Abstract
Transient receptor potential vanilloid type 1 (TRPV1) is a plasma membrane Ca2+ channel involved in transduction of painful stimuli. Dorsal root ganglion (DRG) neurons express ectopic but functional TRPV1 channels in the endoplasmic reticulum (ER) (TRPV1ER). We have studied the properties of TRPV1ER in DRG neurons and HEK293T cells expressing TRPV1. Activation of TRPV1ER with capsaicin or other vanilloids produced an increase of cytosolic Ca2+ due to Ca2+ release from the ER. The decrease of [Ca2+]ER was directly revealed by an ER-targeted aequorin Ca2+ probe, expressed in DRG neurons using a herpes amplicon virus. The sensitivity of TRPV1ER to capsaicin was smaller than the sensitivity of the plasma membrane TRPV1 channels. The low affinity of TRPV1ER was not related to protein kinase A- or C-mediated phosphorylations, but it was due to inactivation by cytosolic Ca2+ because the sensitivity to capsaicin was increased by loading the cells with the Ca2+ chelator BAPTA. Decreasing [Ca2+]ER did not affect the sensitivity of TRPV1ER to capsaicin. Disruption of the TRPV1 calmodulin-binding domains at either the C terminus (Δ35AA) or the N terminus (K155A) increased 10-fold the affinity of TRPV1ER for capsaicin, suggesting that calmodulin is involved in the inactivation. The lack of TRPV1 sensitizers, such as phosphatylinositol 4,5-bisphosphate, in the ER could contribute to decrease the affinity for capsaicin. The low sensitivity of TRPV1ER to agonists may be critical for neuron health, because otherwise Ca2+ depletion of ER could lead to ER stress, unfolding protein response, and cell death.
Introduction
TRPV1 (transient receptor potential vanilloid type 1) belongs to the transient receptor potential family of cation channels and is responsible for detection of painful stimuli (1). Neurons from several sensory ganglia, including dorsal root ganglion (DRG),4 express TRPV1. Activation of TRPV1 produces entry of Na+ and Ca2+ and membrane depolarization. TRPV1 can be selectively activated with capsaicin (2) and is blocked by micromolar concentrations of ruthenium red (3). Like other Ca2+ channels, TRPV1 is subjected to inactivation by Ca2+ (4). Calmodulin (CaM) seems to be involved in this process through two CaM binding sites located one at the C-terminal (5) and the other at the ankyrin repeat domain in the N-terminal end of the protein (6, 7). Deletion of these sites relieves TRPV1 desensitization and tachyphylaxis. Other factors that can modulate the activity of TRPV1 are changes of pH or temperature, inflammatory mediators, and phosphorylation (8). These modulators may be involved in sensitization to pain.
A large part of TRPV1 naturally expressed in DRG neurons locates in endomembranes rather than in the plasma membrane (9–12), and a similar situation has been reported for other TRP channels. The function of these endomembrane channels is not known, although it has been speculated that they may represent a reserve pool that could be rapidly mobilized to the plasma membrane when required (13). In this direction, it has been reported that activation of protein kinase C (14), nerve growth factor-induced phosphorylation via Src kinase (15) or coupling with phosphoinositide 3-kinase (16) promotes insertion of ER-located TRPV1 channels (TRPV1ER) into the plasma membrane (TRPV1PM). This mobilization could be the basis of inflammatory sensitization and hyperalgesia.
On the other hand, previous work has shown that TRPV1ER channels are functional (9–12, 17) and that its activation leads to alterations of ER and mitochondria, followed by cell death (9, 18). Cell death due to ER stress following ER Ca2+ emptying by TRPV1ER stimulation has also been documented in human lung cells (19). Transfection of HEK293T cells with TRPV1 reproduces the neuronal model with expression of functional TRPV1ER and TRPV1PM channels (9, 16, 18, 20, 21).
In all of the previous studies, the effects of TRPV1 on [Ca2+]ER were inferred from the changes of the cytosolic Ca2+ concentration ([Ca2+]C). We can now monitor directly [Ca2+]ER in living cells using ER-targeted aequorins (22–24). Here we have studied in detail the release of Ca2+ from ER induced by activation of TRPV1 in DRG neurons and in HEK293T cells expressing TRPV1 channels.
EXPERIMENTAL PROCEDURES
Plasmids
The original rat TRPV1 plasmid, cloned in pcDNA3, was a generous gift from Dr. D. Julius. For tracing the subcellular distribution of TRPV1, the plasmid containing the green fluorescent protein-TRPV1 fusion gene was used (pEF-GFP-TRPV1) (25). The following TRPV1 mutants were generated by a QuikChange site-directed mutagenesis kit (Stratagene), and all clones were verified by sequencing. The N604S mutation (26), which is unglycosylated, was introduced using the following forward oligonucleotide: 5′-ATTGAGGATGGGAAGAGTAACTCTCTGCCTATGG-3′; for the mutant K155A (7), the forward oligonucleotide 5′-AGACCCAGAGACAGGAGCGACCTGTCTGCTAAAAG-3′ was used; for the mutant R181A, the forward oligonucleotide 5′-CTCCTGGACGTTGCCGCGAAGACAGACAGCCTGAAG-3′ was used; and for the mutant lacking amino acids 767–801 near the C terminus (Δ35AA) (5), the forward oligonucleotide 5′-CGAGATAGACATGCCACCCAGCAGGAAGAAGTTC-3′ and the reverse oligonucleotide 5′-ACAGTTGCCTGGGTCCTCGTTGATGATACCCAC-3′ were used. The vector pHSVermutRA containing the red fluorescent protein fused to mutated aequorin was used here as an ER marker (27).
Cell Cultures, Gene Transfection, and Amplicon Infection
HEK293T and HeLa cells (ATCC CRL-11268 and CCL-2, respectively) were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 μg/ml streptomycin, 100 units/ml penicillin, and 5 μg/ml PlasmocinTM (Invivogen) at 37 °C, under an air, 5% CO2 mixture. A stable HEK293 line containing a tetracycline-inducible phosphoinositide-specific inositol polyphosphate 5-phosphatase type IV was used for the experiments with decreased phosphatidylinositol 4,5-bisphosphate (PIP2) levels (28). Induction of 5-phosphatase type IV was achieved by incubation with tetracycline (0.1 μg/ml for 12–20 h).
For aequorin experiments, about 7 × 104 cells were seeded on poly-l-lysine-coated 4-well plates and co-transfected with 0.4 μg of pcDNA3-TRPV1 (wild type or mutants) and 0.1 μg of aequorin cDNA using LipofectamineTM 2000 (Invitrogen). The capsaicin antagonist capsazepine (10 μm) was added after TRPV1 transfection to prevent toxicity. The cells were better preserved in this culture medium. For localization experiments, pEF-GFP-TRPV1 (0.4 μg) and pHSVermutRA (0.1 μg) were cotransfected. All of the experiments were performed 24 h after transfections.
DRG neurons were isolated from neonatal (1–5-day-old) rats, as described previously (29). Briefly, ganglia from lumbar region were removed in Ca2+/Mg2+-free Hanks' balanced salt solution at 4 °C and digested with trypsin-EGTA (0.25%; Invitrogen) and DNase (0.1 mg/ml) for 20 min at 37 °C. Neurons were dissociated from digested ganglia by manual trituration with a fire-polished, silanized glass pipette. Neurons were resuspended in Neurobasal-A medium (Invitrogen) supplemented with 1% B-27TM (Invitrogen), 0.5 mm GlutamaxTM, 50 ng/ml nerve growth factor, 100 μg/ml streptomycin, and 100 units/ml penicillin and plated on 12-mm diameter glass coverslips coated with laminin (10 μg/ml) and poly-d-lysine (100 μg/ml) at a density of 3 × 104 cells/coverslip.
