Abstract
Expression of the arginine/lysine transporter Cat-1 is highly induced in proliferating and stressed cells via mechanisms that include transcriptional activation. A bifunctional INE (intronic element) within the first intron of the Cat-1 gene was identified and characterized in this study. The INE had high sequence homology to an amino acid response element and was shown to act as a transcriptional enhancer in unstressed cells by binding the transcription factor, purine-rich element binding protein A (Purα). During endoplasmic reticulum stress, binding of Purα to the INE decreased; the element acted as a positive regulator in early stress by binding of the transcription factor ATF4 and as a negative regulator in prolonged stress by binding the stress-induced C/EBP family member, CHOP. We conclude that transcriptional control of the Cat-1 gene is tightly controlled by multiple cis-DNA elements, contributing to regulation of cationic amino acid transport for cell growth and proliferation. In addition, we propose that genes may use stress-response elements such as the INE to support basal expression in the absence of stress.
Introduction
The Cat-1 (cationic amino acid transporter-1) gene encodes the high affinity transporter for the essential amino acids arginine and lysine. Cat-1 supports vital metabolic functions, such as synthesis of proteins, polyamines, and nitric oxide (reviewed in Ref. 1). Together with three other proteins (Cat-2a, Cat-2b, and Cat-3), it is a part of the system y+ transporter family (1). All family members transport the same amino acids, but they differ in their expression patterns and substrate affinities. Cat-1 is ubiquitously expressed and localizes in the plasma membrane of all mammalian cells except the adult liver (1). Mice lacking Cat-1 are 25% smaller than normal littermates, anemic, and die shortly after birth (2). These findings suggest that Cat-1 plays critical roles in both hematopoiesis and growth control during mouse development (2, 3). In addition, alterations in Cat-1 expression have been linked to diseases. For example, some patients with congestive heart failure have an abnormality in l-arginine transport due to a decreased level of Cat-1 mRNA (4). Furthermore, a recently identified cytosine to uracil polymorphism in the 3′-untranslated region (3′-UTR)2 of the human Cat-1 mRNA seems to attenuate its expression level and contributes to hypertension and endothelial dysfunction (5). Elevated Cat-1 expression has also been reported to contribute to the pathogenesis of kidney hyperfiltration in diabetic rats. Despite the importance of Cat-1 in cellular metabolism, the determinant(s) for basal expression of the Cat-1 gene are not known.
We have previously shown that transcriptional control is an important mechanism for induction of Cat-1 gene expression under different hormonal or nutritional needs, including stress conditions that increase the phosphorylation of translation initiation factor eIF2α (eukaryotic initiation factor 2α) and decrease global protein synthesis (1, 6). Under stress conditions, expression of the Cat-1 gene is regulated at multiple levels: (i) transcriptional control via an amino acid response element (AARE) located in the first exon of the gene (7, 8); (ii) control of mRNA decay via the binding of the HuR protein to an AU-rich element within the 3′-UTR (9); and (iii) translational control of Cat-1 mRNA via a cap-independent initiation mechanism involving an internal ribosome entry site (10, 11). Additionally, miR-122, a liver specific microRNA has been suggested to post-transcriptionally inhibit translation of the human Cat-1 mRNA via the 3′-UTR (12). miR-122-mediated repression was relieved under different stress conditions.
Little is known about the mechanisms that regulate Cat-1 mRNA levels under normal/unstressed conditions. This study addresses the mechanism of basal Cat-1 gene transcription and its adaptive regulation during ER stress. We show that an enhancer element in the first intron of the gene regulates the TATA-less Cat-1 promoter. In addition, the purine-rich element binding protein A (Purα) was identified as a transcription factor that modulates Cat-1 gene expression. Purα is a member of the Pur protein family, which has four known members (reviewed in Ref. 13). It interacts with both DNA and RNA either directly or via regulatory proteins to control processes in DNA replication, gene transcription, RNA localization, and mRNA translation (reviewed in Ref. 13). Vascular smooth muscle α-actin, cardiac α-myosin heavy chain, and androgen receptor are some of the genes transcriptionally regulated by Purα (13, 14).
We also show that the intronic enhancer element (INE) within intron 1 of the Cat-1 gene plays a key role in regulating Cat-1 transcription. It increased promoter activity in unstressed conditions by binding Purα. During ER stress the INE played a bifunctional role. In early ER stress, it stimulated transcription by binding ATF4, whereas, it inhibited transcription by binding the transcription factor CHOP during prolonged stress. Our findings suggest that the regulated binding of Purα, ATF4, and CHOP to the INE plays a key role in Cat-1 gene expression in physiological and pathological states.
MATERIALS AND METHODS
Cell Culture and DNA Transfection
Cells were cultured in high glucose Dulbecco's modified Eagle's medium supplemented with penicillin (100 units/ml), streptomycin (100 μg/ml), and 2 mm l-glutamine under a humidified atmosphere of 5% CO2 at 37 °C. C6 rat glioma cells were cultured in media supplemented with 5% heat-inactivated fetal bovine serum and 5% calf serum. Mouse embryonic fibroblasts (MEFs) with or without homozygous deletions of the Purα gene (15) or the CHOP gene (16) were grown in media supplemented with 10% fetal bovine serum. FuGENE 6 HD (Roche Applied Science) was used to transfect cultured cells according to the manufacturer's instructions. Expression plasmids for β-galactosidase or Renilla luciferase were co-transfected to control for transfection efficiency (17). Luciferase and β-galactosidase activities were measured as described previously (17). Lipofectamine 2000 transfection reagent (Invitrogen) was used for transfections with small interfering RNAs (siRNAs) (Dharmacon) according to the manufacturer's protocol. Briefly, cells were transfected with 50 nm siRNA using 5 μl/ml Lipofectamine, and used for experiments after 48 h or as indicated. Induction of ER stress was performed as described (17, 18).
