Abstract
Rad53 is an essential protein kinase governing DNA damage and replication stress checkpoints in budding yeast. It also appears to be involved in cellular morphogenesis processes. Mass spectrometry analyses revealed that Rad53 is phosphorylated at multiple SQ/TQ and at SP/TP residues, which are typical consensus sites for phosphatidylinositol 3-kinase-related kinases and CDKs, respectively. Here we show that Clb-CDK1 phosphorylates Rad53 at Ser774 in metaphase. This phosphorylation event does not influence the DNA damage and replication checkpoint roles of Rad53, and it is independent of the spindle assembly checkpoint network. Moreover, the Ser-to-Asp mutation, mimicking a constitutive phosphorylation state at site 774, causes sensitivity to calcofluor, supporting a functional linkage between Rad53 and cellular morphogenesis.
Introduction
Protein kinases are fundamental regulators of cellular metabolism and cell cycle progression. Therefore, understanding how they are regulated is a very important challenge for the comprehension of several cellular pathways.
Saccharomyces cerevisiae Rad53 is a serine/threonine/tyrosine kinase, and it is well established that Rad53 family members, including the Chk2 protein kinase in human cells, play a central role in the signal transduction pathway activated in response to DNA lesions and help prevent genome rearrangements and cancer (1, 2). Rad53 is phosphorylated by the upstream phosphatidylinositol 3-kinase-related kinases Mec1 and Tel1 (ATR and ATM in human cells); this triggers autophosphorylation events, leading to full activation of the kinase (3). In addition to the kinase domain, Rad53 presents two FHA domains through which the protein interacts with substrates and regulators (4). Moreover, Rad53 also contains a bipartite NLS domain in the C-terminal region (5, 6), required for efficient translocation of Rad53 into the nucleus, where it exerts its checkpoint functions as a guardian of the genome.
Rad53 has been reported to be also phosphorylated in a Mec1-independent manner in response to spindle damage, but the kinase responsible for this modification has not been described (7). However, it is unlikely that Rad53 kinase activity plays a role in response to spindle damage because Rad53, prepared from cells treated with nocodazole (an agent that cause spindle depolimerization and triggers the spindle assembly checkpoint), does not exhibit autophosphorylation activity, as determined by in situ kinase assay (8); moreover, rad53Δ cells are not sensitive to spindle-damaging agents.
Finally, Rad53 appears to be involved in cellular morphogenesis (9, 10). In fact, rad53Δ cells have an abnormal shape and are sensitive to morphogenesis-stressing agents (9). Genetic evidence suggests that the roles of Rad53 in morphogenesis are not dependent on Mec1-dependent phosphorylation, and it is unknown whether the role of Rad53 in morphogenesis is regulated during the cell cycle.
Mass spectrometry analysis revealed that Rad53 is phosphorylated at many serine and threonine sites, and, interestingly, it is phosphorylated at proline-directed Ser175, Ser375, and Ser774 sites in exponentially growing cells, without any DNA and/or spindle damage (5, 11). These findings suggested that the CDK1 cell cycle kinase may directly phosphorylate Rad53 and influence its activity, as supported by recent observations implicating CDK1 in DNA damage checkpoint activation (12, 13).
Here, we provide genetic and biochemical evidence that CDK1 phosphorylates Rad53 in metaphase, without any damage to the spindle or genomic DNA, and that most of the modification that had been previously described as induced by spindle damage (7) is instead due to the accumulation of cells in metaphase induced by the spindle-damaging agent. The Ser774 residue is the main target for CDK1-dependent Rad53 modification in metaphase. Interestingly, the Ser-to-Asp mutation of residue 774, mimicking a constitutively phosphorylation state, causes sensitivity to calcofluor (a morphogenetic stress agent), further suggesting a role for Rad53 in controlling cellular morphogenesis. Our findings raise the possibility that the role of Rad53 in morphogenesis (9) may be modulated during the cell cycle by CDK1. Furthermore, none of the CDK1-dependent phosphorylation events occurring on Rad53 at metaphase appear to have any role in promoting Rad53 activation in response to DNA damage or replication stress.
EXPERIMENTAL PROCEDURES
Yeast Strains and Cultures
All strains are isogenic derivatives of W303. The genotypes of the yeast strains utilized in this study are listed in supplemental Table S1. To obtain strains Y849 and Y850, plasmid pVF6 (generously provided by F. Vanoli and M. Foiani), carrying the cdc28-as1 mutant allele, was cut with ClaI and integrated in the CDC28 locus.
All the experiments were done in YPD medium containing 10 g yeast extract, 20 g peptone, glucose 2% final concentration, H2O to 1 liter; the pH was adjusted to 5.4 with HCl. E. coli strain (DH5α) was used for the production of mutants and cloning; E. coli cultures were grown in LD medium containing 10 g bactotryptone, 5 g yeast extract, 5 g NaCl, H2O to 1 liter, and the pH was adjusted to 7.25.