Herpes simplex virus 1 (HSV-1)-based amplicons carrying the ER-targeted low Ca2+ affinity mutated GFP-aequorin were packaged and titrated as described previously (23). For the bioluminescence-measuring experiments, 3 × 104 neurons were seeded on treated coverslips and infected at a multiplicity of infection ranging between 0.01 and 0.5 with the ER-targeted aequorin amplicons (23, 24).
Expression of Fluorescent Proteins and Immunofluorescence
DRG neurons were fixed with 4% paraformaldehyde and blocked with 10% goat serum and 0.5% Triton X-100 in Tris-buffered saline for 20 min. The fixed cells were incubated overnight at 4 °C with the primary antibody diluted in 1% goat serum and 0.5% Triton X-100 in Tris-buffered saline. A polyclonal antibody recognizing the N-terminal end of TRPV1 was used (1:1000 rabbit anti-TRPV1 (Chemicon)). After washing with 10% goat serum and 0.5% Triton X-100 in Tris-buffered saline, the cells were incubated (45 min at room temperature) with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (1:1000; Sigma) in 1% goat serum and 0.5% Triton X-100 in Tris-buffered saline. Nuclei were stained with 4′,6-diamidino-2-phenylindole. Cells were mounted in Vectashield® (Vector) and observed with a C-Apochromat ×63 water immersion objective (numerical aperture 1.20) in a Zeiss Axioplan Z microscope. The Zeiss ApoTomeTM system was used for optical sectioning. Expression of GFP and red fluorescent protein were analyzed by imaging of the green (excitation, 490/20 nm; emission, 540/50 nm) and the red fluorescence (excitation, 560/40 nm; emission, 615/45 nm) in fixed cells. For ER colocalization analysis, living cells were labeled with ER-TrackerTM (Molecular Probes; preloaded by incubation at 400 nm in culture medium for 30 min at 37 °C) and fixed with paraformaldehyde.
Measurements of Aequorin Bioluminescence
GFP-aequorin fusion proteins targeted to the cytosol (cytGA) (27) or to the ER (erGA and ermutGA) were used. Cells expressing cytGA were incubated for 1 h at room temperature with 1 μm coelenterazine in a standard incubation medium with the following composition: 145 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 10 mm glucose, 10 mm sodium-HEPES, pH 7.4. For [Ca2+]ER measurements, cells expressing either ER-targeted wild type GFP-aequorin (erGA) reconstituted with 1 μm coelenterazine or ER-targeted low affinity mutated GFP-aequorin (ermutGA) (30) reconstituted with 1 μm coelenterazine n (22), were incubated for 1 h at room temperature in Ca2+-free medium containing 0.5 mm EGTA and 10 μm sarcoendoplasmic reticulum Ca2+-ATPase inhibitor 2,5-di-tert-butyl-benzohydroquinone (24).
In the experiments with permeabilized cells, perfusion was performed with intracellular-like medium with the following composition: 140 mm KCl, 1 mm KH2PO4, 1 mm MgCl2, 1 mm Mg-ATP, 2 mm sodium succinate, 20 mm sodium-HEPES, pH 7.0, and different Ca2+ concentrations and other additions as detailed below. The cells were perfused at about 5 ml/min with Ca2+-free medium for 3 min and then permeabilized by perfusion with 20 or 60 μm digitonin in Ca2+-free (containing 2 mm EGTA) intracellular-like medium for 1 min. The solution was then switched to intracellular-like medium without digitonin and containing 20 or 100 nm Ca2+, buffered with EGTA (31) for 3–5 min to allow Ca2+ refilling of the ER. Then the effects of different test solutions were investigated by perfusion in intracellular-like medium.
Aequorin photoluminescence was measured as described previously (32), and calibrations in [Ca2+] were done using the constant values published before (32, 33). All of the measurements were performed at 22 °C.
Calcium Imaging
The procedure used was as described previously (34, 35). Briefly, DRGs were loaded with 4 μm fura2-acetoxymethyl ester (Molecular Probes) for 1 h at room temperature in standard incubation medium (see above). Cells were then washed with fresh medium, and the cell-containing coverslips were mounted under the microscope (Nikon Diaphot). Test solutions were applied by continuous perfusion at 2–3 ml/min. For fluorescence measurements, cells were alternately epi-illuminated at 340 and 380 nm, and light emitted above 520 nm was recorded using an extended ISIS-M camera (Photonic Science) and analyzed using an Applied Imaging Magical image processor (Sunderland, Tyne and Wear, UK). Consecutive frames obtained at 340- and 380-nm excitation were ratioed pixel by pixel, and the [Ca2+]C was estimated as described previously (36).
Data are expressed as mean ± S.E. Statistical significance was evaluated by Student's t test.
RESULTS
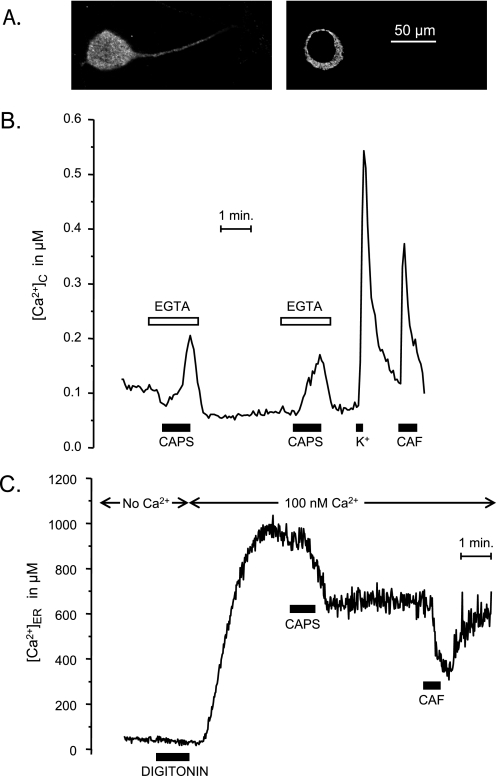
Fig. 1A illustrates the distribution of TRPV1 in rat DRG neurons. Consistently with previous reports (9–12), VR1 expression was not restricted to plasma membrane, but it was also present in the cytoplasmic area, where it showed a staining pattern compatible with ER localization. TRPV1 immunoreactivity was observed both in the soma and along the neuronal processes. Calcium imaging experiments performed with fura-2 on DRG neurons showed that stimulation with capsaicin in Ca2+-free medium produced a transient increase of [Ca2+]C, demonstrating that TRPV1 activation is able to produce Ca2+ release from the intracellular calcium stores (Fig. 1B). Concentrations of capsaicin above 5 μm were necessary in order to induce clear responses in Ca2+-free medium. After allowing replenishment of the stores by a 3-min incubation with 1 mm Ca2+, a second pulse of capsaicin induced a second Ca2+ transient similar to the first one, indicating the reversibility of the effect. The increase of [Ca2+]C obtained by stimulation with 20 μm capsaicin in Ca2+-free medium was generally smaller than the one obtained by Ca2+ entry (in Ca2+-containing medium) upon depolarization with high K+ or by release from the intracellular Ca2+ stores with caffeine (Fig. 1B). Stimulation with 1 μm capsaicin in Ca2+-containing medium produced Ca2+ entry in about 50% of all of the neurons present in the microscopic field. Application of 20 μm capsaicin in Ca2+-free medium produced a measurable Ca2+ release response in 93 of 133 DRG neurons studied, which did also respond to high K+. Ruthenium red (10 μm) blocked capsaicin-induced Ca2+ entry but not Ca2+ release from the stores (results not shown).