Plasmid Constructs
Construct 1 was constructed from CMVmin pUHD 10-3 containing luciferase (LUC) and the Cat-1 promoter vector, PA1.4/Cat-1 5′-UTR as described (7, 17). PCR amplification was used to generate two fragments: (i) a fragment containing the Cat-1 exon 1 and 296 nucleotides from the 5′-splice site of intron 1 including the splice donor site and (ii) a fragment containing exon 2 and 316 nucleotides upstream of the 3′-splice site of intron 1 including the splice acceptor site. Fragments i and ii were introduced into construct 1 to generate construct 2. Construct 3 was generated by a PCR-based strategy to replace the chimeric intron 1 in construct 2 with the intron from the pCAT-3 control vector (Promega). Construct 2del was generated by PCR looping, removing nucleotides 64–298 downstream of the exon 1/intron 1 junction. Construct 3ins was generated by PCR, introducing the same nucleotides that were removed from construct 2del into construct 3 near the 5′-splice site of the heterologous intron. Constructs 2mut and 3ins/mut were generated by PCR-directed mutagenesis of constructs 2 and 3ins, respectively, using a primer containing a mutation of the INE sequence (TGATGCAAC to TGTAACCAC). Constructs 4 and 4mut were prepared by ligating an oligonucleotide containing either 3xWT-INE or 3xMUT-INE (supplemental Table 1) into the SalI enhancer site of the pGL3-promoter vector (Promega). Constructs 5 and 5mut were described previously (8). Mouse CHOP cDNA was cloned into pcDNA3.1 (Invitrogen) via the EcoRI site to generate the CHOP expression vector. The expression vector for ATF4 was from D. Ron (19).
Chromatin Immunoprecipitation (ChIP) Analysis
Chromatin immunoprecipitation analysis was performed as described (8) using a Sonic Dismembrator 550 (Fisher Scientific) at setting 4 and 15–20% duty cycle. Nuclear extracts were sonicated with 8 cycles of 1-s pulse and 1-s rest for 2 min with 2-min cooling between cycles. Antibodies (2 μg) were added for immunoprecipitation. ATF4 and CHOP antibodies were from Santa Cruz Biotechnology. Purα antibodies were from Dr. R. J. Kelm (20, 21). Immunoprecipitated and purified DNA fragments were analyzed by PCR and qPCR. Regions encompassing the Cat-1 exon 1 AARE and intronic element were amplified. The PCR primers used are listed in supplemental Table 3.
DNA Affinity Pulldown Assays
HeLa nuclear extracts were loaded onto a phosphocellulose column and eluted with increasing KCl concentration as described (22). The fraction eluted with 0.5 m KCl was used to purify proteins that interact with the intronic element. The eluted nuclear extracts and biotinylated double-stranded DNA oligonucleotides containing three copies of WT-INE or MUT-INE (supplemental Table 1) were incubated in binding buffer (20 mm Tris, pH 8.0, 20% glycerol, 0.2 mm EDTA, pH 8.0, 100 mm KCl, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, and 3 μg poly(dI-dC)). The complexes were captured by incubation for 20 min at room temperature with 25 μl magnetic beads (Dynabeads® M-280 streptavidin, Invitrogen). Beads were washed 4× with binding buffer, and the associated proteins were eluted in 50 μl 1× SDS-PAGE sample buffer and resolved by SDS-PAGE. The regions of interest were excised from the Coomassie Brilliant Blue-stained gel and analyzed by liquid chromatography/mass spectrometry at the Cleveland Clinic Foundation Mass Spectrometry Facility. Alternatively, nuclear extracts from Purα−/− or Purα−/+ MEFs were incubated with 3xWT-INE or 3xMUT-INE oligonucleotide as described above, and isolated proteins were separated by SDS-PAGE and analyzed by Western blotting.
RT (Reverse Transcription)-PCR and Quantitative Real-time RT-PCR (qRT-PCR) Analysis
cDNAs were synthesized from RNA samples using Superscript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen) as described (8, 18). Real-time PCR was performed using iCycler (Bio-Rad) and SYBR GreenER qPCR Supermix for the iCycler (Invitrogen) according to the manufacturer's instructions. PCR primers used are listed in supplemental Table 3.
Other Methods
Total cell and nuclear extracts were prepared as described (18, 23). Proteins were detected on Western blots with primary antibodies specific for Purα (E. M. Johnson) or Purα and Purβ (R. J. Kelm), tubulin (Sigma), and CHOP (Santa Cruz Biotechnology) and visualized after incubation with horseradish peroxidase-conjugated secondary antibody (Perkin Elmer).
RESULTS
The First Intron of the Cat-1 Gene Contains an Enhancer Element
During work on a related research project, we identified a putative enhancer element in the first intron of the Cat-1 gene. To characterize its function, we constructed a truncated version of intron 1 in its natural position between exons 1 and 2 (Fig. 1A, construct 2).
FIGURE 1.