Construction of Rad53 Mutants
The plasmid pCH10, carrying RAD53-9myc under the control of its own promoter (14), was cut with Not1 and ligated to obtained the pCla6 plasmid, carrying RAD53 without the 9myc epitope cassette. pCla6 was used in PCR-based site-directed mutagenesis to generate the mutations rad53-S175A/S175D, rad53-S375A/S375D, and rad53-S774A/S774D. Two complementary oligonucleotides (listed in supplemental Table S3) containing the selected mutation were used to amplify the plasmid pCla6. The PCR product was subsequently digested with DpnI to eliminate the wild type template, and the obtained DNA was used to transform E. coli DH5α cells. The resulting plasmids (listed in supplemental Table S2) were verified by DNA sequencing. This kind of mutagenesis has been described previously (15). To construct yeast strains, standard genetic procedures for transformation and tetrad analysis were followed.
Western Blot Analysis
The TCA protein extraction and the Western blot procedures have been described previously (15). The Rad53 protein was analyzed using the specific monoclonal antibodies (Mab.EL7) that we have recently produced (15). In some experiments (Fig. 3), the SDS-PAGE analysis was performed in a larger apparatus and at 4 °C, conditions that improved the separation of the Rad53 phosphorylated isoforms.
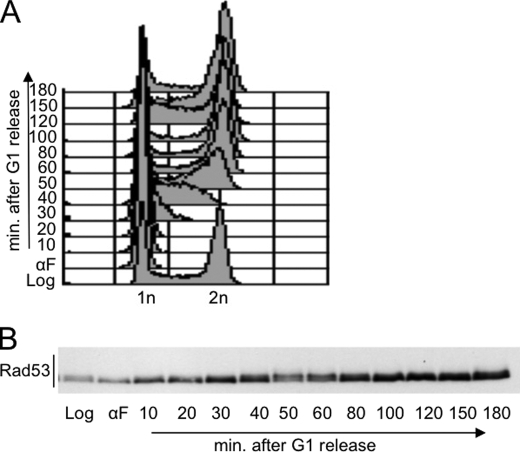
FIGURE 3.
Analysis of Rad53 phosphorylation throughout the cell cycle. Exponentially growing wild type cells were presynchronized in G1 by α-factor (αF) treatment and released from the G1 block in fresh YPD medium. Samples were taken at the indicated time points to test FACS profiles (A) and Rad53 phosphorylation by Western blot (B) with Mab.EL7 monoclonal antibodies.
In Vitro Dephosphorylation Assay
Crude extracts were prepared as described (14) and resuspended in λ phosphatase buffer with or without 4000 units of λ phosphatase (Biolabs). Samples were incubated for 30 min at 30 °C and resuspended in Laemmli buffer.
Cell Synchrony and Flow Cytometry
Cells were presynchronized in G1 with α-factor (2 μg/ml) and then released in fresh medium. Cells were arrested in G1 and G2/M with α-factor (10 μg/ml) or nocodazole (15 μg/ml), respectively. DNA content was analyzed by FACSCalibur (Bekton-Dickinson) and CellQuest software (Bekton-Dikinson).
RESULTS
CDK1 Promotes Rad53 Phosphorylation in Nocodazole-treated Cells
Several amino acid residues of the Rad53 protein have been found to be phosphoryated in vivo. Most of these modifications occur in cells treated with DNA damaging agents and likely drive full activation of the kinase toward specific substrates (5, 11). However, mass spectrometry analysis has revealed that some Rad53 residues are phosphorylated even in cells growing in unperturbed conditions, raising the possibility that Rad53 protein is regulated throughout a normal cell cycle (5, 11).
Interestingly, we observed a slower migrating Rad53 isoform in nocodazole-treated cells in the absence of any DNA damage (Fig. 1A). This nocodazole-induced Rad53 isoform is due to phosphorylation events because it is reverted by in vitro treatment with λ phosphatase (Fig. 1A). Our results, together with previous findings (7, 8, 12), indicate that Rad53 is phosphorylated in cells responding to spindle defects caused by the nocodazole treatment.
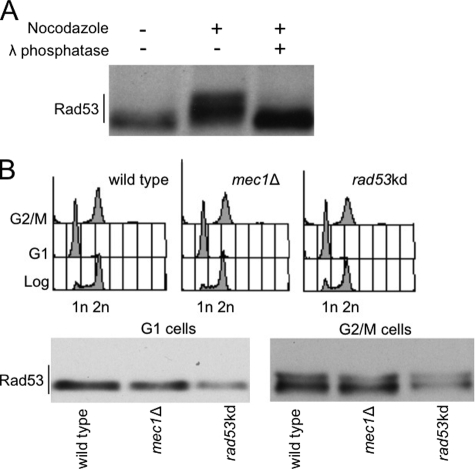
FIGURE 1.
Analysis of Rad53 phosphorylation in nocodazole-blocked cells. A, Western blot analysis of protein extracts prepared from wild type cells 3 h after treatment with/without nocodazole (15 μg/ml). Samples were treated with/without λ phosphatase before gel electrophoresis. Rad53 protein was tested with Mab.EL7 monoclonal antibodies. B, exponentially growing cells of wild type, mec1Δ, rad53-kd strains were treated for 3 h with α-factor (10 μg/ml) or nocodazole (15 μg/ml) to block cell cycle in G1 or G2/M, respectively. Samples were taken and processed to test FACS profiles and Rad53 phosphorylation by Western blot with Mab.EL7 monoclonal antibodies.
