Abstract
Adrenoceptors receptors (ARs) play a pivotal role in regulating cardiovascular response to catecholamines during stress. β2ARs, prototypical G protein-coupled receptors (GPCRs), expressed in animal hearts, display dual coupling to both Gs and Gi proteins to control the adenylyl cyclase-cAMP dependent protein kinase A (PKA) pathway to regulate contraction responses. Here, we showed that the β2AR coupling to Gi proteins was agonist dose-dependent and occurred only at high concentrations in mouse cardiac myocytes. Both the β2AR-induced PKA activity, measured by fluorescence resonance energy transfer (FRET) imaging, and the increase in myocyte contraction rate displayed sensitivity to the Gi inhibitor pertussis toxin (PTX). Further studies revealed that activated β2ARs underwent PKA phosphorylation at a broad range of agonist concentrations. Disruption of the PKA phosphorylation sites on the β2AR blocked receptor/Gi coupling. However, a sufficient β2AR/Gi coupling was also dependent on the G protein-coupled receptor kinase (GRK)-mediated phosphorylation of the receptors, which only occurred at high concentrations of agonist (≥100 nm). Disruption of the GRK phosphorylation sites on the β2AR blocked receptor internalization and coupling to Gi proteins, probably by preventing the receptor's transportation to access Gi proteins. Furthermore, neither PKA nor GRK site mutated receptors displayed sensitivity to the Gi-specific inhibitor, GiCT. Together, our studies revealed distinct roles of PKA and GRK phosphorylation of the β2AR for agonist dose-dependent coupling to Gi proteins in cardiac myocytes, which may protect cells from overstimulation under high concentrations of catecholamines.
Introduction
Adrenoceptors (ARs)3 play a pivotal role in regulating cardiovascular response to catecholamines during stress. Both β1- and β2ARs are prototypical GPCRs expressed in animal hearts, which mediate the increases in cardiac contraction upon agonist stimulation through the Gs-adenylyl cyclase-cAMP-dependent PKA pathway (1). β2ARs are also known to uniquely couple to inhibitory Gi proteins, which inhibits adenylyl cyclases to reduce cardiac contraction and initiates anti-apoptotic and cell growth signaling (2, 3). Although β2AR coupling to Gi has been studied in reconstituted systems and in fibroblasts (4, 5), little is known about the regulation process and the circumstances under which this coupling occurs in cardiac cells, along with its physiological consequences.
Various studies indicate that phosphorylation of β2AR plays a critical role in regulating differential G protein coupling. The β2AR phosphorylation by PKA mediates the switch of coupling from Gs to Gi (6, 7), presumably operating in a feedback after receptor activation. Our previous studies have revealed that β2AR/Gi coupling is also dependent on receptor internalization and recycling (8, 9). Meanwhile, Wang et al. have shown that direct inhibition of GRK2 prevents β2AR/Gi coupling in mouse cardiac myocytes (8), supporting a role of the β2AR phosphorylation by GRK in receptor/Gi coupling.
Studies have also revealed that β2ARs are differentially phosphorylated by PKA and GRK in fibroblasts in an agonist dose-dependent manner (10–13). Although the PKA-mediated phosphorylation is sensitive to stimulation with subnanomolar concentrations, the GRK-mediated phosphorylation happens only when β2ARs are stimulated with saturated concentrations (10–13). This differential regulation of receptor phosphorylation by different kinases and their effects on subsequent receptor trafficking indicate that the β2AR/Gi coupling may be finely regulated in an agonist concentration-dependent manner.
Here, using a fluorescence resonance energy transfer (FRET) assay with a biosensor for PKA activity and a physiological cardiac contraction rate assay, we show for the first time that β2AR/Gi coupling occurs only at a saturated concentration of isoproterenol (Iso) in cardiac cells. Stimulation induces the PKA-mediated phosphorylation of the β2AR over a wide range of agonist concentrations, whereas the GRK-mediated phosphorylation occurs only at sufficiently high concentrations (≥100 nm) of Iso and is necessary for receptor internalization. We further demonstrate that mutations of the specific PKA or GRK phosphorylation sites on the β2AR abolish receptor coupling to Gi protein. Our data suggest that the PKA-mediated phosphorylation is necessary for β2AR/Gi coupling, but a sufficient receptor/Gi coupling is also dependent on the GRK-mediated phosphorylation and subsequent trafficking of the receptors.
MATERIALS AND METHODS
Site-directed Mutagenesis and Recombinant Adenoviruses
pcDNA3.1-FLAG-tagged murine β2AR was used as a template for mutagenesis. A protein kinase A mutant (PKAmut) β2AR lacking protein kinase A (PKA) phosphorylation sites (serines 261, 262, 345, and 346) was generated by replacing the four serines with alanines. A GRK mutant (GRKmut) β2AR lacking the GRK phosphorylation sites (serines 355, 356, and 358) was also created by replacing serines with alanine. These phosphorylation sites are determined by Tran et al. (13). Both β2AR mutants were sequenced and transformed into adenovector for producing adenoviruses, as described previously (14). The titers of the viruses were assessed by comparison of receptor expression levels with both Western blot and ligand binding, as described previously (15). The GFP-GiCT virus used in contraction assays was a kind gift from Dr. Walter Koch (Thomas Jefferson University, Philadelphia, PA).
