereby most ends are rejoined successfully without any alteration of the DNA sequence (8). The alternative pathway for NHEJ is the highly mutagenic and deletional NHEJ (D-NHEJ), which results in short deletions after use of imperfect microhomology of about 1–10 bp at the repair junctions. D-NHEJ activity has been demonstrated in the budding yeast Saccharomyces cerevisiae. In addition, D-NHEJ is independent of Rad52, Rad1, or Ku80 but depends on Mre11 in yeast (13, 14). However, the genetic determinants of this subpathway have not been well established in mammalian cells.
Mre11 is the core subunit of the Mre11·Rad50·Nbs1 complex (called the MRN complex), which is conserved throughout all kingdoms of life. The MRN complex is a central player in most aspects of the cellular response to DSBs, including HR, NHEJ, telomere maintenance, and DNA damage checkpoints (15–17). Loss of Mre11 results in increased radiosensitivity and chromosomal instability (17). Patients with germ line mutations of Mre11 have clinical presentations similar to those of ataxia telangiectasia patients (ataxia telangiectasia-like disorder) (18).
After DNA damage, the MRN complex is recruited to the sites of damage via zinc hooks at the ends of the long, flexible arms of Rad50 (19, 20). Mre11 contains both single-stranded DNA endonuclease and 3′-5′ exonuclease activities in vitro, but in vivo Mre11 is also implicated in 5′-3′ DSB resection. The MRN complex also interacts with BRCA1 and CtIP, which may be essential for DSB end resection to generate 3′ overhanging single-stranded DNA during initiation of HR (21, 22).
Mre11 has an N-terminal nuclease domain, which contains five phosphoesterase motifs, and a C-terminal glycine–arginine-rich domain (GAR). Arthur et al. (23) showed that an H85L mutation completely abrogated exonuclease activity, whereas binding to Rad50 and Nbs1 was retained. Complementation of ataxia telangiectasia-like disorder cells with this mutant, called Mre11-3, restored the localization of the MRN complex to DSBs in IR-induced foci (23, 24). Methylation of the GAR region has also been shown to be important for the DNA binding and exonuclease activity of Mre11 in vitro (25, 26). Both the crystal structure of yeast Mre11 and data from conditional knock-out mice (Mre11H129N/Δ) reveal that the nuclease activity of Mre11 is required for HR repair of DSBs (22, 27). However, the role of Mre11 in NHEJ is not well defined (27, 28). Most recently, Mre11 was reported to support NHEJ in mammalian cell (29–31). However, whether Mre11 regulates both NHEJ subpathways or only D-NHEJ is controversial, and the mechanisms by which Mre11 is involved in NHEJ remain to be established.
To address these questions, we have established a system that can analyze the accuracy and efficiency of rejoining of two adjacent DSB ends at chromosomal level in human embryonic kidney 293 (HEK293) cells. We show here that Mre11 siRNA knockdown in these cells results in significant reduction of the overall NHEJ efficiency. Upon sequencing the repair junctions, we found that Mre11 siRNA knockdown suppressed D-NHEJ by ∼10-fold, reflected by a reduction of small deletions in the repair junction, but it had no effect on the efficiency of C-NHEJ. Mutation of Mre11 in either the phosphoesterase domain (Mre11-3) or the GAR region (Mre11-R/A) to produce abnormal exonuclease activity impaired the D-NHEJ pathway only. The D-NHEJ deficiency is significantly more severe in cells with Mre11-R/A than that in cells with Mre11-3. Therefore, our data suggest that Mre11 is required specifically for D-NHEJ repair of DNA DSBs and that its exonuclease activity is at least one of the important mechanisms for this DNA end joining subpathway.
EXPERIMENTAL PROCEDURES
Cell Line and Plasmid Construction
HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin/streptomycin, and glutamine. The NHEJ reporter plasmid pPHW1 was described previously (9, 32). pPHW1 was linearized with PvuI and transfected into HEK293 cells using FuGENE 6 (Roche Applied Science). Puromycin (5 μg/ml; Sigma) was added 48 h after transfection for selection. Southern blotting was performed to confirm that a single copy of pPHW1 was integrated at a single locus. This cell line (HEK293/pPHW1) was maintained in complete Dulbecco's modified Eagle's medium containing 2 μg/ml puromycin. To create DSB within the chromosomally integrated NHEJ substrate, meganuclease I-SceI was expressed in the HEK293/pPHW1 cells by transient transfection of the expression plasmid pCMV-I-SceI.
Wild-type or mutant Mre11 cDNA was inserted into pcDNA3-Myc-HisC vector between the KpnI and NotI restriction sites, creating plasmids pcDNA3-Myc-Mre11-WT, pcDNA3-Myc-Mre11-R/A, and pcDNA3-Myc-Mre11-3. The Mre11-3 protein has a double mutation in its third phosphoesterase domain, specifically, His129-Asp130 was converted to Leu129-Val130. The Mre11-R/A protein has a R587A mutation in its GAR region (23, 25). Both mutant proteins lack exonuclease activity and showed resistance to Mre11 siRNA silencing.
siRNA Knockdown and Western Blotting
The sequences for Mre11 siRNA and scrambled control siRNA were as follows: Mre11 siRNA, 5′-GAACCUGGUCCCAGAGGAGdTdT-3′; and control siRNA, 5′-GGUUUGGCUGGGGUGUUAUdTdT-3′. The target for Mre11 siRNA oligonucleotides is located in the 5′-untranslated region of Mre11 mRNA, so the HEK293/pPHW1 cell line containing Mre11 cDNA (pcDNA3-Myc-Mre11-WT, Mre11-R/A, or Mre11-3) can be resistant to Mre11 siRNA silencing. The oligonucleotides were synthesized by Dharmacon, Inc. (Lafayette, CO). Transfection of siRNA duplexes was carried out by Oligofectamine (Invitrogen). Expression levels of Mre11 were measured at different time points after transfection by Western blotting. The following antibodies were used: rabbit anti-Mre11 (1:1000; Oncogene, Real Carpinteria, CA), rabbit anti-Myc (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), and mouse anti-actin (1:1000; Sigma). Proteins were visualized with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (1:5000; Santa Cruz, CA) followed by the use of an ECL chemiluminescence system (Amersham Biosciences).