Because the genetic requirements for the nocodazole-induced Rad53 modification has not been extensively studied, we then analyzed whether such Rad53 modification was due to in trans phosphorylation events, mediated by specific kinases, or whether it had to be ascribed to autophosphorylation. Taking advantage of the biochemical features of the Rad53-K227A (Rad53-kd) kinase-defective protein variant (6), which receives in trans phosphorylation from the upstream kinase Mec1 but is defective in the autophosphorylation reaction (14), we analyzed Rad53 modification in mec1Δ or rad53-kd cells arrested with α-factor or nocodazole. Exponentially growing cells (Fig. 1B) have been blocked with α-factor or nocodazole in G1 or M phase, respectively; cell cycle arrest was confirmed by FACS analysis (Fig. 1B). By Western blotting, Rad53 does not show any gel mobility shift in G1-blocked cells. On the other hand, the protein is clearly phosphorylated in nocodazole-treated cells, and this modification is not mediated by Mec1, in agreement with previous indications (7), or by autophosphorylation events. In fact, the retarded form of Rad53 observed in metaphase-arrested cells is still evident in mec1Δ and rad53-kd cells.
It is known that nocodazole treatment causes spindle damage and cell cycle block in metaphase due to activation of the spindle assembly checkpoint (SAC)3 (16). SAC prevents activation of the anaphase-promoting complex, whose activity is required to inhibit Clb-CDK1; indeed, nocodazole-treated cells are characterized by high activity of the Clb-CDK1 complex (17). We thus decided to explore the possibility that Clb-CDK1 may be responsible for Rad53 phosphorylation in metaphase.
In S. cerevisiae, the catalytic subunit of the Clb-CDK1 complex is expressed by the essential gene CDC28 (18). We took advantage of the cdc28-as1 mutant, sensitive to the ATP analogue 1NMPP1 (19), to inhibit CDK1 activity in nocodazole-treated cells. Presently, this mutation, used in conjunction with the 1NMPP1 molecule, is the most tunable way to specifically switch off CDK1 activity. Indeed, it has been demonstrated that 1NMPP1 does not inhibit wild type CDK1 or other kinases at the experimental concentrations used, while specifically inhibiting CDK1 activity in the cdc28-as1 mutant background, that has been engineered to become a specific target of 1NMPP1 (19). cdc28-as1 exponentially growing cells were treated with nocodazole for 3 h to induce cell cycle arrest and phosphorylation of Rad53 protein. We then divided the cell culture in two parts and added 1NMPP1 to one-half to inhibit Cdc28 kinase. Western blot analysis (Fig. 2A) revealed that inhibition of CDK1 rapidly leads to the disappearance of phosphorylated Rad53 isoforms, indicating that CDK1 activity is required to maintain Rad53 phosphorylation in nocodazole-treated cells.
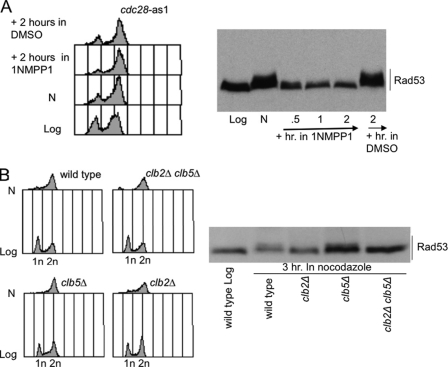
FIGURE 2.
CDK1 is required for nocodazole-induced Rad53 phosphorylation. A, exponentially growing cdc28-as1 cells were treated for 3 h (lane N) with nocodazole (15 μg/ml). 1NMPP1 (dissolved in dimethyl sulfoxide (DMSO)) was then added to one-half of the culture. Samples were taken at the indicated time points to test FACS profiles and Rad53 phosphorylation by Western blot with Mab.EL7 monoclonal antibodies. B, exponentially growing cell cultures of wild type and isogenic clb2Δ, clb5Δ, and clb2Δclb5Δ strains were treated for 3 h (N) with nocodazole (15 μg/ml). Samples were taken to test FACS profiles and Rad53 phosphorylation by Western blot with Mab.EL7 monoclonal antibodies.
CDK1 activity is cell cycle-regulated and its specificity toward substrates is determined by the association with various cyclin subunits (18). Cyclins Cln1–3 are specific for G1 phase, Clb5 and 6 are mainly required for DNA replication, and Clb1–4 are more important in G2/M and exit from mitosis. To determine which cyclin is involved in cells responding to nocodazole, we studied Rad53 phosphorylation in clb5Δ, clb2Δ, and clb2Δclb5Δ double mutants. Cells were treated with nocodazole for 3 h, and Rad53 phosphorylation was analyzed by Western blotting. We found that Rad53 phosphorylation is prevented in clb2Δ and clb2Δclb5Δ cells, but it is maintained in clb5Δ cells (Fig. 2B). Taken together, the results described in Fig. 2 indicate that CDK1 kinase, mainly associated with cyclin Clb2, is required for Rad53 phosphorylation in cells treated with nocodazole.
