Abstract
The Runx2 transcription factor is required for commitment of mesenchymal cells to bone lineages and is a major regulator of osteoblast-specific gene expression. Runx2 is subject to a number of post-transcriptional controls including selective proteolysis and phosphorylation. We previously reported that Runx2 is phosphorylated and activated by the ERK/MAPK pathway (Xiao, G., Jiang, D., Thomas, P., Benson, M. D., Guan, K., Karsenty, G., and Franceschi, R. T. (2000) J. Biol. Chem. 275, 4453–4459). In this study, we used a combination of in vitro and in vivo phosphorylation analysis, mass spectroscopy, and functional assays to identify two sites at Ser301 and Ser319 within the proline/serine/threonine domain of Runx2 that are required for this regulation. These sites are phosphorylated by activated ERK1 in vitro and in cell culture. In addition to confirming ERK-dependent phosphorylation at Ser319, mass spectroscopy identified two other ERK-phosphorylated sites at Ser43 and Ser510. Furthermore, introduction of S301A,S319A mutations rendered Runx2 resistant to MAPK-dependent activation and reduced its ability to stimulate osteoblast-specific gene expression and differentiation after transfection into Runx2-null calvarial cells and mesenchymal cells. In contrast, S301E,S319E Runx2 mutants had enhanced transcriptional activity that was minimally dependent on MAPK signaling, consistent with the addition of a negative charge mimicking serine phosphorylation. These results emphasize the important role played by Runx2 phosphorylation in the control of osteoblast gene expression and provide a mechanism to explain how physiological signals acting on bone through the ERK/MAPK pathway can stimulate osteoblast-specific gene expression.
Introduction
The bone cell lineage is controlled by a hierarchy of transcription factors that are expressed in a defined temporal sequence. Runx2, an essential factor for both hypertrophic cartilage and bone formation, is expressed very early in skeletal development, first appearing coincident with the formation of mesenchymal condensations (1). Subsequent development of the osteoblast lineage requires at least two additional factors; Osterix, which is essential for subsequent progression of the osteoblast lineage, and ATF4, which regulates osteoblast activity, particularly in postnatal animals (2, 3). Runx2 expression continues during the later stages of bone development and persists in regions of active bone remodeling throughout life. Skeletal development in Runx2-deficient mice fails to progress beyond the cartilage anlage stage, whereas dominant-negative suppression of Runx2 even in postnatal animals inhibits osteoblast activity and bone formation (4). Thus, Runx2 is required for both the initial formation of osteoblasts and hypertrophic chondrocytes during development and for sustained osteoblast differentiation during bone remodeling.
Consistent with its multiple roles in bone formation, Runx2 is highly regulated. In addition to transcriptional control by factors such as bone morphogenetic proteins (5), Runx2 activity is controlled both by its interaction with a number of accessory nuclear factors and by post-translational modifications, including phosphorylation. We have been particularly interested in this latter regulation and proposed that Runx2 is phosphorylated and activated by a ERK3/MAPK-dependent pathway initiated by the interaction of osteoprogenitors with a type I collagen-containing extracellular matrix (ECM) via α2β1 integrins (6, 7). This collagen-integrin interaction is necessary for subsequent osteoblast-specific gene expression and differentiation (7–9). Consistent with this model, steady-state Runx2 phosphorylation and DNA binding activity increase with osteoblast differentiation, whereas pharmacological inhibition of the ERK/MAPK pathway rapidly inhibits ECM and BMP-induced gene expression (10–12). In related studies, FGF2 treatment of osteoblasts, which is known to stimulate both ERK/MAPK and protein kinase C pathways, increases Runx2 phosphorylation and Ocn expression in a MAPK-dependent manner (13). Furthermore, manipulation of the MAPK pathway by overexpression of constitutively active or dominant-negative mutants of MEK1, respectively, increases or decreases osteocalcin gene expression and Runx2 phosphorylation (6). ERK/MAPK signaling is also important for in vivo bone development. Transgenic overexpression of constitutively active or dominant-negative MEK1 in mouse osteoblasts, respectively, stimulates or inhibits Runx2 phosphorylation and skeletal maturation. Furthermore, the cleidocranial dysplasia phenotype of Runx2 heterozygous null mice can be partially rescued by crossing these animals with mice expressing constitutively active MEK1, consistent with the in vivo actions of the ERK/MAPK pathway being at least in part mediated by Runx2 (14).
In addition to the work from our laboratory cited above (6–14), a number of studies from other groups support the concept that ECM-integrin binding, MAPK activation, and Runx2 phosphorylation are important for osteoblast differentiation. The requirement for α1β1 and α2β1 collagen-binding integrins in osteoblast differentiation and BMP responsiveness was demonstrated by both in vitro and in vivo analysis (15–18). Also, ERK/MAPK signaling was shown to be necessary for differentiation of human osteoblasts and marrow stromal cells (19, 20). A number of groups also confirmed that Runx2 can be phosphorylated and activated by MAPK inducers. During the osteoblastic differentiation of human marrow stromal cells, Runx2 levels remain relatively unchanged, but DNA binding increases as does Runx2 phosphorylation (21). Also, mechanical loading of osteoblasts, mediated in part through α2β1 integrins, induces MAPK activity (22, 23). Similarly, loading of periodontal ligament cells (osteoprogenitor-like cells) increases Runx2 phosphorylation and binding to OSE2 DNA via an ERK/MAPK-dependent process (24). In osteomimetic prostate cancer cells, differentiation is accompanied by ERK1/2 activation, increased Runx2-DNA binding, and Ocn expression, responses that were all blocked by MAPK inhibition (25). Last, insulin-like growth factor-1, which activates phosphatidylinositol 3-kinase and, subsequently, ERK/MAPK pathways, stimulates Runx2-OSE2 binding and phosphorylation in vascular endothelial cells (26) as well as differentiation of marrow stromal cells (27, 28). Thus, ERK/MAPK-dependent phosphorylation of Runx2 likely plays an important role in the response of osteoblasts to a variety of signals initiated by cell-ECM binding, hormone/growth factor signaling, and mechanical loading.
To further understand how the ERK/MAPK pathway regulates Runx2 transcriptional activity, in the present study we identify amino acid residues in Runx2 that are phosphorylated in a ERK/MAPK-dependent manner and show that these sites are necessary for osteoblast-specific gene expression and differentiation.
EXPERIMENTAL PROCEDURES
Reagents
The reagents used in this study were obtained from the following sources: tissue culture medium and fetal bovine serum from Invitrogen; U0126 from Calbiochem; mouse anti-Runx2 antibody from MBL; phosphoserine antibody from ABCam; and M2 and M2 horseradish peroxidase-conjugated antibody from Sigma.
DNA Constructs and Viral Expression Vectors
The 0.6-kb mouse osteocalcin gene 2-Luc reporter plasmid and a constitutively active MEK1 expression vector were previously described (29–31). A series of Runx2 expression plasmids encoding HA- or FLAG-tagged full-length type II Runx2 (N-terminal sequence: MASN) or several N- and C-terminal deletions were generated by PCR and subcloning into the pCMV5 expression vector. Serine mutants of full-length and aa 1–330 Runx2 were generated using the QuikChange site-directed mutagenesis kit (Stratagene). A cDNA encoding Runx2 with a biotinylation tag was generated by adding the sequence, MASSLRQILDSQKMEWRSNAGGS, to the N terminus of mouse Runx2. This sequence is specifically recognized by bacterial BirA biotin ligase (32). Plasmids containing cDNAs encoding the biotinylation tag and BirA were a generous gift from Dr. John Strouboulis (Alexander Fleming Biomedical Sciences Research Center, Athens, Greece). Adenoviruses encoding wild type and mutant Runx2, and biotinylation tagged Runx2, BirA, and Mek(sp) (constitutively active MEK1) were constructed by first subcloning the respective cDNA into pAdloxp and then generating viruses using Cre-Lox recombination as previously described (33).
Cell Culture
C3H10T1/2, COS7, and HEK293 cells were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium containing 10% FBS and 1% antibiotics. An mTERT-immortalized calvarial cell line from Runx2−/− mice (34) was a generous gift from Drs. Jane Lian and Gary Stein (University of Massachusetts Medical Center, Worcester, MA) and maintained in minimal essential medium-α, 10% FBS. MC3T3-E1 clone 4 (MC-4) cells, previously developed in this laboratory (35), were also maintained in minimal essential medium-α, 10% FBS. To induce differentiation, C3H10T1/2, mTERT cells, and MC-4 cells were grown in minimal essential medium-α, 10% FBS containing 50 μg/ml ascorbic acid as previously described (12, 34, 36).
