Abstract
The biogenesis of lipid droplets (LD) induced by serum depends on group IVA phospholipase A2 (cPLA2α). This work dissects the pathway leading to cPLA2α activation and LD biogenesis. Both processes were Ca2+-independent, as they took place after pharmacological blockade of Ca2+ transients elicited by serum or chelation with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester). The single mutation D43N in cPLA2α, which abrogates its Ca2+ binding capacity and translocation to membranes, did not affect enzyme activation and formation of LD. In contrast, the mutation S505A did not affect membrane relocation of the enzyme in response to Ca2+ but prevented its phosphorylation, activation, and the appearance of LD. Expression of specific activators of different mitogen-activated protein kinases showed that phosphorylation of cPLA2α at Ser-505 is due to JNK. This was confirmed by pharmacological inhibition and expression of a dominant-negative form of the upstream activator MEKK1. LD biogenesis was accompanied by increased synthesis of ceramide 1-phosphate. Overexpression of its synthesizing enzyme ceramide kinase increased phosphorylation of cPLA2α at Ser-505 and formation of LD, and its down-regulation blocked the phosphorylation of cPLA2α and LD biogenesis. These results demonstrate that LD biogenesis induced by serum is regulated by JNK and ceramide kinase.
Introduction
Intracellular lipid droplets (LD)5 are cytosolic inclusions present in most eukaryotic cells, containing a core of triacylglycerols (TAG) and cholesteryl esters, surrounded by a phospholipid monolayer and by specific proteins, among which the best characterized belong to the perilipin family (1–3). In the last few years, the biology of LD has received increasing interest, due to the close relationship between an excess of lipid storage in certain tissues and pathologies such as obesity, diabetes, or atherosclerosis (3–5). Cellular stress has been related to the generation of LD, which might play a cytoprotective role (6, 7). Therefore, dissecting the signaling pathways leading to LD formation may have important clinical applications in metabolic diseases and also in neurodegenerative diseases such as Parkinson and Alzheimer disease (8, 9).
Cells generate LD from exogenous sources, free fatty acids or lipoproteins, but LD also appear in the absence of external lipids when cells are under stress, apparently after the recycling of membrane phospholipids into TAG (7). We have shown recently that group IVA PLA2 (also termed cPLA2α) is required for LD biogenesis either after exogenous lipid loading (10) or during stress (7). Regardless of the LD-inducing situation, the enzyme is not involved in the synthesis of neutral lipids; rather, it allows the formation of LD from TAG-containing membranes. cPLA2α can be activated by several mechanisms (reviewed in Refs. 11, 12). It contains a Ca2+ binding domain (C2) that allows interaction with membrane phospholipids in response to increased intracellular Ca2+ concentrations, and the single substitution D43N in this domain abrogates the Ca2+-dependent translocation of cPLA2α to membranes (13–15). However, the increase of intracellular Ca2+ is not universally required, as several reports have shown cPLA2α activation in vivo under resting Ca2+ conditions (13, 16–19). In this regard, anionic phospholipids like phosphatidylinositol 4,5-bisphosphate (PtdIns-4,5-P2) (20, 21) and ceramide 1-phosphate (Cer-1-P) (22–25) bind specific regions of the enzyme and decrease its Ca2+ requirement to interact with membranes. Also, phosphorylation of cPLA2α at Ser residues 505, 515, and 727 appears to play a role in enzyme activation (11, 12). Among these sites, it is generally accepted that Ser-505 is the one that leads to increased catalytic activity (26), and phosphorylation of Ser-505 depends on MAPK, among which most reports implicate ERK and p38 (11, 12).
Recently, we have shown that phosphorylation of cPLA2α at Ser-505 is key for LD biogenesis, as evidenced by the inability of the S505A mutant to recapitulate the effect of the endogenous enzyme (10). This work was undertaken to delineate the mechanism of cPLA2α activation that generates LD in the cell after serum addition. In essence, we show that the activation of cPLA2α is independent of increases of intracellular Ca2+; furthermore, we identify JNK as the MAPK implicated in cPLA2α activation. Finally, we show that ceramide kinase (CERK), the enzyme responsible for the synthesis of Cer-1-P, mediates LD biogenesis by inducing the phosphorylation of cPLA2α by JNK.
EXPERIMENTAL PROCEDURES
Materials
Nile red, primuline, trypsin, U73122, and Cer-1-P were from Sigma. SP600125, SB20358, PD98059, and N-acetyl-d-sphingosine (C2-ceramide) were from Calbiochem; and methylarachidonyl fluorophosphonate (MAFP) was from Cayman Chemical Co. [5,6,8,9,11,12,14,15-3H]Arachidonic acid ([3H]AA) (200 Ci/mmol) was purchased from American Radiolabeled Chemicals, and [9,10-3H]palmitic acid (49 Ci/mmol) from Amersham Biosciences. Opti-MEM was from Invitrogen. Rabbit anti-cPLA2α, anti-phospho-Ser-505-cPLA2α, anti-JNK, anti-phospho-Thr-183/Tyr-185-JNK, anti-p38, anti-phospho-Thr-180/Tyr-182-p38, anti-p44/42, and anti-phospho-Thr-202/Tyr-204-p44/p42 antibodies were from Cell Signaling; chicken anti-ADRP was from GenWay Biotech; rabbit anti-glyceraldehyde-3-phosphate dehydrogenase was from Ambion, and rabbit anti-CERK from Abcam.
Cells
CHO-K1 cells were cultured in Ham's F-12 medium (Sigma) containing 7.5% fetal bovine serum (FBS, from Sigma), 100 units/ml penicillin, and 100 μg/ml streptomycin (both from Invitrogen). Cell passages were made once a week by trypsinization. For the experiments, cells were seeded at a density of 30,000 cells/ml in 24-well (0.5 ml) or 6-well plates (2 ml) and maintained in FBS-containing medium during 48 h. Before LD induction, cells were switched to serum-free culture medium for 24 h to set control conditions with minimal occurrence of LD (7). When indicated, cells (40–70% confluence) were transfected with 1 μg of plasmid/ml using Lipofectamine PlusTM, following the manufacturer's instructions.
