Abstract
Cyclic AMP analogs containing hydrophobic modification of C8 at the adenine ring such as 8-(4-chlorophenylthio)-cAMP (8-pCPT-cAMP) and 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT-2′-O-methyl-cAMP) can penetrate membranes due to their high lipophilicity and directly activate intracellular cAMP effectors. Therefore, these cAMP analogs have been used in numerous studies, assuming that their effects reflect the consequences of direct activation of cAMP effectors. The present study provides evidence that 8-pCPT-modified cAMP analogs and their corresponding putative hydrolysis products (8-(4-chlorophenylthio)-adenosine (8-pCPT-ado) and 8-(4-chlorophenylthio)-2′-O-methyl-adenosine (8-pCPT-2′-O-methyl-ado)) inhibit the equilibrative nucleoside transporter 1 (ENT1). In PC12 cells, in which nucleoside transport strongly depended on ENT1, 8-pCPT-ado, 8-pCPT-2′-O-methyl-ado, and, to a smaller extent, 8-pCPT-2′-O-methyl-cAMP caused an increase of protein kinase A substrate motif phosphorylation and anti-apoptotic effect by an A2A adenosine receptor (A2AR)-dependent mechanism. In contrast, the effects of 8-pCPT-cAMP were mainly A2AR-independent. In HEK 293 showing little endogenous ENT1-dependent nucleoside transport, transfection of ENT1 conferred A2AR-dependent increase in protein kinase A substrate motif phosphorylation. Together, the data of the present study indicate that inhibition of ENT1 and activation of adenosine receptors have to be considered when interpreting the effects of 8-pCPT-substituted cAMP/adenosine analogs.
Introduction
The second messenger cAMP binds to different types of effectors, i.e. protein kinase A (PKA),3 the cAMP binding guanine nucleotide exchange factors Epac1 and -2, ion channels, and cAMP-regulated phosphodiesterases and thereby exerts its various important effects in cells (for review, see Refs. 1–3). A large number of studies have used membrane-permeable cAMP analogs containing the hydrophobic 4-chlorophenylthio (pCPT) or bromine modification of C8 at the adenine ring to study the function of intracellular cAMP effectors (4). However, cAMP analogs may affect cells independently from their ability to directly activate cAMP effectors. Recently, 8-pCPT-2′-O-methyl-cAMP and its hydrolysis-resistant Sp analog have been shown to inhibit cyclic nucleotide phosphodiesterases (5), which may facilitate activation of PKA and protein kinase G. Another source of cAMP effector-independent effects of cAMP analogs involves the formation of metabolites. Thus, 8-Cl-cAMP inhibits the viability of tumor cell lines (6, 7).
Given the important role of cAMP in cell proliferation (1), we initially studied the effect of membrane-permeable Epac-selective and non-selective cAMP analogs on cell proliferation. We found that 8-pCPT-substituted cAMP analogs as well as their potential hydrolysis products strongly inhibit [3H]thymidine incorporation into PC12 and other cells without affecting the cell number. Further experiments demonstrate that this decrease in [3H]thymidine incorporation is due to an inhibition of [3H]thymidine uptake into cells.
The transport of nucleosides into mammalian cells is accomplished by two types of transporters: concentrative nucleoside transporters, which use the Na+ gradient to actively transport nucleosides into the cells, and equilibrative nucleoside transporters (ENTs), which transport nucleosides in both directions and toward equal intra- and extracellular adenosine concentration (8, 9). ENTs play key roles in nucleoside and nucleobase uptake for salvage pathways of nucleotide synthesis and are also responsible for the cellular uptake of nucleoside analogues used in the treatment of cancers and viral diseases. By regulating the concentration of adenosine available to ubiquitously expressed cell surface adenosine receptors, nucleoside transporters appear to play important regulatory roles in many physiological processes ranging from cardiovascular activity to neurotransmission and immune function (8–11). Here we show that 8-pCPT-modified cAMP analogs, and more potently, 8-pCPT-modified adenosine analogs, cause inhibition of the equilibrative nucleoside transporter 1 (ENT1) and activation of Gs protein-coupled A2A adenosine receptors (A2AR) by an ENT1-dependent mechanism.
MATERIALS AND METHODS
Reagents
Adenosine, cAMP, SCH 58261, 5-(4-nitrobenzyl)-6-thioinosine (NBMPR), and anti-actin IgG were obtained from Sigma-Aldrich. Adenosine deaminase (ADA) was from Roche Applied Science. Horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG were from Bio-Rad. Lipofectamine 2000, Dulbecco's modified Eagle's medium (DMEM), RPMI 1640, fetal calf serum, and horse serum were from Invitrogen. 8-pCPT-cAMP was from Calbiochem. The antibody recognizing phosphorylated PKA substrate motif RRXp(S/T) was from Cell Signaling (Beverly, MA). Anti-ERK2 (sc-154) was from Santa Cruz Biotechnology (Santa Cruz, CA). 8-pCPT-ado, 8-pCPT-cAMP, Sp-8-pCPT-cAMPS, 8-pCPT-2′-O-methyl-cAMP, 8-pCPT-2′-O-methyl-ado, Sp-8-pCPT-2′-O-methyl-cAMPS, 8-Br-ado, 8-Cl-ado, 8-Br-cAMP, 8-OH-cAMP, and N6-benzoyl-cAMP were from BIOLOG (Bremen, Germany). [3H]Thymidine was purchased from Hartmann Analytic (Braunschweig, Germany).
Cell Culture
PC12 cells were grown in DMEM supplemented with 5% fetal calf serum, 5% horse serum, and penicillin/streptomycin. HEK 293 and Panc-1 cells were cultured in DMEM supplemented with 10% fetal calf serum and penicillin/streptomycin. Primary human mesangial cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum and antibiotics (12).
Fluorescence-activated Cell Sorting Analysis
PC12 cells were serum-starved for 16 h followed by a 48-h incubation in normal growth medium in the presence or absence of 8-pCPT-modified cAMP analogs. Cell cycle distribution was analyzed using a FACSCalibur apparatus (BD Biosciences) as described (13).
Generation of ENT1 Overexpressing HEK 293 Cells
HEK 293 cells were transfected with a plasmid encoding human ENT1 (hENT1) fused to green fluorescent protein (15) using Lipofectamine 2000 according to the instructions of the manufacturer. Stable clones were obtained by G418 selection.