Chromosomal NHEJ Assay
Colony Formation Assay
For the quantitative NHEJ assay, the HEK293/pPHW1 cells were transfected with Mre11 siRNA or the scrambled control siRNA duplexes. The transfection was repeated after 24 h. After 72 h, the cells were transfected with pCMV-I-SceI or a control vector plasmid. pcDNA3.1 and pEGFP-N1 were used for mock and transfection efficiency controls, respectively. To allow for I-SceI expression and recombination to proceed, cells were grown for 24 h in nonselective medium. Cells were then replated at densities between 104 and 105 cells/100 cm2, and after 16 h of incubation the medium was changed to XHATM (xanthine, hypoxanthine, aminopterin, thymidine, and mycophenolic acid at 10, 13.6, 0.17, 3.87, and 10 mg/ml respectively; Sigma). Cells were grown for another 2–3 weeks with medium exchanges twice a week. Control cells were grown in puromycin without XHATM. Clones containing more than 50 cells were scored. The NHEJ frequency was derived from the number of XHATM-resistant colonies/the number of cells seeded, corrected for transfection efficiency and plating efficiency.
PCR and Junction Sequence Analysis
To analyze the molecular feature of the junction and the fidelity joined ends, transfection of siRNA and pCMV-I-SceI was performed as before. To allow recombination to proceed, cells were grown for 24 h in nonselective medium. Cells were then replated at densities of 100 cells/100 cm2. Cells were grown for another 2–3 weeks with either nonselective or selective medium containing XHATM. The genomic DNA was extracted. The types of NHEJ repair junctions after I-SceI cleavage were measured by PCR with primers flanking the two I-SceI sites. The primers were as follows: forward primer, 5′-TCTCCGCCCCATGGCTGACTA-3′; and reverse primer, 5′-CTGCATGGATCTGCAACATGTC-3′. The genomic DNA was PCR-amplified, The PCR products were either subjected to agarose electrophoresis or subcloned into a pGEM-T vector (Promega, Madison, WI) for sequencing. The sequencing primers were as follows: forward primer, 5′-TCTCCGCCCCATGGCTGACT-3′; and reverse primer, 5′-AGATTAGCGACCGGAGATTG-3′.
Quantitative Real-time PCR Analysis
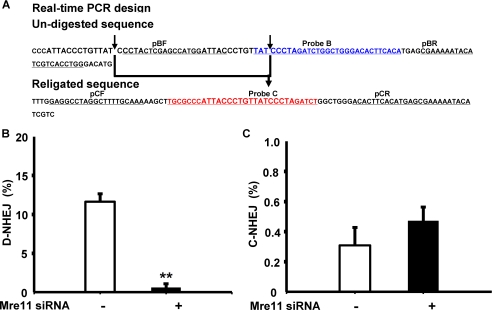
To quantify the C-NHEJ efficiency, the HEK293/pPHW1 cells were transfected with siRNA and I-SceI as before. Genomic DNA was extracted. Quantitative real-time PCRs were carried out with the TaqMan® Gene Expression Master Mix (Applied Biosystems, Foster City, CA). Probe B was designed to amplify only the intact NHEJ substrate, and probe C was designed to amplify only the accurate religation (C-NHEJ) product. The probe B sequence was 5′-TATCCCTAGATCTGGCTGGGACACTTCACA-3′. The probe C sequence was 5′-TGCGCCCATTACCCTGTTATCCCTAGATCT-3′. Both sequences contain I-SceI cut sites. The primer sequences for the intact substrate were as follows: probe B forward, 5′-CCTACTCGAGCCATGGATTAC-3′; and probe B reverse, 5′-CCAGGTGACGATGTATTTTTCG-3′. The primer sequences for the religation substrate were as follows: probe C forward, 5′-GAGGCCTAGGCTTTTGCAAA-3′; and probe C reverse, 5′-TGTATTTTTCGCTCATGTGAAGTGT-3′. RNaseP probe was used for an internal control. For quantification of precise NHEJ repair products, we isolated a stable cell line designated RHW1, in which the I-SceI-induced DSBs was repaired by precise end joining event (C-NHEJ). The standard curve for the number of C-NHEJ events measured using real-time PCR was generated from series dilution of the genomic DNA from RHW1. The values for C-NHEJ repair events were obtained from the standard curve generated using genomic DNA from RHW1. D-NHEJ efficiency was calculated as follows: D-NHEJ = 100% − value of religation (probe C) − value of uncut (probe B). All probes were purchased from ABI (Applied Biosystems).
Statistical Analysis
The data were analyzed via two-way analysis of variance followed by a Bonferroni post test using GraphPad Prism version 4.02 for Windows (GraphPad Software, San Diego, CA) (*, p < 0.05; **, p < 0.001).