Transfections
COS7 cells were plated at a density of 5 × 104 cells/cm2 on 35-mm dishes and transfected using Lipofectamine (Invitrogen). Each transfection contained 0.5 μg of the indicated plasmid and 0.05 μg of pRL-SV40 containing a cDNA for Renilla reniformis luciferase to control transfection efficiency. Cells were harvested and assayed using a dual luciferase assay kit (Promega) with a Monolight 2010 luminometer (Pharmingen). For mTERT cells, transfection was accomplished using FuGENE 6 reagent (Roche). For studies where effects of more sustained Runx2 expression were analyzed, C3H10T1/2 cells were transduced with adenovirus expression vectors as previously described (36).
Western Blot Analysis
Whole cell extracts were prepared by dissolving cell layers in SDS sample buffer. Samples were fractionated by SDS-PAGE on 4–12% precast minigels (Invitrogen) and electrophoretically transferred to nitrocellulose membranes (Schleicher & Schuell). Primary antibodies were used at the following dilutions (Runx2, 1:500; M2, 1:2000; phosphoserine, 1:500). Secondary antibody was used at a dilution of 1:10,000. Immunoreactivity was detected using ECL chemiluminescence reagents (Amersham Biosciences).
RNA Analysis
RNA was isolated using TRIzol reagent (Invitrogen) and further purified by DNase I treatment and an RNeasy kit (Qiagen). Reverse transcriptase reactions were conducted with 2 μg of total RNA, TaqMan reverse transcriptase reagents, and an oligo(dT) primer (Applied Biosystems)). PCR was performed using an ABI Prism 7700 sequence detection system. Glyceradehyde-3-phosphate dehydrogenase mRNA was used as an endogenous control.
Metabolic Labeling and Immunoprecipitation of Runx2
COS7 cells were transfected with Runx2 expression plasmids, cultured for 30 h, and preincubated in phosphate-free Dulbecco's modified Eagle's medium, 0.1% FBS for 12 h. Labeling was conducted for 4 h in phosphate-free Dulbecco's modified Eagle's medium containing 200 μCi/ml [32P]orthophosphate (phosphorus-32 or [35S]methionine/cysteine Tran35S-label, Amersham Biosciences). Nuclear extracts were prepared as previously described (12) and precleared twice with 50 μl of protein A/G-agarose beads. Appropriate antibodies were added and incubated for 2 h at 4 °C with gentle rocking. Immune complexes were then collected by the addition 30 μl of protein A/G-agarose beads and incubation for 1 h at 4 °C followed by centrifugation. Precipitates were washed five times with 1× washing buffer (20 mm HEPES, pH 7.6, 50 mm KCl, 1 mm dithiothreitol, 0.25% Nonidet P-40, 5 mm sodium fluoride, 1 mm EGTA, 5 mm MgCl2). The immunoprecipitated complexes were suspended in SDS sample buffer and analyzed by SDS-PAGE and autoradiography or Western blot analysis using the indicated antibodies. 32P incorporation was measured using a Packard A2024 InstantImager.
In Vitro Phosphorylation
Synthetic peptides were prepared by the University of Michigan Protein Structure Facility. Each peptide (1 μg) was incubated with 10 μCi of [γ-32P]ATP and 1 unit of activated MAPK (Calbiochem) in a buffer containing 25 mm Tris-HCl, pH 7.4, 10 mm MgCl2, 1 mm dithiothreitol, 40 μm ATP, and 0.5 mm EGTA in a final volume of 25 μl. Samples were incubated at 25 °C for 30 min. Reactions were terminated by addition of 25 μl of 2× SDS sample buffer and samples were analyzed by electrophoresis on 15% SDS gels. For in vitro Runx2 phosphorylation, biotinylated Runx2 was purified on streptavidin beads as described below and directly phosphorylated by incubation of beads under the same conditions used for peptide phosphorylation, except that [γ-32P]ATP was omitted.
Runx2 Purification and Identification of Phosphorylation Sites by Mass Spectroscopy
An adenovirus expression system for biotinylation tagging of Runx2 was developed (32). COS7 cells were transduced with adenovirus encoding Runx2 cDNA with an N-terminal biotinylation sequence and AdBirA (adenovirus expressing bacterial biotin protein ligase) with or without AdMek(sp). After 48 h, cell lysates were adsorbed to streptavidin magnetic beads and purified Runx2 was resolved by SDS-PAGE using a 4–12% gradient gel. The Runx2 gel band was alkylated with iodoacetamide and peptide fragments were generated by in-gel digestion with pepsin. Samples were analyzed by LC/MS/MS using a ThermoFisher LTQ Orbitrap XL (NextGenSciences, Ann Arbor, MI). The Orbitrap MS scan was performed at 60,000 full-width at half-maximum resolution and searched using a local copy of Mascot. Phosphorylated peptides containing P-serine were then identified. In certain cases, product ion data were used to confirm identification of the phosphorylation site.
Statistical Analysis
All statistical analyses were performed using SPSS 16.0 Software. Unless indicated otherwise, each reported value is the mean ± S.D. of triplicate independent samples. Statistical significance was assessed using a one-way analysis of variance.
RESULTS
ERK/MAPK-dependent Phosphorylation and Activation of Runx2 Require a Specific Region of the C-terminal Pro/Ser/Thr Domain
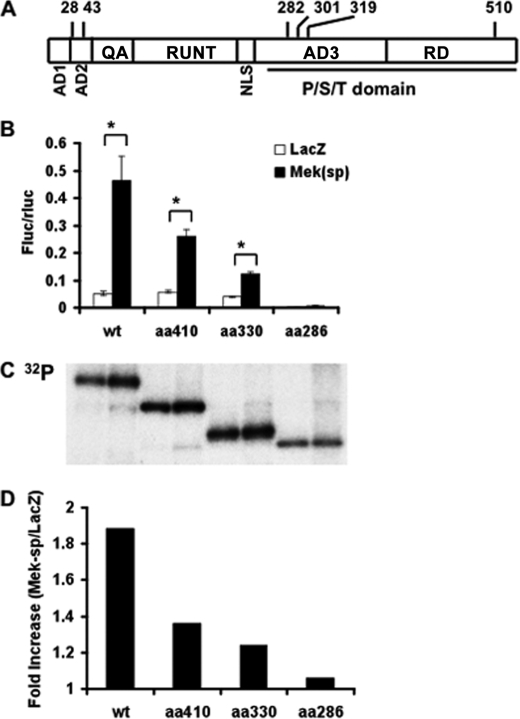
Fig. 1A shows a schematic of the domain structure of Runx2 with positions of potential phosphorylation sites to be discussed in this study. Activation of the ERK/MAPK pathway by overexpression of a constitutively active form of MEK1 or by treatment with FGF2 was previously shown to stimulate Ocn mRNA expression and promoter activity via a mechanism requiring Runx2 (6, 13). An initial deletion analysis of the Runx2 coding sequence showed that removal of the entire C-terminal proline/serine/threonine-rich (Pro/Ser/Thr) domain (contains AD3 and RD regions encompassing amino acid residues 258–528 in the mouse sequence) rendered Runx2 completely resistant to MAPK regulation and phosphorylation. In contrast, deletion of the N-terminal AD1, AD2, and QA-rich regions (amino acids 1–108) lowered basal transcriptional activity without affecting MAPK-dependent activation (6). Similarly, deletion of the Pro/Ser/Thr domain also rendered Runx2 unresponsive to activation by FGF2 (13).
FIGURE 1.