Cardiac Myocyte Contraction Assay
Measurement of spontaneous myocyte contraction was performed as described previously (16). Neonatal cardiac myocytes were isolated from mice lacking either β1AR (β1AR-knock-out; β1AR- KO) or both β1 and β2AR genes (β1β2AR-KO). β1β2AR-KO myocytes were infected with murine β2AR, PKAmut β2AR, or GRKmut β2AR at a multiplicity of infection of 100 and stimulated with 10 μm Iso. Cardiac myocytes were treated with Gi inhibitor pertussis toxin (PTX) at 0.3 μg/ml, 3 h before stimulation. GFP-GiCT virus was co-infected at a multiplicity of infection of 100 or, as indicated, along with the wild type or mutant β2AR viruses. Contraction rate assays were performed the next day as described previously (16).
Receptor Phosphorylation
Neonatal cardiac myocytes were infected with FLAG-tagged wild type or mutant β2ARs at a multiplicity of infection of 100. After 48 h of expression, cells were serum-starved for 2 h and stimulated with different doses of Iso for 5 min. For the time course experiments, cells were stimulated with 10 μm Iso for different times as indicated. Cells were placed into lysis buffer (10 mm Tris, pH 8.0, 1% Nonidet P-40, 150 mm NaCl, 2 mm EDTA, and protease and phosphatase inhibitor mixture (Thermo Scientific)) for 30 min at 4 °C. Samples were centrifuged, and the supernatant was resolved by SDS-PAGE for Western blotting. The β2AR phosphorylation by PKA at Ser(P)261/262 was detected with a monoclonal antibody from Dr. Richard Clark (University of Texas Health Science Center, Houston, TX). The β2AR phosphorylation by GRK at Ser(P)355/356 was detected with a polyclonal antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). IRDye 680CW goat anti-mouse and IRDye 800CW goat anti-rabbit secondary antibodies were used to detect Ser(P)261/262 and Ser(P)355/356 phosphorylation, respectively. Western blots were visualized with an Odyssey scanner (Li-cor Biosciences, Lincoln, NE) and quantified before normalization against the base-line levels.
Receptor Internalization and Recycling
After expressing the FLAG-β2AR, the β1β2AR-KO myocytes were serum-starved for 2 h and stimulated with 10 nm or 10 μm Iso for different times. For receptor recycling, cells were stimulated with Iso for 10 min, rinsed, and refed with serum-free medium for different times. Cells were then fixed, permeabilized, and stained with anti-FLAG antibody, which was revealed with an Alexa-488 conjugated goat anti-mouse antibody (Invitrogen). Fluorescence images were taken with a CCD camera on a Zeiss microscope with Metamorph software. HEK293 cells were transfected with plasmids expressing the FLAG-β2AR, PKAmut β2AR, and GRKmut β2AR. Receptor internalization and recycling was quantified in HEK293 cells by using a fluorescence-linked immunosorbent assay (FLISA) to determine cell surface receptor levels as previously described (17). In brief, the FLAG-tagged receptors were labeled with anti-FLAG M1 antibody, which was revealed by the Alexa-488 goat-anti-mouse secondary antibody and detected by Spectramax M2 fluorometer.
PKA Activity Measurement through FRET Assay
β1AR-KO or β1β2AR-KO cardiac myocytes were infected with virus expressing A-kinase activity reporter (AKAR2.2) as previously described (18). For β1β2AR-KO myocytes, cells were also infected with receptor virus for overnight expression. The procedure for PKA FRET activity was carried out as previously described (18).
Statistical Analysis
Curve fitting and statistical analyses were performed using Prism software (GraphPad Software, Inc. San Diego, CA).
RESULTS
Stimulation of the β2AR Induces an Agonist Concentration-dependent Receptor/Gi Coupling in Cardiac Myocytes
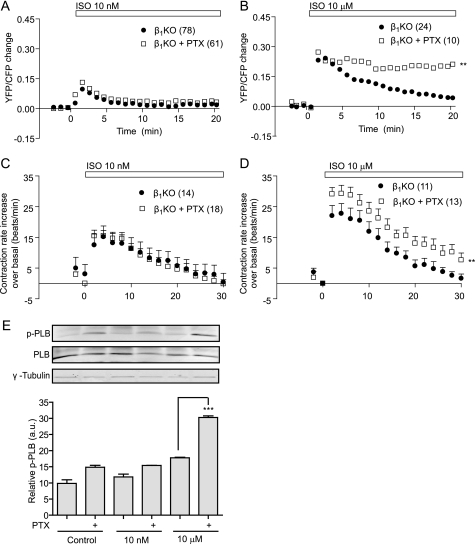
By activation of the endogenous β2AR with Iso in β1AR- KO cardiac myocytes, we analyzed the receptor-induced PKA activity through a FRET assay using the fluorescent biosensor AKAR2.2. Activation of the β2AR with 10 nm Iso induced a transient peak in PKA activity, which returned to base-line levels within 5 min (Fig. 1A). Pretreatment with PTX, which specifically inhibits Gi proteins, did not significantly change the β2AR-induced FRET response. However, a saturated concentration of 10 μm Iso resulted in a more sustained PKA FRET response, which was further enhanced with PTX pretreatment (Fig. 1B). This finding indicates that 10 μm Iso, but not 10 nm Iso, selectively induces β2AR/Gi coupling for PKA activity in cardiac myocytes. We then used a cardiac contraction rate assay to examine the physiological consequences of this dose-dependent receptor/Gi protein coupling. We found that at 10 nm Iso, the β2AR-induced increase in contraction rate was not PTX-sensitive (Fig. 1C), whereas PTX significantly enhanced the increase in contraction rate induced by 10 μm Iso (Fig. 1D). These data again suggest that the β2AR couples to Gi proteins only after stimulation with high concentrations of agonist. Consistent with this notion, the PKA-mediated phosphorylation of phospholamban, a PKA substrate that is critical for the βAR-induced cardiac contractile response, was enhanced by PTX only after stimulation with 10 μm Iso (Fig. 1E). Previous reports have suggested that the PKA-mediated phosphorylation of the β2AR enhances the receptor coupling to Gi protein (6, 7), and the GRK-dependent phosphorylation of the β2AR occurs only after stimulation with high concentrations of agonist (13), making the phosphorylation of β2AR a potential regulator of this dose-dependent Gi coupling in cardiac myocytes.