RESULTS
Strategy and Generation of the HEK293/pPHW1 Cell Line
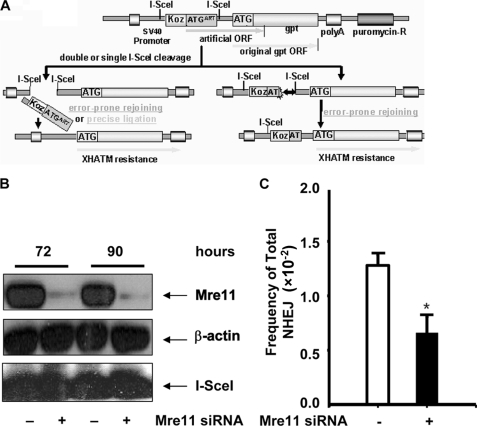
To investigate the mechanisms that control the accuracy of NHEJ-mediated DNA DSB repair in mammalian cells, we generated a cell line in which a single copy of the NHEJ substrate pPHW1 was stably integrated into the genome (HEK293/pPHW1 cells). The structure of the NHEJ substrate and the strategy to measure NHEJ were described previously (9, 32, 33) and are depicted in Fig. 1A. Briefly, an artificial ATG (ATGART)-driven proximal open reading frame (ORF) is dominant over the downstream gpt ORF, thereby preventing gpt translation. The two I-SceI sites, which are separated by 30 bases containing the first ATGART, are in the same orientation, creating fully complementary cohesive 3′-OH single-stranded ends of 4 bases upon double cleavage and loss (“pop-out”) of the intervening nucleotides, including ATGART. Rejoining of the double-stranded ends by either precise C-NHEJ or error-prone D-NHEJ will reconstitute translation of the gpt ORF, permitting cell survival under XHATM selection and detection of recombinants as colonies grown in XHATM selection medium. Alternatively, error-prone D-NHEJ at either of the single I-SceI sites can also disrupt ATGART, allowing again for selection of recombinants with XHATM. By contrast, precise religation at a single I-SceI site cannot be detected directly with XHATM selection using this system.
FIGURE 1.
Mre11 is required for overall NHEJ repair. A, diagram of construction of NHEJ substrate pPHW1 (adapted from ref. 32). An ORF is dominant over the downstream gpt ORF. Reconstituted translation of the gpt ORF will be resistant to XHATM selection. Both precise and error-prone rejoining as well as deletion during NHEJ can be detected. The total NHEJ efficiency is obtained from the formation efficiency of XHATM-resistant colonies. The repair events, i.e. D-NHEJ or C-NHEJ, are then assessed by analysis of the end junction sequence. B, Mre11 protein knockdown with siRNA. Levels of Mre11 protein and I-SceI expression were determined by Western blotting. β-Actin was included as a loading control. C, overall I-SceI-induced DSB end-rejoining efficiency in HEK293/pPHW1 cells with or without Mre11 knockdown was measured by cells resistant to XHATM selection. *, statistical difference in repair efficiency between control siRNA versus Mre11 siRNA (p < 0.05).
Suppression of Mre11 Reduces Total NHEJ Efficiency
To define the role of Mre11 in the NHEJ pathway, we first estimated the overall NHEJ frequency after Mre11 was knocked down with siRNAs in HEK293/pPHW1 cells. The expression of Mre11 was almost completely inhibited between 72 and 90 h after Mre11 siRNA transfection (Fig. 1B). Induction of DSBs was achieved through transfection of pCMV-I-SceI at 48 h after Mre11 knockdown. As indicated in Fig. 1B, siRNA knockdown did not affect I-SceI expression. When NHEJ occurs, the cells will survive and form colonies in XHATM medium. As shown in Fig. 1C, suppression of Mre11 protein expression resulted in more than 2-fold reduction of the overall NHEJ efficiency (1.28 × 10−2 in control siRNA versus 0.66 × 10−2 in Mre11 siRNA), which indicates that Mre11 is involved in the repair of DNA DSBs by NHEJ. However, it was not clear whether Mre11 status affected C-NHEJ, or D-NHEJ, or both.
Mre11 Knockdown Suppresses Small Deletion Events during DNA DSB End Joining
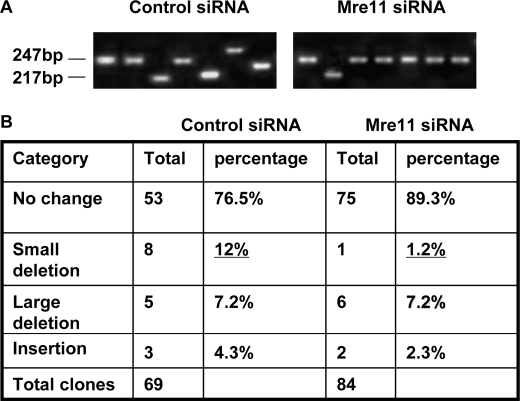
To characterize the repair product in repaired clones, we analyzed profiles of the repair junction by PCR with primers flanking the two I-SceI sites. To avoid selection bias, the colonies did not undergo XHATM selection for gpt expression. According to the DNA sequence surrounding the I-SceI sites in the HEK293/pPHW1 cell line, we categorized PCR products into four groups: (i) uncut or precise rejoining after I-SceI digestion at either of the single I-SceI cut sites or at both of the I-SceI cut sites, the PCR product is 247 bp; (ii) small deletion, we define small deletion as less than 30 bp that clones with the PCR fragments between 217 bp and 247 bp; (iii) large deletion, the PCR fragment is shorter than 217 bp; and (iv) insertion, the PCR fragment is longer than 247 bp. Representative profiles of PCR products are shown in Fig. 2A, and the distribution of the repair products is summarized in Fig. 2B. Without Mre11 silencing, 8 of 69 (12.0%) clones adapted small deletions, but in Mre11 knockdown cells, only 1 of 84 (1.2%) clones had a small deletion. Therefore, Mre11 siRNA knockdown suppressed D-NHEJ repair events compared with control siRNA clones by 10-fold (from 12% to 1.2%). Mre11 knockdown had no effect on insertions and large deletions (Fig. 2B). These results suggest that Mre11 mainly controls small deletions during NHEJ but has little role in either large deletions or insertion mutations during DNA damage repair.
FIGURE 2.
Silencing of Mre11 inhibits small deletion of NHEJ in HEK293/pPHW1 cells. A, profiles of NHEJ junctions with or without Mre11 silencing. PCR was performed with primers flanking the two I-SceI sites. B, quantitation of changes in NHEJ. To avoid selection bias, the colonies did not undergo XHATM selection for gpt expression.