[3H]Thymidine Incorporation into DNA
Cells grown on 96-well plates were incubated with the cAMP/adenosine analogs for the indicated time. Subsequently, 0.1 μCi of [3H]thymidine was added to each well, and the incubation was continued for 4 h. The cells were washed extensively, and the radioactivity incorporated into DNA was determined using a Wallac TriLux 1450 scintillation counter (PerkinElmer Life Sciences) (14).
MTT Assay
To determine the number of viable cells, we employed the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) colorimetric assay (16).
[3H]Thymidine Uptake
Cells were grown on 24-well plates and were switched to serum-free DMEM 4 h prior to the experiment. After preincubation of the cells with the nucleotide/nucleoside analogs for 30 min, 0.3 μCi of [3H]thymidine was added, and the incubation was continued for 1.5 min (PC12 and renal mesangial cells) or 15 min (HEK 293 cells). The cells were washed with phosphate-buffered saline, and the radioactivity associated with the cells was determined using a Wallac TriLux 1450 scintillation counter. Where indicated, the uptake experiment was performed in Krebs bicarbonate-buffered solution containing 25 mm NaHCO3 and 116 mm NaCl or 116 mm N-methyl-d-glucamine, 0.8 mm KH2PO4, 2.5 mm MgCl2, 4.6 mm KCl, 1.2 mm MgSO4, 2.5 mm CaCl2, and 2 mg/ml d-glucose, pH 7.4.
Immunoblotting
Lysates were obtained from the cells by sonication in lysis buffer (50 mm Hepes, pH 7.0, 100 mm NaCl, 0.2 mm MgSO4, 0.5 mm Na3VO4, 1% Triton X-100, 10 μg/ml leupeptin, 10 μg/ml aprotinin). The lysates were separated on SDS-polyacrylamide gels as described previously (17, 18). Gel-resolved proteins were electrotransferred to nitrocellulose membranes and incubated with antibodies raised against phospho-PKA substrate motif RXXp(S/T), anti-β-actin, or anti-ERK2. Antigen-antibody complexes were visualized using appropriate horseradish peroxidase-conjugated antibodies and the enhanced chemiluminescence system (Pierce).
cAMP Assay
Cells were incubated with cAMP/adenosine analogs for the indicated time. The medium was removed, and the cells were extracted with 0.1 m HCl. The suspension was pipetted up and down until the lysate became homogeneous. The lysate was then centrifuged, and the supernatant was transferred for the determination of the concentration of cAMP using a cAMP enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) according to the instructions of the manufacturer.
Apoptosis Assay
Cells grown in 24-well dishes were switched to serum-free DMEM, and 8-pCPT-cAMP/ado and/or ADA were added to the medium. After 24 h, apoptotic cells were assessed by Hoechst 33342 staining. Normal nuclei show faint delicate chromatin staining, nuclei at the early stage of apoptosis display increased condensation and brightness, and nuclei at the late stage of apoptosis exhibit chromatin condensation and nuclear fragmentation.
Adenosine Deaminase Assay
The measurement of the deamination of adenosine and 8-pCPT-cAMP/adenosine analogs was performed as described by Martinek (19).
Data Presentation and Statistical Analysis
The data displayed are means ± S.D. All experiments were repeated at least three times with a similar result. Data from different treatment groups were analyzed by the Student's t test for unpaired samples. p values smaller than 0.05 were considered to indicate statistical significance (*) (see Figs. 1–6).
FIGURE 1.
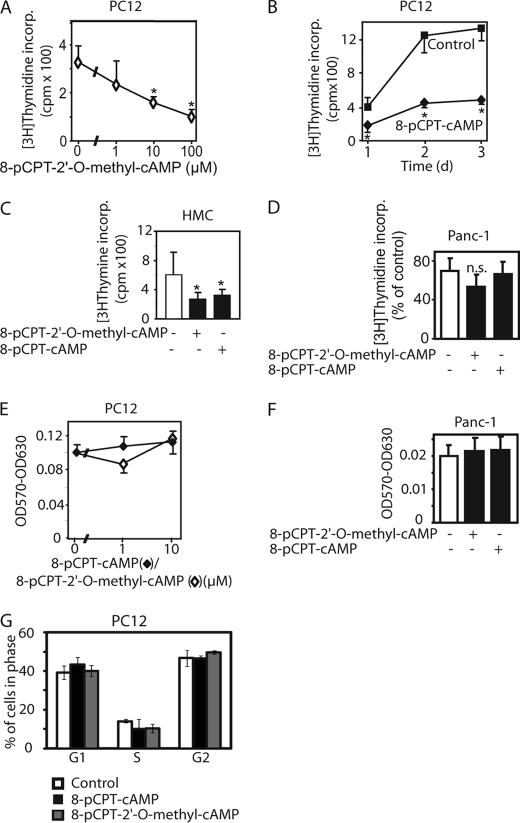
8-pCPT-modified cAMP analogs inhibit [3H]thymidine incorporation (incorp.) into the DNA in several different cell types. PC12 (A, B, E, and G), human renal mesangial (HMC, C), and Panc-1 cells (D and F) were exposed to 8-pCPT-modified cAMP analogs at the indicated concentrations (A and E) or 10 μm (B, C, D, F, and G) for 48 h (A and C–G) or the indicated duration (B). In A–D, 0.1 μCi of [3H]thymidine per well was added during the last 4 h of the incubation. The incorporation of radioactivity into the cells was determined by liquid scintillation counting. E and F, after the 48-h incubation, the viable cell mass was determined by the MTT assay. G, fluorescence-activated cell sorting analysis of PC12 cells after treatment with 8-pCPT-cAMP, 8-pCPT-2′-O-methyl-cAMP (10 μm each), or vehicle for 48 h. Values are means ± S.D. (error bars) of three independent determinations. Asterisks indicate a significant difference between cAMP analog-treated and vehicle-treated cells; n.s., not significant as compared with the control.
FIGURE 2.