FIGURE 1.
Stimulation of the β2AR induces agonist dose-dependent coupling to Gi in cardiac myocytes. A and B, β1AR-KO cardiac myocytes expressing the PKA biosensor AKAR2.2 were stimulated with 10 nm (A) or 10 μm (B) Iso. Myocytes were treated with Gi protein inhibitor PTX for 2 h before stimulation to examine the effect on PKA FRET activity induced by agonist. C and D, β1AR-KO cardiac myocytes were stimulated with 10 nm (C) or 10 μm (D) Iso with and without PTX pretreatment, and the increases in contraction rate over base-line levels were measured as a function of time. E, β1AR-KO myocytes were stimulated with 10 nm or 10 μm Iso with and without PTX treatment. The phosphorylation of phospholamban was detected and normalized against total phospholamban (n = 4). **, p < 0.01 when compared with the group without PTX treatment by two-way ANOVA. ***, p < 0.001 when compared with the group without PTX treatment by Student's t test.
Agonist Stimulation Induces a Dose-dependent Distinct PKA and GRK Phosphorylation for β2AR Internalization and Signaling in Cardiac Myocytes
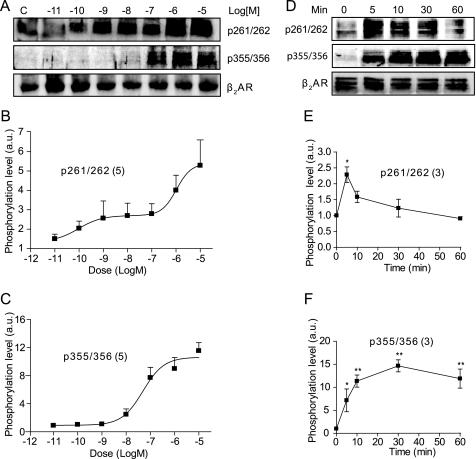
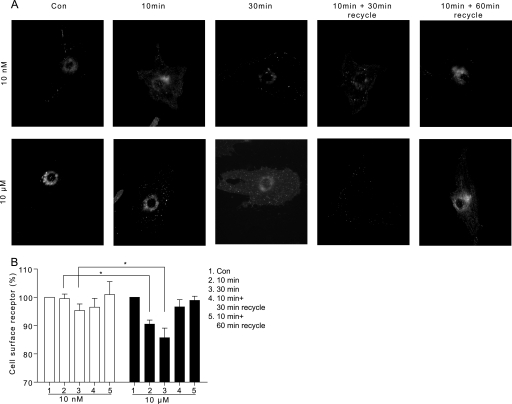
Analysis of the phosphorylation of β2AR in cardiac myocytes showed that the PKA-dependent phosphorylation at serines 261/262 occurred at subnanomolar concentrations of Iso and displayed a dose-dependent increase (Fig. 2, A and B). In contrast, the β2AR phosphorylation by GRK at serines 355/356 occurred only at 100 nm Iso or at higher doses (Fig. 2, A and C). After stimulation with saturated 10 μm Iso, the kinetics of the GRK- and PKA-mediated phosphorylation of the β2AR differed (Fig. 2D). The PKA-mediated phosphorylation showed a rapid increase, followed by a slow decrease, whereas the GRK-mediated phosphorylation was slower but more sustained over time (Fig. 2, E and F). It has been well documented that the GRK-mediated phosphorylation is involved in the endocytosis of β2AR in fibroblasts (19, 20), prompting us to explore the role of β2AR phosphorylation in receptor internalization in cardiac myocytes. The activated β2ARs showed little punctuate staining upon stimulation with 10 nm Iso in cardiac myocytes (Fig. 3A). However, stimulation with 10 μm Iso induced a time-dependent clustering of β2ARs in the cells, similar to those reported previously (8), which is a characteristic of receptor internalization (Fig. 3A). Moreover, the removal of Iso after 10 min of stimulation allowed the internalized receptor to return to the cell surface (Fig. 3A). These observations were further confirmed by quantification with a FLISA assay (Fig. 3B).
FIGURE 2.