Mre11 Enhances D-NHEJ but Has No Effect on C-NHEJ
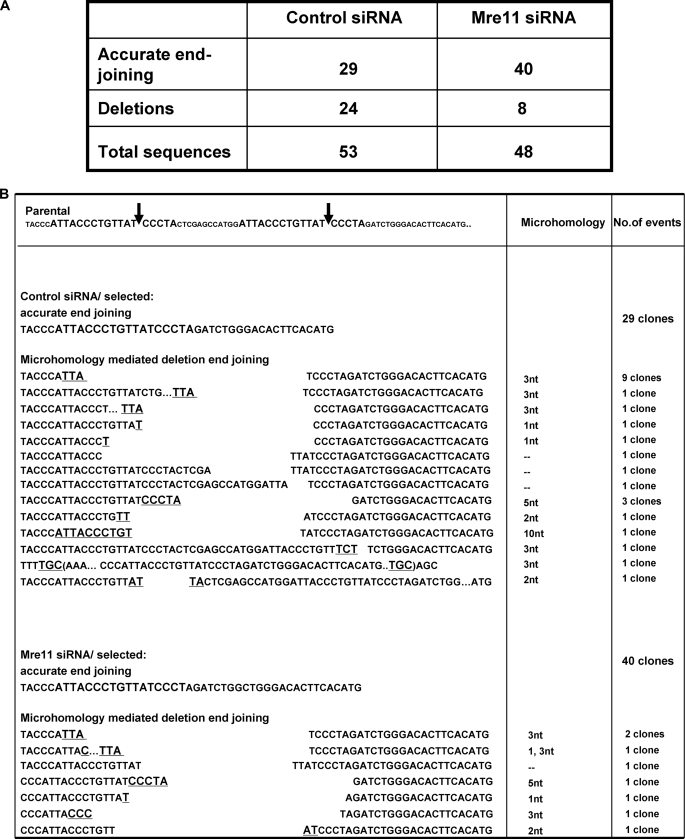
The electrophoresis of the PCR fragments by agarose gel did not allow us to define whether the distal ends were rejoined through precise relegation or via imprecise end joining with deletion/insertion of few base pairs. To define how Mre11 affects DNA rearrangement at the junction site and to determine whether Mre11 regulates both D-NHEJ and C-NHEJ, we analyzed the DNA sequences in the repaired products. Fifty-three repair junctions from control siRNA knockdown clones and 48 repair junctions from Mre11 siRNA knockdown clones were sequenced (Fig. 3A). In clones with control siRNA knockdown, 29 of 53 repair events (54.7%) were accurately religated, and 24 of 53 repair events (45.3%) were involved in microhomologies ranging from 1 to 10 nucleotides (Fig. 3B). In contrast, in clones with Mre11 knockdown, precise religation occurred in 40 of 48 clones (83.3%), whereas only 8 of 48 clones (16.7%) were repaired using microhomologies (Fig. 3B). Importantly, all rearrangements were restricted to the sequence around the I-SceI site, indicating that these mutations did not arise from the PCR or bacteria amplification steps, in which mutations should be distributed all along the sequenced DNA. Hence, as shown in Fig. 3C, the loss of Mre11 resulted in a shift in the molecular spectrum of end joining from comparable proportions of precise religation versus microhomology-mediated deletional end joining (54.7% versus 45.3%) in control siRNA-treated cells to predominantly precise religation and diminished deletional end joining using microhomology (83.3% versus 16.7%). Based on the molecular spectrum of the rejoined ends and the overall efficiency of end joining, we calculated the frequency of D-NHEJ and C-NHEJ. As shown in Fig. 3D, the frequency of D-NHEJ was significantly suppressed by 6.2-fold by siRNA-mediated Mre11 silencing (p < 0.01), whereas the frequency of C-NHEJ was not changed significantly by Mre11 silencing (p > 0.05) (Fig. 3D). Overall, these data indicate that Mre11 promotes D-NHEJ repair using microhomology but does not have a significant effect on C-NHEJ, which is consistent with previous data in yeast (13) and the recent report from in human cells using similar approaches to assess the role of Mre11 in NHEJ subpathways (30). In addition, a small percentage of rejoining involved the insertion events associated with deletion, which were not effected by Mre11 status.
FIGURE 3.
Mre11 promotes deletional D-NHEJ but does not affect the level of precise C-NHEJ. A, junction sequence analysis in the clones undergoing XHATM selection for gpt. B, junction sequences in the XHATM-resistant clones with or without Mre11 knockdown. Imperfect microhomology is displayed in bold and underlined. C, spectrum of end joining obtained from sequencing. D, frequency of end joining based on the spectrum of end joining obtained from sequencing. The frequency of D-NHEJ and C-NHEJ is based on the spectrum of end joining (C) but corrected for the overall end-joining efficiency (Fig. 1C). **, statistical difference in repair efficiency between control siRNA versus Mre11 siRNA (p < 0.001).
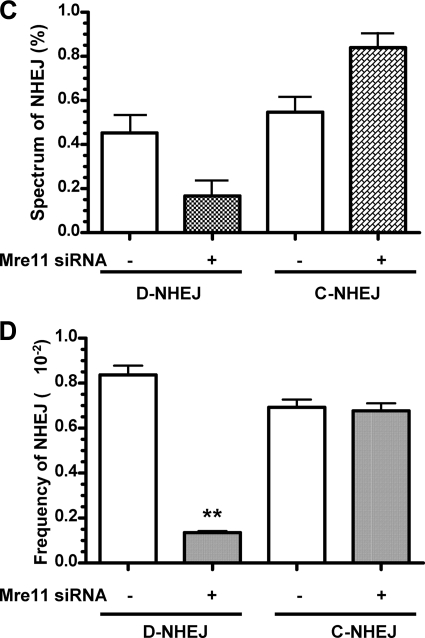
During this study, we also developed a quantitative real-time PCR method to detect C-NHEJ events directly using primers flanking the dual I-SceI sites and a probe complementary to the sequence specific for precisely religated distal ends after simultaneous I-SceI cleavage (Fig. 4A). The presence of D-NHEJ is then derived from the difference between total NHEJ and C-NHEJ events. We first validated the sensitivity and specificity of quantitative real-time PCR as an alternative method in the assessment of the efficiency of C-NHEJ directly and D-NHEJ indirectly. Consistent with the sequencing results above and the results from others (29–31), NHEJ analysis using quantitative real-time PCR demonstrated that silencing of Mre11 by siRNA led to a 20-fold suppression of D-NHEJ efficiency, but had no significant effect on the actual C-NHEJ efficiency (30), which was consistent with the junction-sequencing results. Importantly, the transient expression of I-SceI nuclease was similar in these experiments and not affected by Mre11 status. In addition, we did not see a distinguishable effect of siRNA or I-SceI transfection on cell cycle distribution or cell death during this study, ruling out the possibility that the effects of Mre11 on D-NHEJ were caused by cell cycle changes or cell death (data not shown). Taken together, our data from repair junction sequencing and real-time PCR assay implicate Mre11 in NHEJ-mediated repair of DNA DSBs and specifically in the promotion of D-NHEJ.