Identification of a region in Runx2 necessary for ERK/MAPK-dependent transcriptional activation and phosphorylation. A, schematic of the domain structure of Runx2 with relevant serine residues indicated. AD1–3, transcriptional activation domains; QA, glutamine/alanine-rich domain; RUNT, runt/DNA-binding domain; NLS, nuclear localization sequence; P/S/T domain, proline/serine/threonine-rich domain; RD, repressor domain (from Ref. 30). B, MAPK-dependent transcriptional activity. COS7 cells were transfected with wild type (WT) Runx2 or the indicated C-terminal deletions in the presence of control (LacZ) or Mek(sp) expression vectors and a 6OSE2-luc reporter as described under “Experimental Procedures.” Firefly luciferase activity was normalized for transfection efficiency using a R. reinformis luciferase plasmid. Asterisk, significantly different from corresponding control, p < 0.01; brackets indicate comparisons made; error bars, ±S.D. C and D, Runx2 phosphorylation. COS7 cell cultures treated as in B were metabolically labeled with [32P]orthophosphate or Tran35S-label as described under “Experimental Procedures.” Runx2 was immunoprecipitated (C) and 32P incorporation was normalized to total 35S-labeled protein in each group and expressed as fold-increase with Mek(sp) stimulation (D).
To more precisely define regions of Runx2 necessary for MAPK responsiveness, we carried out a more detailed deletion analysis of the Pro/Ser/Thr domain (Fig. 1, B–D). Wild type Runx2 or several C-terminal deletions (to residues 410, 330, and 286) were transfected into COS7 cells in the presence or absence of constitutively active MEK1 (Mek(sp)) and a p6OSE2-luc Runx2 reporter gene. Samples were either assayed for luciferase activity (panel B) or were metabolically labeled with [32P]orthophosphate and assayed for Runx2 phosphorylation by immunoprecipitation and autoradiography (panels C and D). C-terminal Runx2 deletions gradually reduced MAPK activation of Runx2 transcriptional activity from ∼9-fold with wild type Runx2 to ∼3-fold with the amino acid 330 deletion. Mek(sp) stimulation was completely lost with deletion to residue 286. Similarly, Mek(sp) stimulated total 32P incorporation into wild type Runx2 by ∼2-fold (panel C). This stimulation gradually decreased in the 410 and 330 deletions and was completely lost after deletion to residue 286. These results indicate that the minimal region for MAPK phosphorylation and activation of Runx2 is between amino acids 286 and 330. Subsequent analysis was restricted to this region although it is possible that more C-terminal sites may also participate in this regulation.
Identification of Runx2 Phosphorylation Sites
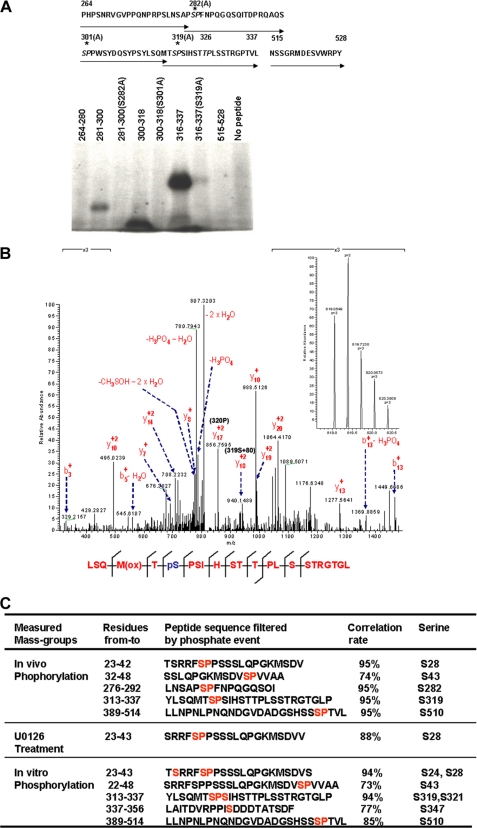
Inspection of the Runx2 peptide sequence in the 286–330 region (Fig. 2A) identified two putative proline-directed serine phosphorylation sites at residues 301 and 319 and an adjacent site at residue 282. A similar proline-directed threonine site was also seen (Thr326). Incubation of peptides spanning the 264 to 337 region with active ERK1 and [γ-32P]ATP revealed that Ser282, Ser301, and Ser319 were all phosphorylated in vitro, whereas Ser/Ala substitution at each site prevented phosphorylation. In contrast, Thr326 was not phosphorylated under these conditions (i.e. Introduction of an S319A mutation in the 316–337 peptide blocked phosphorylation). Peptides containing residues 264–280 and 515–528 were also not phosphorylated.
FIGURE 2.
Runx2 phosphorylation sites. A, in vitro peptide phosphorylation. Synthetic peptides were prepared containing amino acid residues 264–337 of the Runx2 sequence (indicated by arrows) or the indicated amino acid substitutions (top panel). Peptides were labeled with [γ-32P]ATP using activated ERK1 and resolved by SDS-PAGE as described under “Experimental Procedures” (lower panel). B, MS/MS analysis of ERK/MAPK-related phosphorylation sites. COS7 cells were transduced with adenoviruses encoding biotinylation-tagged Runx2, BirA biotin transferase, and Mek(sp) with (U0126 treatment) or without (in vivo phosphorylation) MAPK inhibitor. Runx2 as then purified as described under “Experimental Procedures” and subjected to MS/MS analysis. For in vitro phosphorylation, an aliquot of Runx2 purified from U0126-treated cells was incubated with the activated MAPK before MS/MS analysis. Phosphopeptides were identified using Mascot software. Correlation rate indicates the probability that the indicated peptide identification in the Runx2 sequence is correct. C, verification of Ser319 phosphorylation. The peptic peptide LSQMTSPSIHSTTPLSSTRGRGL was selected to manually validate the phosphorylation event. Mascot search results indicated that acquired MS/MS spectra contain product ion data for both [M + 2H]2+ and [M + 3H]3+ charged states for this peptide. The full MS spectra of the [M + 3H]3+ charged state is shown in the left panel. The right panel shows the annotated MS/MS spectra of m/z = 819.05 (+3). Diagnostic Y17+2 and Y18+2 ions are indicated with m/z values of 856.8 and 940.2, respectively, which confirms the presence of a phosphate on Ser319.
More extensive analysis of Runx2 phosphorylation was conducted using mass spectroscopy. COS7 cells were transduced with adenovirus vectors expressing a biotinylation tagged Runx2, BirA (bacterial biotin protein ligase), and Mek(sp). Runx2 was then purified from cell lysates using streptavidin magnetic beads and LC/MS/MS analysis was carried out on pepsin-digested samples. As shown in Fig. 2B, five peptides were identified containing phosphoserine at residues 28, 43, 282, 319, and 510. The probability of a correct identification for each peptide was 95% with the exception of the peptide containing Ser43, which had a correlation of 74%. In contrast, only phosphoserine 28 was identified in Runx2 purified from cells pretreated with the MAPK inhibitor, U0126. This indicates that other sites at Ser43, Ser282, Ser319, and Ser510 are directly or indirectly dependent on ERK/MAPK activity for phosphorylation. To identify direct MAPK targets, nondenatured Runx2 purified from U0126-treated cells was phosphorylated directly on streptavidin beads with activated P-ERK in vitro and MS was repeated. In this case, phosphate was detected on Ser24, Ser43, Ser319, Ser347, and Ser510. From this analysis, we conclude that the in vivo phosphorylation sites identified by MS, Ser43, Ser319, and Ser510 are probably direct ERK substrates, whereas Ser282 is likely phosphorylated by a second kinase activated by ERK. Because we also obtained functional evidence for the importance of Ser319 in Runx2 regulation (below), more extensive confirmation that this site is phosphorylated was obtained by analyzing product ion data for the LSQMTpSPSIHSTTPLSSTRGTGL peptide (residues 313–337). Both [M + 2H]2+ and [M + 3H]3+ charge states were analyzed ([M + 3H]3+ is shown in the left panel of Fig. 2C). Analysis of product ion data (Fig. 2C, right) confirmed that this peptide was phosphorylated at Ser6 (Ser319) within a mass accuracy of 2 ppm.
Surprisingly, although MS analysis consistently identified peptides containing 56–69% of the entire Runx2 sequence (62–76% if the N-terminal Gln/Ala region is excluded), peptides were never identified spanning the Ser301 region. Identified peptides contained 11 of the 12 proline-directed serine/threonine sites in Runx2, making Ser301 the only site not included in our analysis. Similar results were obtained using alternative protease digestions (trypsin/AspN versus pepsin). This suggests that the Ser301 region contains some abnormality in secondary structure, possibly due to post-translational modification, which prevents normal fragmentation and identification.