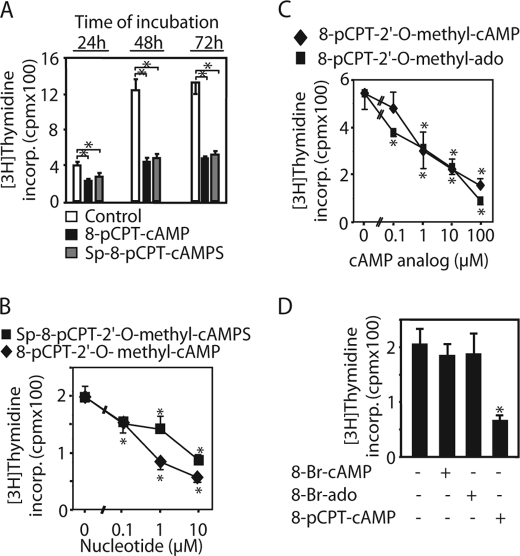
Hydrolysis-resistant 8-pCPT-modified cAMP analogs as well as 8-pCPT-modified adenosine analogs inhibited [3H]thymidine incorporation (incorp.) into the DNA in PC12 cells, whereas 8-Br-cAMP/8-Br-adenosine did not. PC12 cells were incubated with 10 μm (A and D) or the indicated concentration (B and C) of Sp-8-pCPT-2′-O-methyl-cAMPS, Sp-8-pCPT-cAMPS, 8-pCPT-ado, 8-pCPT-2′-O-methyl-ado, 8-Br-cAMP, or 8-Br-ado for the indicated time (A) or for 48 h (B–D). Then, [3H]thymidine incorporation into the cellular DNA was determined as described in the legend for Fig. 1. Values are means ± S.D. (error bars) of three independent determinations. Asterisks indicate a significant difference between cAMP analog-treated and vehicle-treated cells.
FIGURE 3.
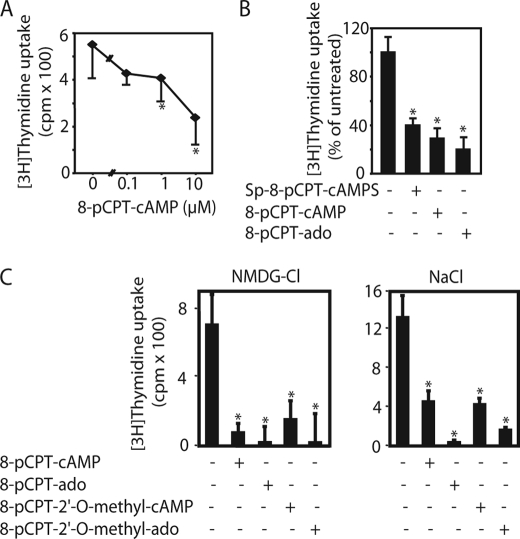
8-pCPT-modified cAMP analogs inhibit [3H]thymidine uptake. PC12 cells were preincubated with the indicated concentration (A) or 10 μm 8-pCPT-cAMP, 8-pCPT-ado, Sp-8-pCPT-cAMPS, 8-pCPT-2′-O-methyl-cAMP, or 8-pCPT-2′-O-methyl-ado (B and C) for 30 min. Then, [3H]thymidine uptake into the cells was determined as described under “Materials and Methods.” Values are means ± S.D. (error bars) of three independent determinations. Asterisks indicate a significant difference between cAMP/ado analog-treated and vehicle-treated cells; n.s., not significant as compared with the control.
FIGURE 4.
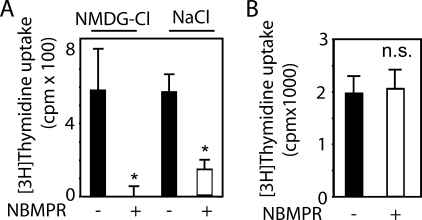
NBMPR inhibits [3H]thymidine uptake into PC12 cells, but had no effect in Panc-1 cells. PC12 (A) or Panc-1 cells (B) were preincubated with NBMPR (200 nm) for 30 min in Krebs bicarbonate-buffered solution containing either NaCl or N-methyl-d-glucamine (NMDG-Cl) as indicated. Then, [3H]thymidine uptake into the cells was determined as described under “Materials and Methods.” Values are means ± S.D. (error bars) of three independent determinations. Asterisks indicate a significant difference between NBMPR-treated and vehicle-treated cells; n.s.; not significant.
FIGURE 5.
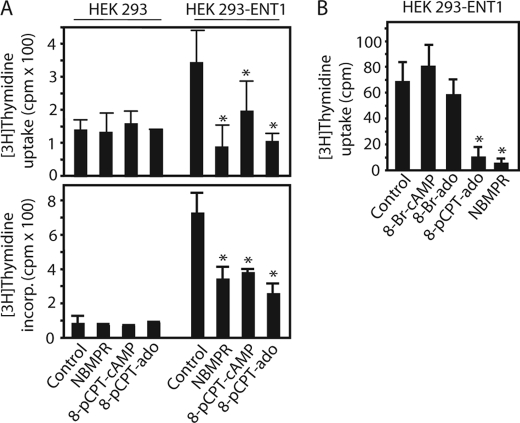
Expression of ENT1 confers 8-pCPT-substituted cAMP/ado analog-sensitive [3H]thymidine transport to HEK 293 cells. A and B, [3H]thymidine uptake as well as [3H]thymidine incorporation into the DNA was measured in HEK 293 cells stably expressing human ENT1 (HEK 293-ENT1) or parental cells in the presence or absence of 8-pCPT-cAMP, 8-pCPT-ado, 8-Br-cAMP, 8-Br-ado (100 μm each), or NBMPR (200 nm). Values are means ± S.D. (error bars) of three independent determinations. Similar results were obtained in three different ENT1-expressing clones. Asterisks indicate a significant difference between treated and vehicle-treated cells.
FIGURE 6.