Nile Red Staining and Fluorescence Microscopy
Cells cultured on glass bottom culture dishes were washed with phosphate-buffered saline, fixed with 3% paraformaldehyde for 10 min, and washed twice with phosphate-buffered saline. Cells were overlaid with 0.5 ml of phosphate-buffered saline, to which 2.5 μl of a stock solution of Nile red in acetone (0.2 mg/ml) were added, so the final concentrations of Nile red and acetone were 1 μg/ml and 0.5%, respectively. Samples were kept in the dark until photographed in a Leica Qwin 500 microscope with a Leica DC200 camera, using the Leica DCviewer 3.2.0.0 software.
Flow Cytometry
Indirect quantification of LD by flow cytometry in Nile red-stained cells was performed as described previously (7, 10). Briefly, paraformaldehyde-fixed cells were stained with 1 μg/ml Nile red during 45 min and analyzed with a Cytomics FC 500 (Beckman Coulter) equipped with an argon laser (488 nm), in the FL1 channel (505–545 nm). After gating out cellular debris, 30,000 events were acquired in all the assays, in linear scale. Fluorescence intensities were quantified as the median value of each distribution of events.
[3H]Arachidonic Release
Stimulated [3H]AA release from cells, which is an estimate of cPLA2α activity, was measured as described (7, 10, 27).
Ca2+ Imaging
Cells grown onto polylysine-coated coverslips were incubated with the Ca2+ indicator Fura-2/AM at 4 μm in Krebs buffer of the following composition (in mm): 119 NaCl, 4.75 KCl, 5 NaHCO3, 1.2 MgSO4, 1.18 KH2PO4, 1.3 CaCl2, 20 Hepes, and 5 glucose, pH 7.4. After 1 h, cells were washed and coverslips mounted in a static chamber on an inverted Nikon TE2000U microscope with a conventional epifluorescence system. Cells were excited alternatively at 340 and 380 nm, and emission light was collected at 510 nm every 4–10 s using a 12-bit CCD ERG ORCA Hamamatsu camera. Ratio image of cells was analyzed using the Metafluor software (Universal Imaging). 14–20 cells were analyzed in each experiment.
Western Blots
Cells were lysed with 62.5 mm Tris-HCl buffer, pH 6.8, containing 2% SDS, 10% glycerol, 50 mm dithiothreitol, and 0.01% bromphenol blue, and around 20 μg of protein were separated by standard 10% SDS-PAGE and transferred to nitrocellulose membranes. Primary (1:1,000) and secondary antibodies (1:5,000) were diluted in 25 mm Tris-HCl buffer, pH 7.4, containing 140 mm NaCl, 10% defatted dry milk, 0.1% bovine serum albumin, and 0.1% Tween 20. Antibody binding was detected using the ECL detection kit (Amersham Biosciences) and visualized using a GeneGenome HR chemiluminescence detection system coupled to a CCD camera or with high performance chemiluminescence films (Amersham Biosciences).
siRNA Transfection
We used three short interfering RNA (siRNA) duplexes from Gene Link, targeting accession number NM_022766 (human CERK mRNA): CERK1-(537), CERK2-(1377), and CERK3-(1679), with the following sequences: 5′- GGACAAGGCAAGCGGATAUTT (sense) and 5′-TTCCUGUUCCGUUCGCCUAUA (antisense) for siRNA CERK1; 5′-CGGAAAUGCUCCAGGUUCATT (sense) and 5′-TTGCCUUUACGAGGUCCAAGU (antisense) for siRNA CERK2; and 5′-ACGAGGAAUUGAAGAGAAUTT (sense) and 5′- TTUGCUCCUUAACUUCUCUUA (antisense) for siRNA CERK3. Cells were transfected at 60% confluence with 30 nm siRNA, by adding to each 35-mm culture well 1 ml of Opti-MEM (Invitrogen) containing 1.5 μl of the stock siRNA solution (20 μm) and 5 μl of Lipofectamine PlusTM (1 mg/ml, from Invitrogen). After 5 h, 1 ml of Ham's F-12 medium containing 7.5% FBS was added, and the cells were incubated for 72 h and then changed to serum-free medium during 24 h prior to treatments. When appropriate, labeling with [3H]palmitate to monitor the formation of Cer-1-P was done in the last 24 h.
Formation of [3H]Cer-1-P
[3H]Palmitate-prelabeled cells were stimulated with 7.5% FBS during 15 min, harvested on ice, washed with 1 ml of phosphate-buffered saline, and centrifuged prior to extraction of lipids (28). To separate Cer-1-P, 0.2-ml aliquots of the chloroform phases were evaporated under vacuum, dissolved in 15 μl of chloroform/methanol (3:1, v/v), and spotted onto Silica Gel G thin layer chromatography plates (Merck), which were developed once in chloroform/methanol/water (67.5:28.4:4, v/v) and three times in hexane/diethyl ether/formic acid (55:45:1, v/v). After staining with primuline spray (5 mg of primuline in 100 ml of acetone/water (80:20, v/v), identification of ceramide and ceramide 1-phosphate was made by co-migration with authentic standards. To quantify [3H]Cer-1-P, silica in regions of the TLC plates co-migrating with standard Cer-1-P was scraped into vials, which after addition of scintillation mixture were counted in a Tri-Carb 2810TR (PerkinElmer Life Sciences) at 40% efficiency.
Confocal Microscopy
Transiently transfected CHO-K1 cells were treated for 10 min with 5 μm ionomycin. To monitor relocation of EGFP-cPLA2α, EGFP-D43N-cPLA2α, or EGFP-S505A-cPLA2α, images were acquired every 60 s with a Leica TCS SP2 AOBS confocal microscope.
Constructs
The construct encoding for the expression of a fusion protein containing N-terminal enhanced green fluorescent protein followed by the entire sequence of the human cPLA2α (EGFP-cPLA2α) was described elsewhere (21, 27). Those for EGFP-D43N-cPLA2α and EGFP-S505A-cPLA2α were described in Refs. 27 and 10, respectively. Transfection of pGFP-C3 (Clontech) was used as control. Constructs pCMV5-ΔMEKK1 and pSRα-K432M-MEKK1, encoding constitutively active and dominant-negative forms of MEKK1, respectively, were kindly provided by Dr. Pura Muñoz-Cánoves (Centre de Regulació Genòmica, Barcelona, Spain). Constructs pCMV5-myc-MEKK3-EE and pcDNA3-MEK1-EE, encoding constitutively active MEKK3 and MEK1, were gifts from Dr. Ana Cuenda (Centro Nacional de Biotecnología, Consejo Superior de Investigaciones Científicas-Universidad Autónoma de Madrid, Madrid, Spain) and Dr. Piero Crespo (Instituto de Investigaciones Biomédicas, Consejo Superior de Investigaciones Científicas-Universidad de Cantabria, Santander, Spain), respectively. The construct for CERK expression (pcDNA3.1 HisTOPO-hCERK) is described elsewhere (29).