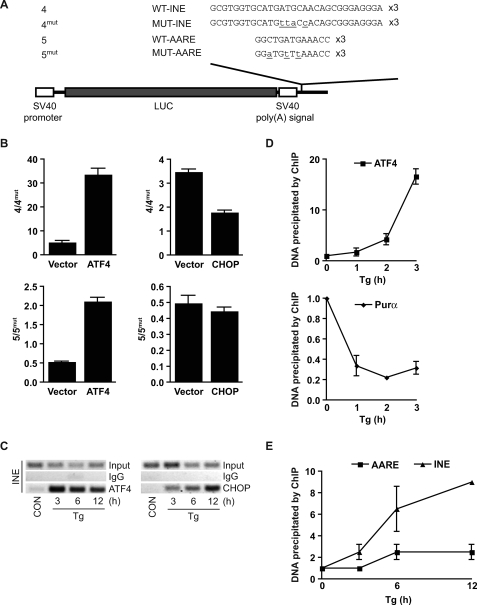
Identification of a regulatory element in the first intron of the Cat-1 gene. A, constructs used in this study. B, alignment of the conserved sequence in intron 1 of mammalian Cat-1 genes. An asterisk denotes a conserved nucleotide. The distance from the 5′-splice junction is indicated. Shading denotes the site of mutations in construct 2mut and 3ins/mut. C and D, transient transfections of the indicated vectors into C6 and MEFs. LUC activity normalized to β-galactosidase (β-GAL) activity is shown. C, inset, RT-PCR of LUC, β-galactosidase, and GAPDH mRNA in the indicated transfected cells. Means ± S.E. from triplicate experiments are shown.
Construct 2, which contains the Cat-1 mini-intron, was generated by inclusion of a 618-bp fragment that combined the 5′- and 3′-ends of intron 1 (intron 1 is >20 kb) in construct 1. Construct 2 produced a 5-fold higher LUC activity and mRNA in C6 cells compared with the intronless construct 1 (Fig. 1C). RT-PCR detected the mRNA species derived from splicing of the mini-intron (data not shown). It was possible that the enhanced expression of construct 2 was due to a cryptic promoter element in intron 1 or pre-mRNA splicing, which may stabilize the chimeric Cat-1-LUC mRNA and enhance its translation (24). Alternatively, intronic sequences may contain enhancer elements that support transcription from the upstream promoter. We first determined that the intronic sequence does not contain a cryptic promoter. Transfection of C6 cells with promoterless vectors containing the mini-intron did not produce any LUC activity (data not shown). To assess the possible influence of splicing, we generated construct 3, in which the Cat-1 mini-intron was replaced with a heterologous intron (Fig. 1A, construct 3). Although the LUC activities from cells transfected with constructs 1 and 3 showed a 2-fold difference, this is significantly lower than the 5-fold increase observed for the construct containing the mini-intron (Fig. 1C). Splicing of the introns was confirmed by RT-PCR (data not shown). The difference in activity between the Cat-1 intron and the heterologous intron was maintained in all cell lines tested (Fig. 1C and data not shown). Our results indicate that the induced transcription observed in the presence of the Cat-1 intron 1 is likely the result of the recruitment of sequence-specific DNA-binding proteins.
To identify potential enhancer elements within the Cat-1 intronic sequence, we compared this region from human, mouse, and rat. Sequence alignment for intron 1 revealed a conserved 24-nt region containing the sequence (TGATGCAAC) that resembled an ATF site, a known target for transcription factors such as ATF4 (Fig. 1B) (25). We hypothesized that this conserved region contributes to the enhancement of transcription via the mini-intron. To test our hypothesis, we performed deletion and mutation analyses (Fig. 1D). Deletion of the conserved region from construct 2 (Fig. 1A, construct 2del) abolished the increase in both LUC mRNA and protein levels (Fig. 1D and data not shown). Furthermore, introduction of the consensus sequence into the construct containing the heterologous intron (Fig. 1A, construct 3ins) increased both LUC mRNA and protein levels (Fig. 1D and data not shown). mRNAs from these constructs were correctly spliced; they produced the expected PCR fragments using primers flanking the splice junction of exons 1 and 2 (data not shown). Mutation of the TGATGCAAC motif within the Cat-1 mini-intron (Fig. 1A, construct 2mut) or within the heterologous intron (Fig. 1A, construct 3ins/mut) abolished the stimulatory effect of the intronic enhancer (Fig. 1D). Furthermore, similar results were observed with these constructs in MEFs and other cell lines (Fig. 1D and data not shown). These results indicate that sequence-specific binding of proteins to DNA, rather than mRNA splicing, is the major contributor for the increased gene expression via the mini-intron. We conclude that expression of the Cat-1 gene is regulated via an enhancer element that contains an AARE-like site within the first intron of the gene (INE).
Identification of Purα as a Potential INE-binding Protein
To determine possible interacting proteins, biotinylated DNA oligonucleotides containing three tandem repeats of the INE sequence were tested in binding experiments using fractionated HeLa nuclear extracts. Oligonucleotides with WT (3xWT-INE) or the mutated INE sequence (3xMUT-INE) were used. We used the HeLa cell nuclear fraction that bound to phosphocellulose and eluted with 0.5 m KCl. This fraction was used because (i) transfection of constructs 2 and 3 in HeLa cells showed a 6-fold difference in LUC activities, suggesting that the INE is functional in these cells (data not shown); (ii) EMSA analysis using radiolabeled WT-INE oligonucleotide and HeLa nuclear extracts showed shifted bands, which were competed with the WT-INE but not with the MUT-INE oligonucleotides (data not shown); and (iii) shifted bands with this fraction were similar to ones seen with unfractionated extract and were stronger than bands seen with fractions eluted with different KCl concentrations (data not shown).