FIGURE 1.
Effects of activation of endogenous TRPV1 channels on Ca2+ release from the ER in DRG neurons. A, expression of TRPV1 revealed by TRPV1 antibody (see “Experimental Procedures”). Two different z sections of the same neuron are shown. B, effects of stimulation with capsaicin (CAPS; 20 μm) on [Ca2+]C in fura-2-loaded cells. In order to avoid Ca2+ entry, the stimulation with capsaicin was performed in Ca2+-free medium containing 10 μm ruthenium red (EGTA). The effects of depolarization with high K+ solution (70 mm; K+) and stimulation with caffeine (50 mm; CAF) are also shown for comparison. C, effects of stimulation with capsaicin (20 μm) on [Ca2+]ER. DRGs were infected with the HSV-ermutGA amplicon virus, and aequorin was reconstituted with coelenterazine n in Ca2+-free medium prior to the experiment. Cells were permeabilized with 20 μm digitonin in intracellular-like Ca2+-free medium (No Ca2+), and then 100 nm Ca2+ (buffered with EGTA) was added, followed by capsaicin (CAPS; 20 μm) or caffeine (CAF; 50 mm), as shown.
In order to demonstrate that the effect of capsaicin was really taking place in the ER, we performed direct measurements of [Ca2+]ER using an ER-targeted aequorin. A representative experiment is shown in Fig. 1C. The DRG neurons were infected with the amplicon virus pHSVerGA 1 day prior to the measurements. The plasma membrane of DRG neurons was permeabilized by a brief treatment with digitonin in Ca2+-free medium, and then the cells were perfused with intracellular-like medium containing 100 nm Ca2+ and 1 mm Mg-ATP. This allowed Ca2+ refilling of the ER by Ca2+ pumping through the sarcoendoplasmic reticulum Ca2+-ATPase. The ER refilled within 2–3 min to a [Ca2+]ER near 10−3 m, a value similar to the one found in cells such as chromaffin, pituitary GH3, or PC12 cells (23). The addition of 20 μm capsaicin at this point produced a decrease of [Ca2+]ER. A pulse of caffeine (50 mm) emptied further the ER. The decrease of [Ca2+]ER induced by 20 μm capsaicin in DRG neurons reverted very slowly. Reversion in TRPV1-transfected HEK293T cells was faster (see Fig. 3). Kinetics of reversibility of capsaicin-induced Ca2+ entry was quite variable from neuron to neuron (supplemental Fig. S1A), but reversibility was generally observed even at high capsaicin concentrations (supplemental Fig. S1B).
FIGURE 3.
Capsaicin-induced Ca2+ release from the ER in TRPV1-expressing HEK293T cells. The effects of different concentrations of capsacin (CAPS; followed by concentration in μm) are shown. The ER release is evidenced by either the increase in [Ca2+]C in cells expressing cytosolic aequorin (A), the decrease of [Ca2+]ER in intact cells expressing ER-targeted aequorin (B), or the decrease of [Ca2+]ER in digitonin-permeabilized cells expressing ER-targeted aequorin (C). Ruthenium red was used to avoid entry of Ca2+ through plasma membrane-located TRPV1 in intact cells (A and B). Permeabilization with digitonin in C was performed as in Fig. 1C, except that the concentration of digitonin was 60 μm.
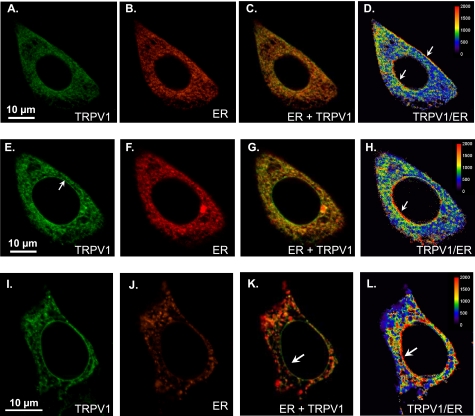
Further investigation on ER Ca2+ release through TRPV1ER was pursued in a model system, by expressing GFP-TRPV1 in HeLa (Fig. 2, A–H) or HEK293T (Fig. 2, I–L) cells. Localization of the expressed protein was similar to the one found in DRG neurons and included both plasma membrane and ER locations. Most of the TRPV1 fluorescence (in green) co-localized with the ER marker (in red in Fig. 2). The level of TRPV1 expression tended to be somewhat more intense at the plasma membrane and at the nuclear membrane, as indicated by the relative dominance of the green fluorescence (Fig. 2, C, G, and K) or the higher ratio of green to red fluorescence (Fig. 2, D, H, and L). The arrows point to areas where the dominance of TRPV1 was more apparent. The same relation was also found between TRPV1 and an ER-tracker distribution (results not shown). It has been reported that prolonged incubation with the vanilloid receptor agonist resiniferatoxin in Ca2+-free medium results in ER vesiculation and fragmentation (9). No pattern of vesicle staining close to the plasma membrane was observed in our experiments (Fig. 2). On the other hand, the cytoplasmic pattern of TRPV1 distribution was similar before and after the aequorin reconstitution, which involves a 60-min incubation in Ca2+-free medium (supplemental Fig. S2).
FIGURE 2.
Co-localization of TRPV1 with an ER marker in HeLa (A–H) and HEK293T (I–L) cells. Cells were co-transfected with GFP-TRPV1 and erRA as described under “Experimental Procedures.” A–D, comparison of the expression of both proteins in a HeLa cell. From left to right, GFP-TRPV1 (A), erRA (B), merge image (C), and TRPV1/ER ratio (D; pseudocolor-coded, scale at right). E–H, a more equatorial section of the same cell as in the top row. I–L, co-expression in HEK293T cells. The arrows indicate areas where TRPV1 expression predominates over ER expression.