Stimulation of the β2AR induces agonist dose-dependent and dynamic receptor phosphorylation by PKA and GRK. A, the β1β2AR-KO cardiac myocytes expressing FLAG-β2ARs were stimulated with different concentrations of Iso and blotted for phosphorylation by PKA (Ser(P)261/262) or GRK (Ser(P)355/356). The Western blots in A were quantified and plotted as -fold increases over base-line levels for the PKA-dependent phosphorylation (B) and GRK-dependent phosphorylation (C). D, myocytes expressing FLAG-β2ARs were stimulated with 10 μm Iso for the indicated time to examine the PKA- and GRK-mediated phosphorylation. E and F, the Western blots in D were quantified and plotted as -fold increases over base-line levels. *, p < 0.05; **, p < 0.01 when compared with the base-line levels by one-way ANOVA. a.u., arbitrary units.
FIGURE 3.
Stimulation of the β2AR induces agonist dose-dependent internalization in cardiac myocytes. A, the β1β2AR-KO cardiac myocytes expressing FLAG-β2AR were stimulated with 10 nm or 10 μm Iso for 10 or 30 min or 10-min Iso stimulation followed by drug removal for 30 or 60 min. The FLAG-β2ARs were visualized through immunofluorescence imaging. These data are representative of at least three experiments. B, the cell surface FLAG-β2ARs were quantified by FLISA in HEK293 cells after stimulation up to 10 or 30 min or after 10 min Iso stimulation followed by drug removal for 30 or 60 min. Receptor levels were normalized against base-line levels (n = 5). **, p < 0.01 by Student's t test. Con, control.
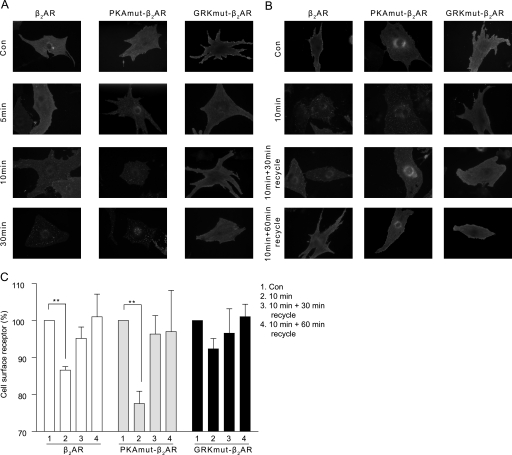
We then directly tested the role of phosphorylation in receptor internalization and signaling by using mutant β2ARs with the PKA or GRK phosphorylation sites replaced by alanines. The expressions of both PKAmut and GRKmut were quantified and equalized through Western blot and ligand binding (supplemental Fig. S1). Western blots of the mutant β2ARs also confirmed abolishment of the specific receptor phosphorylation events upon stimulation with 10 μm Iso (supplemental Fig. S2). Immunostaining of the wild type and PKAmut β2ARs showed time-dependent internalization of the receptors after stimulation with 10 μm Iso (Fig. 4A). However, the GRKmut β2ARs failed to sufficiently internalize under Iso stimulation. Washout of Iso after 10 min of stimulation showed that the internalized receptors underwent recycling to the cell surface (Fig. 4B). Quantification through the FLISA assay confirmed that the wild type and PKAmut β2ARs but not the GRKmut β2ARs were significantly internalized upon 10 min of stimulation with 10 μm Iso (Fig. 4C), supporting the necessary role of GRK phosphorylation in the internalization of the β2AR in cardiac myocytes. Together, the characterization of the mutant β2ARs revealed distinct actions of PKA and GRK on the receptor phosphorylation and trafficking in cardiac myocytes upon agonist stimulation, deeming the phosphorylation-deficient mutants suitable for closer examination on the effects on the receptor signaling in these cells.
FIGURE 4.
Stimulation of wild type and mutant β2ARs induces different receptor internalization and recycling. A, the FLAG-tagged β2AR, PKAmut β2AR, and GRKmut β2AR expressed in cardiac myocytes were stimulated with 10 μm Iso for different times before they were visualized by immunofluorescence imaging. B, the wild type and mutant β2ARs were visualized for recycling after stimulation for 10 min, followed by drug removal for the indicated times. These data are representative of at least three experiments. C, the cell surface wild type and mutant β2ARs were quantified through FLISA after 10 min of Iso stimulation and recycling after drug removal for 30 and 60 min in HEK293 cells (n = 3). Receptor levels were normalized against base-line levels. **, p < 0.01 by Student's t test. Con, control.
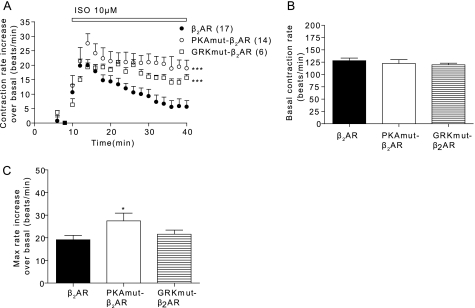
By introducing either wild type or mutant β2AR into β1β2AR-KO cardiac myocytes, we were able to examine the physiological effects of the phosphorylation of the β2AR upon receptor activation. Stimulation of the PKAmut β2AR induced a stronger contraction rate increase over that of the wild type (Fig. 5A). Stimulation of the GRKmut β2AR showed a contraction rate increase similar to that of the wild type, but this increase was much more sustained (Fig. 5A). Although the base-line contraction rates between the wild type and mutant β2ARs were not significantly different (Fig. 5B), the maximal contraction rate increase induced by the PKAmut β2AR was significantly higher than that of wild type (Fig. 5C). These data strongly suggest that the phosphorylation of the β2AR has significant consequences in regulating myocyte contraction rate responses.