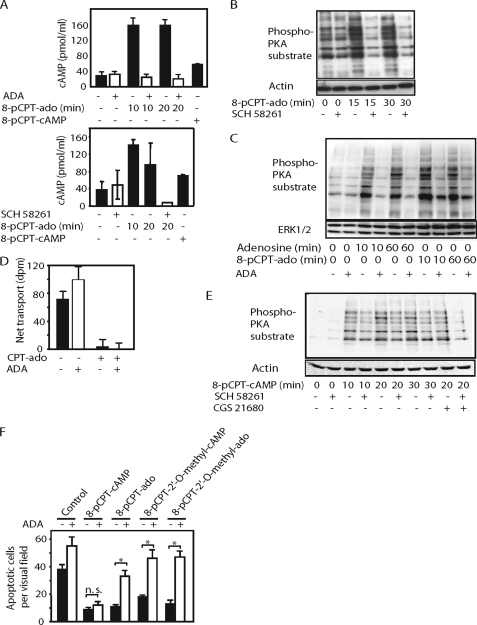
8-pCPT-modified cAMP/ado analogs elicit activation of adenylyl cyclase, PKA, and anti-apoptotic effect by an ENT1- and A2AR-dependent mechanism in PC12 cells. A, PC12 cells were incubated with 8-pCPT-cAMP or 8-pCPT-ado (100 μm each) in the presence or absence of ADA (2 units/ml) for 10 or 20 min followed by the determination of [cAMP]i. Values are means ± S.D. (error bars) of three independent determinations. B, PC12 cells were stimulated with 8-pCPT-ado (100 μm) or vehicle (DMSO) in the presence of absence of SCH 58261 (100 nm) for the indicated time followed by immunoblotting of the cell lysates with an anti-PKA substrate motif or anti-β-actin. C, PC12 cells were stimulated with adenosine (10 μm) or 8-pCPT-ado (100 μm) in the presence or absence of ADA (2 units/ml) for the indicated time followed by immunoblotting of cell lysates with anti-PKA substrate motif or anti-ERK1/2. D, the determination of [3H]thymidine uptake by PC12 cells in the presence and absence of 8-pCPT-ado (100 μm) or ADA (2 units/ml). Values are means ± S.D. (error bars) of three independent determinations. E, PC12 cells were stimulated with 8-pCPT-cAMP (100 μm), CGS 21680 (1 μm), or vehicle (DMSO) in the presence of absence of SCH 58261 (100 nm) for the indicated time followed by immunoblotting of the cell lysates with an anti-PKA substrate motif or anti-ERK1. F, PC12 cells were incubated with 8-pCPT-cAMP, 8-pCPT-ado, 8-pCPT-2′-O-methyl-cAMP, or 8-pCPT-2′-O-methyl-ado (10 μm each) in the presence or absence of ADA in serum-free DMEM for 24 h followed by the determination of apoptotic cells by Hoechst staining. Values are means ± S.D. (error bars) of three different wells from one representative experiment. Asterisks indicate a significant difference; n.s., not significant.
RESULTS
8-pCPT-modified cAMP Analogs Inhibit [3H]Thymidine Incorporation into the DNA of Different Cell Types
8-pCPT-cAMP, 8-(4-methoxyphenylthio)-2′-O-methyl-cAMP, or 8-pCPT- 2′-O-methyl-cAMP have been reported to inhibit [3H]thymidine or bromodeoxyuridine incorporation into MCF-7, PC12, thyroid, or MCR-5 cells (7, 14, 20, 21). To investigate whether the decrease in [3H]thymidine incorporation is accompanied by a reduction of the cell number, we compared the effects of the cAMP analogs on [3H]thymidine incorporation with those on the viable cell mass using the MTT assay in different cell types. 8-pCPT-cAMP as well as 8-pCPT-2′-O-methyl-cAMP strongly reduced [3H]thymidine incorporation into PC12 and human renal mesangial cells (Fig. 1, A–C) but not in Panc-1 cells (Fig. 1D). The reduction of [3H]thymidine incorporation remained detectable after several days of incubation with the cAMP analogs (Fig. 1B). [3H]Thymidine incorporation normally parallels DNA synthesis. However, 8-pCPT-modified cAMP analogs had no significant effect on the viable cell mass (Fig. 1, E and F) and on the cell cycle (Fig. 1G).
Similar to 8-pCPT-2′-O-methyl-cAMP and 8-pCPT-cAMP, the phosphodiesterase-resistant Sp-8-pCPT-2′-O-methyl-cAMPS, Sp-8-pCPT-cAMPS, as well as 8-pCPT-ado and 8-pCPT-2′-O-methyl-ado inhibited [3H]thymidine incorporation into PC12 cells (Fig. 2, A–C). 8-pCPT-ado tended to exert the strongest inhibitory effect, but this was not statistically significant. Different from the 8-pCPT-modified cAMP analogs, 8-Br-cAMP, another cAMP analog frequently used to activate PKA and Epac in intact cells, as well as 8-Br-ado, had no effect on [3H]thymidine incorporation into PC12 cells (Fig. 2D).
8-pCPT-modified cAMP/Ado Analogs Inhibit ENT1
Because of the discrepancy of the effect of 8-pCPT-modified cAMP analogs on [3H]thymidine incorporation and cell number, we examined whether the inhibitory effect of C8-modified cAMP/ado analogs on [3H]thymidine incorporation could be due to inhibition of [3H]thymidine transport into the cells. 8-pCPT-cAMP, 8-pCPT-2′-O-methyl-cAMP, as well as their Sp and adenosine analogs strongly inhibited [3H]thymidine uptake into PC12 cells (Fig. 3, A–C), 8-pCPT-ado having the strongest effect. In contrast, unmodified cAMP, 8-Cl-ado, 8-OH-cAMP, 8-Br-cAMP, 8-Br-ado (supplemental Fig. 1, A and B), and N6-benzoyl-cAMP (data not shown) did not. In Panc-1 cells, 8-pCPT-cAMP and 8-pCPT-2′-O-methyl-cAMP had no effect (supplemental Fig. 1C). Thus, there was a close correlation between the inhibitory effects of 8-pCPT-substituted cAMP/ado analogs on [3H]thymidine uptake and [3H]thymidine incorporation into the DNA.
The data described so far indicated that 8-pCPT-substituted cAMP/ado analogs inhibited the nucleoside transport across the plasma membrane. To differentiate whether 8-pCPT-substituted cAMP/ado analogs affected equilibrative or concentrative nucleoside transporters, we studied the effect of replacement of Na+ in the incubation medium by N-methyl-d-glucamine on [3H]thymidine uptake. As illustrated in Fig. 3C, replacement of Na+ in the incubation medium by N-methyl-d-glucamine did not alter [3H]thymidine uptake into PC12 cells, suggesting that the major part of [3H]thymidine uptake in these cells was Na+-independent. The inhibitory effects of 8-pCPT-modifed cAMP/ado analogs on [3H]thymidine uptake were similar in the presence and absence of Na+ in the incubation medium (Fig. 3C), indicating that their effects were on equilibrative rather than concentrative nucleoside transporters.