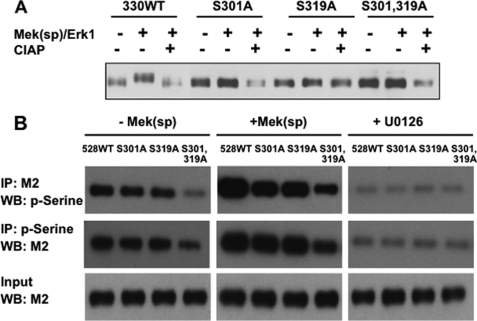
To further explore the possibility that the Ser301 site is phosphorylated in vivo, we used an indirect approach that takes advantage of the observation that activation of MAPK signaling stimulates a shift in the electrophoretic mobility of a truncated Runx2-(1–330) (Fig. 3A). For this experiment, wild type truncated Runx2-(1–330) or Runx2 containing an S301A mutation, an S319A mutation, or combined S301A,S319A mutations was transfected into COS7 cells with or without constitutively active Mek(sp) and ERK1 expression vectors and analyzed by SDS-PAGE. Activation of MAPK signaling clearly reduced the electrophoretic mobility of wild type Runx2-(1–330) and this mobility change was eliminated by treatment of samples with alkaline phosphatase. In contrast, no detectable MAPK-dependent change in mobility was seen with S301A, S319A mutations or the S301A,S319A double mutation. This is the result that would be expected if a detectable mobility shift requires phosphorylation on both Ser301 and Ser319.
FIGURE 3.
Additional evidence for Runx2 phosphorylation at Ser301. A, analysis of electrophoretic mobility of wild type (WT) truncated Runx2-(1–330) and Ser301 and Ser319 mutants. The indicated truncated Runx2 mutants were expressed in COS7 cells in the presence or absence of Mek(sp) and ERK1 expression vectors. Cell lysates were analyzed by SDS-PAGE with or without prior treatment with calf intestinal alkaline phosphatase (CIAP). Runx2 was detected by Western blotting. B, anti-P-serine antibody reactivity with full-length Runx2. FLAG-tagged WT Runx2 or the indicated mutants were expressed in COS7 cells in the presence (+) or absence (−) of Mek(sp) or in the presence of the MAPK inhibitor (+U0126). Nuclear extracts were then either immunoprecipitated with M2 antibody and probed with an anti-P-serine monoclonal antibody or immunoprecipitated (IP) with anti-P-serine and probed with M2.
Although the larger size of full-length Runx2 (528 amino acid residues) precluded conducting mobility shift analysis, we obtained additional evidence for Ser301 and Ser319 phosphorylation using immunoprecipitation/immunoblotting with Runx2 and Ser(P) antibodies (Fig. 3B). COS7 cells were transfected with FLAG-tagged wild type, S301A, S319A, or S301A,S319A full-length Runx2 mutants in the presence or absence of Mek(sp) expression vector or in the presence of the MAPK inhibitor, U0126. Samples were then either immunoprecipitated with a specific anti-Runx2 (M2) antibody followed by Western blotting with antiphosphoserine antibody or, alternatively, immunoprecipitated with antiphosphoserine followed by probing with anti-Runx2 antibody. Cells transfected with WT Runx2 displayed a strong Runx2-associated P-serine signal that was further increased by Mek(sp). S301A or S319A mutations each reduced the P-serine signal to a similar extent, whereas the combined S301A,S319A double mutant displayed even weaker P-serine immunoreactivity. MAPK inhibition (+U0126) greatly reduced the P-serine signal in all groups and eliminated differences between WT and mutant Runx2 as would be expected if Ser301 and Ser319 were both phosphorylated in a MAPK-dependent manner. However, the fact that the double mutant still displayed a Mek(sp)-dependent increase in P-serine indicates that Runx2 contains additional direct or indirect MAPK phosphorylation sites, in agreement with our MS data.
In summary, multiple phosphorylation sites were identified in Runx2 including two sites in the aa 286–330 region. Definitive identification of Ser319 as a direct ERK substrate was established using a combination of in vitro peptide phosphorylation, MS/MS, and electrophoresis mobility shift analysis. Strong evidence was also obtained that Ser301 is phosphorylated by ERK (in vitro peptide phosphorylation, electrophoresis mobility shift analysis, and Ser(P)/Runx2 co-precipitation). However, it was not possible to detect peptide fragments containing the Ser301 region in MS/MS, precluding its identification using this approach.
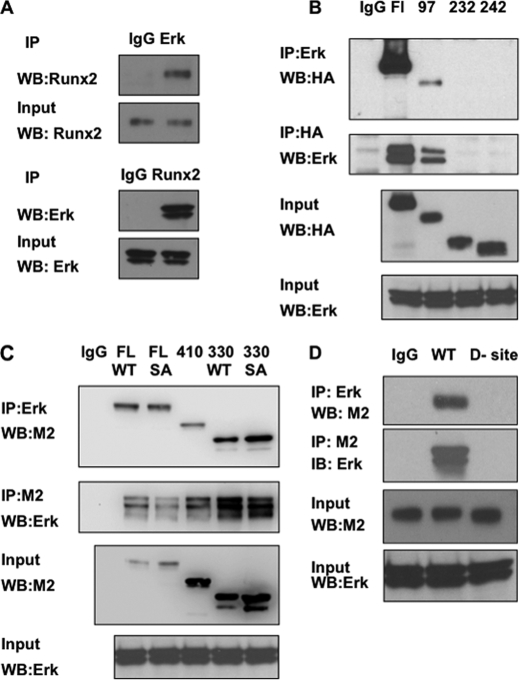
Identification of an ERK Binding Region in Runx2
Consistent with the concept that Runx2 is an ERK/MAPK substrate, ERK was shown to bind Runx2 in co-immunoprecipitation assays (Fig. 4). ERK-Runx2 complexes were detected in nuclear extracts from MC-4 cells that have high endogenous levels of both proteins (panel A) as well as in COS7 cells transfected with affinity-tagged Runx2 (panels B–D). To identify the Runx2 region responsible for ERK binding, COS7 cells were transfected with wild type HA-tagged Runx2, Runx2 with S301A,S319A mutations, or Runx2 with several N-terminal deletions and interactions with endogenous ERK1/2 were examined. Cell lysates were immunoprecipitated with an anti-ERK1/2 antibody and precipitates were probed on Western blots with an anti-HA antibody (Fig. 4B, top). Wild type Runx2 was clearly coprecipitated with the anti-ERK antibody. Of the N-terminal deletions examined, only the Δ97 fragment was precipitated, whereas Δ232 and 242 deletions failed to interact with ERK. The reciprocal experiment (immunoprecipitation with HA antibody and probing blots with anti-ERK antibody, Fig. 4B, second panel from top) gave equivalent results. Similarly, when C-terminal deletions were examined using FLAG-tagged Runx2 (Fig. 4C), Runx2-ERK binding was retained down to and including the Δ330 deletion. This indicates that the ERK binding site is in the runt domain of Runx2. Interestingly, the S301A,S319A mutant Runx2 bound ERK normally, indicating that this interaction does not require intact phosphorylation sites.
FIGURE 4.
Association of Runx2 with ERK. A, co-immunoprecipitation of endogenous Runx2 and ERK. MC-4 cell nuclear extracts were immunoprecipitated with IgG, ERK, or Runx2 antibodies and blots were probed as indicated. B–D, identification of the ERK binding domain in Runx2. Wild type (WT) Runx2 or the indicated N-terminal (HA-tagged Runx2, B) or C-terminal Runx2 deletions (FLAG-tagged Runx2, C) were expressed in COS7 cells and immunoprecipitated (IP) with the indicated antibodies. Blots were then probed for Runx2 (HA or M2 antibodies) or total ERK. Panel C also shows immunoprecipitation results using either full-length or the 1–330 truncated Runx2 containing either wild type sequence (WT) or the S301A,S319A double mutation (SA). Panel D compares immunoprecipitation activity of WT Runx2 with an internal deletion containing a consensus ERK-binding D site (amino acid residues 201–215).
Inspection of the runt domain region of Runx2 revealed that it contains a consensus ERK docking “D” site (GKSFTLTITVFTNPP) at aa 201–215 (37). To confirm its role in ERK complex formation, this region of Runx2 was deleted, resulting in complete loss of ERK binding (Fig. 4D). The D site deletion also almost completely blocked the ability of Mek(sp) to stimulate Runx2-dependent transcription of a 6OSE2-luc reporter (supplemental Fig. S1).