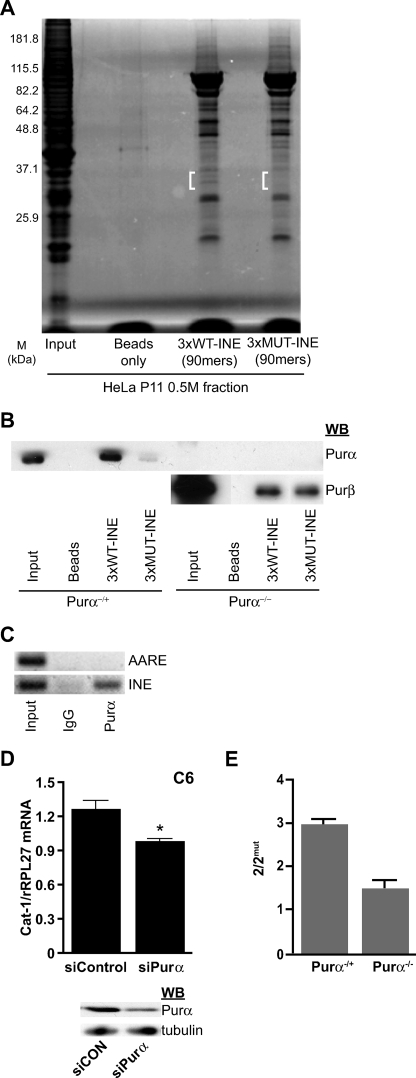
Proteins bound to the streptavidin-containing magnetic beads alone, 3xWT-INE, or 3xMUT-INE from DNA affinity pulldown were resolved using SDS-PAGE and stained with Coomassie Brilliant Blue. Several bands were observed in 3xWT-INE and 3xMUT-INE samples (Fig. 2A). In the 3xWT-INE lane, there was a band of 30–37 kDa that was absent in the 3xMUT-INE lane (Fig. 2A, white bracket). This region from both lanes was excised, and proteins were analyzed by mass spectrometric analysis. Three proteins were identified (supplemental Table 2). Of interest, the purine-rich element-binding protein A (Purα) was identified as interacting with the 3xWT-INE but not the 3xMUT-INE oligonucleotide. This was further confirmed by performing DNA affinity pulldown experiments using extracts from Purα−/− and Purα−/+ MEFs (Fig. 2B). Western blot analyses with antibodies specific for Purα showed strong binding to the 3xWT-INE in Purα−/+ extracts. The specificity of the antibody was confirmed in Purα−/− cells. The related purine-rich element-binding protein B (Purβ) was also identified via mass spectrometry, but in contrast to Purα, it bound to both the 3xWT-INE and 3xMUT-INE in extracts from Purα−/− MEFs (Fig. 2B and supplemental Table 2). These data emphasize the specificity of the Purα binding to the INE of Cat-1. Because this sequence is required for enhancer activity (Fig. 1), it suggests that binding of Purα to the INE facilitates the enhancer activity of this region.
FIGURE 2.
Purα binds the INE to increase Cat-1 gene transcription. A, shown is a Coomassie Brilliant Blue staining of an SDS-PAGE gel used for mass spectrometry analysis. Proteins from HeLa cell nuclear extracts that bind to oligonucleotides containing 3xWT-INE or 3xMUT-INE were isolated and analyzed as described under “Materials and Methods.” White brackets indicate the region used for mass spectrometry analysis. M, molecular weight. B, the same oligonucleotides as in A were used for DNA affinity pulldown analysis using nuclear extracts from Purα−/+ and Purα−/− MEFs as described under “Materials and Methods.” Samples were analyzed for Purα and Purβ on Western blots (WB). C, ChIP was performed using antibodies against Purα or with normal IgG as control. PCR was performed with primer sets specific for the regions of interest (supplemental Table 3). D, upper panel, C6 cells were transfected with either control (siCON) or Purα-specific siRNAs. The level of Cat-1 mRNA was analyzed using qRT-PCR and normalized to rRPL27. D, lower panel, Purα and tubulin protein levels in total cell extracts were assessed by Western blotting. E, constructs 2 and 2mut were transiently transfected into Purα−/+ and Purα−/− MEFs. LUC activity was normalized to the activity of the co-transfected Renilla luciferase as described under “Materials and Methods.” The normalized LUC values were expressed as the ratio of 2 to 2mut. Expression from constructs 2 and 2mut were 10- and 5-fold lower, respectively, in Purα−/− than in Purα−/+ MEFs. Means ± S.E. for triplicate determinations are shown. *, p < 0.05.
We next tested whether Purα also regulates Cat-1 transcription. We first determined whether Purα binds to the INE in vivo using ChIP analysis. Chromatin from C6 glioma cells was immunoprecipitated with antibodies to Purα or normal IgG, and DNA fragments containing the INE and the AARE of the Cat-1 gene were amplified by PCR (Fig. 2C). We found that Purα was present at the INE but not the AARE (Fig. 2C). The absence of signals in either region when normal IgG was used further confirmed the specificity of ChIP results. To test whether Purα is an important determinant of Cat-1 gene transcription, we examined the effect of siRNA knockdown of Purα on Cat-1 mRNA levels. Cells transfected with siRNA against Purα showed a 57% decrease in Purα protein levels compared with control siRNA cells (Fig. 2D, lower panel). This decrease in Purα level produced a 22% decrease in the abundance of the Cat-1 mRNA (Fig. 2D, upper panel). We next determined if the levels of Purα influence the enhancer activity of the INE. To this end, we transfected Purα−/− and Purα−/+ MEFs with constructs 2 and 2mut. The resulting LUC/β-galactosidase values were expressed as the ratio of 2/2mut (Fig. 2E). This ratio was 2-fold higher in Purα−/+ than in Purα−/− MEFs, suggesting a role of Purα in the function of the INE as an enhancer (Fig. 2E). Taken together, these results demonstrate the importance of Purα in regulation of Cat-1 gene transcription via the INE.