The ENT1-selective inhibitor NBMPR strongly inhibited [3H]thymidine transport into PC12 cells (Fig. 4A), indicating that the nucleoside transport was mainly mediated by ENT1 in these cells. In Panc-1 cells, NBMPR had no effect (Fig. 4B). Thus, there was a close correlation between the effect of NBMPR and 8-pCPT-modified cAMP/ado analogs on [3H]thymidine uptake, suggesting that these cAMP analogs inhibited [3H]thymidine uptake by inhibiting ENT1. Similar to the 8-pCPT-modified cAMP/ado analogs, NBMPR had no effect on the proliferation of PC12 cells as determined by the MTT test despite its inhibitory effect on [3H]thymidine transport as well as [3H]thymidine incorporation.
In HEK 293 cells, NBMPR had no effect on [3H]thymidine incorporation (Fig. 5A). To confirm the suggestion that 8-pCPT-modified cAMP/ado analogs inhibited ENT1, we generated HEK 293 cells stably expressing hENT1 and examined the effects of 8-pCPT-cAMP, 8-pCPT-ado, and NBMPR on [3H]thymidine uptake as well as [3H]thymidine incorporation into the cells. The expression of hENT1 strongly increased [3H]thymidine uptake as well as its incorporation into the DNA as compared with the parental cells (Fig. 5A). NBMPR, 8-pCPT-cAMP, or 8-pCPT-ado inhibited [3H]thymidine uptake as well as its incorporation into the DNA in hENT1-expressing HEK 293 cells (Fig. 5A). These data support the notion that 8-pCPT-ado and 8-pCPT-cAMP inhibited ENT1. Similar to PC12 cells, 8-Br-cAMP and 8-Br-ado had no effect on [3H]thymidine uptake in hENT1-overexpressing HEK 293 cells (Fig. 5B), supporting the suggestion that 8-Br-cAMP and 8-Br-ado did not inhibit ENT1.
8-pCPT-modified cAMP/Ado Analogs Elicit Activation of Adenylyl Cyclase and PKA by an ENT1- and A2AR-dependent Mechanism in PC12 Cells
Adenosine transport inhibition may increase the amount of endogenous adenosine available to bind cell surface adenosine receptors, leading to adenosine receptor activation (22, 23). In PC12 cells, the Gs protein-coupled A2AR is the only functionally relevant adenosine receptor expressed endogenously (24). Its activation elicits an increase of the intracellular cAMP concentration ([cAMP]i) and activation of PKA. To investigate the possibility that 8-pCPT-modified cAMP/ado analogs may cause activation of A2AR signaling, we tested their effects on [cAMP]i and PKA substrate motif phosphorylation RRXp(S/T), reflecting PKA activity. 8-pCPT-ado and, to a smaller extent, 8-pCPT-cAMP, caused an increase in [cAMP]i (Fig. 6A). Accordingly, 8-pCPT-ado and 8-pCPT-cAMP elicited increases in PKA substrate motif phosphorylation (Fig. 6, B, C, and E). The increases in [cAMP]i and PKA substrate motif phosphorylation by 8-pCPT-ado were completely inhibited in the presence of ADA (Fig. 6, A and C), which converts adenosine to inosine, a poor activator of adenosine A2AR (25). Similar results were obtained with the A2AR antagonist SCH 58261 (Fig. 6, A, B, and E). In contrast, ADA (Fig. 6D) and SCH 58261 did not affect the inhibitory effects of 8-pCPT-ado on [3H]thymidine uptake. 8-pCPT-2′-O-methyl-ado, a potential hydrolysis product of 8-pCPT-2′-O-methyl-cAMP, also increased PKA substrate motif phosphorylation by an ADA- and SCH 58261-sensitive mechanism, whereas 8-pCPT-2′-O-methyl-cAMP had only a small, if any, effect (supplemental Fig. 2).
ADA or SCH 58261 had considerable lower inhibitory effects on 8-pCPT-cAMP-induced PKA substrate motif phosphorylation than on the effects of 8-pCPT-ado (Fig. 6E), which can be reconciled to the fact that 8-pCPT-cAMP, different from 8-pCPT-ado, can also activate PKA directly. As expected, PKA substrate motif phosphorylation in response to the A2AR agonist CGS 21680 was completely inhibited by SCH 58261 (Fig. 6E). NBMPR also caused an increase in PKA substrate motif phosphorylation, which was reduced by SCH 58261 (supplemental Fig. 3), supporting the suggestion that inhibition of ENT1 leads to A2AR-dependent activation of PKA.
8-pCPT-modified cAMP/ado Analogs Inhibit Apoptosis upon Serum Withdrawal by a Mechanism Depending on Extracellular Adenosine in PC12 Cells
Activation of A2AR protects PC12 cells from apoptotic cell death upon serum withdrawal (26). To strengthen the hypothesis that 8-pCPT-cAMP/ado analogs may cause activation of A2AR, we studied the effects of 8-pCPT-modified cAMP/ado analogs in the presence and absence of ADA on serum withdrawal-induced apoptosis of PC12 cells. As illustrated in Fig. 6F, 8-pCPT-cAMP, 8-pCPT-ado, 8-pCPT- 2′-O-methyl-cAMP, and 8-pCPT-2′-O-methyl-ado inhibited apoptosis upon serum withdrawal, 8-pCPT-2′-O-methyl-cAMP being least potent. The effects of 8-pCPT-ado, 8-pCPT-2′-O-methyl-cAMP, and 8-pCPT-2′-O-methyl-ado were prevented by ADA (Fig. 6F) or SCH 58261 (data not shown). In contrast, the effect of 8-pCPT-cAMP was ADA- and SCH 58261-insensitive. These data are in agreement with the suggestion that 8-pCPT-ado, 8-pCPT-2′-O-methyl-ado, and 8-pCPT-2′-O-methyl-cAMP caused anti-apoptotic effects due to their ability to activate A2AR, whereas 8-pCPT-cAMP can elicit this effect by direct stimulation of PKA.