Statistical Analysis
Data analysis was carried out with Prism software (GraphPad). Responses among different treatments were analyzed with one-way analysis of variance followed by Bonferroni's multiple comparison test. All experiments were carried out with determinations in triplicate. Most results are presented as means ± S.E. of three independent experiments, except those in Fig. 8B, which are means ± range of two experiments. Results shown as fluorescence Ca2+ signals, Western blots, or microphotographs are representative of at least three independent experiments with essentially the same outcome.
FIGURE 8.
CERK is required for the activation of JNK and cPLA2α and for LD biogenesis. CHO-K1 cells were transfected with three siRNA-CERK duplexes as described under “Experimental Procedures” and maintained in 7.5% FBS-containing medium during 72 h and then in medium without FBS for 24 h. During these last 24 h, cells were prelabeled with [3H]palmitate or with [3H]AA to monitor synthesis of Cer-1-P and cPLA2α activity, respectively (B and C). A, Western blots showing that siRNA-CERK1 and siRNA-CERK3 knocked down the expression of CERK, precluded phosphorylation of JNK and cPLA2α induced by a 6-h treatment with FBS, and decreased ADRP content. B, [3H]palmitate-prelabeled cells were stimulated with FBS during 15 min. Lipid extracts were separated by TLC, and areas co-migrating with Cer-1-P standard were scraped onto vials and counted. C, [3H]AA-prelabeled cells were stimulated with FBS during 15 min, and radioactivity in the medium was counted. D, cells were stimulated with FBS during 6 h, fixed, and stained with Nile red, and LD were quantified by flow cytometry. Results in C and D are means ± S.E. of three experiments. Results in B are means ± range of two experiments. *, significantly different (p < 0.01) from controls. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
RESULTS
Activation of cPLA2α Required for LD Biogenesis Is Independent of Ca2+
Serum-starved cells challenged with 7.5% FBS produced transient Ca2+ increases (Fig. 1A). As expected, this Ca2+ signal disappeared when cells were pretreated with 45 μm BAPTA-AM during 30 min prior to stimulation with FBS (Fig. 1A). The inhibitory effect of BAPTA-AM was confirmed after the inability of ionomycin to induce a Ca2+ response (data not shown). cPLA2α was phosphorylated upon FBS stimulation when intracellular Ca2+ increases were abrogated by BAPTA-AM (Fig. 1B), and in fact, cPLA2α activity, measured as [3H]AA release to the medium, was unaffected by intracellular Ca2+ chelation (Fig. 1D). This Ca2+-chelating strategy was toxic over 6 h, however, and did not allow us to measure LD levels, which we usually monitor after a 6-h treatment (7, 10). Instead, we used the phosphoinositide phospholipase C inhibitor U73122 to block the Ca2+ signals induced by FBS, which are due to the generation of inositol 1,4,5-trisphosphate (30). As shown in Fig. 1A, pretreatment with 10 μm U73122 blocked the Ca2+ response, but it did not affect phosphorylation of cPLA2α at Ser-505 (Fig. 1C) or [3H]AA releasing activity (Fig. 1D). Importantly, in the presence of the phospholipase C inhibitor, FBS still promoted ADRP expression (Fig. 1C) and LD biogenesis that was sensitive to the cPLA2α inhibitor MAFP (Fig. 1E).
FIGURE 1.
FBS activates cPLA2α and induces LD biogenesis in the presence of BAPTA-AM or U73122. A, Ca2+ responses of serum-starved, fura-2/AM-loaded CHO-K1 cells under control conditions (continuous line) or after pretreatment with 45 μm BAPTA-AM (circles) or 10 μm U73122 (triangles) for 30 min before fluorescence measurements were started. At the time indicated, 7.5% FBS was added. B and C, Western blots of serum-starved CHO-K1 pretreated with 45 μm BAPTA-AM or 10 μm U73122 during 30 min and then stimulated with 7.5% FBS for 15 min (B) or 6 h (C). D, cPLA2 activity measured after the radioactivity was released to the medium of serum-starved CHO-K1 cells that had been prelabeled during 24 h with 0.5 μCi/ml [3H]arachidonic acid, then washed, and treated with 45 μm BAPTA-AM, 10 μm U73122, and/or 10 μm MAFP for 30 min before a 15-min stimulation with 7.5% FBS. E, indirect quantification of LD in serum-starved CHO-K1 cells that had been pretreated for 30 min with 10 μm U73122 and/or MAFP and then stimulated with 7.5% FBS for 6 h. Cells were fixed and stained with Nile red to quantify LD by flow cytometry. Fluorescence intensities in FL1 were quantified as the median values of each event distribution. Results in C and D are means ± S.E. of three independent experiments. *, significantly different (p < 0.01) from controls.