ATF4 and CHOP Regulate INE-mediated Cat-1 Gene Expression during ER Stress
We reported previously that Cat-1 mRNA levels increase during stress via a mechanism that involves binding of the transcription factor ATF4 to the AARE (7, 8). Given that the INE resembles an ATF binding site and its core sequence is identical with the ATF3 AARE, we hypothesize that it can recruit stress-induced transcription factors such as ATF4 and CHOP. To test this hypothesis, we generated expression vectors containing three WT or mutant INEs (constructs 4 and 4mut) and WT or mutant AAREs (constructs 5 and 5mut) in the enhancer region of the pGL3 promoter vector (Fig. 3A). Transient transfection of these constructs with or without co-transfected ATF4 or CHOP showed that ATF4 overexpression increased the ratio of 4/4mut by almost 7-fold, whereas CHOP overexpression decreased this ratio by almost 2-fold (Fig. 3B). Transfection of ATF4 increased transcription mediated by the AARE-containing vectors but transfection of CHOP had no effect (Fig. 3B, 5/5mut). These data suggest that both the AARE and INE can activate transcription by binding ATF4 but only the INE can repress transcription by binding CHOP. To confirm that ATF4 and CHOP bind the INE in vivo, chromatin from C6 cells was immunoprecipitated with antibodies against CHOP, ATF4 or normal IgG, and DNA fragments containing the region encompassing the INE were amplified by PCR (Fig. 3, C–E). In agreement with the functional analyses, we found that ATF4 binding to the INE peaked at ∼3 h of endoplasmic reticulum stress induced by Tg treatment (18) and decreased thereafter. Purα, which binds the INE in unstressed cells, was found to dissociate between 1 to 3 h of ER stress, a time when ATF4 binding to the INE increased (Fig. 3D). CHOP binding to the same region showed low levels of association at 3 h of ER stress and a sharp increase following 12 h (Fig. 3, C and E). A smaller increase in association of CHOP to the AARE was observed at 3–12 h of ER stress (Fig. 3E). This lower binding of CHOP to the AARE is likely the result of binding to the INE on chromatin fragments that contain both the AARE and INE. Because these elements are separated by 355 bp, ChIP may have isolated fragments that contain both elements. Alternatively, weak binding of CHOP to the AARE may occur in vivo. We conclude that during early ER stress, ATF4 enhances transcription by replacing the weak transcriptional activator Purα on the INE, whereas during late ER stress, CHOP binding inhibits transcription. The specificity of ATF4 and CHOP binding was confirmed using normal IgG as negative control or by the lack of binding to the genomic DNA corresponding to the 3′-UTR of the Cat-1 mRNA (Fig. 3C and data not shown).
FIGURE 3.
ATF4 and CHOP bind to the Cat-1 INE in vivo and modulate transcription. A, the 4, 4mut, 5, and 5mut constructs contain three repeats of the sequence in the enhancer site of the pGL3-promoter vector. B, C6 cells were transiently co-transfected with the indicated constructs and expression plasmids or vector. Cell extracts were assayed for LUC activity and normalized to the co-transfected β-galactosidase activity as described under “Materials and Methods.” The normalized values were expressed as the ratio of 4 to 4mut or 5 to 5mut. ATF4 increased expression from construct 4 by 4-fold but decreased expression from 4mut by 30%. ATF4 also increased expression from construct 5 by 2-fold but decreased expression from 5mut by 50%. CHOP decreased expression from constructs 4 and 4mut by 60 and 10%, respectively. CHOP also decreased expression from constructs 5 and 5mut by 25%. C, C6 glioma cells were treated with Tg for the indicated times or untreated (CON), and ChIP was performed with antibodies against ATF4 and CHOP as described under “Materials and Methods.” D and E, shown is a qPCR analysis using primers for the INE (D and E) or AARE (E) of DNA isolated from ChIP analysis using ATF4 and Purα (D) or CHOP (E) antibodies. Values were obtained relative to input DNA and expressed as a fold change relative to untreated cells.
Attenuation of Cat-1 Transcription during Late ER Stress Requires CHOP
A recent report by Su et al. (26) demonstrated that CHOP/ATF4 heterodimers inhibit the stress-dependent induction of the asparagine synthetase (ASNS) gene during amino acid starvation. Interestingly, they also reported that induction of the Cat-1 mRNA during amino acid starvation was not attenuated by CHOP. We hypothesized that CHOP may attenuate Cat-1 transcription during late ER stress via binding to the INE. This idea was supported by the following findings: (i) overexpression of CHOP inhibits transcription of a reporter construct that contains the Cat-1 INE; and (ii) ChIP analysis showed recruitment of CHOP to the INE in C6 cells upon Tg treatment. Therefore, we investigated whether CHOP modulates Cat-1 gene transcription during ER stress by examining the relative levels of Cat-1 mRNA in WT and CHOP-deficient MEFs during ER stress (Fig. 4A). In the absence of CHOP, we found a sustained induction of Cat-1 mRNA between 6 and 18 h of ER stress (Fig. 4A). This trend is opposite from that of the WT MEFs, which showed attenuation of mRNA during this time. Similar results were obtained for the ASNS mRNA, consistent with the recent report documenting the inhibition of ASNS gene transcription by CHOP (26). This sustained induction of both Cat-1 and ASNS mRNAs during late ER stress was partially attenuated by transient overexpression of CHOP in CHOP−/− MEFs (Fig. 4A). The partial attenuation could be explained by the low levels of CHOP protein transfected in the CHOP−/− MEFs, as compared with the high levels of the endogenous CHOP protein in the CHOP+/+ MEFs (Fig. 4A, lower panel). The gradual decrease in the exogenous CHOP protein during ER stress was probably due to the lack of transcriptional control elements in the expression vector for CHOP. The increased levels of the endogenous CHOP protein are the result of transcriptional induction during ER stress.
FIGURE 4.