Expression of hENT1 Confers ADA/SCH 58261-sensitve Activation of PKA by 8-pCPT-cAMP/ado Analogs in HEK 293 Cells
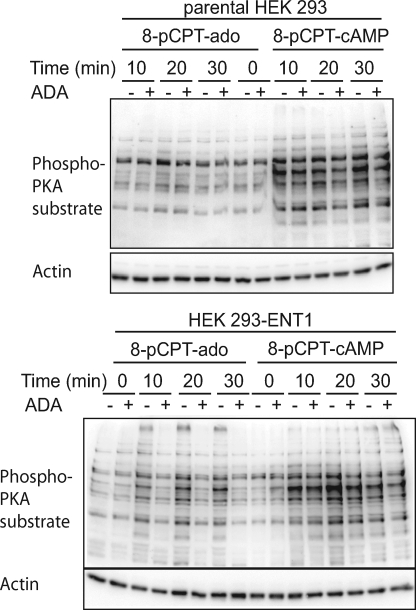
To further investigate the role of ENT1 in activation of adenylyl cyclase/PKA by 8-pCPT-modified cAMP/ado analogs, we studied the effects of 8-pCPT-cAMP and 8-pCPT-ado on PKA substrate motif phosphorylation in parental as well as hENT1-overexpressing HEK 293 cells. 8-pCPT-ado caused an increase in PKA substrate motif phosphorylation in hENT1-overexpressing HEK 293 cells, whereas it had only a small, if any, effect in parental cells (Fig. 7 and supplemental Fig. 4). The increase in PKA substrate motif phosphorylation by 8-pCPT-ado was inhibited by ADA (Fig. 7) or SCH 58261 (supplemental Fig. 4). Thus, the expression of hENT1 conferred ADA/SCH 58261-sensitive activation of PKA by 8-pCPT-ado. Measurement of the deaminase activity of ADA revealed that ADA caused deamination of adenosine itself but not of 8-pCPT-cAMP, 8-pCPT-2′-O-methyl-cAMP, 8-pCPT-ado, and 8-pCPT-2′-O-methyl-ado. This suggests that the inhibitory effects of ADA on 8-pCPT-cAMP/ado-induced increase in PKA substrate motif phosphorylation were not due to deamination of the 8-pCPT-cAMP/ado analogs. These data supported the suggestion that activation of A2AR signaling by 8-pCPT-cAMP/ado analogs depended on endogenous adenosine.
FIGURE 7.
Effect of 8-pCPT-cAMP and 8-pCPT-ado on PKA substrate motif phosphorylation in HEK 293-ENT1 cells. Parental or HEK 293-ENT1 cells were incubated with 8-pCPT-cAMP or 8-pCPT-ado (100 μm each) in the presence or absence of ADA (2 units/ml) for the indicated time followed by immunoblotting of the cell lysates with an anti-PKA substrate motif or anti-β-actin.
DISCUSSION
cAMP analogs bearing hydrophobic substituents at C8 are membrane-permeant, rendering them capable to activate intracellular cAMP effectors in intact cells (4). Therefore, they are widely used for this purpose, assuming that they act mainly by direct activation of cAMP effectors and thus, their biological effects are due to this mechanism of action. In the present study, we found that 8-pCPT-modified cAMP analogs inhibited ENT1 at similar concentrations as required to activate intracellular cAMP effectors. Considering the ubiquitous presence of ENT1, it is feasible to assume that some effects of these cAMP analogs are due to inhibition of ENT1 rather than direct activation of intracellular cAMP effectors.
A frequently used cAMP analog, possessing both 8-pCPT and the 2′-O-methyl modification to achieve membrane permeability as well as Epac selectivity, also inhibited the nucleoside transport. In contrast, bromine, chlorine, or OH modification at C8- or N6-benzoyl modification did not result in cAMP/ado analogs that inhibited ENT1. Thus, the present study emphasizes the importance of using a battery of cAMP analogs with known potencies for their target proteins to enable dissection between cAMP effector-dependent and -independent effects. In particular, 8-Br-modified cAMP analogs may offer the possibility to activate PKA and/or Epac without simultaneously inhibiting ENT1.
The inhibitory effects of 8-pCPT-cAMP or 8-pCPT-2′-O-methyl-cAMP on nucleoside transport were apparently unrelated to their ability to activate cAMP effectors because their potential hydrolysis products, 8-pCPT-ado and 8-pCPT-2′-O-methyl-ado, were at least as potent as their corresponding cAMP analogs to inhibit [3H]thymidine transport. It has recently been reported that 8-pCPT-modified cAMP analogs can be hydrolyzed to the corresponding AMP and adenosine analogs by phosphodiesterases and 5′-nucleotidases (5, 27) and that the hydrolyzable 8-pCPT-substituted cAMP analogs, but not the non-hydrolyzable Sp-8-pCPT-cAMPS analogs, increase cortisol synthesis in adrenal zona fasciculata cells (28) and reduce the proliferation of the protozoan Trypanosoma brucei (27). Thus, the cAMP effector-independent effects of the cAMP analogs in those studies appear to be due to the formation of metabolites. In the present study, however, short term incubation with the hydrolysis-stabilized Sp-8-pCPT-cAMPS or Sp-8-pCPT-2′-O-methyl-cAMPS also inhibited [3H]thymidine uptake, suggesting that hydrolysis of 8-pCPT-substituted cAMP analogs was not essential for their inhibitory effect on [3H]thymidine transport. Nevertheless, in tissues with high phosphodiesterases activity, 8-pCPT-modified cAMP analogs may exert some of its effects through the formation of metabolites, in particular during prolonged incubation.