FIGURE 5.
Activation of wild type and mutant β2ARs induces distinct responses in contraction rates in cardiac myocytes. A, the β1β2AR-KO cardiac myocytes expressing β2AR, PKAmut β2AR, or GRKmut β2AR were stimulated with 10 μm Iso for contraction rate measurement. The base-line levels of contraction rates (B) and the maximal increases in contraction rate after stimulation (C) were plotted. ***, p < 0.001 when compared with the wild type β2AR by two-way ANOVA. *, p < 0.05 by Student's t test.
PKA and GRK Phosphorylation Differentially Regulate β2AR/Gi Coupling in Cardiac Myocytes
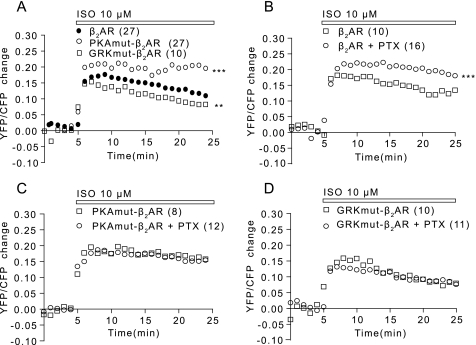
By analyzing PKA activity in cardiac myocytes induced by these mutant β2ARs, we were able to observe the kinetics of upstream signaling, which leads to contraction induced by the receptors. Stimulation of the wild type, PKAmut β2AR, or GRKmut β2AR with 10 μm Iso induced a stronger FRET increase in PKA activity, although the increase induced by the GRKmut β2AR was less than those of the wild type and PKAmut receptors (Fig. 6A). Inhibition of Gi proteins with PTX pretreatment significantly enhanced the PKA FRET increase induced by activation of the wild type β2AR (Fig. 6B). However, the FRET response induced by the PKAmut β2AR was insensitive to PTX (Fig. 6C), indicating that this receptor was unable to couple to Gi protein. Interestingly, the FRET response induced by the GRKmut β2AR was also PTX-insensitive (Fig. 6D), indicating that the GRK-dependent phosphorylation was necessary for β2AR coupling to Gi protein.
FIGURE 6.
Wild type and mutant β2ARs differentially couple to Gi proteins to regulate agonist-induced PKA FRET responses. A, the β1β2AR-KO cardiac myocytes expressing the PKA FRET biosensor AKAR2.2 together with the β2AR, PKAmut β2AR, or GRKmut β2AR were stimulated with 10 μm Iso as indicated. The PKA FRET ratio was measured. B–D, myocytes were also treated with PTX to access the receptor/Gi coupling in regulating PKA FRET response after Iso stimulation. **, p < 0.01; ***, p < 0.001 when compared with the wild type receptor by two-way ANOVA. YFP, yellow fluorescent protein; CFP, cyan fluorescent protein.
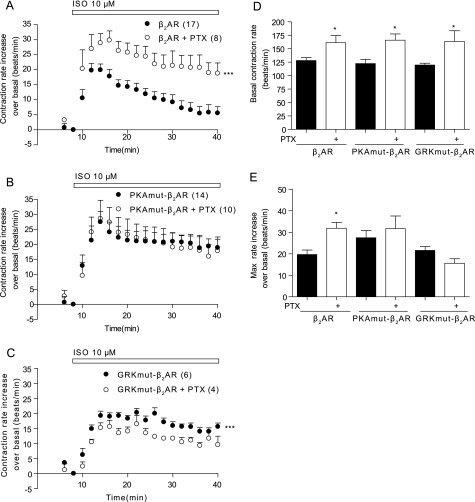
Extending the PKA FRET results into the contraction rate assay reinforced the physiological effects of this phosphorylation-dependent G protein-coupling phenomenon. Although pretreatment with PTX significantly enhanced the wild type β2AR-induced increase in contraction rate upon stimulation with 10 μm Iso (Fig. 7, A and E), it did not significantly affect the PKAmut β2AR-induced increase in contraction rate (Fig. 7, B and E). Moreover, PTX did not enhance the GRKmut β2AR-induced increase in contraction rate; instead, it lowered the contraction rate increase (Fig. 7, C and E), which could be in part due to the enhanced base-line contraction rate by PTX treatment (Fig. 7D). These data suggest that both PKA and GRK phosphorylation are required for β2AR coupling to Gi protein, which thereafter affects myocyte contraction rate response.
FIGURE 7.
Wild type and mutant β2ARs differentially couple to Gi proteins to regulate agonist-induced contraction rate increases. A–C, the β1β2AR-KO cardiac myocytes expressing the β2AR, PKAmut β2AR, or GRKmut β2AR were stimulated with 10 μm Iso in the presence or absence of PTX. The response in myocyte contraction rate was measured. D and E, the base-line levels of contraction rates and the maximal increases in contraction rates after stimulation in the presence or absence of PTX were plotted. ***, p < 0.001 when compared with the group without PTX treatment by two-way ANOVA. *, p < 0.05 when compared with the group without PTX treatment in Student's t test.