CDK1 Kinase Promotes Rad53 Phosphorylation in Metaphase, also in the Absence of Spindle Defects
The results described so far may indicate a direct effect of CDK1 on Rad53, or they might be explained by assuming that nocodazole treatment activates the SAC, and one or more kinases of this pathway may phosphorylate Rad53 in a CDK1-dependent manner. Indeed, mutations in spindle assembly checkpoint factors, such as mad2Δ and bub2Δ, prevent nocodazole-induced Rad53 phosphorylation (7). However, abrogation of the spindle assembly checkpoint through specific mutations also eliminates the cell cycle block at metaphase caused by nocodazole treatment, preventing the possibility to maintain high levels of Clb-CDK1 activity. Moreover, previous work (7) failed to identify a kinase among the spindle assembly checkpoint factors that is responsible for the nocodazole-induced Rad53 phosphorylation. Thus, we investigated the possibility that CDK1 may directly induce Rad53 phosphorylation at the metaphase-anaphase transition independently on SAC activation. To visualize CDK1-dependent Rad53 phosphorylation through a normal cell cycle, wild type cells were synchronized in G1 by α-factor treatment and released into fresh medium without the pheromone. Cell cycle progression after the release from the G1 block was followed by FACS analysis (Fig. 3A). Rad53 protein level and its modifications were analyzed (Fig. 3B) using the highly specific Mab.EL7 monoclonal antibody that we recently described (15).
Fig. 3B shows that the monoclonal antibody Mab.EL7 detects a modification of Rad53 in samples taken at different time points during an unperturbed cell cycle. The slower migrating isoform of Rad53 is accumulated at time points corresponding to the G2/M transition, and it is not visualized in G1 and at the early stage of S phase, as supported by the FACS profiles (Fig. 3A). The possibility that this cell cycle-dependent Rad53 modification may be associated to the kinase activity of Rad53 itself is unlikely. In fact, by using a highly sensitive in situ assay, we and others (8, 14) failed to gain any evidence indicating that Rad53 kinase activity may fluctuate during an unperturbed cell cycle.
The cell cycle-dependent modification of Rad53 at G2/M under unperturbed conditions, suggests that the Rad53 phosphorylated form detected after nocodazole treatment is simply related to a G2/M cell cycle block, rather than to SAC activation. Therefore, we assumed that the accumulation of the CDK1-dependent phosphorylated form of Rad53 could be better visualized in a cell population uniformly arrested in mitosis.
To block cell cycle in metaphase without triggering the SAC, we took advantage of a genetic system in which we can deplete Cdc20 by switching off the expression of the corresponding gene. Indeed, Cdc20 is an unstable protein required to activate anaphase-promoting complex and drive the exit from mitosis (20). In this strain, expression of CDC20 under the control of the inducible GAL1 promoter is repressed by addition of glucose into the culture medium. Because Cdc20 is one of the final targets of the spindle assembly checkpoint cascade, this genetic system is widely used to block cell cycle progression in metaphase, mimicking the effect of the spindle assembly checkpoint activation without generating any damage to the spindle (20).
Addition of glucose to exponentially growing GAL1::CDC20 cells represses the CDC20 expression, leading to a progressive accumulation of cells in metaphase due to the inability to activate anaphase-promoting complex as a consequence of Cdc20 depletion. At different times after glucose addition, samples were taken to monitor FACS profiles and Rad53 protein levels and modifications. Fig. 4A shows that Rad53 phosphorylation correlates with the accumulation of cells in mitosis. Moreover, treatment with nocodazole 6 h after Cdc20 depletion only led to a mild but noticeable increase in the fraction of cells blocked in M phase and in the intensity of the Rad53-phosphorylated band. These results suggest that the SAC is not involved in phosphorylating Rad53 in mitosis, whereas it sustains the idea that CDK1, whose activity is high in CDC20-depleted cells, is directly involved in phosphorylating Rad53.
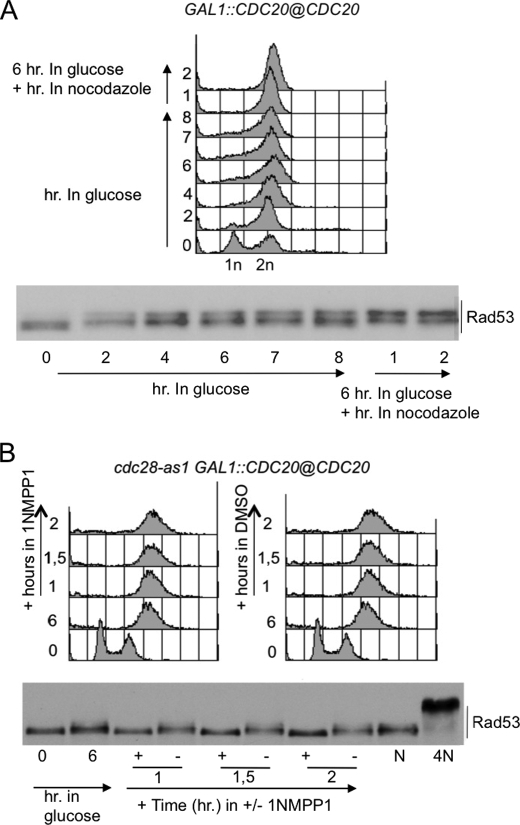
FIGURE 4.
CDC20 shut-off leads to CDK1-dependent Rad53 phosphorylation in metaphase. A, yeast extract peptone and galactose cells of the GAL1::CDC20, carrying endogenous CDC20 gene under GAL1 promoter, were treated with 2% glucose to shut off the GAL1 promoter. 6 h after glucose addition, a small aliquot of the culture was treated with nocodazole (15 μg/ml). Samples were taken at the indicated time points to test FACS profiles and Rad53 phosphorylation by Western blot with Mab.EL7 monoclonal antibodies. B, yeast extract peptone and galactose cells of the cdc28-as1 GAL1::CDC20, were treated with 2% glucose to shut off the GAL1 promoter. 6 h after glucose addition, 1NMPP1 (dissolved in dimethyl sulfoxide (DMSO)) was added to one-half of the culture. Aliquots of the culture were treated with nocodazole (15 μg/ml for 3 h; lane N) or 4NQO (2 μg/ml for 30 min., lane 4N). Samples were taken at the indicated time points to test FACS profiles and Rad53 phosphorylation by Western blot with Mab.EL7 monoclonal antibodies.