Inhibition of Cat-1 transcription via the INE during late ER stress requires CHOP. A, upper, quantification of mRNA levels for the indicated genes in Tg-treated CHOP+/+, CHOP−/−, and CHOP−/− transiently expressing CHOP (CHOP−/− (CHOP)). Data from qRT/PCR analysis of total RNA using gene-specific primers were normalized to the 18S ribosomal RNA signal. *, p < 0.05 compared with CHOP −/− MEFs. A, lower, Western blot analysis of total cell extracts from the indicated Tg-treated MEFs. Blots were probed with antibodies against the indicated proteins. B, CHOP+/+ and CHOP−/− MEFs were transiently transfected with the indicated reporter constructs for 24 h followed by incubation in the presence or absence (CON) of Tg for 18 h. C, CHOP−/− MEFs were transiently co-transfected with the indicated constructs and the CHOP expression plasmid or vector. C, inset, cell extracts were subjected to immunoblot analysis. Cell extracts were assayed for LUC activity and normalized to the activity of the co-transfected Renilla luciferase (B) or β-galactosidase (C) vector as described under “Materials and Methods.” The normalized values were expressed as the ratio of WT to MUT. B, Tg caused 18- and 16-fold increases in expression from constructs 2 and 2mut, respectively, in CHOP+/+ MEFs and 17- and 8-fold increases in expression in CHOP−/− MEFs. C, overexpression of CHOP in CHOP−/− MEFs decreased expression from construct 4 and increased expression from the corresponding mutant.
We next tested whether the lack of inhibition of Cat-1 mRNA expression during late stress was also seen with reporter constructs. We transfected the constructs 2 and 2mut into CHOP+/+ and CHOP−/− MEFs and compared LUC activities from control and Tg-treated cells (Fig. 4B). As expected, we observed a 2-fold increase in the ratio of 2/2mut only in the CHOP−/− MEFs. The reason that we did not observe a change in this ratio in CHOP+/+ MEFs treated with Tg, was that the untreated and 18 h-treated cells have similar levels of Cat-1 mRNA due to transcriptional attenuation (compare Fig. 4, A and B). To further address the CHOP-mediated inhibition of Cat-1 gene transcription via the INE, we examined the effect of expressing CHOP in CHOP−/− MEFs (Fig. 4C, left panel). In agreement with the inhibitory function of CHOP via the INE, CHOP expression caused a ∼60% decrease in the 4/4mut ratio. CHOP expression in the transfected cells was confirmed by Western blotting (Fig. 4C). Taken together, our data suggest that CHOP inhibits transcription of the Cat-1 gene via the INE during late ER stress.
DISCUSSION
In our previous studies, we demonstrated that Cat-1 gene expression is under the control of a TATA-less promoter, consistent with its low expression in most tissues (7, 8). In the present study, we identified a novel regulatory element (INE) within the first intron of the gene. This finding is supported by the following results. (i) Promoter activity was regulated by two DNA elements downstream of the transcription start site: a positive element in the first exon (AARE) and a bifunctional element in the first intron (INE). (ii) Bifunctional regulation by the INE involves activation of the promoter by Purα binding and attenuation during ER stress by CHOP binding. The identification of the bifunctional INE and Purα as an interacting protein reveals novel regulatory features of Cat-1 gene transcription. This interaction may be an important activator of Cat-1 expression in both basal and stress conditions. In fact, Purα is highly expressed in the adult brain (15), the only adult tissue that also expresses Cat-1 at high levels (1). Purα is a multifunctional protein that can bind single-stranded RNA and DNA and modulates DNA replication, transcription, or mRNA translation in a positive or negative manner (reviewed in Ref. 13). Consensus binding sites for the protein are (GGN)n>(GGNN)n>(GGNNN)n, where N is not a G. Purα also regulates transcription via GA/GC-rich regions with the cooperative action of Sp1 and Sp3 (14, 21). There is one report that Purα inhibits cAMP-response element-mediated transcription by binding to a purine-rich element adjacent to the cAMP-response element binding site (27).
The bifunctional INE in the first intron of the Cat-1 gene was an important discovery of this study. The first intron also contains a TG repeat near the INE ((TG)30 in the rat and (TG)12 in the mouse) 59 and 57 bp downstream of the 5′-end of the first intron, respectively. Dinucleotide repeats of length ≥12 are found mostly in the introns of inefficiently transcribed genes. The repeats inhibit transcription because they tend to change the DNA topology from B- to Z-form, thus affecting the movement of RNA polymerase (28, 29). The low levels of Cat-1 gene expression in most cells and tissues might be explained by the presence of such repeats and the >50 kb of genomic sequence between the transcription start site and the AUG initiation codon. The INE might serve to modulate the influence by these factors on transcription.
We show here that the binding of Purα to the Cat-1 INE depends on its AARE-like sequence. The element is identical to the AARE in the ATF3 promoter (30) and has only one mismatch with the Cat-1 AARE. The Cat-1 AARE and INE bind ATF4, but only the INE binds Purα (data not shown), suggesting that Purα binds to regions in the INE adjacent to the AARE-like sequence. A possible mechanism involves binding of Purα to a flanking region (through interactions with single- or double-stranded DNA) stabilized by interaction with a protein that binds the AARE-like site. This mechanism explains the absence of Purα binding to both the mutant INE and the Cat-1 AARE (Fig. 2, B and C). Purα is known to interact with other proteins such as Sp1 and YB-1 (reviewed in Ref. 13), but there are no reports of interactions with ATF family members. However, it was recently shown in prostate cancer cells that Purα overexpression induces ER stress response genes, including ATF3 (31). ATF3 and other stress response genes contain AAREs, which regulate transcription of many genes during stress (32). This is consistent with the idea that Purα acts in concert with ATF family members to regulate stress responses.