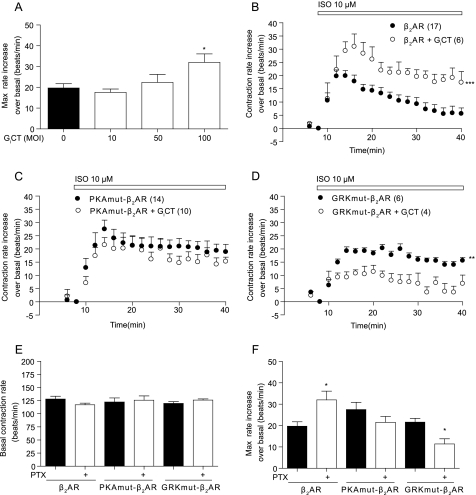
We further employed a specific inhibitor of the Gi protein consisting of the C-terminal portion of Gαi2 (GiCT) (21) to probe receptor/Gi coupling by either wild type or mutant β2AR. We first determined that infection with GiCT virus at a multiplicity of infection of 100 optimally inhibited receptor/Gi coupling and enhanced the β2AR-induced increase in contraction rate (Fig. 8A). We then introduced GiCT along with wild type or mutant β2AR into β1β2AR-KO cardiac myocytes. Overexpressing GiCT significantly enhanced the wild type β2AR-induced increase in contraction rate (Fig. 8, B and F), which was similar to that obtained with PTX treatment. However, GiCT did not affect the PKAmut β2AR-induced increase in contraction rate (Fig. 8, C and F). Similar to that of PTX treatment (Fig. 7C), inhibition of Gi protein with GiCT also reduced the GRKmut β2AR-induced increase in contraction rate (Fig. 8, D and F). Overexpression of GiCT did not affect the base-line contraction rates (Fig. 8E). Together, we showed that inhibition of Gi with GiCT significantly enhanced the maximal contraction rate induced by the wild type but not by the PKAmut and GRKmut β2ARs.
FIGURE 8.
GiCT inhibits β2AR/Gi coupling for myocyte contraction rate response upon agonist stimulation. A, the maximal responses in contraction rate induced by the β2AR were enhanced by expression of GiCT along with the wild type β2AR in β1β2AR-KO cardiac myocytes. B–D, the β1β2AR-KO cardiac myocytes expressing the β2AR, PKAmut β2AR, or GRKmut β2AR were stimulated with 10 μm Iso in the presence or absence of GiCT. The response in myocyte contraction rate was measured. E and F, the base-line levels of contraction rates and the maximal increases in contraction rates after stimulation in the presence or absence of GiCT were plotted. **, p < 0.01; ***, p < 0.001 when compared with the wild type receptor by two-way ANOVA. *, p < 0.05 when compared with the group without GiCT in Student's t test.
DISCUSSION
By measuring PKA activity and cardiac contraction rate increase, we show for the first time that β2AR activation of Gi proteins occurs selectively at high concentrations but not at low concentrations of agonist. Moreover, we show that the receptor coupling to Gi proteins is differentially regulated by the PKA- and GRK-mediated phosphorylation of the activated β2ARs. At both low and high concentrations of agonist, the activated β2ARs undergo the PKA-mediated phosphorylation. In comparison, only high concentrations of agonist induce the GRK-mediated phosphorylation of the β2ARs for subsequent internalization, which is also necessary for sufficient receptor coupling to Gi proteins (8, 9). Our studies link together various components of the β2AR, including receptor phosphorylation, receptor trafficking, and differential receptor/G protein coupling in cardiac cells, which may allow the activation of the β2AR signaling pathway to function as either a stimulatory or protective mechanism for cardiac cells under different levels of stress.
It has been well documented that the PKA-mediated phosphorylation of β2AR enhances the receptor coupling affinity to Gi protein in a reconstituted system, and mutation of the PKA phosphorylation sites blocks receptor coupling to Gi in HEK293 fibroblasts (6, 7). In this study, we have further resolved the necessity of the PKA-dependent phosphorylation of β2AR in coupling to Gi proteins in cardiac myocytes. Activation of the murine β2AR by both low and high concentration of isoproterenol leads to phosphorylation of the receptor by PKA. At high concentrations of isoproterenol, the phosphorylation levels of the β2AR by PKA are further enhanced in comparison with those at low concentrations. This is probably due to dissociation of phosphodiesterase 4D isoforms from the activated receptors (data not shown), which leads to higher local PKA activity for receptor phosphorylation. However, under stimulation with low concentrations of isoproterenol, the activated β2ARs do not couple to Gi, even if the PKA-mediated phosphorylation occurs (Figs. 1 and 2). However, disruption of the PKA phosphorylation sites on the β2AR with mutagenesis blocks the receptor coupling to Gi (Figs. 6–8). Together, these data indicate that PKA phosphorylation is necessary but not sufficient for the activated β2AR coupling to Gi in cardiac myocytes.