To test whether the Rad53 phosphorylation detected in cells blocked by CDC20 depletion was due to CDK1 activity, we repeated the previous experiment in a GAL1::CDC20 strain carrying the cdc28-as1 mutation (Fig. 4B). 6 h after the addition of glucose, the culture was split into two, and the 1NMPP1 inhibitor was added to half of the culture. Fig. 4B clearly shows that inactivation of CDK1 prevents Rad53 phosphorylation in cells arrested in mitosis. The findings described above indicate that high activity of the Clb-CDK1 kinase complex in mitosis promotes Rad53 phosphorylation, similarly to what is found in nocodazole-treated cells, also suggesting that Rad53 phosphorylation induced by nocodazole treatment is due, at least partially, to CDK1 activity and ruling out the possibility that it requires activation of the spindle assembly checkpoint (7).
Mutagenesis of Proline-directed Sites in Rad53
CDK1 consensus phosphorylation sites are serine or threonine residues followed by a proline (SP/TP). Mass spectrometry analysis revealed that Rad53 is phosphorylated at three proline-directed sites: Ser175, Ser375, and Ser774 (5, 11), supporting our data suggesting that CDK1 directly phosphorylates Rad53 in metaphase. In agreement with this hypothesis, Xenopus p34, the frog homolog of Cdc28, phosphorylates Rad53 in vitro (5).
We explored whether one or more of the three proline-directed sites indicated above are responsible for Rad53 phosphorylation in mitotic cells. We mutagenized the three sites to alanine or aspartate to mimic the nonphosphorylatable or the constitutively phosphorylated state, respectively, generating the rad53-S175A, rad53-S375A, rad53-S774A, rad53-S175D, rad53-S375D, and rad53-S774D alleles.
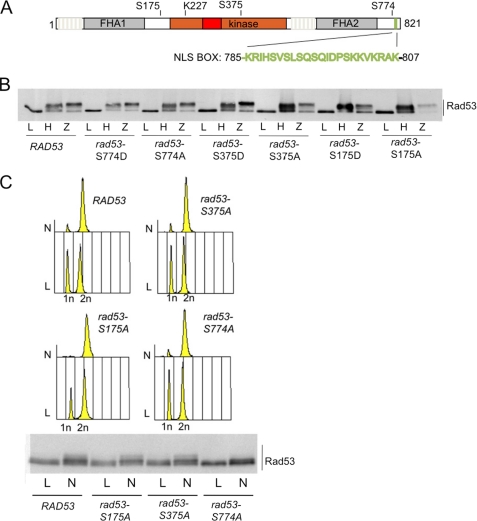
First, we analyzed the level and the activity of the mutant Rad53 proteins in cells growing in normal condition or experiencing DNA damage (zeocine treatment) or replication stress (hydroxyurea treatment). Fig. 5B shows that none of the alleles display any significant change in Rad53 protein level in exponentially growing conditions, and the mutations do not affect Rad53 phosphorylation in response to DNA damage or replication stress. We then tested the effect of these site-specific mutations on the phosphorylation of Rad53 in cells arrested in mitosis by nocodazole treatment. We found that although the Rad53-S175A and Rad53-S375A mutant forms are phosphorylated as the wild type protein in nocodazole-treated cells, the Rad53-S774A form is not (Fig. 5C). In nocodazole-blocked cells, Rad53-S774A protein variant lost the hyperphosphorylation shift in the Western blotting analysis, although we cannot rule out completely the possibility that the Rad53-S774A variant may still retain a minor modification that proved to be difficult to be visualized under our gel electrophoresis conditions.
FIGURE 5.
CDK1 phosphosites mutagenesis of RAD53. A, schematic representation of Rad53 protein showing the phosphorylatable proline-directed serine sites (Ser175, Ser375, and Ser774). Relevant Rad53 domains are indicated as follows: gray boxes indicate the fork head domains (FHA1 and FHA2); the red box inside the kinase domain (brown) represents the activation segment; the green box at the carboxyl end of the protein represents a bipartite nuclear localization signal. B, analysis of the phosphorylation state of Rad53 protein in various rad53 alleles, as indicated. Exponentially growing cell cultures of rad53Δ sml1Δ strains, carrying the indicated rad53 allele under its own promoter on a centromeric plasmid, were treated for 3 h with hydroxyurea (200 mm, lane H) or for 30 min with zeocine (50 μg/ml, lane Z). Samples were taken to test Rad53 phosphorylation by Western blot with Mab.EL7 monoclonal antibodies. Please note that lane Z for rad53-S175D was accidentally underloaded. C, exponentially growing cell cultures of rad53Δ sml1Δ strains, carrying the indicated rad53 allele under its own promoter on a centromeric plasmid, were treated for 3 h (N) with nocodazole (15 μg/ml). Samples were taken to test FACS profiles and Rad53 phosphorylation by Western blot with Mab.EL7 monoclonal antibodies.