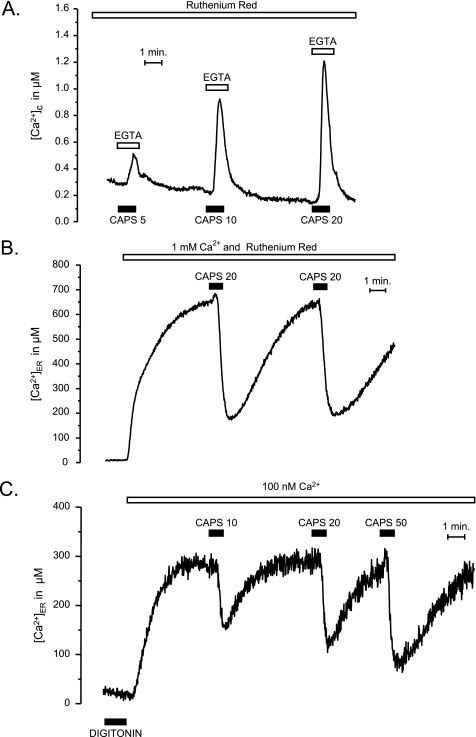
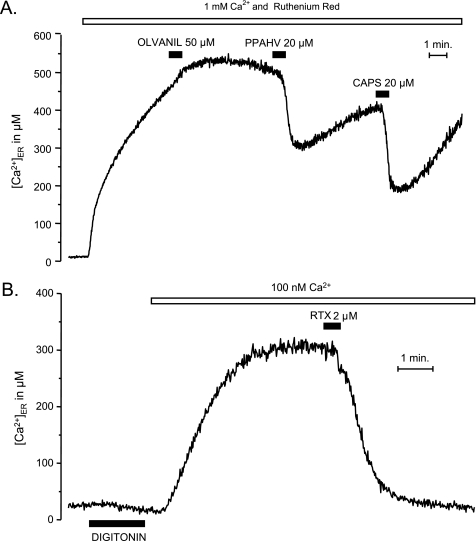
The functional behavior of the TRPV1 channels expressed in HeLa or HEK293T cells was similar to the one found in DRG neurons. Stimulation with capsaicin in Ca2+-free medium produced a concentration-dependent increase in [Ca2+]C, most probably due to Ca2+ release from the ER (Fig. 3A). As in DRG neurons, stimulation of HEK293T cells expressing TRPV1 in Ca2+-containing medium produced a large Ca2+ entry (results not shown) (supplemental Fig. S1B). Stimulation of Ca2+ entry required smaller concentrations of capsaicin than stimulation of Ca2+ release from the intracellular calcium stores (see below). In cells transfected with ER-targeted aequorin, the release of Ca2+ could be directly evidenced by a decrease of [Ca2+]ER (Fig. 3B) when the cells were stimulated with capsaicin in the presence of 10 μm ruthenium red, used here to avoid Ca2+ entry through TRPV1PM. If the cells that had been treated with capsaicin were then washed with regular Ca2+-containing medium (without capsaicin) the ER stores refilled; a second capsaicin application at this time yielded a response of similar amplitude as the first one, demonstrating the reversibility of the ER-depleting effect evoked by capsaicin (Fig. 3B). Finally, the concentration-dependent Ca2+-releasing effect of capsaicin from the ER could also be demonstrated in digitonin-permeabilized cells (Fig. 3C). In these cells, after refilling the stores by incubation in intracellular-like solution with Ca2+ concentrations similar to those observed in the cytosol of resting cells (100 nm), a concentration-dependent and reversible Ca2+-depleting effect of capsaicin could be demonstrated (Fig. 3C). Other TRPV1 agonists, such as phorbol 12-phenylacetate 13-acetate 20-homovanillate (37), at 20 μm produced ER emptying in TRPV1-transfected HEK293T cells (Fig. 4A). Resiniferatoxin (2 μm), a potent agonist of TRPV1 in DRGs (9, 18), produced Ca2+ release from the ER in TRPV1-transfected HEK293T cells, both intact (not shown) and permeabilized cells (Fig. 4B). We did not find an effect of other agonists, such as the endocannabinoid receptor agonists olvanil (50 μm; Fig. 4A) or anandamide (50 μm; not shown).
FIGURE 4.
Effects of different TRPV1 channel agonists on Ca2+ release from the ER in TRPV1-transfected HEK293T cells. A, intact cells stimulated with either olvanil, phorbol 12-phenylacetate 13-acetate 20-homovanillate (PPAHV), or capsaicin (CAPS). B, digitonin-permeabilized cells stimulated with 2 μm resiniferatoxin (RTX). Details are as in Fig. 3C.
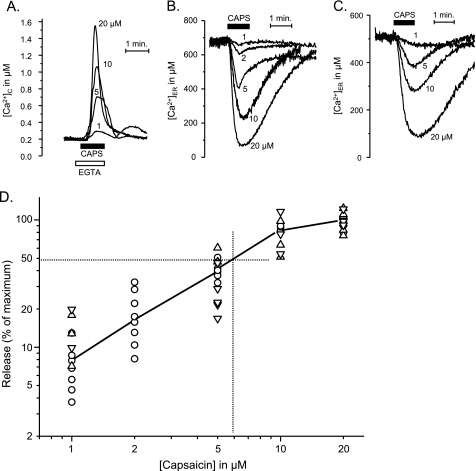
Whereas plasma membrane TRPV1 channels show high affinity for capsaicin, with K50 well below 1 μm (12), Ca2+ release from ER required higher capsaicin concentrations. Affinity measurements in HEK293T cells transfected with TRPV1 are shown in Fig. 5. Three different situations were studied: the increase of [Ca2+]C that results from stimulation with capsaicin in Ca2+-free medium (Fig. 5A) and the decrease of [Ca2+]ER induced by capsaicin, either in intact (Fig. 5B) or in digitonin-permeabilized cells (Fig. 5C). In the last case, the intracellular Ca2+ stores were first refilled by incubation in intracellular-like medium containing 100 nm Ca2+ (buffered with EGTA; see “Experimental Procedures”). The effects of concentrations of capsaicin in the 1–20 μm range were studied in all of the three cases with similar results; there was hardly any effect at 1 μm capsaicin, whereas a nearly maximal release was reached at 20 μm. Results from a total of 89 measurements are summarized in Fig. 5D, where release is expressed as a percentage of the maximum. The half-maximal release was attained at 5–10 μm capsaicin.
FIGURE 5.
Concentration dependence of the effects of capsaicin on the release of Ca2+ from the ER in TRPV1-expressing HEK293T cells. The ER release was evidenced by either the increase in [Ca2+]C in cells expressing cytosolic aequorin (A), the decrease of [Ca2+]ER in intact cells expressing ER-targeted aequorin (B), or the decrease of [Ca2+]ER in digitonin-permeabilized cells expressing ER-targeted aequorin ([Ca2+]C maintained at 100 nm by a EGTA/Ca2+ buffer) (C). The extracellular medium contained 10 μm ruthenium red in A and B. Other details were as in Fig. 3. D, dose-response curve. Data were taken from A (Δ[Ca2+]C, inverted triangles), B (−Δ[Ca2+]ER; circles) and C (−Δ[Ca2+]ER; triangles) and were normalized as percentage of the maximum effect.
Some reports have shown that sensitivity of TRPV1 to capsaicin is enhanced by activation of some signaling pathways involving the activation of key kinases (14, 15). We investigated the actions of several kinases on the affinity of capsaicin by testing the effects on the ER Ca2+ release induced at 1 and 20 μm capsaicin. Forskolin (10 μm), an agonist of protein-kinase A, had no detectable effect. We also tested the following protein kinase C agonists: oleyl-acetyl-glycerol (20 μm), phorbol-12-myristate 13-acetate (200 nm), and phorbol-dibutyrate (200 nm) alone or in combination with docosahexaeonic acid (50 μm) or eicosapentaoic acid (50 μm) (38). The results were negative in all cases (results not shown).
PIP2 has been reported to inhibit (39, 40), to activate (7, 16, 41–43), or to have a dual effect on (44, 45) TRPV1. We tested here the effects of decreasing PIP2 in our experimental system by inducing 5-phosphatase type IV activity with tetracycline in HEK293 cells expressing TRPV1 (see “Experimental Procedures”). This procedure decreases 15-fold the PIP2 levels (28). The increase of [Ca2+]C induced by stimulation with extracellular ATP, which is mediated by IP3 production, was almost completely prevented (84% reduction), confirming a drastic depletion of the PIP2 pool (supplemental Fig. S3, A and B). However, Ca2+ entry induced by capsaicin was much less inhibited (by 39%) (Fig. S3, C and D). These results confirm that PIP2 affects TRPV1PM, but they do also show that the channel can still allow Ca2+ entry even with very low PIP2 levels. This is consistent with previous results showing that the lack of expression of Pirt, the phosphoinositide-binding protein responsible for the effect of PIP2 on TRPV1, prevented only partially (by about 39%) the TRPV1-induced inward current in the DRG neurons of the Pirt−/− mice (42). On the other hand, stably expressing TRPV1 HEK cells (which do not express Pirt) do show a capsaicin-induced inward current, which was increased by 59% by Pirt overexpression (42). Finally, the affinity of TRPV1 for capsaicin was not significantly modified by either Pirt deficiency or overexpression (42). These results indicate that, although PIP2 positively modulates TRPV1, it may be not absolutely necessary for function of this channel.