FIGURE 4.
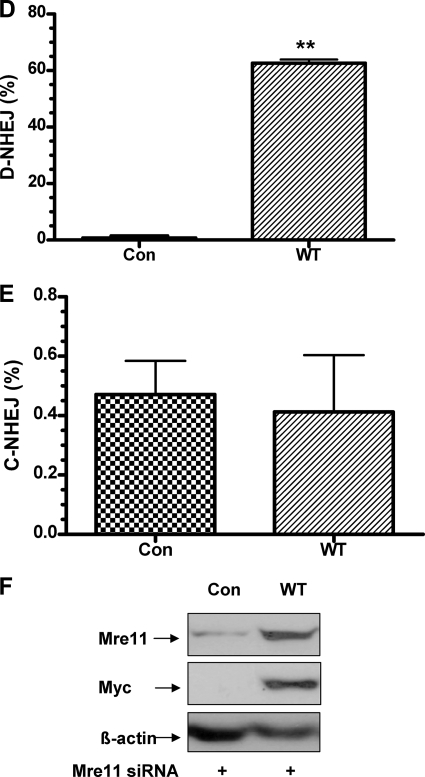
Rescue of suppressed D-NHEJ by expression of siRNA-resistant wild-type Mre11. A, quantitative real-time PCR to determine the effect of Mre11 knockdown on end joining. The arrows indicate the I-SceI cleavage sites, and the bold arrow indicates the precise religation product as well as the probe C complementary sequence. B, Mre11 knockdown inhibits D-NHEJ. C, Mre11 knockdown does not affect C-NHEJ. **, statistical difference in D-NHEJ repair efficiency between control siRNA versus Mre11 siRNA (p < 0.001). D, wild-type Mre11 rescues D-NHEJ after endogenous Mre11 is knocked down in HEK293/pPHW1 cells. **, statistical difference between control cells and wild-type Mre11-rescued cells (p < 0.001). E, wild-type Mre11 has no effects on C-NHEJ after endogenous Mre11 is knocked down in HEK293/pPHW1 cells. F, protein expression of endogenous Mre11 and exogenous Myc-Mre11 after rescue by transfection of siRNA-resistant Myc-tagged wild-type Mre11. β-Actin was included as a loading control.
siRNA-resistant Wild-type Mre11 Rescues Suppressed D-NHEJ in Cells with Mre11 Knockdown
To exclude off-target, nonspecific effects in our siRNA experiments, we performed phenotypical rescue experiments by expression of siRNA-resistant Mre11. Because silencing of Mre11 suppresses D-NHEJ, reintroduction of Mre11 should rescue the phenotype. We established stable cell lines with Myc-tagged wild-type Mre11 that were resistant to Mre11 siRNA silencing. As expected, we observed a dramatic increase in deletional NHEJ in cells rescued with Myc-tagged wild-type Mre11 (Fig. 4D). However, we did not see any obvious effects of Mre11 rescue on the C-NHEJ efficiency (Fig. 4E). Western blotting confirmed that the Mre11 knockdown was rescued by exogenous Myc-tagged Mre11 in HEK293/pPHW1 cells (Fig. 4F). Of note, we noticed a significant increase of D-NHEJ levels in cells rescued with wild-type Mre11 compared with the levels seen in cells without rescue under control siRNA conditions. This might have been caused by overexpression of exogenous Mre11 (data not shown), as reported previously (31). These results further support our observations and a recent report (30) indicating that Mre11 actively promotes chromosomal D-NHEJ but has no more than a limited function in C-NHEJ in mammalian cells.
Promotion of D-NHEJ by Mre11 Depends on Its Exonuclease Activity
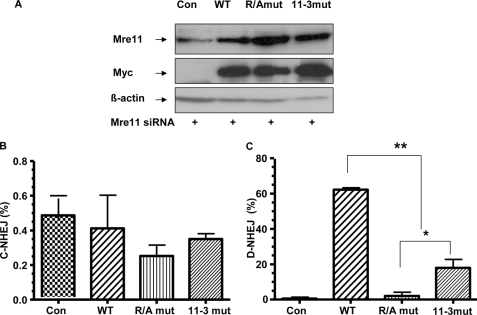
Mre11 contains an N-terminal nuclease domain as well as a C-terminal DNA binding region (24–27). It has both 3′ and 5′ endonuclease and 3′-5′ exonuclease activity. The 3′-5′ exonuclease activity is one of the most important biochemical features of Mre11, which is found important for DNA repair of DSBs (22). To determine the mechanisms by which Mre11 controls D-NHEJ repair, we reasoned that the function of Mre11 in D-NHEJ using microhomology may rely on Mre11 exonuclease activity. In recent studies, the Mre11 GAR domain important for the checkpoint response was reported to regulate the exonuclease activity through arginine methylation. The exonuclease activity was impaired with inhibition of arginine methylation in the GAR domain (Mre11-R/A) (25, 26). In addition, the exonuclease function can be disrupted by N-terminal mutation (Mre11-3), whereas binding to Rad50 and Nbs1 is maintained. To determine whether the exonuclease activity as well as the GAR domain accounts for the role of Mre11 in D-NHEJ, we stably expressed plasmids of wild-type or mutant Mre11 (Mre11-3 or Mre11-R/A) resistant to siRNA silencing in HEK293/pPHW1 cells. After endogenous Mre11 was knocked down (Fig. 5A), we found that neither Mre11-3 nor Mre11-R/A had an effect on C-NHEJ events (Fig. 5B) because the levels of C-NHEJ in cells with wild-type Mre11 and mutant Mre11 were almost the same. However, although exogenous expression of wild-type Mre11 fully rescued the suppressed levels of D-NHEJ in cells with knocked-down Mre11, the exogenous Mre11-3 mutant partially rescued D-NHEJ repair (Fig. 5C), indicating a residual activity of Mre11-3 mutant in mediating D-NHEJ. Interestingly, the Mre11-R/A mutant was completely incapable of rescuing D-NHEJ events. These findings reveal that impairment of the exonuclease activity of Mre11 by mutations in either its phosphoesterase domain (Mre11-3) or its GAR region (Mre11-R/A) compromised D-NHEJ, and therefore, the exonuclease activity of Mre11 accounts, at least in part, for its role in D-NHEJ. In addition, the data suggest that the exonuclease activity of Mre11 is not the only mechanism involved in promoting D-NHEJ.