Functional Analysis of Phosphorylation Sites
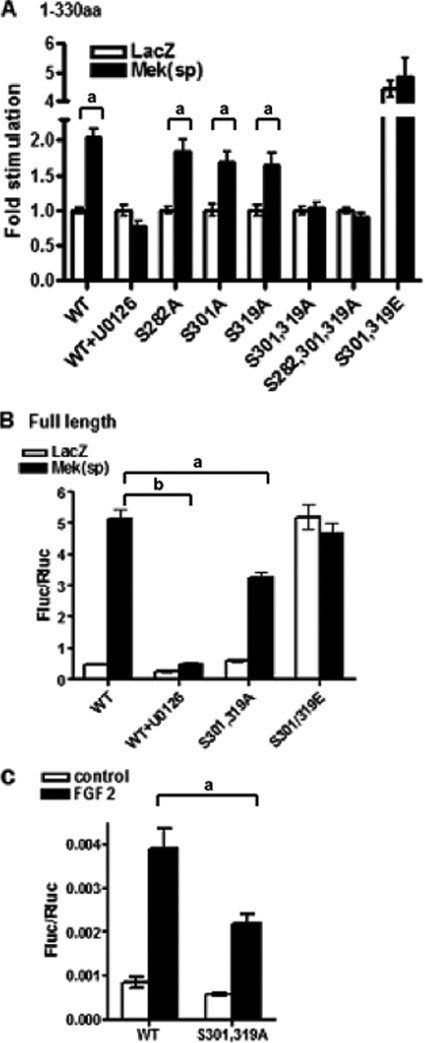
We initially assessed the functionality of the above phosphorylation sites in the context of the Runx2-(1–330) fragment because this contained the minimal sequence for MAPK responsiveness. Ser/Ala mutants described above or Ser/Glu mutations were generated at each site individually or in combination, and wild type or mutated expression plasmids were transfected into COS7 cells with 6OSE2-luc +/−Mek(sp). As shown in Fig. 5A, the ability of Mek(sp) to stimulate transcriptional activity of Runx2-(1–330) was totally blocked with the MEK1/2 inhibitor, U0126. However, individual Ser/Ala mutations at residues 301 or 319 only slightly inhibited MAPK stimulation, whereas the S282A mutation was without effect. On the other hand, introduction of a S301A,S319A double mutation completely eliminated MAPK responsiveness. In contrast, the S301E,S319E mutant exhibited high basal transcriptional activity in the absence of MAPK stimulation that was not affected by Mek(sp). This is consistent with previous studies showing that addition of the charged amino acid is able to mimic a phosphorylated serine residue (38).
FIGURE 5.
Functional analysis of Runx2 phosphorylation sites. A, identification of phosphorylation sites necessary for MAPK responsiveness using Runx2-(1–330). Specific mutations were created in Runx2-(1–330) to generate S282A, S301A, or S319A mutants or the indicated combinations as well as an S301E,S319E mutant. Runx2 expression plasmids were transfected into COS7 cells in the presence or absence of Mek(sp) vector and luciferase reporters as described in the legend to Fig. 1. B, evaluation of requirement for Ser301 and Ser319 sites in the context of full-length Runx2 protein. S301A,S319A or S301E,S319E mutations were generated in full-length Runx2 and evaluated for Mek(sp) responsiveness as in panel A. C, FGF2-responsiveness of wild type (WT) and mutant Runx2. COS7 cells were transfected with wild type full-length Runx2 or the S301A,S319A mutant. After 24 h, cells were treated for an additional 24 h with FGF2 (50 ηg/ml) before luciferase activity was measured. Statistically significant differences are indicated: a, p < 0.05; b, p < 0.01. Error bars, ±S.D.
To evaluate the role of phosphorylation sites in the context of the native Runx2 protein, S301A,S319A or S301E,S319E mutations were also introduced into full-length Runx2 and evaluated for Mek(sp) (Fig. 5B) or FGF2 responsiveness (Fig. 5C) using the same 6OSE2-luc reporter used above. As was the case with Runx2-(1–330), the Mek(sp)-dependent induction of luciferase activity was blocked with U0126, whereas the S301E,S319E mutation resulted in constitutive activation of reporter activity that was not further stimulated by Mek(sp). In contrast to the result obtained with Runx2-(1–330), S301A,S319A mutations only partially blocked the MAPK response (approximately 40% inhibition).
As noted above, FGF2 induction of Ocn expression also requires ERK/MAPK activity and is associated with Runx2 phosphorylation (13). A preliminary deletion analysis (not shown) also indicated that FGF2 responsiveness was lost after deletion of the aa 286–330 Runx2 region. To evaluate whether Ser301 and Ser319 are required for the FGF2 response, wild type or S301A,S319A mutant Runx2 were transfected into COS7 cells together with 6OSE2-luc, and luciferase activity was measured after a 24-h treatment with FGF2. Consistent with our previous report (13), FGF2 stimulated 6OSE2-luc activity in a Runx2-dependent manner. However, growth factor activity was significantly reduced, although not eliminated, in cells transfected with the S301A,S319A mutant (Fig. 5C). Taken together, studies with full-length Runx2 indicate that Ser301 and Ser319 phosphorylation sites are important for MAPK responsiveness although more distal sites outside the 1–330 region may also be needed for full transcriptional activation.
Requirement for Ser301 and Ser319 Phosphorylation Sites for Osteoblast-specific Gene Expression and Differentiation
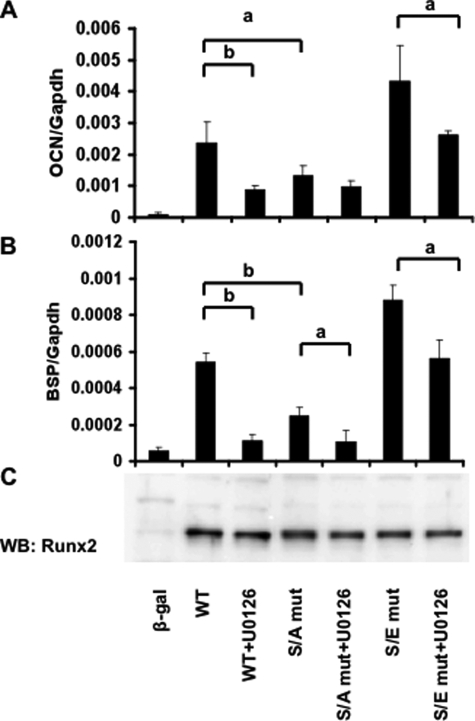
The studies shown in Fig. 5 demonstrated that S301A,S319A mutations in Runx2-(1–330) completely blocked Mek(sp)-dependent activation of transcriptional activity, whereas the same mutations in the context of the full-length Runx2 molecule only partially eliminated MAPK responsiveness. Because results from transcription assays using constitutively active kinases and artificial reporter constructs often do not mimic those obtained with endogenous genes, we considered it important to show that these mutations also reduce the ability of Runx2 to stimulate endogenous osteoblast gene expression and differentiation. Two approaches were taken to address this issue. In the first, mTERT-immortalized calvarial cells from Runx2−/− mice (34) were transfected with wild type Runx2, S301A,S319A, or S301E,S319E mutants (Fig. 6). After 5 days of growth in ascorbic acid-containing medium to stimulate ECM-dependent differentiation, total RNA was isolated and levels of osteocalcin (A) and bone sialoprotein mRNAs (B) were measured by quantitative reverse transcription-PCR. As expected, wild type Runx2 transfection strongly induced both mRNAs and this induction was suppressed by the MAPK inhibitor, U0126. In contrast, the S301A,S319A mutant only weakly stimulated mRNA expression and its activity was resistant to further inhibition by U0126. The S301E,S319E mutant, on the other hand, induced Ocn and Bsp mRNAs to higher levels than those obtained with wild type Runx2 via a mechanism that was largely resistant to MAPK inhibition. As shown in panel C, these results cannot be explained by differences in expression levels of wild type and mutant Runx2 proteins.
FIGURE 6.