This finding suggests that in mitotic cells, Rad53 is mainly phosphorylated by CDK1 on serine 774 and that S774 phosphorylation is responsible for the major Rad53 electrophoretic mobility shift observed in nocodazole-treated cells. To explore the functional role of the CDK1-dependent phosphorylation of Rad53, we analyzed the sensitivity of the rad53 mutant strains to different toxic agents.
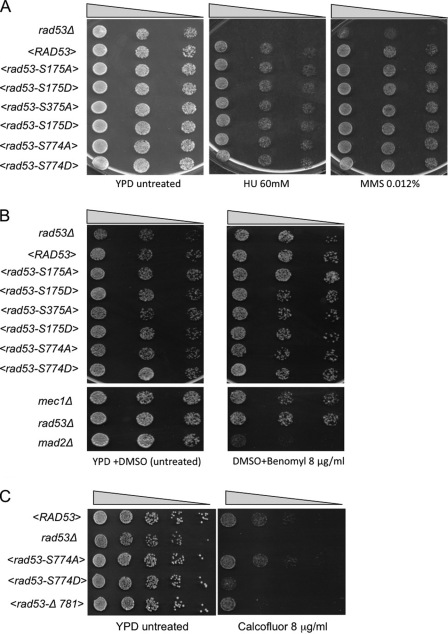
Serial dilutions of cells of the various rad53 strains were plated in the presence of different concentrations of hydroxyurea treatment or methyl metanesulfonate (a DNA-alkylating agent). Wild type or rad53Δ strains were used as controls in the plates. The plates were analyzed after 3 days of incubation at 28 °C (Fig. 6A). We found that none of the rad53 alleles tested were sensitive to hydroxyurea treatment or methyl metanesulfonate. This result is in agreement with our previous finding (Fig. 5B), indicating that the various Rad53 mutations do not prevent Rad53 activation following DNA damage and replication stress.
FIGURE 6.
Viability of CDK1-phosphosites rad53 mutants in different stress conditions. Drop test analysis of serial 5-fold dilutions of exponentially growing cell cultures of rad53Δ sml1Δ strains, carrying the indicated rad53 allele under its own promoter on a centromeric plasmid. Isogenic mec1Δ, mad2Δ, and rad53-Δ781 strains were also tested as indicated. A, B, and C, cells were plated on YPD and YPD plus hydroxyurea (HU), methyl methanesulfonate (MMS), benomyl, or calcofluor white at the indicated concentrations. Plates were incubated 3 days at 28 °C (A and C) or 16 °C (B).
By a similar approach, we then tested the sensitivity to benomyl, a spindle-damaging agent. In this case, as controls we used mec1Δ and mad2Δ strains (Fig. 6B). As shown previously (21), mad2Δ cells are sensitive to spindle damage, whereas strains mutated in the MEC1 and RAD53 kinases do not cause any sensitivity. We found also that the CDK1-phosphosite mutants of Rad53 are not sensitive to benomyl, ruling out the possibility that CDK1-dependent phosphorylation of Rad53 might have a functional role in the spindle assembly checkpoint.
Recently, Rad53 was implicated in morphogenesis (9). The relationship between the function of Rad53 in the DNA damage checkpoint and its role in cellular morphogenesis is not yet understood. Moreover, the observation that the Mec1 kinase and other DNA damage checkpoint factors are not involved in cellular morphogenesis (9, 10), leads to the hypothesis that Rad53 function in this pathway is separated from its role in response to DNA damage. Because serine 774 is the main CDK1-dependent modification of Rad53 in metaphase, we tested the sensitivity of the rad53-S774A and rad53-S774D mutant strains to calcofluor white. This agent interferes with cell wall synthesis and cellular morphogenesis (9). Serial dilutions of cells were plated in the presence of various concentrations of calcofluor white, and the plates were incubated at 28 °C for 3 days. rad53Δ mutants are very sensitive to morphogenetic stress, as reported previously (9). Surprisingly, we found that phospho-mimicking rad53-S774D mutant cells are sensitive to calcofluor white, albeit less than rad53Δ cells, whereas rad53-S774A cells are not (Fig. 6C). We also found that rad53-Δ781, expressing a truncated variant of Rad53 protein that lacks the NLS motif (5, 6), is also sensitive to calcofluor white.
The results described in Fig. 6 suggest that CDK1-dependent phosphorylation of Rad53 does not play any role in the response to DNA or spindle damage. Moreover, the sensitivity of rad53-S774D mutant to calcofluor rises the possibility that CDK1-dependent phosphorylation of Rad53 may influence Rad53 function during morphogenesis and/or in the response to morphogenesis stress.
DISCUSSION
Biochemical and genetic analysis have shown that Mec1 and/or Tel1, the upstream kinases in the checkpoint cascade, phosphorylate Rad53 at multiple SQ/TQ residues, through the action of a mediator protein (Rad9 or Mrc1), which likely promote the optimal interaction between Rad53 and the phosphatidylinositol 3-kinase-related kinases (1, 3, 22). The in trans phosphorylation of Rad53 is followed by its autophosphorylation, leading to full activation of the kinase in the presence of DNA damage or replication stress.