FIGURE 5.
Exonuclease activity in Mre11 is critical for mutagenic D-NHEJ. A, generation of stable HEK293/pPHW1 cell lines with vector alone (Con), Myc-tagged wild-type Mre11 (WT), Myc-tagged Mre11-R/A (R/Amut), or Mre11-3 (11-3mut), which were resistant to Mre11 siRNA. Expression of total endogenous and exogenous Mre11 or Myc-tagged exogenous Mre11 protein was determined by Western blotting. B, mutation in the nuclease domain or the GAR region of Mre11 diminishes D-NHEJ. Results were analyzed by real-time PCR. Asterisks represent statistically significant differences between wild-type Mre11, the mutants, as well as Mre-11-R/A versus Mre11-3 (*, p < 0.05; **, p < 0.001). C, nuclease activity of Mre11 has no effect on C-NHEJ. Results were analyzed by real-time PCR.
DISCUSSION
We and others have demonstrated that there are two distinct, but probably interrelated, NHEJ subpathways that are characterized by different protein requirements: Mre11-dependent D-NHEJ, which produces deletions during microhomology searching, and Ku80/XRCC4-dependent C-NHEJ, which precisely rejoins the DSB ends with no or minimal modifications (9, 29–33). To explore further the mechanisms that regulate NHEJ subpathways, we have used an assay to create site-specific DSBs within the chromosomally integrated NHEJ substrate pPHW1 in HEK293 cells (32, 33), which produces fully complementary cohesive ends. We showed that both precise C-NHEJ and imprecise D-NHEJ pathways are utilized to repair the DSBs in this setting, consistent with a previous observation (33). When the DNA damage response protein Mre11 was silenced, there was an ∼10–12-fold reduction in small sequence deletions at the break junction compared with the levels of D-NHEJ in siRNA control cells (Figs. 2B and 4D). Most recently, three studies have reported similar findings regarding the role of Mre11 in the imprecise D-NHEJ (29–31). However, whether Mre11 regulates both D-NHEJ and C-NHEJ pathways or D-NHEJ only was not clear. After further analysis of the molecular features at the break junctions by either direct sequencing or quantitative real-time PCR, we found that Mre11 predominantly promoted microhomology-mediated deletional mutations, but had little influence if any on the precise rejoining of DSB ends via C-NHEJ repair, suggesting that a scaffolding or synapsis function by Mre11 is not essential for the error-free religation of two adjacent double-stranded ends. Notably, in our plasmid substrate, the double-stranded ends were only ∼30 bp apart, a distance that could well be bridged by local assembly of NHEJ proteins, and those DNA ends are more likely treated as a single DSB.
Because both C-NHEJ and D-NHEJ subpathways are available and efficient for rejoining of the DSB ends, do cells have preference in choosing NHEJ subpathways? Through sequence analysis of PCR products from NHEJ repair junctions and real-time PCR quantification, we found that D-NHEJ is quite efficient compared with C-NHEJ despite, with a frequency of 5.89 × 10−2 for C-NHEJ versus 7.02 × 10−2 for D-NHEJ (Figs. 3D and 4, B and C). A previous study reported that C-NHEJ is mediated by repair complex including protein Ku70/80 because accurate repair of cohesive ends is decreased dramatically in Ku80-, or XRCC4-defective cells (8), indicating that Ku80 and XRCC4 may influence the cell's choice of the NHEJ subpathways. In contrast, suppression of Mre11 expression severely suppressed D-NHEJ efficiency (from 8.2 × 10−2 to 0.7 × 10−2) (Fig. 3D), indicating that Mre11 may also influence the choice between the two NHEJ subpathways. Previously, we showed that BRCA1 promotes precise rejoining of DSBs in human breast carcinoma cells while suppressing microhomology-mediated error-prone end joining and restricting sequence deletion at the break junction during repair (34). In addition, BRCA1 interacts with Ku80, which may be required for BRCA1-Ku80 foci formation in response to laser-induced DSBs (35). BRCA1 also interacts with the Mre11·Rad50·Nbs1·CtIP supercomplex and is critical for the function of CtIP in HR. Thus, whether BRCA1 also controls the switch during choice of Ku80/XRCC4-dependent C-NHEJ and Mre11-dependent D-NHEJ through inhibition of Mre11 nuclease activity is a very interesting question (36).