Induction of osteoblast differentiation markers by wild type (WT) Runx2 and phosphorylation site mutants. An mTERT-immortalized cell line derived from Runx2−/− calvaria was transfected with wild type and mutant Runx2 expression vectors and grown for 5 days in ascorbic acid-containing medium before measurement of Ocn (A) and Bsp (B) mRNAs by real-time reverse transcription-PCR. Indicated samples were also treated with U0126 12 h before harvest. The mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA in each sample. Runx2 protein in each group was measured on Western blots (WB) (C). S/A mut, S301A,S319A mutant; S/E mut, S301E,S319E mutant. Statistically significant differences are indicated: a, p < 0.05; b, p < 0.01. β-gal, β-galactosidase. Error bars, ±S.D.
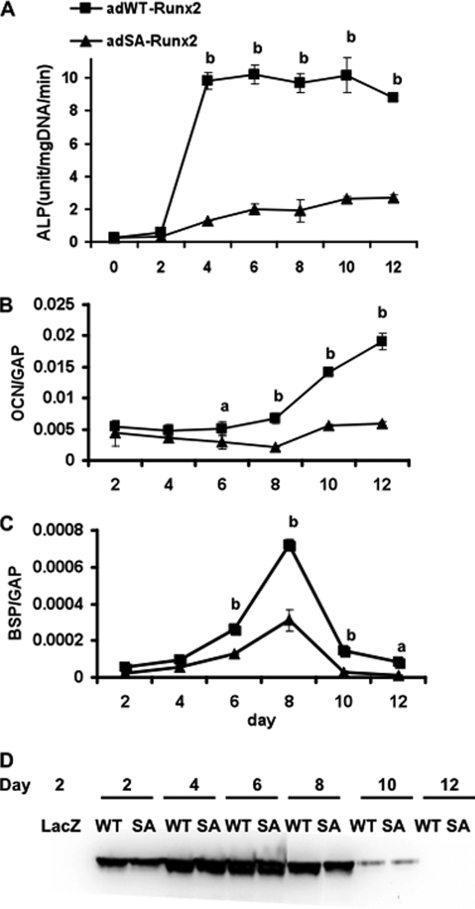
To examine the requirement for Runx2 phosphorylation sites in osteoblast differentiation over a more prolonged time period, we used an adenovirus expression system in C3H10T1/2 cells. This mesenchymal cell line contains no detectable endogenous Runx2, but will undergo osteoblast differentiation after transduction with an adeno-Runx2 expression vector. Previous studies showed that AdRunx2 vectors continue to produce active transcription factors for at least 7–10 days in this system (36). Wild type and S301A,S319A Runx2 adenovirus were constructed and titered in C3H10T1/2 cells to produce equivalent amounts of Runx2 protein as measured on Western blots. As shown in Fig. 7, wild type Runx2 clearly induced osteoblast differentiation in this system. Sustained induction of alkaline phosphatase activity (panel A) as well as Ocn and Bsp mRNAs (panels B and C) was observed over a 12-day period. In contrast, cells expressing the Ser/Ala mutant had less than 25% the alkaline phosphatase activity of wild type at all times examined. Induction of Ocn and Bsp mRNAs was similarly attenuated. Western blot results revealed little or no differences in Runx2 protein levels in the two groups at all times examined, making it unlikely that differences in Runx2 expression or stability could explain these results.
FIGURE 7.
Induction of osteoblast differentiation by wild type Runx2 and the S301A,S319A phosphorylation site mutant. Adenovirus expression vectors containing wild type and S301A,S319A Runx2 mutants (SA) were used to transduce C3H10T1/2 mesenchymal cells. Cells were then grown in differentiation medium for the indicated times before measurement of alkaline phosphatase activity (ALP; A) or Ocn/Bsp mRNA levels (OCN and BSP; normalized to glyceraldehyde-3-phosphate dehydrogenase (GAP); C and D). Runx2 protein in each sample was measured on Western blots (D). Statistically significant differences are indicated: a, p < 0.05; b, p < 0.01. SA, S301A,S319A mutant. Error bars, ±S.D.
DISCUSSION
In this study, we identified two phosphorylation sites in Runx2 at Ser301 and Ser319 that are required for MAPK-dependent activation of Runx2 transcriptional activity and osteoblast differentiation. These sites are phosphorylated in a MAPK-dependent manner in intact cells. As shown by MS/MS analysis, at least one, Ser319, is a direct ERK1 substrate. Furthermore, Runx2 can bind ERK using a D site between amino acids 201 and 215 in the runt domain region. Consistent with Ser301 and Ser319 being important for osteoblast function, inactivating Ser to Ala mutations greatly reduced the ability of Runx2 to stimulate expression of Ocn and Bsp mRNAs in Runx2−/− calvarial cells and blocked Runx2-dependent induction of osteoblast gene expression and differentiation in a mesenchymal cell line. In contrast, Ser to Glu mutations, which mimic the charge density of phosphorylated amino acids, activated Runx2-dependent transcription. Taken together, these studies emphasize the importance of MAPK-dependent phosphorylation as a means of controlling Runx2 transcriptional activity in bone.
As one of the major signal transduction pathways in bone, the ERK/MAPK pathway is able to integrate stimuli from growth/differentiation factor binding to receptor tyrosine kinases (39), ECM-integrin binding and focal adhesion kinase activation (40), certain non-genomic actions of estrogens (41), and mechanical stimulation mediated by FAK activation (42) and connexin 43 up-regulation (43). It also has important functions in the differentiation of post-mitotic mesenchymal and neuronal cells (44, 45) and regulates the activity of several tissue-specific transcription factors including MyoD (muscle (46)), Sox9 (cartilage (47)) and peroxisome proliferator-activated receptor γ (adipose (48)). Furthermore, as previously shown by this laboratory, in vivo transgenic stimulation of ERK/MAPK signaling in osteoblasts accelerates bone development and is able to partially rescue the cleidocranial dysplasia phenotype of Runx2 haploinsufficient mice (14). Based on this work, it is likely that the phosphorylation sites we identified at Ser301 and Ser319 are critical for regulating Runx2 activity in vivo and may function to integrate the osteogenic response to hormonal, mechanical, and environmental stimuli.
Although Ser301 and Ser319 are clearly very important for Runx2 transcriptional activity, it is possible that other sites also participate in the MAPK response. Specifically, progressively larger C-terminal deletions of Runx2 gradually reduced MAPK-dependent activation of the 6OSE2-luc reporter gene, with the truncated Runx2-(1–330) being the minimal Runx2 truncation that still retained MAPK responsiveness (Fig. 1). It is, therefore, possible that phosphorylation sites in the region between residues 330 and 528 may also participate in the MAPK response, perhaps functioning as secondary sites after priming phosphorylations occur at Ser301 and Ser319. Consistent with this idea, our MS/MS analysis, which identified peptides containing all proline-directed serines in Runx2 except Ser301, detected two additional direct MAPK phosphorylation sites at Ser43 and Ser510 as well as an indirect MAPK site at Ser282. Also, the S301A,S319A mutant Runx2 still retained a MAPK-dependent increase in total P-serine on Western blots as would be expected if additional sites were present (Fig. 3). However, the observation that the high constitutive activity of the S301E,S319E Runx2 mutant was refractory to MAPK inhibition (Fig. 6) argues against these other sites being major contributors to the MAPK response because inhibiting their phosphorylation was not able to prevent the transcriptional activation seen when 301 and 319 sites were in an activated state.
MAPK-dependent phosphorylation of Runx2 is also clearly required for FGF2-dependent induction of Ocn expression (13). However, S301A,S319A mutations only partially blocked FGF2 activation of the 6OSE2-luc reporter (Fig. 5C). This partial inhibition may be explained by the involvement of additional MAPK sites as well as other kinase sites. In this regard, a recent study by Kim and co-workers (49) reported that FGF2 also activates Runx2 via phosphorylation by PKCγ at Ser247. Thus, it is possible that FGF2 activates Runx2 transcriptional activity by phosphorylating both ERK/MAPK and PKC sites.
Runx family members exhibit a high degree of amino acid sequence conservation, particularly in the DNA binding or Runt domain. Although the C-terminal Pro/Ser/Thr domain of Runx proteins is not highly conserved, the two phosphorylation sites we identified in Runx2 are also present in Runx1 (but not in Runx3). Interestingly, EGF and phorbol ester activation of the ERK/MAPK pathway can stimulate Runx1 transcriptional activity via phosphorylation on these sites (50, 51). Runx1 is essential for hematopoietic cell differentiation (52) and is also a frequent site for chromosomal translocations in acute myelogenous leukemia. Consistent with this oncogenic activity, transfection of Runx1 into fibroblasts stimulates anchorage-independent growth and transformation. Interestingly, Ser/Ala mutations at Ser249 and Ser266 in Runx1 (equivalent to Ser301 and Ser319 in Runx2) inhibit growth of NIH 3T3 fibroblasts in soft agar, a common assay for cell transformation (50). Runx2 can also function as an oncogene under certain conditions and has been associated with cell proliferation and migration of breast cancer cells (53, 54). It is, therefore, possible that ERK/MAPK-dependent phosphorylation of Runx2 at Ser301 and Ser319 could also be associated with this metastasis-related behavior.