In contrast, the β2ARs undergo GRK-dependent phosphorylation only under stimulation with high concentrations of Iso (Fig. 2). Disruption of the GRK sites prolongs the Iso-induced myocyte contraction rate response (Fig. 5A), which is consistent with the notion that the phosphorylation of β2AR by GRK is required for rapid receptor desensitization (22). We do not observe obvious change in the overall PKA activity induced by the GRKmut (Fig. 6A), probably because the change is small and occurs in the local environment of the receptor and thus is not sensed by PKA probes presented throughout the cytosol. However, similar to those of the PKAmut, the PKA FRET and contraction rate responses induced by the GRKmut β2AR do not show sensitivity to the inhibition of Gi protein (Figs. 6–8), indicating that the GRK-mediated phosphorylation is required for sufficient coupling to Gi. One possibility is that the GRK-phosphorylated receptors undergo internalization to promote the access of the receptor to Gi protein. Another possibility is that the GRK-mediated phosphorylation of the β2AR directly enhances the binding affinity of the receptor to Gi protein. However, evidence so far argues that the GRK phosphorylation-dependent receptor trafficking is more likely the key for sufficient receptor/Gi coupling. To support this notion, either blocking receptor internalization or disrupting receptor recycling through point mutation on the PDZ motif of the β2AR blocks the receptor/Gi coupling, which yields enhanced myocyte contraction responses (9, 15). Moreover, Wang et al. (8) have shown that direct inhibition of GRK2 activity blocks the receptor coupling to Gi proteins in myocytes. Furthermore, the GRK phosphorylation-dependent recruitment of arrestin-PDE4D complexes to the activated β2AR has been implicated in receptor/Gi coupling (23). These studies, together with our data, suggest that the GRK-mediated phosphorylation of β2AR and subsequent receptor trafficking are critical for sufficient access of the receptor to Gi proteins. Consistent with this notion, a Gi-specific inhibitor, GiCT, selectively blocks the receptor coupling to Gi without affecting the receptor coupling to Gs. These data suggest that the GiCT peptide did not compete against the unphosphorylated receptors in binding to Gs protein but blocks the phosphorylated receptor in binding to Gi protein, which reinforces the idea that β2AR/Gi coupling is dependent on the agonist-induced phosphorylation of the receptor. Together, our data suggest that although the PKA-mediated phosphorylation is required for high affinity binding to Gi protein, the GRK-mediated phosphorylation is necessary for receptor trafficking that appears to control the accessibility of β2AR to Gi protein in cardiac myocytes.
Cardiac contraction in response to adrenergic stimulation is a tightly controlled process to ensure steady increases in cardiac output without overstimulation. Our observation of an agonist concentration-dependent switch of the activated β2AR coupling from Gs and Gi may be critical for such fine regulation. At low concentrations of catecholamines, the activated receptors will couple only to Gs for a stimulatory effect on cAMP/PKA activities for increasing cardiac contraction. However, in the presence of high concentrations of catecholamines, the activated β2ARs will switch from Gs to Gi to reduce cAMP/PKA activities in cardiac tissues while preventing the overstimulation of cardiac contraction. The mechanism for how high concentrations of agonists can induce receptor phosphorylation by different kinases for subsequent switch of G protein coupling is currently not known. Structural studies reveal that higher concentrations of agonists induce conformational changes distinct from those induced by low concentrations (17), which may enhance the receptor binding affinity to GRK. Alternatively, low concentrations may selectively activate the Gs protein-precoupled pool of receptors with high affinity binding, whereas higher concentrations of agonists activate both precoupled and non-coupled pools of receptors. The latter pool of receptors may be involved in coupling to Gi proteins. The mechanism thus remains to be addressed. This protective mechanism by receptor/Gi coupling may be essential for preventing a high frequency of contractions, such as fibrillation during stress. In addition, β2AR/Gi coupling can play a protective role against the apoptotic effects induced by adrenergic/Gs stimulation (24–27).
More than a simple extension of the biochemical characterization of the β2AR, this study provides insightful information on the physiological effects of receptor phosphorylation by PKA and GRK. More importantly, it opens up questions about the nature of phosphorylation and its role in receptor desensitization, a concept that has been established in the last couple of decades. Undoubtedly, phosphorylation seems to play a role in diminishing the effects of receptor activation in cardiac myocytes, since abolishing those phosphorylation sites enhanced the contraction rate over the wild type upon stimulation (Fig. 4). However, we find that receptor phosphorylation appears to be critical in regulating contraction through coupling to different G proteins to protect cardiac cells from overwhelming agonist stimulation. Our study provides a clear step toward understanding the nature of receptor regulation by PKA- and GRK-mediated phosphorylation in a physiological context.
Supplementary Material
Acknowledgments
We thank members of the Xiang laboratory for critical reading and comments, Dr. Jin Zhang of John Hopkins University for the AKAR2.2 constructs, Dr. Walter Koch for the GiCT adenovirus, and Dr. Richard Clark for the pS261/262 antibodies.
This work was supported, in whole or in part, by National Institutes of Health Grants HL082846 and AHA 0635331N (to Y. X.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- AR
- adrenoceptor
- Iso
- isoproterenol
- PKA
- protein kinase A
- GRK
- G protein-coupled receptor kinase
- GiCT
- Gi C terminus
- FRET
- fluorescence resonance energy transfer
- FLISA
- fluorescence-linked immunosorbent assay
- PTX
- pertussis toxin
- PKAmut
- PKA mutant
- GRKmut
- GRK mutant
- KO
- knock-out
- ANOVA
- analysis of variance.