Whether Rad53 is activated in unperturbed exponentially growing cells was an argument of discussion: on one hand, mutations abrogating the catalytic activity of Rad53 cause cell lethality (23), suggesting that its kinase activity is essential in unperturbed conditions; on the other hand, the methods currently used to measure Rad53 kinase activity do not reveal any active Rad53 form in unperturbed exponentially growing cells (14, 15). One possible interpretation is that the procedures to test Rad53 kinase activity are not sensitive enough to detect a very low level of activity. Hence, most of the details leading to phosphorylation and activation of Rad53 have been analyzed in response to DNA damage and replication stress, and the regulation of Rad53 in untreated exponentially growing cells is much less studied.
Recently, accurate mass spectrometry analysis revealed that Rad53 is phosphorylated at SP/TP residues in exponentially growing cells (5, 11), suggesting that during an unperturbed cell cycle, Rad53 may be the target of a kinase different from Mec1 and Tel1 phosphatidylinositol 3-kinase-related kinases. Here, we provide genetic and biochemical evidence that CDK1 phosphorylates Rad53 at the G2/M transition in an unperturbed cell cycle, and this modification is clearly visible in metaphase-arrested cells (Figs. 2 and 3). These findings support the hypothesis that CDK1 is at least one of the kinases modifying Rad53 in exponentially growing conditions.
Previous observations (7) led to the hypothesis that an undescribed kinase, likely activated by the SAC pathway, phosphorylates Rad53 in response to spindle damage. Our findings indicate that the Rad53 phosphorylation detected in mitotic cells does not require DNA damage or spindle checkpoint pathways but, instead, it is a metaphase-anaphase event carried out by CDK1, which targets the serine 774 residue of Rad53. We also found that inactivation of CDK1 abrogates Rad53 phosphorylation observed after spindle damage (Fig. 2). Although alternative hypothesis can be suggested, we favor the idea that CDK1 phosphorylates Rad53 at metaphase and that the Rad53 phosphorylation observed in cells with damaged spindles is possibly due to the fact that the SAC pathway blocks the cell cycle at the metaphase-anaphase transition with high Clb-CDK1 activity. Moreover, we failed to observe any sensitivity to benomyl in various rad53 mutant cells (Fig. 6), and there is no evidence that Rad53 activity is required in response to spindle damage.
What is the functional significance of the cell cycle-dependent phosphorylation of Rad53 mediated by CDK1? Interestingly, rad53-S774D mutant cells, in which the main CDK1 phosphorylation site mimics a constitutively phosphorylated state, are sensitive to calcofluor white (Fig. 6), suggesting morphogenetic dysfunctions. Because the Ser774 residue is the main target for the CDK1-dependent Rad53 phosphorylation at metaphase, it is tempting to speculate that CDK1 may regulate Rad53 to orchestrate cellular morphogenesis during the cell cycle. Surprisingly, the nonphosphorylatable rad53-S774A mutant allele does not show any calcofluor sensitivity. Opposing phenotypes of mutations altering the phosphorylation of a specific residue have been observed in the regulation of kinases (24) and of other relevant proteins (for an example, see Ref. 25). Such an effect has been extensively characterized biochemically for certain protein kinases (24), whereas for most proteins, the biochemical details remain obscure. Concerning the effect of opposite mutations at the Ser774 residue of Rad53, an attractive hypothesis may be related to the location of the phosphorylatable residue, which is positioned near the NLS motif (amino acids 781–801; see Fig. 4A). We speculate that such a residue may influence the subcellular localization of Rad53 or its interaction with specific cellular structures. Interestingly, the Rad53-Δ781 mutant protein, which lacks the NLS (5, 6), is not phosphorylated in metaphase and in the presence of DNA damage (supplemental Fig. S1). Moreover, rad53-Δ781 cells are sensitive to calcofluor white, DNA damage, and replication stress (Fig. 6C and see Ref. 10). It is possible that mimicking the constitutive phosphorylation of Ser774, the rad53-S774D mutation may deregulate the dynamics of Rad53 localization or intracellular interactions. However, because Rad53-S774D is properly activated in response to genotoxic stress (Fig. 5), and mutant cells are not sensitive to DNA damage (Fig. 6), we have to infer that in this mutant, a sufficient amount of Rad53 is localized in the nucleus. Direct microscopic examination of wild type and mutant Rad53 tagged with green fluorescent protein or other epitopes suitable for immunofluorescence analysis, failed to detect any evident alteration in the partition of Rad53 between the cytoplasm and the nucleus.4 Rad53 is an abundant protein that appears diffusely stained both in the cytoplasm and in the nucleus (10). Partial relocalization or accumulation of Rad53 to specific cellular compartments or structures is thus difficult to test by microscopic analysis.
Recently, it was shown that Rad53 interacts with septins (10) and regulates the phosphorylation and timely degradation of Swe1 (9), which is the main kinase involved in the morphogenesis checkpoint controlling bud emergence and growth (26). It will be a challenge for further studies to investigate whether the rad53-S774A/S774D mutations will influence Swe1 level and function in the cell, perhaps causing abnormalities in the cell wall and/or bud formation.
The interaction of Rad53 with other proteins localized at specific cellular structures may be addressed by genetic screenings to identify synthetic interactions between the rad53-S774D/S774A mutant alleles and mutations in other yeast genes. Alternatively, the Rad53 interactors so far identified by tandem affinity purification-tagging methods (10), can be challenged for their biochemical and genetic interactions with the Rad53-S774D/S774A protein variants. In conclusion, our findings indicate that CDK1 phosphorylates Rad53 in metaphase, and such modification may influence its role in modulating morphogenetic events, also supporting the notion of a molecular link between cell growth and genome integrity checkpoints (9, 10).