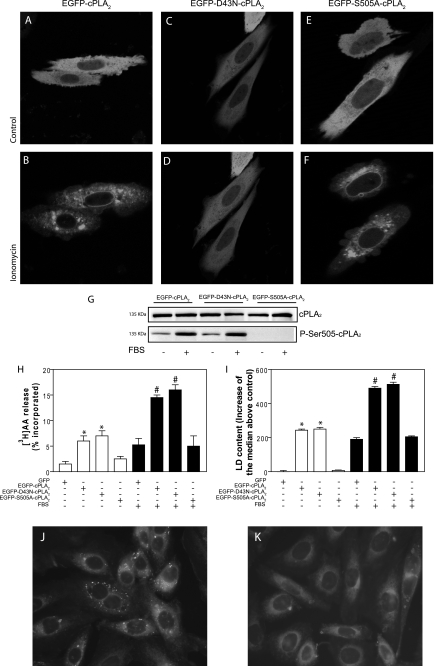
Additional evidence for a Ca2+-independent activation of cPLA2α during LD biogenesis came from the transfection of a D43N-cPLA2α mutant. Asp-43 resides in the Ca2+-binding loop 1 at the membrane binding face of the C2 domain of the enzyme and participates in the coordination of the two Ca2+ ions that drive membrane binding (11). Substitution of this residue for Asn increases 100-fold the Ca2+ requirement for activity in vitro (31), and it prevents translocation of the enzyme to membranes in response to Ca2+ ionophores (15, 27). In agreement with this, EGFP-D43N-cPLA2α did not relocate in CHO-K1 cells stimulated with 5 μm ionomycin, unlike the wild type enzyme, which translocated to perinuclear membranes and structures resembling the Golgi apparatus (Fig. 2, A–D). In contrast, EGFP-S505A-cPLA2α, which lacks the Ser-505 phosphorylation site but retains its Ca2+ binding capacity, translocated to membranes in response to ionomycin just as the wild type enzyme (Fig. 2, E and F). Importantly, FBS treatment induced phosphorylation of wild type and D43N cPLA2α, at Ser-505 (Fig. 2G). Furthermore, transfection of EGFP-D43N-cPLA2α resulted in increased release of [3H]AA (Fig. 2H), both under basal conditions and upon FBS stimulation, to the same levels achieved by transfection of EGFP-cPLA2α. Regarding LD occurrence, the effect of the D43N mutant was identical to the wild type enzyme; there was a 2.5-fold increase of LD upon FBS stimulation, monitored either by flow cytometry (Fig. 2I), ADRP content (data not shown), or by fluorescence microscopy (Fig. 2, J and K). Taken together, these results demonstrate that the activation of cPLA2α and biogenesis of LD induced by FBS are independent of Ca2+ and do not require massive relocation of the enzyme. Furthermore, the results confirm our previous finding that phosphorylation of cPLA2α at Ser-505 is essential for its activation by FBS, although there is no apparent relocation in response to the faint Ca2+ signal elicited by the stimulus (10).
FIGURE 2.
EGFP-D43N-cPLA2α is as effective as EGFP-cPLA2α to induce LD biogenesis in response to FBS. A–F, CHO-K1 cells were transiently transfected with plasmids encoding EGFP-cPLA2α (A and B), EGFP-D43N-cPLA2α (C and D), or EGFP-S505A-cPLA2α (E and F). Confocal images were obtained before (A, C, and E) or 10 min after addition of 5 μm ionomycin. G, Western blots of total cPLA2α and phospho-Ser-505-cPLA2α of transiently transfected cells. H, [3H]AA release from serum-starved CHO-K1 cells transfected with EGFP-cPLA2α, EGFP-D43N-cPLA2α, or EGFP-S505A-PLA2α and kept unstimulated (open bars) or stimulated with 7.5% FBS for 15 min (filled bars). I, indirect quantification of LD by flow cytometry in cells after 6 h under control conditions (open bars) or with 7.5% FBS (filled bars). J and K, serum-starved CHO-K1 cells transiently transfected with EGFP-D43N-cPLA2α (J) or with EGFP-S505A-PLA2α (K) were stained with Nile red for epifluorescence microscopy. Results in H and I are means ± S.E. of three independent experiments. *, significantly different (p < 0.01) from green fluorescent protein-transfected cells; #, significantly different (p < 0.01) from FBS-stimulated, green fluorescent protein-transfected cells.
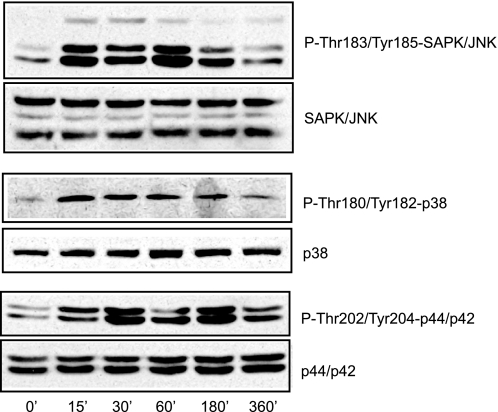
JNK, but Not ERK or p38 MAPK, Is Responsible for cPLA2α Phosphorylation and LD Biogenesis
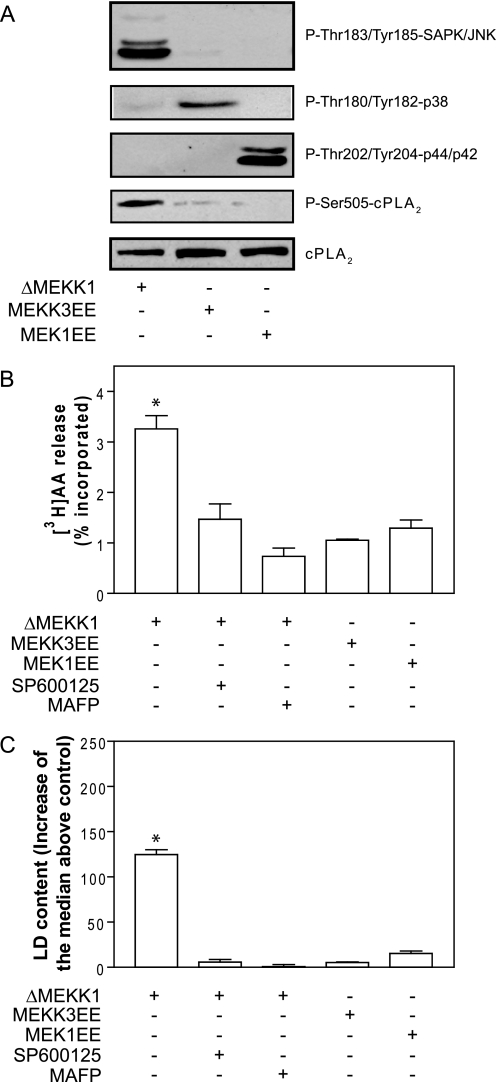
cPLA2α is phosphorylated at Ser-505 by MAPKs (11, 12), and to dissect the pathway during LD biogenesis, we monitored JNK, p38, and ERK MAPKs at different times after FBS replacement. As shown in Fig. 3, all three MAPKs became activated after stimulation with 7.5% FBS. We therefore activated each MAPK individually by transfecting constitutively active upstream activators of each pathway. As shown in Fig. 4A, transfection of the catalytic domain of MEKK1, which encodes a constitutively active kinase (ΔMEKK1), induced phosphorylation of JNK in the absence of FBS stimulus, but not of ERK, and only a slight increase of p38. Likewise, transfection of MKK3EE and MEK1EE resulted in the specific phosphorylation of p38 and ERK, respectively, in the absence of FBS. Importantly, only the activation of JNK increased phosphorylation of cPLA2α at Ser-505 (Fig. 4A), and this was inhibited in the presence of the JNK inhibitor SP600125 at 10 μm (data not shown). ΔMEKK1-JNK-mediated phosphorylation of cPLA2α was mirrored by an increased [3H]AA-releasing activity in the absence of FBS stimulus, which again was inhibited by SP600125 and also by MAFP (Fig. 4B). In contrast, [3H]AA released from cells transfected either with MKK3EE or MEK1EE was not different from that in control cells (Fig. 4B). In close agreement with data on cPLA2α phosphorylation and activity, Fig. 4C shows that cells transfected with ΔMEKK1 had increased LD content as monitored by flow cytometry. In contrast, stimulation of p38 or ERK did not induce the formation of LD. Again, LD biogenesis induced by expression of ΔMEKK1 in the absence of FBS stimulus was sensitive to inhibition by SP600125 and MAFP.