REFERENCES
- 1.Lefkowitz R. J. (2007)Acta Physiol. (Oxf.) 190,9–19 [DOI] [PubMed] [Google Scholar]
- 2.Singh K., Xiao L., Remondino A., Sawyer D. B., Colucci W. S. (2001)J. Cell Physiol. 189,257–265 [DOI] [PubMed] [Google Scholar]
- 3.Xiao R. P. (2001)Sci. STKE 2001,RE15. [DOI] [PubMed] [Google Scholar]
- 4.Asano T., Katada T., Gilman A. G., Ross E. M. ( 1984) J. Biol. Chem. 259, 9351– 9354 [PubMed] [Google Scholar]
- 5.Tepe N. M., Liggett S. B. (2000)J. Recept. Signal Transduct. Res. 20,75–85 [DOI] [PubMed] [Google Scholar]
- 6.Daaka Y., Luttrell L. M., Lefkowitz R. J. (1997)Nature 390,88–91 [DOI] [PubMed] [Google Scholar]
- 7.Zamah A. M., Delahunty M., Luttrell L. M., Lefkowitz R. J. (2002)J. Biol. Chem. 277,31249–31256 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., De Arcangelis V., Gao X., Ramani B., Jung Y. S., Xiang Y. (2008)J. Biol. Chem. 283,1799–1807 [DOI] [PubMed] [Google Scholar]
- 9.Xiang Y., Rybin V. O., Steinberg S. F., Kobilka B. (2002)J. Biol. Chem. 277,34280–34286 [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff W. P., Lohse M. J., Bouvier M., Liggett S. B., Caron M. G., Lefkowitz R. J. (1990)Symp. Soc. Exp. Biol. 44,225–240 [PubMed] [Google Scholar]
- 11.Iyer V., Tran T. M., Foster E., Dai W., Clark R. B., Knoll B. J. (2006)Br. J. Pharmacol. 147,249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohse M. J., Benovic J. L., Caron M. G., Lefkowitz R. J. (1990)J. Biol. Chem. 265,3202–3211 [PubMed] [Google Scholar]
- 13.Tran T. M., Friedman J., Qunaibi E., Baameur F., Moore R. H., Clark R. B. (2004)Mol. Pharmacol. 65,196–206 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Lauffer B., Von Zastrow M., Kobilka B. K., Xiang Y. (2007)Mol. Pharmacol. 72,429–439 [DOI] [PubMed] [Google Scholar]
- 15.Xiang Y., Kobilka B. (2003)Proc. Natl. Acad. Sci. U.S.A. 100,10776–10781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devic E., Xiang Y., Gould D., Kobilka B. (2001)Mol. Pharmacol. 60,577–583 [PubMed] [Google Scholar]
- 17.Swaminath G., Xiang Y., Lee T. W., Steenhuis J., Parnot C., Kobilka B. K. (2004)J. Biol. Chem. 279,686–691 [DOI] [PubMed] [Google Scholar]
- 18.Soto D., De Arcangelis V., Zhang J., Xiang Y. (2009)Circ. Res. 104,770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan D. J., Millman E. E., Godines V., Friedman J., Tran T. M., Dai W., Knoll B. J., Clark R. B., Moore R. H. (2006)J. Biol. Chem. 281,7684–7692 [DOI] [PubMed] [Google Scholar]
- 20.Ferguson S. S., Barak L. S., Zhang J., Caron M. G. (1996)Can. J. Physiol. Pharmacol. 74,1095–1110 [DOI] [PubMed] [Google Scholar]
- 21.DeGeorge B. R., Jr., Gao E., Boucher M., Vinge L. E., Martini J. S., Raake P. W., Chuprun J. K., Harris D. M., Kim G. W., Soltys S., Eckhart A. D., Koch W. J. (2008)Circulation 117,1378–1387 [DOI] [PubMed] [Google Scholar]
- 22.Premont R. T., Gainetdinov R. R. (2007)Annu. Rev. Physiol. 69,511–534 [DOI] [PubMed] [Google Scholar]
- 23.Baillie G. S., Sood A., McPhee I., Gall I., Perry S. J., Lefkowitz R. J., Houslay M. D. (2003)Proc. Natl. Acad. Sci. U.S.A. 100,940–945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Chesley A., Lundberg M. S., Asai T., Xiao R. P., Ohtani S., Lakatta E. G., Crow M. T. (2000)Circ. Res. 87,1172–1179 [DOI] [PubMed] [Google Scholar]
- 25.Geng Y. J., Ishikawa Y., Vatner D. E., Wagner T. E., Bishop S. P., Vatner S. F., Homcy C. J. (1999)Circ. Res. 84,34–42 [DOI] [PubMed] [Google Scholar]
- 26.Zhu W. Z., Wang S. Q., Chakir K., Yang D., Zhang T., Brown J. H., Devic E., Kobilka B. K., Cheng H., Xiao R. P. (2003)J. Clin. Invest. 111,617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu W. Z., Zheng M., Koch W. J., Lefkowitz R. J., Kobilka B. K., Xiao R. P. (2001)Proc. Natl. Acad. Sci. U.S.A. 98,1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.