It is not presently understood how phosphorylation of Runx2 stimulates transcription. We and others previously observed that the apparent affinity of Runx2 for OSE2-containing DNA increases with differentiation and this increase can be blocked with MAPK inhibition (12, 26, 55). However, it is not known if this is a direct consequence of Runx2 phosphorylation. Interestingly, using chromatin immunoprecipitation assays, we find Runx2 associated with Ocn and Bsp chromatin in both differentiated and undifferentiated MC3T3-E1 cells even in the presence of MAPK inhibition (56). Thus, Runx2 does not dissociate from its binding sites on chromatin even though its in vitro affinity for DNA may be lower in the unphosphorylated state. Runx2 is also known to serve as a docking site for many nuclear factors that can form active or inactive transcription complexes on chromatin (57). In this regard, we recently showed that the physical association of Runx2 with ERK reported in the present study can also be detected on the chromatin of Runx2 target genes in vivo (56). In this case, P-ERK binding to Ocn and Bsp chromatin required Runx2 and intact Runx2 binding sites in the DNA. Furthermore, this binding was dependent on the elevated MAPK activity associated with osteoblast differentiation. Runx2 can therefore be viewed as providing a docking site for P-ERK on the chromatin of target genes. In addition to phosphorylating Runx2, chromatin-bound P-ERK may also initiate subsequent events such as phosphorylation of other chromatin substrates or the recruitment of additional factors including histone acetyltransferases like p300/cAMP-response element-binding protein to modify chromatin structure, thereby allowing the initiation of transcription. Interestingly, the ERK/ MAPK-dependent phosphorylation of Runx1 discussed above is associated with the dissociation of the histone deacetylase co-factor, mSin3a, from Runx1, thereby allowing subsequent increases in histone acetylation (58). Because Runx1 phosphorylation sites are conserved in Runx2, this observation provides a plausible mechanism for how ERK/MAPK phosphorylation could alter Runx2-dependent transcription. This possibility is currently being pursued by this laboratory. Last, Afzal and co-workers (59) showed that MAPK-mediated phosphorylation of Runx2 is also necessary for complex formation with Smads.
In addition to the ERK/MAPK-dependent regulation of Runx2 described herein, several other types of post-translational modifications have been described for this molecule. Phenylthiohydantoin/protein kinase A-mediated phosphorylation of a C-terminal Runx2 site was correlated with induction of MMP13 (60). More recently, Cdk4-mediated phosphorylation at Ser472 was shown to target Runx2 for ubiquitination and proteosomal degradation during the cell cycle (61), whereas cdc2 phosphorylation at Ser451 was shown to be necessary for cell cycle progression of endothelial cells (62). Also, glycogen synthase kinase 3β-dependent phosphorylation of Runx2 at Ser369-Ser373-Ser377 was shown to reduce transcriptional activity (63). Last, Runx2 can be acetylated on critical lysine residues by p300 acetyltransferase. This modification, which is stimulated by BMP2, increases transcription and stabilizes Runx2 against proteosomal degradation (64). Thus, post-translational modification appears to be a common mechanism for regulating Runx2 activity and stability.
In summary, phosphorylation of Runx2 at Ser301 and Ser319 clearly has an important regulatory role in Runx2-dependent transcription because mutation of these sites in the context of the intact Runx2 molecule severely attenuated the ability of Runx2 to stimulate osteoblast-specific gene expression during differentiation. Ongoing in vivo studies will be necessary to assess the full impact of Runx2 phosphorylation to the overall activity of this molecule during skeletal development and remodeling.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants DE11723 and DE12211 (to R. T. F.) and Grant DK072230 and Department of Defense Grant W81XWH-07-1-0160 (to G. X.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ERK
- extracellular signal-regulated kinase
- MAPK
- mitogen-activated protein kinase
- ECM
- extracellular matrix
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- OSE2
- osteoblast-specific element 2
- Mek(sp)
- constitutively active MEK1 mutant
- FGF
- fibroblast growth factor
- HA
- hemagglutinin
- aa
- amino acid
- FBS
- fetal bovine serum
- MS
- mass spectrometry.
REFERENCES
- 1.Ducy P., Zhang R., Geoffroy V., Ridall A. L., Karsenty G. (1997) Cell 89, 747–754 [DOI] [PubMed] [Google Scholar]
- 2.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J. M., Behringer R. R., de Crombrugghe B. (2002) Cell 108, 17–29 [DOI] [PubMed] [Google Scholar]
- 3.Yang X., Matsuda K., Bialek P., Jacquot S., Masuoka H. C., Schinke T., Li L., Brancorsini S., Sassone-Corsi P., Townes T. M., Hanauer A., Karsenty G. (2004) Cell 117, 387–398 [DOI] [PubMed] [Google Scholar]
- 4.Ducy P., Starbuck M., Priemel M., Shen J., Pinero G., Geoffroy V., Amling M., Karsenty G. (1999) Genes Dev. 13, 1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tou L., Quibria N., Alexander J. M. (2003) Mol. Cell Endocrinol. 205, 121–129 [DOI] [PubMed] [Google Scholar]
- 6.Xiao G., Jiang D., Thomas P., Benson M. D., Guan K., Karsenty G., Franceschi R. T. (2000) J. Biol. Chem. 275, 4453–4459 [DOI] [PubMed] [Google Scholar]
- 7.Xiao G., Wang D., Benson M. D., Karsenty G., Franceschi R. T. (1998) J. Biol. Chem. 273, 32988–32994 [DOI] [PubMed] [Google Scholar]
- 8.Franceschi R. T., Iyer B. S. (1992) J. Bone Miner. Res. 7, 235–246 [DOI] [PubMed] [Google Scholar]
- 9.Franceschi R. T., Iyer B. S., Cui Y. (1994) J. Bone Miner. Res. 9, 843–854 [DOI] [PubMed] [Google Scholar]
- 10.Franceschi R. T., Ge C., Xiao G., Roca H., Jiang D. (2007) Ann. N. Y. Acad. Sci. 1116, 196–207 [DOI] [PubMed] [Google Scholar]
- 11.Xiao G., Gopalakrishnan R., Jiang D., Reith E., Benson M. D., Franceschi R. T. (2002) J. Bone Miner. Res. 17, 101–110 [DOI] [PubMed] [Google Scholar]
- 12.Xiao G., Cui Y., Ducy P., Karsenty G., Franceschi R. T. (1997) Mol. Endocrinol. 11, 1103–1113 [DOI] [PubMed] [Google Scholar]
- 13.Xiao G., Jiang D., Gopalakrishnan R., Franceschi R. T. (2002) J. Biol. Chem. 277, 36181–36187 [DOI] [PubMed] [Google Scholar]
- 14.Ge C., Xiao G., Jiang D., Franceschi R. T. (2007) J. Cell Biol. 176, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman D., Jin F., Leboy P., Hardy S., Damsky C. (2000) Dev. Biol. 220, 2–15 [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi Y., Nakayama K., Matsumoto T. (1996) J. Biol. Chem. 271, 3938–3944 [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi Y., Suzawa M., Kikuchi T., Nishida E., Fujita T., Matsumoto T. (1997) J. Biol. Chem. 272, 29309–29316 [DOI] [PubMed] [Google Scholar]
- 18.Jikko A., Harris S. E., Chen D., Mendrick D. L., Damsky C. H. (1999) J. Bone Miner. Res. 14, 1075–1083 [DOI] [PubMed] [Google Scholar]