FIGURE 3.
FBS induces the phosphorylation of JNK, p38, and ERK in CHO-K1 cells. Serum-starved CHO-K1 cells were treated with 7.5% FBS for the indicated times. Total and phosphorylated JNK, p38, and ERK were monitored by Western blot.
FIGURE 4.
Activation of JNK, but not of p38 or ERK, is sufficient to phosphorylate cPLA2α and induce LD biogenesis in the absence of FBS. CHO-K1 cells were transiently transfected with plasmids encoding ΔMEKK1, MEKK3EE, or MEK1EE and switched to culture medium without FBS. A, phosphorylation of JNK, p38, ERK, and cPLA2α in the absence of FBS. B, cells were prelabeled with 0.5 μCi/ml [3H]AA during 24 h and then washed, and the release of radioactivity to the medium was quantified after 15 min. C, indirect quantification of LD by flow cytometry. Results in B and C are means ± S.E. of three independent experiments. *, significantly different (p < 0.01) from control.
The preceding results show that the JNK inhibitor SP600126 antagonizes the activation of cPLA2α and the biogenesis of LD in the absence of an FBS stimulus. To validate this pharmacological evidence in a more physiological setting, we compared the ability of MAPK inhibitors to block cPLA2α activation and LD biogenesis induced by FBS. As shown in Fig. 5A, phosphorylation of cPLA2α induced by 7.5% FBS was inhibited in the presence of 10 μm SP600125, but it was not affected by the same concentration of the p38 inhibitor SB20358 or the MAPK/ERK kinase inhibitor PD98059. In close agreement with these results, [3H]AA release stimulated by FBS was only decreased in the presence of the JNK inhibitor but was unaffected by SB20358 or PD98959 (Fig. 5B). Furthermore, the occurrence of LD induced by FBS, monitored either by flow cytometry (Fig. 5C) or after the expression of ADRP (Fig. 5A), was sensitive only to the JNK inhibitor.
FIGURE 5.
JNK inhibitor SP600125 prevents cPLA2α activation and LD biogenesis induced by FBS. Serum-deprived CHO-K1 cells were pretreated for 30 min with 10 μm concentrations of SP600125, SB20358, or PD98059 and then challenged with 7.5% FBS for 15 min (B) or 6 h (A and C). A, Western blots of total cPLA2α, phospho-Ser-505-cPLA2α, and ADRP. B, radioactivity released from serum-deprived CHO-K1 cells that were prelabeled during 24 h with 0.5 μCi/ml [3H]AA, washed, and pretreated with drugs for 30 min before a 15-min stimulation with 7.5% FBS. C, serum-starved cells were treated for 30 min with drugs, then stimulated with FBS during 6 h, fixed, and stained with Nile red to quantify LD by flow cytometry. Results in B and C are means ± S.E. of three independent experiments. *, p < 0.01 compared with control in the absence of FBS; #, p < 0.01 compared with FBS-treated cells.
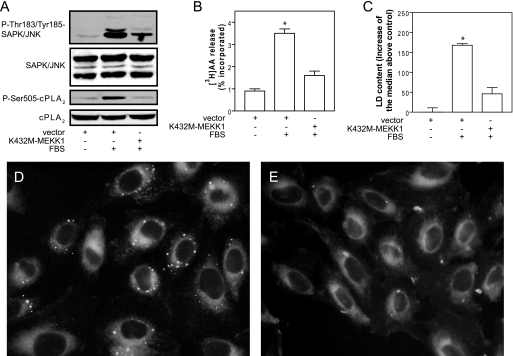
We also inhibited JNK by transfecting a dominant-negative form of MEKK1, the upstream activator of JNK. Fig. 6A shows that phosphorylation of JNK in response to FBS stimulation was partially inhibited in cells transfected with K432M-MEKK1, as compared with cells transfected with empty vector. The partial inhibition of JNK was enough to fully inhibit cPLA2α phosphorylation induced by FBS (Fig. 6A). In close agreement with this, cPLA2α activity monitored after the release of [3H]AA (Fig. 6B) and LD biogenesis measured by flow cytometry (Fig. 6C) were clearly inhibited by transfection of K432M-MEKK1. Results on the inhibition of LD were confirmed by ADRP expression levels (data not shown) and by fluorescence microscopy. Fig. 6, D and E, shows that, unlike cells transfected with empty vector, which generate LD after FBS replacement, those transfected with K432M-MEKK1 do not contain LD. Taken together, these results demonstrate that the MAPK that activates cPLA2α during LD biogenesis is JNK.
FIGURE 6.
Transfection of a dominant-negative form of MEKK1 (K432M-MEKK1) inhibits JNK phosphorylation, activation of cPLA2α, and LD biogenesis. CHO-K1 cells were transfected with empty vector or vector encoding K432M-MEKK1. Cells were serum-starved for 24 h and then challenged for 15 min (A and B) or 6 h (C) with 7.5% FBS. A, phosphorylation levels of JNK and cPLA2α. B, radioactivity released from cells that had been prelabeled during 24 h with 0.5 μCi/ml [3H]AA. C, indirect quantification of LD by flow cytometry. D and E, cells were transfected with empty vector (D) or with vector encoding K432M-MEKK1 (E), stimulated for 6 h with 7.5% FBS, and stained with Nile red for epifluorescence microscopy. Results in B and C are means ± S.E. of three independent experiments. *, significantly different (p < 0.01) from control.