Rapid recruitment of an Mre11 complex to DSBs activates ataxia telangiectasia mutated protein kinase and triggers DNA damage response (37). Mre11 is a conserved protein with an N-terminal nuclease domain as well as a C-terminal DNA binding region (24). Accumulating evidence shows that Rad50/Mre11 hooks the broken DNA ends after induction of DSBs (38) and that the 3′-5′ exonuclease activity is one of the most important biochemical features of Mre11 (28, 36, 38–40). Biochemically, the Mre11 complex from both yeast and humans exhibits enzyme activity on double-stranded DNA as well as single-stranded DNA. The crystal structure of the nuclease domain from Pyrococcus furiosus Mre11 reveals that the phosphoesterase domains form the nuclease active site (41). Two metal ions are coordinated by five conserved phosphoesterase motifs of the protein. In S. cerevisiae, mutations of conserved residues in the phosphoesterase domains in Mre11 increased sensitivity to IR and showed defects in DSB repair (24). On the other hand, the GAR domain is suggested to regulate the exonuclease activity through Mre11 methylation, which is also important for the checkpoint response (25, 26). In our study, two exonuclease-inactive mutants, one via alteration of the phosphoesterase domain (Mre11-3) and one via mutation of the GAR domain (Mre11-R/A), were unable to rescue the loss of endogenous Mre11 expression fully. This evidence strongly suggests that the exonuclease activity of Mre11 is important to D-NHEJ in human cells and that the GAR domain plays a critical role in the regulation of that activity.
In a most recent study on the role of MRN in end-joining pathways during isotype class switching, it is reported that the deficiency in isotype switching is relatively minor in the absence of Mre11 nuclease activity (Mre11−/H129) compared with that in cells with loss of Mre11 (Mre11−/−) (29). Interestingly, we found that the phenotype of the Mre11-R/A mutant is significantly more severe than for the Mre11-3 mutant (p < 0.05) in that Mre11-3 was able to rescue the Mre11 knockdown partially (Fig. 5B). It has been reported that the GAR region, in addition to regulation of the Mre11 nuclease, also facilitates DNA binding (25). The nuclease-deficient Mre11-3·Rad50·Nbs1 complex could still localize to DSBs in IR-induced foci, whereas inhibition of arginine methylation in the GAR region impaired both Mre11 nuclease activity and Mre11 foci formation in vivo, even though the Mre11-R/A mutant is proficient in MRN complex formation (25). An intriguing possibility is that the more severe D-NHEJ deficiency phenotype of Mre11-R/A-expressing cells may be due to the loss of both its exonuclease activity and its DNA binding properties.
Recent studies also suggest that the Mre11 complex coordinates with CtIP to remove small nucleotides from DNA ends to form an early intermediate, which is not an ideal structure for HR, during the first step of DNA end resection (42, 43). It was shown that Mre11 is required for CtIP recruitment at DSBs (44, 45), and CtIP also promotes microhomology-mediated end joining (46, 47). We speculate that CtIP-involved D-NHEJ may be partially mediated by the GAR domain of Mre11. Future studies to determine the functional interaction of CtIP and MRN complex in the context of D-NHEJ efficiency would shed light on the mechanisms control the NHEJ subpathways and the fidelity of end joining-mediated DSB repair.
Our data indicate that Mre11 controls the D-NHEJ subpathway, but it does not affect C-NHEJ if the DNA ends are complementary. Furthermore, the exonuclease activity in the Mre11 phosphoesterase domain, which is regulated by its GAR region, may modulate the extent of D-NHEJ at the break site. This study provides new insights into the mechanisms of Mre11 during NHEJ in human cells.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA118158-02 (to F. X.).
- DSB
- double-strand break
- GAR
- glycine–arginine-rich
- HR
- homologous recombination
- MRN complex
- Mre11·Rad50·Nbs1 complex
- NHEJ
- nonhomologous end joining
- C-NHEJ
- conservative NHEJ
- D-NHEJ
- deletional NHEJ
- siRNA
- small interfering RNA
- ORF
- open reading frame
- WT
- wild type
- XHATM
- xanthine, hypoxanthine, aminopterin, thymidine, and mycophenolic acid.
REFERENCES
- 1.Hoeijmakers J. H. (2001) Nature 411, 366–374 [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer P., Thode S., Hancke J., Vielmetter W. (1994) Mol. Cell. Biol. 14, 888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willers H., McCarthy E. E., Wu B., Wunsch H., Tang W., Taghian D. G., Xia F., Powell S. N. (2000) Oncogene 19, 632–639 [DOI] [PubMed] [Google Scholar]
- 4.Modesti M., Kanaar R. (2001) Genome Biol. 2, R1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West S. C. (2003) Nat. Rev. Mol. Cell Biol. 4, 435–445 [DOI] [PubMed] [Google Scholar]
- 6.Lieber M. R., Ma Y., Pannicke U., Schwarz K. (2003) Nat. Rev. Mol. Cell Biol. 4, 712–720 [DOI] [PubMed] [Google Scholar]
- 7.Weterings E., van Gent D. C. (2004) DNA Repair 3, 1425–1435 [DOI] [PubMed] [Google Scholar]
- 8.Guirouilh-Barbat J., Huck S., Bertrand P., Pirzio L., Desmaze C., Sabatier L., Lopez B. S. (2004) Mol. Cell 14, 611–623 [DOI] [PubMed] [Google Scholar]
- 9.Schulte-Uentrop L., El-Awady R. A., Schliecker L., Willers H., Dahm-Daphi J. (2008) Nucleic Acids Res. 36, 2561–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker J. R., Corpina R. A. (2001) Nature 412, 607–614 [DOI] [PubMed] [Google Scholar]