Supplementary Material
Acknowledgments
We thank M. Foiani and S. Piatti for material and reagents. We also thank A. Nespoli, P. Grianti, F. Lazzaro, F. Puddu, and M. Giannattasio for discussions and Istituto FIRC di Oncologia Molecolare-Istituto Europeo di Oncologia Campus facilities and services for the production of monoclonal antibodies and DNA sequencing.
This work was supported by grants from Associazione Italiana Ricerca sul Cancro, Fondazione Cariplo, the European Union FP6 Integrated Project DNA Repair, and Ministero dell'Istruzione, dell'Universita' d della Ricerca (to A. P., M. M.-F., and P. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3 and Fig. S1.
L. Diani and A. Pellicioli, unpublished observations.
- SAC
- spindle assembly checkpoint
- FACS
- fluorescence-activated cell sorter
- kd
- kinase defective.
REFERENCES
- 1.Stracker T. H., Usui T., Petrini J. H. (2009) DNA Repair 8, 1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartek J., Falck J., Lukas J. (2001) Nat. Rev. Mol. Cell Biol. 2, 877–886 [DOI] [PubMed] [Google Scholar]
- 3.Pellicioli A., Foiani M. (2005) Curr. Biol. 15, R769–771 [DOI] [PubMed] [Google Scholar]
- 4.Hammet A., Pike B. L., McNees C. J., Conlan L. A., Tenis N., Heierhorst J. (2003) IUBMB Life 55, 23–27 [DOI] [PubMed] [Google Scholar]
- 5.Smolka M. B., Albuquerque C. P., Chen S. H., Schmidt K. H., Wei X. X., Kolodner R. D., Zhou H. (2005) Mol. Cell Proteomics 4, 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng P., Fay D. S., Burton J., Xiao H., Pinkham J. L., Stern D. F. (1993) Mol. Cell. Biol. 13, 5829–5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clémenson C., Marsolier-Kergoat M. C. (2006) Mol. Cell. Biol. 26, 9149–9161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tercero J. A., Longhese M. P., Diffley J. F. (2003) Mol. Cell 11, 1323–1336 [DOI] [PubMed] [Google Scholar]
- 9.Enserink J. M., Smolka M. B., Zhou H., Kolodner R. D. (2006) J. Cell Biol. 175, 729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smolka M. B., Chen S. H., Maddox P. S., Enserink J. M., Albuquerque C. P., Wei X. X., Desai A., Kolodner R. D., Zhou H. (2006) J. Cell Biol. 175, 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweeney F. D., Yang F., Chi A., Shabanowitz J., Hunt D. F., Durocher D. (2005) Curr. Biol. 15, 1364–1375 [DOI] [PubMed] [Google Scholar]
- 12.Ira G., Pellicioli A., Balijja A., Wang X., Fiorani S., Carotenuto W., Liberi G., Bressan D., Wan L., Hollingsworth N. M., Haber J. E., Foiani M. (2004) Nature 431, 1011–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enserink J. M., Hombauer H., Huang M. E., Kolodner R. D. (2009) J. Cell Biol. 185, 423–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pellicioli A., Lucca C., Liberi G., Marini F., Lopes M., Plevani P., Romano A., Di Fiore P. P., Foiani M. (1999) EMBO J. 18, 6561–6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorani S., Mimun G., Caleca L., Piccini D., Pellicioli A. (2008) Cell Cycle 7, 493–499 [DOI] [PubMed] [Google Scholar]
- 16.Lew D. J., Burke D. J. (2003) Annu. Rev. Genet. 37, 251–282 [DOI] [PubMed] [Google Scholar]
- 17.Hoyt M. A., Totis L., Roberts B. T. (1991) Cell 66, 507–517 [DOI] [PubMed] [Google Scholar]
- 18.Nasmyth K. (1996) Trends Genet. 12, 405–412 [DOI] [PubMed] [Google Scholar]
- 19.Bishop A. C., Ubersax J. A., Petsch D. T., Matheos D. P., Gray N. S., Blethrow J., Shimizu E., Tsien J. Z., Schultz P. G., Rose M. D., Wood J. L., Morgan D. O., Shokat K. M. (2000) Nature 407, 395–401 [DOI] [PubMed] [Google Scholar]
- 20.Lim H. H., Goh P. Y., Surana U. (1998) Curr. Biol. 8, 231–234 [DOI] [PubMed] [Google Scholar]
- 21.Li R., Murray A. W. (1991) Cell 66, 519–531 [DOI] [PubMed] [Google Scholar]
- 22.Stracker T. H., Usui T., Petrini J. H. (2009) DNA Repair (Amst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fay D. S., Sun Z., Stern D. F. (1997) Curr. Genet. 31, 97–105 [DOI] [PubMed] [Google Scholar]
- 24.Johnson L. N., Noble M. E., Owen D. J. (1996) Cell 85, 149–158 [DOI] [PubMed] [Google Scholar]
- 25.Downs J. A., Lowndes N. F., Jackson S. P. (2000) Nature 408, 1001–1004 [DOI] [PubMed] [Google Scholar]
- 26.Lew D. J. (2003) Curr. Opin. Cell Biol. 15, 648–653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.