Ceramide 1-Phosphate Induces LD Biogenesis in the Absence of FBS
Next, we assessed the possible implication of Cer-1-P in cPLA2α activation in our system. Cer-1-P drives the translocation of cPLA2α to membranes and is absolutely required for its activation in response to inflammatory agonists (25), but it also might contribute to the phosphorylation state of the enzyme (32). We treated serum-starved cells with 2.5 μm Cer-1-P in ethanol/dodecane (98:2) at a final vehicle dilution of 1:1000 (33). Cer-1-P induced both JNK and cPLA2α phosphorylation (Fig. 7A). Also, Cer-1-P stimulated the release of [3H]AA in an SP600125- and MAFP-sensitive fashion, to levels that were similar to those attained with FBS stimulation (Fig. 7B). In contrast to Cer-1-P, C2-ceramide at the same concentration did not affect JNK and cPLA2α phosphorylation or cPLA2α activity. In agreement with these results, Cer-1-P induced LD biogenesis that was inhibited by SP600125 and MAFP (Fig. 7C). LD quantification with flow cytometry agreed with ADRP expression levels and epifluorescence microscopy (data not shown). To confirm results on exogenously supplied Cer-1-P, we overexpressed CERK, the enzyme responsible for Cer-1-P synthesis. Expression of CERK in CHO-K1 cells induced the phosphorylation of JNK and cPLA2α (Fig. 7D). Furthermore, the synthesis of Cer-1-P was enhanced, and this was accompanied by the stimulation of cPLA2α activity and the biogenesis of LD (Fig. 7, E–G). These results indicate that an increase of Cer-1-P can induce LD formation through JNK activation and subsequent cPLA2α phosphorylation at Ser-505.
FIGURE 7.
Cer-1-P and CERK stimulate cPLA2α and induce LD biogenesis. A, Western blots of phospho-JNK and phospho-cPLA2α from serum-starved CHO-K1 cells that were treated with vehicle, 2.5 μm C2-ceramide, 2.5 μm Cer-1-P, or 7.5% FBS for 15 min. B, radioactivity released from [3H]AA prelabeled, serum-starved cells that were pretreated for 30 min with MAFP or SP600125, then stimulated for 15 min with C2-Cer, Cer-1-P, or FBS. C, quantification of LD by flow cytometry after a 6-h treatment with C2-Cer and Cer-1-P of FBS. D–G, cells were transiently transfected with a plasmid encoding CERK and then maintained in the absence of FBS. Overexpression of CERK increased the phosphorylation of JNK and cPLA2α (D). CERK-transfected cells were prelabeled for 24 h with [3H]palmitate or with [3H]AA to monitor synthesis of Cer-1-P (E) and cPLA2α activity (F), respectively. CERK also increased basal levels of LD in the absence of FBS (G). *, significantly different (p < 0.01) from control.
FBS Increases Cer-1-P, and Silenced Expression of CERK Inhibits the Formation of LD Induced by FBS
To tackle the relevance of the latter set of results, we knocked down the expression of CERK with an siRNA approach. Among the three different siRNA sequences used, two of them (siRNA CERK1 and siRNA CERK3) effectively down-regulated the enzyme, whereas siRNA CERK2 had no effect (Fig. 8A). Fig. 8B shows that FBS stimulation increased the synthesis of [3H]Cer-1-P in [3H]palmitate-prelabeled cells, and this was blocked by siRNA CERK1 and siRNA CERK3 but not by siRNA CERK2. We did not observe changes in [3H]ceramide content under any condition (data not shown). CERK down-regulation also inhibited the phosphorylation of JNK and cPLA2α induced by FBS (Fig. 8A). Furthermore, knocked down expression of CERK inhibited [3H]AA release (Fig. 8C) and LD biogenesis monitored by flow cytometry (Fig. 8D), ADRP expression (Fig. 8A), or by fluorescence microscopy (data not shown). In conclusion, the above results show that serum-induced LD biogenesis is mediated by CERK and formation of Cer-1-P.
DISCUSSION
This study addresses the mechanism of cPLA2α activation needed for the biogenesis of LD induced by FBS. Our findings can be summarized in three major points. First, cPLA2α activation and LD biogenesis are independent of intracellular Ca2+ rises, with no apparent relocation of the enzyme. Second, JNK is the MAPK that activates cPLA2α in our experimental model. Third, the Cer-1-P-generating enzyme CERK is required for the activation of JNK, and hence of cPLA2α.
This study shows that the pathway leading to cPLA2α activation and LD biogenesis is Ca2+-independent. Intracellular chelation with BAPTA-AM or blocking of the inositol 1,4,5-trisphosphate-induced Ca2+ transient elicited by FBS does not alter cPLA2α activation and LD biogenesis. Moreover, the single mutation D43N in the C2 domain of the enzyme, which abrogates its Ca2+ binding capacity, does not affect activity in response to FBS. Several reports have established the dispensability of Ca2+ rises in terms of full enzyme activation in vivo (13, 16–19) while putting forward the requirement of the signaling lipids PtdIns-4,5-P2 (17, 20, 21, 34) and Cer-1-P to allow interaction with membranes (22–25). The D43N mutation in cPLA2α renders the enzyme refractory to physiological Ca2+ concentrations (31), but it is fully active on PtdIns-4,5-P2-containing micelles (34). We have not addressed the possible role of PtdIns-4,5-P2 in LD biogenesis, but in light of the present results this is certainly a goal in the near future. It is now clear that cPLA2α activity is essential for the generation of LD after TAG present in the endoplasmic reticulum (7, 10); however, we have consistently found no enzyme translocation to the nuclear envelope and nearby membranes in response to FBS (10). Bearing in mind that the D43N mutation does not suppress interfacial activity of the C2 domain (34), in our experimental model the enzyme could interact with the diffuse endoplasmic reticulum membrane system and/or nascent LD. This would probably escape detection in our studies, which were carried out within minutes following FBS stimulation (10).