- 19.Lai C. F., Chaudhary L., Fausto A., Halstead L. R., Ory D. S., Avioli L. V., Cheng S. L. (2001) J. Biol. Chem. 276, 14443–14450 [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal R. K., Jaiswal N., Bruder S. P., Mbalaviele G., Marshak D. R., Pittenger M. F. (2000) J. Biol. Chem. 275, 9645–9652 [DOI] [PubMed] [Google Scholar]
- 21.Shui C., Spelsberg T. C., Riggs B. L., Khosla S. (2003) J. Bone Miner. Res. 18, 213–221 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt C., Pommerenke H., Dürr F., Nebe B., Rychly J. (1998) J. Biol. Chem. 273, 5081–5085 [DOI] [PubMed] [Google Scholar]
- 23.Pavalko F. M., Chen N. X., Turner C. H., Burr D. B., Atkinson S., Hsieh Y. F., Qiu J., Duncan R. L. (1998) Am. J. Physiol. Cell Physiol. 275, C1591–C1601 [PubMed] [Google Scholar]
- 24.Ziros P. G., Gil A. P., Georgakopoulos T., Habeos I., Kletsas D., Basdra E. K., Papavassiliou A. G. (2002) J. Biol. Chem. 277, 23934–23941 [DOI] [PubMed] [Google Scholar]
- 25.Zayzafoon M., Abdulkadir S. A., McDonald J. M. (2004) J. Biol. Chem. 279, 3662–3670 [DOI] [PubMed] [Google Scholar]
- 26.Qiao M., Shapiro P., Kumar R., Passaniti A. (2004) J. Biol. Chem. 279, 42709–42718 [DOI] [PubMed] [Google Scholar]
- 27.Celil A. B., Hollinger J. O., Campbell P. G. (2005) J. Cell. Biochem. 95, 518–528 [DOI] [PubMed] [Google Scholar]
- 28.Celil A. B., Campbell P. G. (2005) J. Biol. Chem. 280, 31353–31359 [DOI] [PubMed] [Google Scholar]
- 29.Ducy P., Karsenty G. (1995) Mol. Cell. Biol. 15, 1858–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thirunavukkarasu K., Mahajan M., McLarren K. W., Stifani S., Karsenty G. (1998) Mol. Cell. Biol. 18, 4197–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng C. F., Guan K. L. (1993) J. Biol. Chem. 268, 23933–23939 [PubMed] [Google Scholar]
- 32.de Boer E., Rodriguez P., Bonte E., Krijgsveld J., Katsantoni E., Heck A., Grosveld F., Strouboulis J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7480–7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy S., Kitamura M., Harris-Stansil T., Dai Y., Phipps M. L. (1997) J. Virol. 71, 1842–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae J. S., Gutierrez S., Narla R., Pratap J., Devados R., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B., Javed A. (2007) J. Cell. Biochem. 100, 434–449 [DOI] [PubMed] [Google Scholar]
- 35.Wang D., Christensen K., Chawla K., Xiao G., Krebsbach P. H., Franceschi R. T. (1999) J. Bone Miner. Res. 14, 893–903 [DOI] [PubMed] [Google Scholar]
- 36.Yang S., Wei D., Wang D., Phimphilai M., Krebsbach P. H., Franceschi R. T. (2003) J. Bone Miner. Res. 18, 705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akella R., Moon T. M., Goldsmith E. J. (2008) Biochim. Biophys. Acta 1784, 48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansour S. J., Matten W. T., Hermann A. S., Candia J. M., Rong S., Fukasawa K., Vande Woude G. F., Ahn N. G. (1994) Science 265, 966–970 [DOI] [PubMed] [Google Scholar]
- 39.Cobb M. H., Boulton T. G., Robbins D. J. (1991) Cell Regul. 2, 965–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franceschi R. T., Xiao G. (2003) J. Cell. Biochem. 88, 446–454 [DOI] [PubMed] [Google Scholar]
- 41.Kousteni S., Han L., Chen J. R., Almeida M., Plotkin L. I., Bellido T., Manolagas S. C. (2003) J. Clin. Invest. 111, 1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moalli M. R., Wang S., Caldwell N. J., Patil P. V., Maynard C. R. (2001) J. Appl. Physiol. 91, 912–918 [DOI] [PubMed] [Google Scholar]
- 43.Lecanda F., Warlow P. M., Sheikh S., Furlan F., Steinberg T. H., Civitelli R. (2000) J. Cell Biol. 151, 931–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Y., Li W., Wu J., Germann U. A., Su M. S., Kuida K., Boucher D. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12759–12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kao S., Jaiswal R. K., Kolch W., Landreth G. E. (2001) J. Biol. Chem. 276, 18169–18177 [DOI] [PubMed] [Google Scholar]
- 46.Zetser A., Frank D., Bengal E. (2001) Dev. Biol. 240, 168–181 [DOI] [PubMed] [Google Scholar]
- 47.Murakami S., Kan M., McKeehan W. L., de Crombrugghe B. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams M., Reginato M. J., Shao D., Lazar M. A., Chatterjee V. K. (1997) J. Biol. Chem. 272, 5128–5132 [DOI] [PubMed] [Google Scholar]
- 49.Kim B. G., Kim H. J., Park H. J., Kim Y. J., Yoon W. J., Lee S. J., Ryoo H. M., Cho J. Y. (2006) Proteomics 6, 1166–1174 [DOI] [PubMed] [Google Scholar]
- 50.Tanaka T., Kurokawa M., Ueki K., Tanaka K., Imai Y., Mitani K., Okazaki K., Sagata N., Yazaki Y., Shibata Y., Kadowaki T., Hirai H. (1996) Mol. Cell. Biol. 16, 3967–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y., Biggs J. R., Kraft A. S. (2004) J. Biol. Chem. 279, 53116–53125 [DOI] [PubMed] [Google Scholar]
- 52.Tanaka T., Tanaka K., Ogawa S., Kurokawa M., Mitani K., Nishida J., Shibata Y., Yazaki Y., Hirai H. (1995) EMBO J. 14, 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnes G. L., Hebert K. E., Kamal M., Javed A., Einhorn T. A., Lian J. B., Stein G. S., Gerstenfeld L. C. (2004) Cancer Res. 64, 4506–4513 [DOI] [PubMed] [Google Scholar]
- 54.Pratap J., Lian J. B., Javed A., Barnes G. L., van Wijnen A. J., Stein J. L., Stein G. S. (2006) Cancer Metastasis Rev. 25, 589–600 [DOI] [PubMed] [Google Scholar]
- 55.Fujita T., Azuma Y., Fukuyama R., Hattori Y., Yoshida C., Koida M., Ogita K., Komori T. (2004) J. Cell Biol. 166, 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Ge C., Franceschi R. (2009) J. Bone Miner. Res., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lian J. B., Javed A., Zaidi S. K., Lengner C., Montecino M., van Wijnen A. J., Stein J. L., Stein G. S. ( 2004) Crit. Rev. Eukaryot. Gene Expr. 14, 1– 41 [PubMed] [Google Scholar]
- 58.Imai Y., Kurokawa M., Yamaguchi Y., Izutsu K., Nitta E., Mitani K., Satake M., Noda T., Ito Y., Hirai H. (2004) Mol. Cell. Biol. 24, 1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Afzal F., Pratap J., Ito K., Ito Y., Stein J. L., van Wijnen A. J., Stein G. S., Lian J. B., Javed A. (2005) J. Cell. Physiol. 204, 63–72 [DOI] [PubMed] [Google Scholar]
- 60.Selvamurugan N., Pulumati M. R., Tyson D. R., Partridge N. C. (2000) J. Biol. Chem. 275, 5037–5042 [DOI] [PubMed] [Google Scholar]
- 61.Shen R., Wang X., Drissi H., Liu F., O'Keefe R. J., Chen D. (2006) J. Biol. Chem. 281, 16347–16353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiao M., Shapiro P., Fosbrink M., Rus H., Kumar R., Passaniti A. (2006) J. Biol. Chem. 281, 7118–7128 [DOI] [PubMed] [Google Scholar]
- 63.Kugimiya F., Kawaguchi H., Ohba S., Kawamura N., Hirata M., Chikuda H., Azuma Y., Woodgett J. R., Nakamura K., Chung U. I. (2007) PLoS ONE 2, e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeon E. J., Lee K. Y., Choi N. S., Lee M. H., Kim H. N., Jin Y. H., Ryoo H. M., Choi J. Y., Yoshida M., Nishino N., Oh B. C., Lee K. S., Lee Y. H., Bae S. C. ( 2006) J. Biol. Chem. 281, 16502– 16511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.