We also examined the possibility that the low capsaicin affinity of the ER-releasing effect could be due to inactivation/desensitization by Ca2+. This hypothesis was tested in two different series of experiments designed to test the effects of [Ca2+]C and [Ca2+]ER on the capsaicin-induced Ca2+ release. In the first series, the increase of [Ca2+]C was dampened by adding BAPTA, which was loaded into the cells by incubation with the acetoxymethyl ester form. Although the steady state levels of [Ca2+]C and [Ca2+]ER were unaffected, quick [Ca2+]C changes are almost completely abolished due to Ca2+ buffering by the cytosolic BAPTA (supplemental Fig. S4).
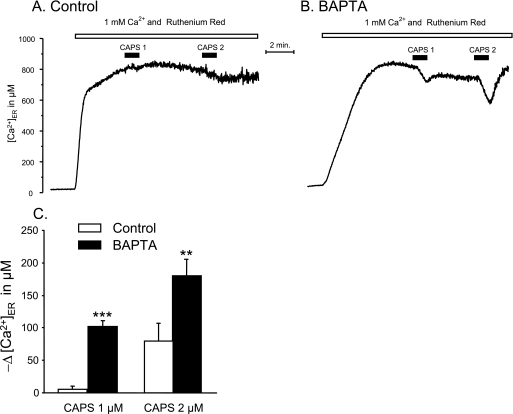
Fig. 6 compares typical results of capsaicin-induced Ca2+ release in control (A) and BAPTA-loaded cells (B). In the BAPTA-loaded cells, the filling of the ER, reflected by the rate of the increase of [Ca2+]ER, was slower (Fig. 6, compare B with A). This probably reflects the slower increase of [Ca2+]C after the addition of external Ca2+ resulting from the buffering capacity of the cytosolic BAPTA. However, the [Ca2+]ER attained at the steady state was, as expected, similar in the control cells. Stimulation with low capsaicin concentrations, 1 and 2 μm, had little or no effect in the control condition (Fig. 6A). In contrast, in the BAPTA-loaded cells, the ER depletion induced by a pulse of 2 μm or even 1 μm capsaicin was measurable (Fig. 6B). The average values obtained in six similar experiments are shown in Fig. 6C. At 1 μm capsaicin, the Ca2+ release increased almost 20-fold, whereas at 2 μm, the increase was only 2.3-fold. It seem then clear that buffering the [Ca2+]C rise decreases the threshold of capsaicin for Ca2+ release from ER, although emptying of the ER is not complete.
FIGURE 6.
Effects of preloading with BAPTA on capsaicin-induced Ca2+ release from the ER in TRPV1-expressing HEK293T cells. BAPTA was loaded into the cytosol by incubation of the cells with 10 μm BAPTA, acetoxymethyl ester for 60 min at 20 °C, and then the ER was allowed to refill with Ca2+, and cells were stimulated with low capsaicin concentrations (CAPS; 1 or 2 μm). The results obtained in control (A) and BAPTA-loaded cells (B) are compared. Ruthenium red was added to prevent entry of Ca2+ from the extracellular medium through the plasma membrane-located TRPV1 channels. C, means ± S.E. of six similar experiments. **, p < 0.01; ***, p < 0.001 (Student's t test).
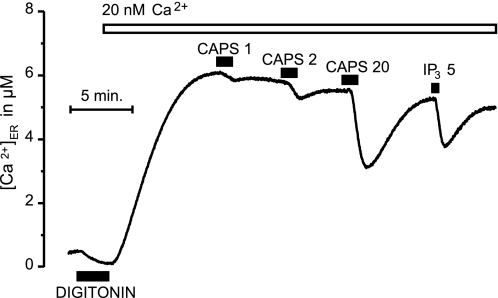
To test a possible inhibitory effect of Ca2+ inside the ER, we designed a second set of experiments in which the steady state level of [Ca2+]ER was maintained at a very low level. For measuring this concentration range of [Ca2+]ER, the ermutGA used in the rest of the experiments shown here was inadequate, and ER-targeted wild-type aequorin was used instead to measure precisely concentrations in the low micromolar range. TRPV1-expressing HEK293T cells were then permeabilized with digitonin and incubated with 20 nm Ca2+ in intracellular-like medium (Fig. 7). Under these conditions, the steady-state level of [Ca2+]ER was about 6 μm, 2 orders of magnitude smaller than in the control cells incubated with 100 nm Ca2+. Under these conditions, stimulation with 1 or 2 μm capsaicin had little effect, whereas 20 μm capsaicin produced a large Ca2+ release. The effect of 5 μm inositol trisphosphate is also shown for comparison. These results suggest that Ca2+ inside the ER does not inactivate the TRPV1ER channels.
FIGURE 7.
Capsaicin-induced release of Ca2+ from the ER at very low [Ca2+]ER in TRPV1-expressing HEK293T cells. Cells were transfected with ER-targeted native aequorin. After permeabilization with 60 μm digitonin in Ca2+-free medium, refilling of the ER was effected at low [Ca2+]C (20 nm). Consequently, the refilling proceeded to steady-state [Ca2+]ER levels much lower than when the usual refilling (at 100 nm [Ca2+]C) was performed (compare with Figs. 3C, 4A, and 6). Stimulation with capsaicin (CAPS; 1, 2, or 20 μm) or inositol trisphospate (IP3) was as shown.
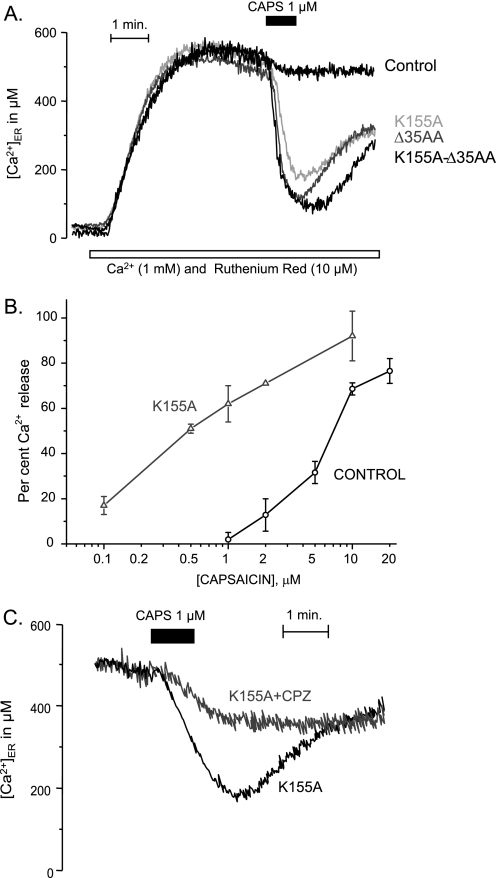
TRPV1, as other Ca2+ channels, inactivates by Ca2+, and CaM is thought to be involved in the inactivation process (46). It has been reported that CaM binds to the N-terminal region of TRPV1 (6) and also to the C-terminal region (5). The CaM binding site at the N terminus seems to be the same that binds PIP2 (7). Mutations in the ankyrin repeats at the N terminus (6, 7) or deletion mutants in the C terminus of TRPV1 (5) prevent Ca2+-dependent desensitization of the channel. We hypothesized that CaM could modulate the TRPV1ER activity by reducing its affinity for capsaicin. Therefore, we mutated the residue Lys-155 (K155A mutant), which has been proposed to be an essential residue of the CaM binding site within the ankyrin domain of the TRPV1. We also used a 35-amino acid deletion (Δ35AA) mutant in the C-terminal region (amino acids 767–801) and the double mutant K155A/Δ35AA. The mutant R181A, in a region outside the CaM binding domain, was also tested as a negative control. The level of expression and the cellular distribution of the TRPV1 mutants were similar to the ones found for the native protein (supplemental Fig. S5). Interestingly, 1 μm capsaicin produced a very strong Ca2+ release in all of the three mutants of the CaM binding sites (Fig. 8A), whereas no effect was seen in the cells expressing the native TRPV1 or in the negative control R181A mutant or in the unglycosylated N604S mutant. These data suggest that CaM is able to bind to TRPV1ER and act as a negative modulator of the gating by decreasing its affinity for capsaicin. It is remarkable that the effects of the two mutations, which affect two different CaM binding sites, are not additive, because the effect of the double mutant, K155A/Δ35AA, does not seem larger than the one obtained with any of the single mutations (Fig. 8A).