We have shown previously that transcription of the Cat-1 gene (8) increases early in the ER stress response and is attenuated at later times via a mechanism that involves the C/EBPβ (CCAAT/enhancer-binding protein β) gene product LIP (8, 18). The AARE plays a role in this attenuation during late ER stress, possibly by the binding of LAP/LIP or ATF4/LIP heterodimers (8). We show here that CHOP is also involved in transcriptional attenuation via binding to the INE. Because CHOP interacts with LAP or LIP during ER stress (33), it is possible that CHOP/LIP or CHOP/LAP heterodimers attenuate Cat-1 gene transcription via binding to the INE.
A recent report by Su and Kilberg (26) showed that CHOP associates with ATF4 during stress to attenuate transcription of the ASNS gene via its AARE. The authors also concluded that the Cat-1 AARE is not a target of CHOP repression because Cat-1 mRNA levels were not affected by CHOP overexpression. We also found that CHOP is an attenuator of ASNS gene transcription during stress (Fig. 4). However, we showed that Cat-1 expression is attenuated by CHOP during Tg treatment. This apparent discrepancy could be due to different times of Tg treatment (4 h compared with 18 h in our study). Consistent with our data, Su et al. (26) found a significant increase in binding of CHOP to the Cat-1 gene in vivo during Tg treatment. However, the interpretation of this finding is difficult because the AARE and INE are separated by 355 bp and could be isolated on the same DNA fragment in this ChIP experiment. Although we agree with this group that CHOP binds to the Cat-1 promoter during ER stress, our interpretation is that CHOP attenuates Cat-1 transcription via the INE and not the AARE (Figs. 3 and 4).
A model is proposed for the role of INE in the regulation of the Cat-1 gene transcription in the absence of stress and during early and late ER stress (Fig. 5). The model includes the following features. 1) Purα interacts with the INE under basal conditions to stimulate transcription. 2) ATF4 acts as an inducer by replacing Purα on the INE during early ER stress. In addition to the ChIP analysis (Fig. 3), we found that overexpression of ATF4 stimulates expression from an INE-containing plasmid relative to controls in C/EBPβ−/− MEFs (data not shown), demonstrating that the effect of ATF4 is independent of C/EBPβ. 3) CHOP acts at the INE to repress transcription during late ER stress. Although the dimerization partner of CHOP is not known, ATF4 binding decreases during prolonged stress and our previous studies suggest that transcriptional attenuation requires LAP or LIP (18). Therefore, C/EBPβ isoforms are likely dimerization partners with CHOP in transcriptional attenuation.
FIGURE 5.
Working model of the role of the INE in the regulation of the Cat-1 gene transcription during ER stress. Purα binds to the INE and supports transcription under basal conditions. Purα dissociates and is replaced by ATF4 during early stress, which stimulates transcription. The dimerization partner of ATF4 is unknown. During late stress, CHOP and one of several dimerization partners replace ATF4, which inhibits expression.
This study introduces Purα and CHOP as novel factors in modulating Cat-1 gene transcription. Because CHOP is viewed as a factor that promotes apoptosis and terminates survival signals during severe stress (33), it will be interesting to determine whether attenuating Cat-1 expression is important for these processes, as has been shown for other nutrient transporters (34). An additional important finding is the binding of Purα to AARE-containing elements. Although AARE flanking sequences from different stress response genes have (GGN)n motifs and there are purine-rich sequences close to AAREs that have inhibitory function in basal gene transcription (35), their regulation by Purα has yet to be proven experimentally. Future experiments using Purα-null cells should define Purα-regulated genes and their impact in the cellular response to physiological stress.
Supplementary Material
Acknowledgments
We thank Drs. David Ron and Edward M. Johnson for providing expression plasmids and antibodies. We also thank Chuanping Wang and Drs. Yi Li, Alex B. Lopez, and Francesca Gaccioli for performing experiments in support of the findings reported here.
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK060596 and R01DK053307 (to M. H.) and R01CA103867 and R01CA124760 (to C.-M. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables I–III.
- 3′-UTR
- 3′-untranslated region
- AARE
- amino acid-response element
- ATF
- activating transcription factor
- ASNS
- asparagine synthase
- CHOP
- C/EBP homologous protein
- ER
- endoplasmic reticulum
- LAP
- liver-enriched transcriptional activating protein
- LIP
- liver-enriched transcriptional inhibitory protein
- MEFs
- mouse embryonic fibroblasts
- WT
- wild-type
- Tg
- thapsigargin
- RT
- reverse transcription
- qRT-PCR
- quantitative real-time PCR
- LUC
- firefly luciferase
- Purα
- purine-rich element binding protein A
- Purβ
- purine-rich element binding protein B
- CMV
- cytomegalovirus
- 5′-UTR
- 5′-untranslated region
- ChIP
- chromatin immunoprecipitation.