There was a close correlation between the inhibitory effects of 8-pCPT-substituted cAMP/ado analogs on [3H]thymidine incorporation into the DNA of different cell types with their inhibitory effect on [3H]thymidine transport, indicating that the inhibitory effects of 8-pCPT-substituted cAMP/ado analogs on [3H]thymidine incorporation could be due to suppression of nucleoside transport. Notably, the reduced incorporation of [3H]thymidine into the DNA did not reflect changes in cell proliferation. Similar observations were obtained in MCF-7 cells, in which 8-pCPT-cAMP also has a stronger inhibitory effect on [3H]thymidine incorporation into the DNA than on the cell number (7), in K562 cells, in which a panel of protein kinase inhibitors reduces [3H]thymidine uptake and its incorporation into the DNA without affecting cell proliferation (29), and in EOC-20 microglial cells, in which cannabidiol inhibits [3H]thymidine transport and incorporation but not cell proliferation (27). Thus, measuring the incorporation of nucleosides such as [3H]thymidine or bromodeoxyuridine may not reflect DNA synthesis and cell proliferation in the presence of 8-pCPT-cAMP/ado analogs.
p38 mitogen-activated protein (MAP) kinase inhibitors as well as cannabidiol competitively inhibit substrate binding to ENT1 (30, 31). Preliminary experiments indicate that 8-pCPT-cAMP and 8-pCPT-ado did not inhibit the binding of [3H]NBMPR to PC12 cells, suggesting that 8-pCPT-substituted cAMP/ado analogs inhibit ENT1 differently from NBMPR or cannabidiol. Thus, 8-pCPT-cAMP/ado-induced inhibition of ENT1 may not involve competitive inhibition of the binding of the substrate. Thus, the mechanism of 8-pCPT-cAMP/ado analog-induced inhibition of ENT1 is presently unclear and could be indirect.
Inhibition of ENT1 may have a number of important biological consequences. The transporters play key roles in nucleoside and nucleobase uptake for salvage pathways of nucleotide synthesis and are also responsible for the cellular uptake of nucleoside analogues used in the treatment of cancers and viral diseases. By regulating the concentration of adenosine available to cell surface adenosine receptors, nucleoside transporters appear to play important regulatory roles in many physiological processes ranging from cardiovascular activity to neurotransmission and immune function (8–11). Thus, the adenosine uptake inhibitor KF24345 as well as cannabidiol, which have been shown to inhibit ENT1, reduce lipopolysaccharide-induced tumor necrosis factor-α response by an A2AR-dependent mechanism in mice (23, 30). This might be due to increasing the amount of endogenous adenosine and A2AR activation. However, direct evidence that ENT1 inhibition causes activation of A2AR was missing.
The data of the present study indicate that 8-pCPT-modified cAMP/ado analogs can activate A2AR signaling in ENT1-expressing cells. Thus, 8-pCPT-modified cAMP/ado analogs caused an increase in [cAMP]i, activation of PKA and prevented apoptosis in PC12 cells, in which [3H]thymidine transport was found to highly depend on ENT1. Similar data were obtained for the effect of 8-pCPT-modified cAMP/ado analogs on PKA activity in HEK 293 cells transfected with hENT1, but not in parental cells, in which [3H]thymidine transport was mainly NBMPR-insensitive. The effects of 8-pCPT-ado, 8-pCPT-2′-O-methyl-cAMP, and 8-pCPT-2′-O-methyl-ado were ablated by SCH 58261, a specific inhibitor of the Gs protein-coupled A2AR, whereas the effects of 8-pCPT-cAMP were mainly SCH 58261-insensitive. The latter can be reconciled to its ability to directly activate PKA independently from A2AR. Because inhibition of ENT1 leads to an increase of the extracellular adenosine concentration (8, 9), a plausible scenario is that 8-pCPT-modified cAMP/ado analogs inhibit ENT1, thereby facilitating activation of A2AR by endogenous adenosine. In support of this, exogenous ADA mimicked the effects of SCH 58261.
Although it cannot completely ruled out that 8-pCPT-modified cAMP/ado analogs affect A2AR directly, our data do not support this possibility. First, ADA completely inhibited PKA substrate motif phosphorylation in response to 8-pCPT-ado, although it did not deaminate 8-pCPT-modified adenosine analogs and had no influence on [3H]thymidine uptake. Second, if 8-pCPT-ado would directly activate A2AR, this effect should not depend on ENT1.
In addition to inhibition of ENT1 and activation of A2AR, other mechanisms may also contribute to the elevation of [cAMP]i and activation of PKA by 8-pCPT-cAMP/ado analogs. A recent study showing that 8-pCPT-2′-O-methyl-cAMP and Sp-8-pCPT-2′-O-methyl-cAMPS inhibit certain cyclic nucleotide phosphodiesterases (5), which facilitates activation of protein kinases A and G, provides one mechanism by which 8-pCPT-modified cAMP analogs may elicit biological effects independently from their ability to activate Epac or PKA. Still other yet unknown mechanisms may participate in the ability of 8-pCPT-modified adenosine analogs to stimulate A2AR-dependent PKA activation.
In summary, the data of the present study indicate that 8-pCPT-substituted cAMP/ado analogs inhibit ENT1 and can elicit activation of A2AR. Thus, the effects of cAMP analogs bearing 8-pCPT modification might be due to ENT1 inhibition and/or adenosine receptor activation in addition to direct activation on cAMP effectors. In addition, the elucidation of the mechanism of inhibition of ENT1 by 8-pCPT-modified cAMP/ado analogs may enable the development of novel ENT1 inhibitors.
Supplementary Material
Acknowledgments
We thank H. Diehl (University of Saarland, Germany) for technical assistance, Dr. L. M. Graves (Department of Pharmacology, University of North Carolina, Chapel Hill, NC) for plasmids encoding hENT1 and valuable discussion, and Dr. A. Menke (University of Ulm, Germany) for donating Panc-1 cells.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Grant Pi 258/7-4) and in part by grants from the University of Saarland (HOMFOR), the Wilhelm Sander foundation (Grant 2003.119.2), and the Marie Christine Held and Erika Hecker foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- PKA
- protein kinase A
- 8-pCPT-cAMP
- 8-(4- chlorophenylthio)-cAMP
- 8-pCPT-ado
- 8-(4-chlorophenylthio)-adenosine
- 8-pCPT-2′-O-methyl-cAMP
- 8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- 8-pCPT-2′-O-methyl-ado
- 8-(4-chlorophenylthio)-2′-O-methyl-adenosine
- Sp-8-pCPT-2′-O-methyl-cAMPS
- Sp-8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- Sp-8-pCPT-cAMPS
- Sp-8-(4-chlorophenylthio)-cAMP
- 8-Br-ado
- 8-bromoadenosine
- 8-Br-cAMP
- 8-bromoadenosine-3′,5′-cyclic monophosphate
- 8-Cl-ado
- 8-chloroadenosine
- 8-OH-cAMP
- 8-hydroxy-3′,5′-cyclic monophosphate
- N6-benzoyl-cAMP
- N6-benzoyl-3′,5′-cyclic monophosphate
- ADA
- adenosine deaminase
- A2AR
- adenosine A2A receptor
- ENT
- equilibrative nucleoside transporter
- hENT1
- human ENT1
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NBMPR
- 5- (4-nitrobenzyl)-6-thioinosine
- DMEM
- Dulbecco's modified Eagle's medium
- DMSO
- dimethyl sulfoxide.