FIGURE 8.
Capsaicin-induced release of Ca2+ from the ER in HEK293T cells expressing different TRPV1 mutants. A, comparison of the Ca2+ release induced by capsaicin on the native TRPV1 (in black) and the mutants K155A, Δ35AA, and K155A/Δ35AA (different gray levels, as shown), all tested at 1 μm capsaicin. The empty plasmid vector, the control mutant R181A, or the unglycosylated N604S mutant did not increase the affinity for capsaicin (not shown). B, comparison of the dose response curves of the control (black) and the K155A mutant (gray). Shown are the average values of 3–14 determinations. Bars, S.E. C, stimulation by 1 μm capsaicin in K155A is antagonized by 10 μm capsazepine.
It has been reported that ATP is also able to bind the N-terminal CaM binding site with the result of preventing desensitization. Consistently, ATP removal blocks the capsaicin-induced inward current (7). In our hands, removal of ATP did not prevent the release of Ca2+ from ER induced by capsaicin in permeabilized cells (supplemental Fig. S6).
Fig. 8B compares the concentration-response curves of the Ca2+ release from the ER induced by capsaicin in HEK293T cells transfected either with the native TRPV1 or with the K155A mutant. The affinity for capsaicin was about 10 times higher for the mutant than for the control, whereas the maximal effect was similar. On the other hand, the Ca2+ release from the ER induced in the K155A mutant by 1 μm capsaicin was blocked (67% inhibition) by 10 μm capsazepine, a competitive antagonist of TRPV1 (Fig. 8C and supplemental Fig. S7).
DISCUSSION
DRG neurons express TRPV1 channels both at the plasma membrane and at the ER, and both TRPV1PM and TRPV1ER are functional, able to induce a [Ca2+]C peak upon stimulation with capsaicin (Fig. 1, A and B). Expression of TRPV1 was nearly as high in the cytoplasmic area as in the plasma membrane in intact DRGs (Fig. 1A) as well as in transiently transfected HeLa (Fig. 2, A and E) or HEK293T cells (Fig. 2I). The cytoplasmic pattern was compatible with an ER distribution (Fig. 1A), and TRPV1 co-localized with ER markers (Fig. 2, B, F, and J), although a relatively higher density of TRPV1 was sometimes evidenced at the plasma membrane (Fig. 2D) and at the nuclear envelope (Fig. 2, D, H, and L). The functional significance of this last localization is intriguing.
Location of TRP channels in endomembranes of neurons has been reported not only for TRPV1 (see Introduction) but also for other TRP channels such as growth factor-regulated channel (47), TRPM8 (48), or TRPC5 (49) It has been proposed that migration of these channels from the endomembranes to the plasma membrane, which could be regulated by agonists or growth factors, is an important control mechanism for Ca2+ entry. TRPM8 has also been found in the ER of prostate cancer epithelial cells, where it could be related to control of proliferation and apoptosis (50). On the other hand, activation of the endomembrane Ca2+ channels results in Ca2+ depletion of the intracellular stores. Maintained depletion can trigger ER stress and unfolded protein response, and finally, it could lead to cell death by activation of apoptotic mechanisms (51). ER stress is associated with the pathogenesis of a variety of maladies, including neurodegenerative diseases, that course with accumulation of aggregated proteins (52). TRPV1 stimulation has been shown to induce cell death not only in neurons (9) but also in prostate (50) and lung cells (19).
By directly measuring Ca2+ inside the ER in DRG neurons infected with an amplicon carrying an ER-targeted aequorin, we demonstrate that capsaicin produces a decrease of [Ca2+]ER similar in size to the one elicited by caffeine (Fig. 1C). Since the effects found in DRGs were reproduced in HEK293T cells transiently transfected with TRPV1 (Fig. 3), further experiments were performed in this cell model. Besides capsaicin, other known TRPV1 agonists, such as phorbol 12-phenylacetate 13-acetate 20-homovanillate (37) or resiniferatoxin (1), were able to induce Ca2+ release from the ER. In contrast, the cannabinoid receptor agonists anandamide and olvanil (18) were not effective. These two agonists have less affinity for TRPV1PM than capsaicin (53), so that it is possible that the concentrations tested here are insufficient to activate TRPV1ER. In the case of anandamide, it has been reported that very large concentrations and acidic pH are required for activation of TRPV1-mediated Ca2+ entry in DRG neurons (18).
The concentration of capsaicin required for releasing Ca2+ from the ER was relatively high, in the 1–50 μm range (K50 ∼5–10 μm) (Fig. 5), whereas the concentration required for inducing Ca2+ entry was much smaller, in the 0.1–1 μm range (18) (results not shown). This discrepancy between the affinities depending on whether TRPV1 is located at the plasma membrane or at the ER is rather intriguing. Is the low capsaicin affinity of TRPV1ER due to the lack of accessory proteins or co-factors or perhaps to inhibition by selective ER components? Are there differences in covalent modulation (e.g. phosphorylation state) or lipidic microenvironment that justify the low capsaicin affinity of TRPV1? We have addressed here some of these questions.
We did not find changes in TRPV1ER sensitivity to capsaicin in cells treated with activators or inhibitors of either protein-kinase A or C, suggesting that the differences between TRPV1PM and TRPV1ER are not due to differences in phosphorylation of the channels.
Modulation of vanilloid receptors and other TRP channels by phosphoinositides has deserved much attention in recent years. PIP2 has been reported to inhibit (39, 40), to activate (7, 16, 41–43), or to have a dual effect (44, 45) on TRPV1. In our hands, decreasing PIP2 levels 15-fold (28) inhibits Ca2+ entry through TRPV1PM by 39% (supplemental Fig. S4, C and D). The range of inhibition we find here is consistent with other reports (42). Therefore, although PIP2 modulates positively TRPV1, it may not be absolutely necessary for function of this channel. According to these results, the lack of PIP2 in the ER could contribute to the decrease of TRPV1 activity observed in this location, but it seems insufficient to explain the large differences observed between TRPV1PM and TRPV1ER. In addition, the changes of PIP2 have been reported to affect the capacity (Vmax) of the TRPV1 inward current rather than the affinity for capsaicin (42), whereas the reverse situation is observed for TRPV1ER (Fig. 5D). However, other studies have found that PIP2 increases the affinity for capsaicin (45, 54). In addition, because the affinity of PIP2 to TRPV1 is high (45, 54), complete PIP2 unbinding from TRPV1 may require a larger decrease than the one obtained in the experiments of supplemental Fig. S3. The interplay between PIP2, calmodulin, and ATP is probably quite complicated (45).