Our results show that JNK regulates cPLA2α activation and LD biogenesis by inducing phosphorylation at Ser-505. Although phosphorylation at this site increases activity in vitro only 2-fold (11), it confers increased membrane affinity at low micromolar Ca2+ concentrations (11, 12), and this tighter binding to the lipid surface could become critical for enzyme activation under our resting Ca2+ conditions. FBS activates ERK, p38, and JNK, and we identify the JNK cascade as the one governing cPLA2α phosphorylation in our system. This also stands probably for oleate-induced LD.6 A literature survey shows that the identity of the MAPK cascade leading to the phosphorylation of cPLA2α at Ser-505 is a matter of cell type and triggering stimulus, often involving ERK or/and p38 MAPK. However, to our knowledge only two recent reports have uncovered a key role of JNK in cPLA2α activation, both related to the microbicidal activity of phagocytes. Lee et al. (35) showed that secretion of monocyte chemoattractant protein-1 induced by stimulation of macrophages with synthetic oligodeoxynucleotides containing CpG motifs depends on cPLA2α activity, which can be blocked by pharmacological inhibition or down-regulation of JNK. Casas et al. (36), on the other hand, showed that translocation of cPLA2α to phagosomal membranes of macrophages, an event required for eicosanoid generation and killing of the ingested microbe, requires enzyme phosphorylation at Ser-505 and is blocked after pharmacological inhibition of JNK. Therefore, this study describes for the first time the crucial role of the JNK cascade in the activation of cPLA2α for LD biogenesis. We showed previously that the implication of cPLA2α in LD biogenesis after serum lipoproteins is not unique to the Chinese hamster ovary cell model (10). It remains to be established whether this generalization also stands for JNK, as it may hold promise for new pharmacological approaches in the treatment of metabolic diseases. In this regard, LD have been shown to interfere with membrane translocation of the insulin-sensitive glucose transporter, an observation that might account for insulin resistance in type 2 diabetes (5). Therefore, it is tempting to speculate that the observations that animal models of obesity have abnormally elevated JNK activity and that JNK1 knock-out mice on obesity-inducing diets are protected against adiposity and insulin resistance (37) are related to cPLA2α-mediated formation of LD.
This study also presents CERK and its product Cer-1-P as new key players in the biogenesis of LD. This is somehow not surprising, as Cer-1-P is a firmly established activator of cPLA2α (32, 38). Cer-1-P also has pro-survival effects (39), in line with the protective role that LD play during nutrient starvation and other stress conditions (6, 7). Cer-1-P interacts with the cationic β-groove (Arg-57, Lys-58, and Arg-59) of the C2 domain of cPLA2α. This enhances interaction with membranes and lowers the EC50 for Ca2+ down to 31 nm, resulting in increased and catalytic activity in vitro (22, 24). Recently, it has been described that CERK is required for cPLA2α activation and translocation to the Golgi in response to Ca2+ increases in the cell (25). Our results clearly show the requirement of CERK for cPLA2α activation and LD biogenesis. However, they do not quite fit with a direct interaction of Cer-1-P and cPLA2α. First, cPLA2α activation for LD biogenesis is independent of Ca2+, and it does not require enzyme translocation to the nuclear envelope and the Golgi. Second, our data show that CERK activates JNK and downstream phosphorylation of cPLA2α at Ser-505. This suggests that Cer-1-P does not solely promote association of the enzyme with membranes. Our data fully support a formerly proposed mechanism (32, 40) whereby Cer-1-P activates the JNK cascade, either directly or after the inhibition of protein phosphatases 1 and 2A. This mechanism is in keeping with our observation that okadaic acid induces LD biogenesis in a JNK- and cPLA2α-dependent fashion.6 Regardless of the mechanism, our results clearly point to CERK as a potential target for the treatment of metabolic diseases.
Acknowledgments
CIBERDEM is an initiative of Instituto de Salud Carlos III. We appreciate the fruitful discussions on MAPK signaling with Dr. José Miguel Lizcano and Dr. Néstor Gómez.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-072925 and CA-117990, Grants SAF 2004-01698, SAF 2007-60055, SAF 2007-67154, and BFU2009-07823 from the Spanish Ministry of Education and Science, and Grant PI05/1723 from the Spanish Ministry of Health.
A. Gubern and E. Claro, unpublished results.
- LD
- lipid droplet(s)
- AA
- arachidonic acid
- ADRP
- adipophilin (adipose differentiation-related protein)
- CERK
- ceramide kinase
- Cer-1-P
- ceramide 1-phosphate
- C2-ceramide
- N-acetyl-d-sphingosine
- FBS
- fetal bovine serum
- JNK
- c-Jun N-terminal kinase
- MAFP
- methylarachidonyl fluorophosphonate
- MAPK
- mitogen-activated protein kinase
- PLA2
- phospholipase A2
- siRNA
- short interfering RNA
- TAG
- triacylglycerol
- PtdIns-4,5-P2
- phosphatidylinositol 4,5-bisphosphate
- ERK
- extracellular signal-regulated kinase
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester).