REFERENCES
- 1.Hatzoglou M., Fernandez J., Yaman I., Closs E. (2004) Annu. Rev. Nutr. 24, 377–399 [DOI] [PubMed] [Google Scholar]
- 2.Perkins C. P., Mar V., Shutter J. R., del Castillo J., Danilenko D. M., Medlock E. S., Ponting I. L., Graham M., Stark K. L., Zuo Y., Cunningham J. M., Bosselman R. A. (1997) Genes Dev. 11, 914–925 [DOI] [PubMed] [Google Scholar]
- 3.Shima Y., Maeda T., Aizawa S., Tsuboi I., Kobayashi D., Kato R., Tamai I. (2006) Blood 107, 1352–1356 [DOI] [PubMed] [Google Scholar]
- 4.Kaye D. M., Ahlers B. A., Autelitano D. J., Chin-Dusting J. P. (2000) Circulation 102, 2707–2712 [DOI] [PubMed] [Google Scholar]
- 5.Yang Z., Venardos K., Jones E., Morris B. J., Chin-Dusting J., Kaye D. M. (2007) Circulation 115, 1269–1274 [DOI] [PubMed] [Google Scholar]
- 6.Komar A. A., Hatzoglou M. (2005) J. Biol. Chem. 280, 23425–23428 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez J., Lopez A. B., Wang C., Mishra R., Zhou L., Yaman I., Snider M. D., Hatzoglou M., Hatzolgou M. (2003) J. Biol. Chem. 278, 50000–50009 [DOI] [PubMed] [Google Scholar]
- 8.Lopez A. B., Wang C., Huang C. C., Yaman I., Li Y., Chakravarty K., Johnson P. F., Chiang C. M., Snider M. D., Wek R. C., Hatzoglou M. (2007) Biochem. J. 402, 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaman I., Fernandez J., Sarkar B., Schneider R. J., Snider M. D., Nagy L. E., Hatzoglou M. (2002) J. Biol. Chem. 277, 41539–41546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez J., Yaman I., Huang C., Liu H., Lopez A. B., Komar A. A., Caprara M. G., Merrick W. C., Snider M. D., Kaufman R. J., Lamers W. H., Hatzoglou M. (2005) Mol. Cell 17, 405–416 [DOI] [PubMed] [Google Scholar]
- 11.Yaman I., Fernandez J., Liu H., Caprara M., Komar A. A., Koromilas A. E., Zhou L., Snider M. D., Scheuner D., Kaufman R. J., Hatzoglou M. (2003) Cell 113, 519–531 [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya S. N., Habermacher R., Martine U., Closs E. I., Filipowicz W. (2006) Cell 125, 1111–1124 [DOI] [PubMed] [Google Scholar]
- 13.Johnson E. M. (2003) Anticancer Res. 23, 2093–2100 [PubMed] [Google Scholar]
- 14.Subramanian S. V., Polikandriotis J. A., Kelm R. J., Jr., David J. J., Orosz C. G., Strauch A. R. (2004) Mol. Biol. Cell 15, 4532–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalili K., Del Valle L., Muralidharan V., Gault W. J., Darbinian N., Otte J., Meier E., Johnson E. M., Daniel D. C., Kinoshita Y., Amini S., Gordon J. (2003) Mol. Cell. Biol. 23, 6857–6875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H. Y., Wek R. C. (2005) J. Biol. Chem. 280, 14189–14202 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez J., Yaman I., Mishra R., Merrick W. C., Snider M. D., Lamers W. H., Hatzoglou M. (2001) J. Biol. Chem. 276, 12285–12291 [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Bevilacqua E., Chiribau C. B., Majumder M., Wang C., Croniger C. M., Snider M. D., Johnson P. F., Hatzoglou M. (2008) J. Biol. Chem. 283, 22443–22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. (2000) Mol. Cell 6, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 20.Kelm R. J., Jr., Cogan J. G., Elder P. K., Strauch A. R., Getz M. J. (1999) J. Biol. Chem. 274, 14238–14245 [DOI] [PubMed] [Google Scholar]
- 21.Ji J., Tsika G. L., Rindt H., Schreiber K. L., McCarthy J. J., Kelm R. J., Jr., Tsika R. (2007) Mol. Cell. Biol. 27, 1531–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meisterernst M., Roy A. L., Lieu H. M., Roeder R. G. (1991) Cell 66, 981–993 [DOI] [PubMed] [Google Scholar]
- 23.Fernandez J., Bode B., Koromilas A., Diehl J. A., Krukovets I., Snider M. D., Hatzoglou M. (2002) J. Biol. Chem. 277, 11780–11787 [DOI] [PubMed] [Google Scholar]
- 24.Le Hir H., Nott A., Moore M. J. (2003) Trends Biochem. Sci. 28, 215–220 [DOI] [PubMed] [Google Scholar]
- 25.Hai T., Hartman M. G. (2001) Gene 273, 1–11 [DOI] [PubMed] [Google Scholar]
- 26.Su N., Kilberg M. S. (2008) J. Biol. Chem. 283, 35106–35117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadakata T., Kuo C., Ichikawa H., Nishikawa E., Niu S. Y., Kumamaru E., Miki N. (2000) Brain Res. Mol. Brain Res. 77, 47–54 [DOI] [PubMed] [Google Scholar]
- 28.Peck L. J., Wang J. C. (1985) Cell 40, 129–137 [DOI] [PubMed] [Google Scholar]
- 29.Sharma V. K., Kumar N., Brahmachari S. K., Ramachandran S. (2007) Physiol. Genomics 31, 96–103 [DOI] [PubMed] [Google Scholar]
- 30.Pan Y. X., Chen H., Thiaville M. M., Kilberg M. S. (2007) Biochem. J. 401, 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue T., Maeno A., Talbot C., Jr., Zeng Y., Yeater D. B., Leman E. S., Kulkarni P., Ogawa O., Getzenberg R. H. (2009) Prostate 69, 861–873 [DOI] [PubMed] [Google Scholar]
- 32.Kilberg M. S., Barbosa-Tessmann I. P. (2002) J. Nutr. 132, 1801–1804 [DOI] [PubMed] [Google Scholar]
- 33.Wang X. Z., Kuroda M., Sok J., Batchvarova N., Kimmel R., Chung P., Zinszner H., Ron D. (1998) EMBO J. 17, 3619–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edinger A. L., Cinalli R. M., Thompson C. B. (2003) Dev. Cell 5, 571–582 [DOI] [PubMed] [Google Scholar]
- 35.Palii S. S., Thiaville M. M., Pan Y. X., Zhong C., Kilberg M. S. (2006) Biochem. J. 395, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.