Another known mode of modification of TRPV1 activity is through Ca2+-induced inactivation/desensitization (8). Because it has been reported that desensitization can be reversed by high concentrations of capsaicin (55), we have tested here the hypothesis that partial inactivation of TRPV1ER could be the cause of its low sensitivity to capsaicin. One could argue that TRPV1PM and TRPV1ER should inactivate similarly, because the protein domain responsible for inactivation is in both cases facing the cytosol. Nevertheless, local modulators could confer different sensitivity to inactivation. Alternatively, the high Ca2+ microdomains may be different near the plasma membrane and near the ER. In any case, in order to antagonize inactivation by Ca2+, we loaded the cells with BAPTA. This increased the effect induced by low (1–2 μm) concentrations of capsaicin, although release was not complete at these capsaicin concentrations (Fig. 6). However, sometimes the inactivation sites are so close to the Ca2+ microdomains that even rapid chelators, such as BAPTA, are not as efficient to prevent the inactivation as one would expect (56).
We tested also the possibility that TRPV1ER could be specifically desensitized by Ca2+ inside the ER, which is much higher than in cytosol. For this purpose, we refilled ER with low Ca2+ concentrations to reach a [Ca2+]ER steady state level 100-fold lower than the usual one, but this decrease in the [Ca2+]ER did not noticeably increase the sensitivity to capsaicin (Fig. 7).
Inhibition of TRPV1 by Ca2+ seems to be dependent on calmodulin (4, 8). Two different CaM binding sites have been described (8), one located at the C terminus (C-terminal CaM binding site (CCB)) (5, 55, 57) and the other at the N terminus (N-terminal CaM binding site (NCB)) (6, 7). NCB includes three ankyrin repeats and can also bind ATP (7). CCB is a more unusual CaM binding motif that includes residues 767–801 of TRPV1 (5), with Arg-785 playing an essential role (57). CCB can also bind PIP2 (7). Interestingly, mutations in the CaM binding sites of TRPV1, either at the C-terminal (Δ35AA) or at the N-terminal end (K155A), increased the apparent affinity for capsaicin by about 1 order of magnitude (Fig. 8). It is remarkable that the double mutation K155A/Δ35AA did not seem to increase the affinity for capsaicin above the individual mutants. This outcome is consistent with the model proposed by Lishko et al. (7), where inhibition results from the interaction of the two CaM molecules bound to NCB and CCB. In this model, the mutation of any of the two CaM binding sites would have the same effect as the simultaneous alteration of both of them.
We conclude that the low affinity of TRPV1ER may be due, at least in part, to a calmodulin-dependent inhibition of the channel. This interpretation should be regarded with caution, because both CaM binding sites, CCB and NCB, have been reported to also bind other ligands (7) and to participate in other functions independent of calmodulin binding (5). It should be recalled that, in the model of Lishko et al. (7), ATP and PIP2 act as “sensitizers,” antagonizing the desensitizing action of the two interacting calmodulins. It is doubtful whether ATP has a modulatory role under physiological conditions because (i) no large changes of ATP are expected to take place, and (ii) ATP binding to TRPV1 is inhibited by divalent cations and most of the ATP is bound to Mg2+ in the living cell. On the other hand, PIP2 is not, under normal conditions, present in the ER membrane.
It is not obvious why TRPV1ER should be more sensitive to down-regulation by the CaM-dependent mechanism than TRPV1PM. It might be attributed perhaps to microenvironmental differences. These may include weaker effects of the sensitizers, PIP2 and ATP, and stronger action of the desensitizers, Ca2+/CaM. It is clear that the level of PIP2 in the ER is much smaller than in the plasma membrane. In the case of ATP, it is not known whether there could be differences between the cytosolic concentrations near plasma membrane or ER. Finally, [Ca2+]C near endomembranes may be higher because of spontaneous random quantal Ca2+ release from the ER. All of these factors would favor a lower capsaicin sensitivity of TRPV1ER. This CaM modulation is not unique for TRPV1 because it has been shown that CaM binds to other ER channels, such as inositol 1,4,5-trisphosphate receptors or ryanodine receptors, and may modulate its function (58). It is known, for example, that ryanodine receptor 1 is modulated by CaM in a biphasic manner, behaving as a partial agonist at low nanomolar Ca2+ concentrations and as an inhibitor at higher Ca2+ concentrations (59).
In summary, our results point out that low sensitivity to capsaicin of the TRPV1ER channels is mainly due to inactivation by Ca2+/calmodulin. The fact that TRPV1ER has low reactivity to agonists is providential, because otherwise the ER could easily become accidentally depleted of Ca2+, this triggering ER stress, unfolded protein response, and, ultimately, cell death.
Supplementary Material
Acknowledgments
We thank Dr. P. Majerus for kindly providing the tetracycline-inducible 5-phosphatase type IV cells. Technical assistance by Miriam García Cubillas and Jesús Fernández is gratefully acknowledged.
This work was supported in part by Spanish Ministerio de Ciencia e Innovación (MICINN) Grant BFI2007-60157, Instituto de Salud Carlos III Grant RD06/0010/0000, and Junta de Castilla y León Grant gr175.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- DRG
- dorsal root ganglion
- ER
- endoplasmic reticulum
- PM
- plasma membrane
- [Ca2+]C
- cytosolic Ca2+ concentration
- [Ca2+]ER
- Ca2+ concentration inside the ER
- CaM
- calmodulin
- HSV
- herpes simplex virus
- GFP
- green fluorescent protein
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- NCB
- N-terminal calmodulin-binding site
- CCB
- C-terminal calmodulin-binding site
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
REFERENCES
- 1.Caterina M. J., Julius D. (2001) Annu. Rev. Neurosci. 24, 487–517 [DOI] [PubMed] [Google Scholar]
- 2.Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- 3.Dray A., Forbes C. A., Burgess G. M. (1990) Neurosci. Lett. 110, 52–59 [DOI] [PubMed] [Google Scholar]
- 4.Zhu M. X. (2005) Pflugers Arch. 451, 105–115 [DOI] [PubMed] [Google Scholar]
- 5.Numazaki M., Tominaga T., Takeuchi K., Murayama N., Toyooka H., Tominaga M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8002–8006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbaum T., Gordon-Shaag A., Munari M., Gordon S. E. (2004) J. Gen. Physiol. 123, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lishko P. V., Procko E., Jin X., Phelps C. B., Gaudet R. (2007) Neuron 54, 905–918 [DOI] [PubMed] [Google Scholar]
- 8.Planells-Cases R., Garcìa-Sanz N., Morenilla-Palao C., Ferrer-Montiel A. (2005) Pflugers Arch. 451, 151–159 [DOI] [PubMed] [Google Scholar]