REFERENCES
- 1.Brasaemle D. L. (2007) J. Lipid Res. 48, 2547–2559 [DOI] [PubMed] [Google Scholar]
- 2.Martin S., Parton R. G. (2006) Nat. Rev. Mol. Cell Biol. 7, 373–378 [DOI] [PubMed] [Google Scholar]
- 3.Wolins N. E., Brasaemle D. L., Bickel P. E. (2006) FEBS Lett. 580, 5484–5491 [DOI] [PubMed] [Google Scholar]
- 4.Murphy D. J. (2001) Prog. Lipid Res. 40, 325–438 [DOI] [PubMed] [Google Scholar]
- 5.Boström P., Andersson L., Rutberg M., Perman J., Lidberg U., Johansson B. R., Fernandez-Rodriguez J., Ericson J., Nilsson T., Borén J., Olofsson S. O. (2007) Nat. Cell Biol. 9, 1286–1293 [DOI] [PubMed] [Google Scholar]
- 6.Du L., Hickey R. W., Bayir H., Watkins S. C., Tyurin V. A., Guo F., Kochanek P. M., Jenkins L. W., Ren J., Gibson G., Chu C. T., Kagan V. E., Clark R. S. (2009) J. Biol. Chem. 284, 2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubern A., Barceló-Torns M., Casas J., Barneda D., Masgrau R., Picatoste F., Balsinde J., Balboa M. A., Claro E. (2009) J. Biol. Chem. 284, 5697–5708 [DOI] [PubMed] [Google Scholar]
- 8.Cole N. B., Murphy D. D., Grider T., Rueter S., Brasaemle D., Nussbaum R. L. (2002) J. Biol. Chem. 277, 6344–6352 [DOI] [PubMed] [Google Scholar]
- 9.Hutter-Paier B., Huttunen H. J., Puglielli L., Eckman C. B., Kim D. Y., Hofmeister A., Moir R. D., Domnitz S. B., Frosch M. P., Windisch M., Kovacs D. M. (2004) Neuron 44, 227–238 [DOI] [PubMed] [Google Scholar]
- 10.Gubern A., Casas J., Barceló-Torns M., Barneda D., de la Rosa X., Masgrau R., Picatoste F., Balsinde J., Balboa M. A., Claro E. (2008) J. Biol. Chem. 283, 27369–27382 [DOI] [PubMed] [Google Scholar]
- 11.Ghosh M., Tucker D. E., Burchett S. A., Leslie C. C. (2006) Prog. Lipid Res. 45, 487–510 [DOI] [PubMed] [Google Scholar]
- 12.Burke J. E., Dennis E. A. (2009) Cardiovasc. Drugs Ther. 23, 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Z. H., Gijón M. A., de Carvalho M. S., Spencer D. M., Leslie C. C. (1998) J. Biol. Chem. 273, 8203–8211 [DOI] [PubMed] [Google Scholar]
- 14.Gijón M. A., Spencer D. M., Kaiser A. L., Leslie C. C. (1999) J. Cell Biol. 145, 1219–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perisic O., Paterson H. F., Mosedale G., Lara-González S., Williams R. L. (1999) J. Biol. Chem. 274, 14979–14987 [DOI] [PubMed] [Google Scholar]
- 16.Gijón M. A., Spencer D. M., Siddiqi A. R., Bonventre J. V., Leslie C. C. (2000) J. Biol. Chem. 275, 20146–20156 [DOI] [PubMed] [Google Scholar]
- 17.Balsinde J., Balboa M. A., Li W. H., Llopis J., Dennis E. A. (2000) J. Immunol. 164, 5398–5402 [DOI] [PubMed] [Google Scholar]
- 18.Balboa M. A., Balsinde J., Dennis E. A. (2000) Biochem. Biophys. Res. Commun. 267, 145–148 [DOI] [PubMed] [Google Scholar]
- 19.Sheridan A. M., Sapirstein A., Lemieux N., Martin B. D., Kim D. K., Bonventre J. V. (2001) J. Biol. Chem. 276, 29899–29905 [DOI] [PubMed] [Google Scholar]
- 20.Mosior M., Six D. A., Dennis E. A. (1998) J. Biol. Chem. 273, 2184–2191 [DOI] [PubMed] [Google Scholar]
- 21.Casas J., Gijón M. A., Vigo A. G., Crespo M. S., Balsinde J., Balboa M. A. (2006) Mol. Biol. Cell 17, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettus B. J., Bielawska A., Subramanian P., Wijesinghe D. S., Maceyka M., Leslie C. C., Evans J. H., Freiberg J., Roddy P., Hannun Y. A., Chalfant C. E. (2004) J. Biol. Chem. 279, 11320–11326 [DOI] [PubMed] [Google Scholar]
- 23.Subramanian P., Stahelin R. V., Szulc Z., Bielawska A., Cho W., Chalfant C. E. (2005) J. Biol. Chem. 280, 17601–17607 [DOI] [PubMed] [Google Scholar]
- 24.Stahelin R. V., Subramanian P., Vora M., Cho W., Chalfant C. E. (2007) J. Biol. Chem. 282, 20467–20474 [DOI] [PubMed] [Google Scholar]
- 25.Lamour N. F., Subramanian P., Wijesinghe D. S., Stahelin R. V., Bonventre J. V., Chalfant C. E. (2009) J. Biol. Chem. 284, 26897–26907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavicevic Z., Leslie C. C., Malik K. U. (2008) J. Lipid Res. 49, 724–737 [DOI] [PubMed] [Google Scholar]
- 27.Casas J., Gijón M. A., Vigo A. G., Crespo M. S., Balsinde J., Balboa M. A. (2006) J. Biol. Chem. 281, 6106–6116 [DOI] [PubMed] [Google Scholar]
- 28.Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 29.Wijesinghe D. S., Massiello A., Subramanian P., Szulc Z., Bielawska A., Chalfant C. E. (2005) J. Lipid Res. 46, 2706–2716 [DOI] [PubMed] [Google Scholar]
- 30.Sun X., Mörk A. C., Helmke R. J., Martinez J. R., Zhang G. H. (1999) J. Cell. Biochem. 73, 458–468 [PubMed] [Google Scholar]
- 31.Bittova L., Sumandea M., Cho W. (1999) J. Biol. Chem. 274, 9665–9672 [DOI] [PubMed] [Google Scholar]
- 32.Lamour N. F., Chalfant C. E. (2005) Mol. Interv. 5, 358–367 [DOI] [PubMed] [Google Scholar]
- 33.Wijesinghe D. S., Lamour N. F., Gomez-Munoz A., Chalfant C. E. (2007) Methods Enzymol. 434, 265–292 [DOI] [PubMed] [Google Scholar]
- 34.Six D. A., Dennis E. A. (2003) J. Biol. Chem. 278, 23842–23850 [DOI] [PubMed] [Google Scholar]
- 35.Lee J. G., Lee S. H., Park D. W., Lee S. H., Yoon H. S., Chin B. R., Kim J. H., Kim J. R., Baek S. H. (2008) Cell. Signal. 20, 105–111 [DOI] [PubMed] [Google Scholar]
- 36.Casas J., Meana C., Esquinas E., Valdearcos M., Pindado J., Balsinde J., Balboa M. A. (2009) J. Immunol. 183, 2767–2774 [DOI] [PubMed] [Google Scholar]
- 37.Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 38.Chalfant C. E., Spiegel S. (2005) J. Cell Sci. 118, 4605–4612 [DOI] [PubMed] [Google Scholar]
- 39.Gómez-Muñoz A. (2006) Biochim. Biophys. Acta 1758, 2049–2056 [DOI] [PubMed] [Google Scholar]
- 40.Pettus B. J., Bielawska A., Spiegel S., Roddy P., Hannun Y. A., Chalfant C. E. (2003) J. Biol. Chem. 278, 38206–38213 [DOI] [PubMed] [Google Scholar]