- 11.Leuther K. K., Hammarsten O. (1999) EMBO J. 18, 1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Trujillo K. (2000) J. Biol. Chem. 275, 26196–26205 [DOI] [PubMed] [Google Scholar]
- 13.Ma J. L., Kim E. M., Haber J. E., Lee S. E. (2003) Mol. Cell. Biol. 23, 8820–8828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McVey M., Lee S. E. (2008) Trends Genet. 24, 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varon R., Vissinga C., Platzer M., Cerosaletti K. M., Chrzanowska K. H., Saar K., Beckmann G., Seemanová E., Cooper P. R., Nowak N. J., Stumm M., Weemaes C. M., Gatti R. A., Wilson R. K., Digweed M., Rosenthal A., Sperling K., Concannon P., Reis A. (1998) Cell 93, 467–476 [DOI] [PubMed] [Google Scholar]
- 16.Carney J. P., Maser R. S., Olivares H., Davis E. M., Le Beau M., Yates J. R., 3rd, Hays L., Morgan W. F., Petrini J. H. (1998) Cell 93, 477–486 [DOI] [PubMed] [Google Scholar]
- 17.Haber J. E. (1998) Cell 95, 583–586 [DOI] [PubMed] [Google Scholar]
- 18.Williams R. S., Williams J. S., Tainer J. A. (2007) Biochem. Cell Biol. 85, 509–520 [DOI] [PubMed] [Google Scholar]
- 19.Wiltzius J. J., Hohl M., Fleming J. C., Petrini J. H. (2005) Nat. Struct. Mol. Biol. 12, 403–407 [DOI] [PubMed] [Google Scholar]
- 20.Hopfner K. P., Craig L., Moncalian G., Zinkel R. A., Usui T., Owen B. A., Karcher A., Henderson B., Bodmer J. L., McMurray C. T., Carney J. P., Petrini J. H., Tainer J. A. (2002) Nature 418, 562–566 [DOI] [PubMed] [Google Scholar]
- 21.Chen L., Nievera C. J., Lee A. Y., Wu X. (2008) J. Biol. Chem. 283, 7713–7720 [DOI] [PubMed] [Google Scholar]
- 22.Williams R. S., Moncalian G., Williams J. S., Yamada Y., Limbo O., Shin D. S., Groocock L. M., Cahill D., Hitomi C., Guenther G., Moiani D., Carney J. P., Russell P., Tainer J. A. (2008) Cell 135, 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arthur L. M., Gustausson K., Hopfner K.-P., Carson C. T., Stracker T. H., Karcher A., Felton D., Weitzman M. D., Tainer J., Carney J. P. (2004) Nucleic Acids Res. 32, 1886–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bressan D. A., Olivares H. A., Nelms B. E., Petrini J. H. (1998) Genetics 150, 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Déry U., Coulombe Y., Rodrigue A., Stasiak A., Richard S., Masson J. Y. (2008) Mol. Cell. Biol. 28, 3058–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boisvert F.-M., Déry U., Masson J.-Y., Richard S. (2005) Genes Dev. 19, 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buis J., Wu Y., Deng Y., Leddon J., Westfield G., Eckersdorff M., Sekiguchi J. M., Chang S, Ferguson D. O. (2008) Cell 135, 85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paull T. T., Gellert M. (1998) Mol. Cell 1, 969–979 [DOI] [PubMed] [Google Scholar]
- 29.Dinkelmann M., Spehalski E., Stoneham T., Buis J., Wu Y., Sekiguchi J. M., Ferguson D. O. (2009) Nat. Struct. Mol. Biol. 16, 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie A., Kwok A., Scully R. (2009) Nat. Struct. Mol. Biol. 16, 814–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rass E., Grabarz A., Plo I., Gautier J., Bertrand P., Lopez B. S. (2009) Nat. Struct. Mol. Biol. 16, 819–824 [DOI] [PubMed] [Google Scholar]
- 32.Dahm-Daphi J., Hubbe P., Horvath F., El-Awady R. A., Bouffard K. E., Powell S. N., Willers H. (2005) Oncogene 24, 1663–1672 [DOI] [PubMed] [Google Scholar]
- 33.Willers H., Husson J., Lee L. W., Hubbe P., Gazemeier F., Powell S. N., Dahm-Daphi J. (2006) Radiat. Res. 166, 567–574 [DOI] [PubMed] [Google Scholar]
- 34.Zhuang J., Zhang J., Willers H., Wang H., Chung J. H., van Gent D. C., Hallahan D. E., Powell S. N., Xia F. (2006) Cancer Res. 66, 1401–1408 [DOI] [PubMed] [Google Scholar]
- 35.Wei L., Lan L., Hong Z., Yasui A., Ishioka C., Chiba N. (2008) Mol. Cell. Biol. 28, 7380–7393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paull T. T., Cortez D., Bowers B., Elledge S. J., Gellert M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6086–6091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J. H., Paull T. T. (2004) Science 304, 93–96 [DOI] [PubMed] [Google Scholar]
- 38.de Jager M., van Noort J., van Gent D. C., Dekker C., Kanaar R., Wyman C. (2001) Mol. Cell 8, 1129–1135 [DOI] [PubMed] [Google Scholar]
- 39.Bleuit J. S., Xu H., Ma Y., Wang T., Liu J., Morrical S. W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 8298–8305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connelly J. C., de Leau E. S., Leach D. R. (2003) DNA Repair 2, 795–807 [DOI] [PubMed] [Google Scholar]
- 41.Hopfner K. P., Karcher A., Craig L., Woo T. T., Carney J. P., Tainer J. A. (2001) Cell 105, 473–485 [DOI] [PubMed] [Google Scholar]
- 42.Mimitou E. P., Symington L. S. (2008) Nature 455, 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Z., Chung W. H., Shim E. Y., Lee S. E., Ira G. (2008) Cell 134, 981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sartori A. A., Lukas C., Coates J., Mistrik M., Fu S., Bartek J., Baer R., Lukas J., Jackson S. P. (2007) Nature 450, 509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda S., Nakamura K., Taniguchi Y., Paull T. T. (2007) Mol. Cell 28, 351–352 [DOI] [PubMed] [Google Scholar]
- 46.Bennardo N., Cheng A., Huang N., Stark J. M. (2008) PLoS Genet. 2008, 4, e1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun M. H., Hiom K. (2009) Nature 459, 460–463 [DOI] [PMC free article] [PubMed] [Google Scholar]