- 9.Olah Z., Szabo T., Karai L., Hough C., Fields R. D., Caudle R. M., Blumberg P. M., Iadarola M. J. (2001) J. Biol. Chem. 276, 11021–11030 [DOI] [PubMed] [Google Scholar]
- 10.Eun S. Y., Jung S. J., Park Y. K., Kwak J., Kim S. J., Kim J. (2001) Biochem. Biophys. Res. Commun. 285, 1114–1120 [DOI] [PubMed] [Google Scholar]
- 11.Liu M., Liu M. C., Magoulas C., Priestley J. V., Willmott N. J. (2003) J. Biol. Chem. 278, 5462–5472 [DOI] [PubMed] [Google Scholar]
- 12.Kárai L. J., Russell J. T., Iadarola M. J., Oláh Z. (2004) J. Biol. Chem. 279, 16377–16387 [DOI] [PubMed] [Google Scholar]
- 13.Montell C. (2004) Nat. Cell Biol. 6, 690–692 [DOI] [PubMed] [Google Scholar]
- 14.Morenilla-Palao C., Planells-Cases R., García-Sanz N., Ferrer-Montiel A. (2004) J. Biol. Chem. 279, 25665–25672 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Huang J., McNaughton P. A. (2005) EMBO J. 24, 4211–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein A. T., Ufret-Vincenty C. A., Hua L., Santana L. F., Gordon S. E. (2006) J. Gen. Physiol. 128, 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner H., Fleig A., Stokes A., Kinet J. P., Penner R. (2003) Biochem. J. 371, 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olah Z., Karai L., Iadarola M. J. (2001) J. Biol. Chem. 276, 31163–31170 [DOI] [PubMed] [Google Scholar]
- 19.Thomas K. C., Sabnis A. S., Johansen M. E., Lanza D. L., Moos P. J., Yost G. S., Reilly C. A. (2007) J. Pharmacol. Exp. Ther. 321, 830–838 [DOI] [PubMed] [Google Scholar]
- 20.Shin J. S., Wang M. H., Hwang S. W., Cho H., Cho S. Y., Kwon M. J., Lee S. Y., Oh U. (2001) Neurosci Lett. 299, 135–139 [DOI] [PubMed] [Google Scholar]
- 21.De Petrocellis L., Harrison S., Bisogno T., Tognetto M., Brandi I., Smith G. D., Creminon C., Davis J. B., Geppetti P., Di Marzo V. (2001) J. Neurochem. 77, 1660–1663 [DOI] [PubMed] [Google Scholar]
- 22.Barrero M. J., Montero M., Alvarez J. (1997) J. Biol. Chem. 272, 27694–27699 [DOI] [PubMed] [Google Scholar]
- 23.Alonso M. T., Barrero M. J., Carnicero E., Montero M., Garcia-Sancho J., Alvarez J. (1998) Cell Calcium 24, 87–96 [DOI] [PubMed] [Google Scholar]
- 24.Alonso M. T., Barrero M. J., Michelena P., Carnicero E., Cuchillo I., García A. G., García-Sancho J., Montero M., Alvarez J. (1999) J. Cell Biol. 144, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jambrina E., Alonso R., Alcalde M., del Carmen Rodríguez M., Serrano A., Martínez-A C., García-Sancho J., Izquierdo M. (2003) J. Biol. Chem. 278, 14134–14145 [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum T., Awaya M., Gordon S. E. (2002) BMC Neurosci. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manjarrés I. M., Chamero P., Domingo B., Molina F., Llopis J., Alonso M. T., García-Sancho J. ( 2008) Pflugers Arch. 455, 961– 970 [DOI] [PubMed] [Google Scholar]
- 28.Kisseleva M. V., Cao L., Majerus P. W. (2002) J. Biol. Chem. 277, 6266–6272 [DOI] [PubMed] [Google Scholar]
- 29.Gilabert R., McNaughton P. (1997) J. Neurosci. Methods 71, 191–198 [DOI] [PubMed] [Google Scholar]
- 30.Montero M., Brini M., Marsault R., Alvarez J., Sitia R., Pozzan T., Rizzuto R. (1995) EMBO J. 14, 5467–5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bers D. M., Patton C. W., Nuccitelli R. (1994) Methods Cell Biol. 40, 3–29 [DOI] [PubMed] [Google Scholar]
- 32.Montero M., Alonso M. T., Carnicero E., Cuchillo-Ibáñez I., Albillos A., García A. G., García-Sancho J., Alvarez J. (2000) Nat. Cell Biol. 2, 57–61 [DOI] [PubMed] [Google Scholar]
- 33.Alvarez J., Montero M. (2002) Cell Calcium 32, 251–260 [DOI] [PubMed] [Google Scholar]
- 34.Villalobos C., Núñez L., Garcia-Sancho J. (1996) FASEB J. 10, 654–660 [DOI] [PubMed] [Google Scholar]
- 35.Núñez L., Senovilla L., Sanz-Blasco S., Chamero P., Alonso M. T., Villalobos C., García-Sancho J. (2007) J. Physiol. 580, 385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grynkiewicz G., Poenie M., Tsien R. Y. (1985) J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 37.Appendino G., Cravotto G., Palmisano G., Annunziata R., Szallasi A. (1996) J. Med. Chem. 39, 3123–3131 [DOI] [PubMed] [Google Scholar]
- 38.Matta J. A., Miyares R. L., Ahern G. P. (2007) J. Physiol. 578, 397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuang H. H., Prescott E. D., Kong H., Shields S., Jordt S. E., Basbaum A. I., Chao M. V., Julius D. (2001) Nature 411, 957–962 [DOI] [PubMed] [Google Scholar]
- 40.Prescott E. D., Julius D. (2003) Science 300, 1284–1288 [DOI] [PubMed] [Google Scholar]
- 41.Liu B., Zhang C., Qin F. (2005) J. Neurosci. 25, 4835–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim A. Y., Tang Z., Liu Q., Patel K. N., Maag D., Geng Y., Dong X. (2008) Cell 133, 475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein R. M., Ufret-Vincenty C. A., Hua L., Gordon S. E. (2008) J. Biol. Chem. 283, 26208–26216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukacs V., Thyagarajan B., Varnai P., Balla A., Balla T., Rohacs T. (2007) J. Neurosci. 27, 7070–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohacs T., Thyagarajan B., Lukacs V. (2008) Mol. Neurobiol. 37, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levitan I. B. (1999) Neuron 22, 645–648 [DOI] [PubMed] [Google Scholar]
- 47.Kanzaki M., Zhang Y. Q., Mashima H., Li L., Shibata H., Kojima I. (1999) Nat. Cell Biol. 1, 165–170 [DOI] [PubMed] [Google Scholar]
- 48.Tsuzuki K., Xing H., Ling J., Gu J. G. (2004) J. Neurosci. 24, 762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bezzerides V. J., Ramsey I. S., Kotecha S., Greka A., Clapham D. E. (2004) Nat. Cell Biol. 6, 709–720 [DOI] [PubMed] [Google Scholar]
- 50.Thebault S., Lemonnier L., Bidaux G., Flourakis M., Bavencoffe A., Gordienko D., Roudbaraki M., Delcourt P., Panchin Y., Shuba Y., Skryma R., Prevarskaya N. (2005) J. Biol. Chem. 280, 39423–39435 [DOI] [PubMed] [Google Scholar]
- 51.Lai E., Teodoro T., Volchuk A. (2007) Physiology 22, 193–201 [DOI] [PubMed] [Google Scholar]
- 52.Zhao L., Ackerman S. L. (2006) Curr. Opin. Cell Biol. 18, 444–452 [DOI] [PubMed] [Google Scholar]
- 53.Lam P. M., Hainsworth A. H., Smith G. D., Owen D. E., Davies J., Lambert D. G. (2007) J. Neurochem. 102, 801–811 [DOI] [PubMed] [Google Scholar]
- 54.Qin F. (2007) Handb. Exp. Pharmacol. 179, 509–525 [DOI] [PubMed] [Google Scholar]
- 55.Novakova-Tousova K., Vyklicky L., Susankova K., Benedikt J., Samad A., Teisinger J., Vlachova V. (2007) Neuroscience 149, 144–154 [DOI] [PubMed] [Google Scholar]
- 56.García A. G., García-De-Diego A. M., Gandía L., Borges R., García-Sancho J. (2006) Physiol. Rev. 86, 1093–1131 [DOI] [PubMed] [Google Scholar]
- 57.Grycova L., Lansky Z., Friedlova E., Obsilova V., Janouskova H., Obsil T., Teisinger J. (2008) Biochem. Biophys. Res. Commun. 375, 680–683 [DOI] [PubMed] [Google Scholar]
- 58.Saimi Y., Kung C. (2002) Annu. Rev. Physiol. 64, 289–311 [DOI] [PubMed] [Google Scholar]
- 59.Tripathy A., Xu L., Mann G., Meissner G. (1995) Biophys. J. 69, 106–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.