REFERENCES
- 1.Dumaz N., Marais R. (2005) FEBS J. 272, 3491–3504 [DOI] [PubMed] [Google Scholar]
- 2.Bos J. L. (2006) Trends Biochem. Sci. 31, 680–686 [DOI] [PubMed] [Google Scholar]
- 3.Beavo J. A., Brunton L. L. (2002) Nat. Rev. Mol. Cell Biol. 3, 710–718 [DOI] [PubMed] [Google Scholar]
- 4.Holz G. G., Chepurny O. G., Schwede F. (2008) Cell. Signal. 20, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poppe H., Rybalkin S. D., Rehmann H., Hinds T. R., Tang X. B., Christensen A. E., Schwede F., Genieser H. G., Bos J. L., Doskeland S. O., Beavo J. A., Butt E. (2008) Nat. Methods 5, 277–278 [DOI] [PubMed] [Google Scholar]
- 6.Van Lookeren Campagne M. M., Villalba Díaz F., Jastorff B., Kessin R. H. (1991) Cancer Res. 51, 1600–1605 [PubMed] [Google Scholar]
- 7.Vintermyr O. K., Bøe R., Brustugun O. T., Maronde E., Aakvaag A., Døskeland S. O. (1995) Endocrinology 136, 2513–2520 [DOI] [PubMed] [Google Scholar]
- 8.Baldwin S. A., Beal P. R., Yao S. Y., King A. E., Cass C. E., Young J. D. (2004) Pflugers Arch. 447, 735–743 [DOI] [PubMed] [Google Scholar]
- 9.Löffler M., Morote-Garcia J. C., Eltzschig S. A., Coe I. R., Eltzschig H. K. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1004–1013 [DOI] [PubMed] [Google Scholar]
- 10.Ralevic V., Burnstock G. (1998) Pharmacol. Rev. 50, 413–492 [PubMed] [Google Scholar]
- 11.Ohta A., Sitkovsky M. (2001) Nature 414, 916–920 [DOI] [PubMed] [Google Scholar]
- 12.Mondorf U. F., Geiger H., Herrero M., Zeuzem S., Piiper A. (2000) FEBS Lett. 472, 129–132 [DOI] [PubMed] [Google Scholar]
- 13.van Noesel M. M., van Bezouw S., Salomons G. S., Voûte P. A., Pieters R., Baylin S. B., Herman J. G., Versteeg R. (2002) Cancer Res. 62, 2157–2161 [PubMed] [Google Scholar]
- 14.Kiermayer S., Biondi R. M., Imig J., Plotz G., Haupenthal J., Zeuzem S., Piiper A. (2005) Mol. Biol. Cell 16, 5639–5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman E. I., Huang M., Leisewitz A. V., Wang Y., Yang J., Graves L. M. (2009) FEBS Lett. 583, 425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosmann T. (1983) J. Immunol. Methods 65, 55–63 [DOI] [PubMed] [Google Scholar]
- 17.Piiper A., Dikic I., Lutz M. P., Leser J., Kronenberger B., Elez R., Cramer H., Müller-Esterl W., Zeuzem S. (2002) J. Biol. Chem. 277, 43623–43630 [DOI] [PubMed] [Google Scholar]
- 18.Piiper A., Elez R., You S. J., Kronenberger B., Loitsch S., Roche S., Zeuzem S. (2003) J. Biol. Chem. 278, 7065–7072 [DOI] [PubMed] [Google Scholar]
- 19.Martinek R. G. (1963) Clin. Chem. 102, 620–625 [PubMed] [Google Scholar]
- 20.Dremier S., Milenkovic M., Blancquaert S., Dumont J. E., Døskeland S. O., Maenhaut C., Roger P. P. (2007) Endocrinology 148, 4612–4622 [DOI] [PubMed] [Google Scholar]
- 21.Haag S., Warnken M., Juergens U. R., Racké K. (2008) Naunyn Schmiedebergs Arch. Pharmacol. 378, 617–630 [DOI] [PubMed] [Google Scholar]
- 22.Dunwiddie T. V., Diao L. (2000) Neuroscience 95, 81–88 [DOI] [PubMed] [Google Scholar]
- 23.Noji T., Takayama M., Mizutani M., Okamura Y., Takai H., Karasawa A., Kusaka H. (2002) J. Pharmacol. Exp. Ther. 300, 200–205 [DOI] [PubMed] [Google Scholar]
- 24.Arslan G., Kull B., Fredholm B. B. (1999) Naunyn Schmiedebergs Arch. Pharmacol. 359, 28–32 [DOI] [PubMed] [Google Scholar]
- 25.Fredholm B. B., IJzerman A. P., Jacobson K. A., Klotz K. N., Linden J. (2001) Pharmacol. Rev. 53, 527–552 [PMC free article] [PubMed] [Google Scholar]
- 26.Huang N. K., Lin Y. W., Huang C. L., Messing R. O., Chern Y. (2001) J. Biol. Chem. 276, 13838–13846 [DOI] [PubMed] [Google Scholar]
- 27.Laxman S., Riechers A., Sadilek M., Schwede F., Beavo J. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19194–19199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enyeart J. A., Enyeart J. J. (2009) PLOS One 4, e6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang M., Wang Y., Cogut S. B., Mitchell B. S., Graves L. M. ( 2003) J. Pharmacol. Exp. Ther. 304, 753– 760 [DOI] [PubMed] [Google Scholar]
- 30.Carrier E. J., Auchampach J. A., Hillard C. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7895–7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang M., Wang Y., Collins M., Gu J. J., Mitchell B. S., Graves L. M. (2002) J. Biol. Chem. 277, 28364–28367 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.