Abstract
Azoles inhibit ergosterol biosynthesis, resulting in ergosterol depletion and accumulation of toxic 14α-methylated sterols in membranes of susceptible yeast. We demonstrated previously that miconazole induces actin cytoskeleton stabilization in Saccharomyces cerevisiae prior to induction of reactive oxygen species, pointing to an ancillary mode of action. Using a genome-wide agar-based screening, we demonstrate in this study that S. cerevisiae mutants affected in sphingolipid and ergosterol biosynthesis, namely ipt1, sur1, skn1, and erg3 deletion mutants, are miconazole-resistant, suggesting an involvement of membrane rafts in its mode of action. This is supported by the antagonizing effect of membrane raft-disturbing compounds on miconazole antifungal activity as well as on miconazole-induced actin cytoskeleton stabilization and reactive oxygen species accumulation. These antagonizing effects point to a primary role for membrane rafts in miconazole antifungal activity. We further show that this primary role of membrane rafts in miconazole action consists of mediating intracellular accumulation of miconazole in yeast cells.
Introduction
The class of azole antimycotics constitutes the largest group of synthetic antifungal therapeutics currently in clinical use. The generally accepted mode of antifungal action of azoles is the inhibition of ergosterol biosynthesis arising from a multimechanistic process initiated by the inhibition of two cytochrome P450 enzymes involved in ergosterol biosynthesis, namely the P450 enzyme that catalyzes the lanosterol 14α-demethylation step and the P450 enzyme that catalyzes Δ22 desaturation (1). Azole treatment results in predominance of 14α-methylated sterols and inhibition of subsequent reactions of the ergosterol biosynthesis pathway (1). Apart from inhibition of ergosterol biosynthesis, miconazole induces accumulation of reactive oxygen species (ROS)3 in susceptible fungi, leading to fungal cell death (2, 3). Moreover, we have demonstrated that miconazole induces actin stabilization prior to this ROS accumulation (4). These data point to an ancillary mode of action for this azole, as was already suggested in the 1970s (5).
To obtain further mechanistic insight in the mode of antifungal action of miconazole, we screened in this study the complete haploid collection of 4853 Saccharomyces cerevisiae deletion mutants, individually deleted for nonessential genes, for resistance to miconazole on solid medium. Using this strategy, we demonstrate that S. cerevisiae mutants affected in sphingolipid and ergosterol biosynthesis are resistant to miconazole, suggesting a possible involvement of membrane rafts in the mode of antifungal action of miconazole. These rafts are membrane patches that are enriched in sphingolipids and ergosterol and that are thought to compartmentalize the plasma membrane and to have an important role in cell signaling (6). We investigated the effect of membrane raft-disturbing compounds on (i) miconazole antifungal activity, (ii) miconazole-induced actin cytoskeleton stabilization, and (iii) miconazole-induced ROS accumulation. Furthermore, using HPLC analysis, we investigated the effect of membrane raft disruption on intracellular accumulation of miconazole in yeast cells.
EXPERIMENTAL PROCEDURES
Materials, Yeast Strains, Plasmids, and Growth Media
Miconazole and methyl-β-cyclodextrin (MβCD) were purchased from Sigma. Edelfosine was a kind gift from Prof. Christopher McMaster (Atlantic Research Centre, Dalhousie University, Halifax, Canada). Acetonitrile was purchased from Fisher (Leicestershire, United Kingdom). The yeast strains used were S. cerevisiae strain BY4741 (wild-type (WT)) and the BY4741-derived deletion mutant library (Invitrogen). These yeast strains were cultivated in yeast/peptone/dextrose (YPD; 1% yeast extract, 2% peptone, and 2% glucose). The plasmid encoding green fluorescent protein (GFP)-tagged Pma1p was a kind gift of Prof. Annick Breton (7). Yeast strains transformed with this plasmid were cultured in 0.8 g/liter complete amino acid supplement mixture minus uracil (Bio 101, Inc.), 6.5 g/liter yeast nitrogen base, and 20 g/liter glucose.
Screening of a Yeast Deletion Mutant Library for Miconazole Resistance
The individual yeast deletion mutants were grown in 96-well microtiter plates containing 100 μl of YPD. After 48 h of incubation at 30 °C, the individual deletion mutants were spotted on YPD-agar plates containing 10 μg/ml miconazole using a 96-pin replicator for identification of miconazole-resistant yeast deletion mutants. After 48–72 h of incubation at 30 °C, plates were scored, and resistant mutants were identified. Miconazole-resistant mutants were reassessed using the assay described below.
Quantification of Miconazole Resistance of the Selected Yeast Mutant
5-μl samples of 5-fold serial dilutions of each yeast cell culture (grown to stationary phase in YPD in microtiter plates) were spotted on YPD plates containing 0 or 10 μg/ml miconazole. Growth was assessed after 48 h of incubation at 30 °C.
Analysis of Membrane Raft-disturbing Activity of Edelfosine and Miconazole
Membrane rafts were monitored using Pma1p as a marker protein (8) by Western blotting and fluorescence microscopy. To this end, membrane rafts were isolated according to a reported isolation method (8–13). Briefly, a logarithmically growing S. cerevisiae culture in YPD (A600 = 2.0) was incubated with miconazole (0 or 10 μg/ml) or edelfosine (50 μg/ml) for 3 h. Ten A600 units of cells were lysed with glass beads, and samples were split into two fractions: a homogenate and a second fraction that was incubated with 1% Triton X-100 for 30 min on ice. The detergent-treated sample was centrifuged at 100,000 × g for 1 h to yield a detergent-resistant pellet and a soluble fraction. Proteins were precipitated with trichloroacetic acid and analyzed by gel electrophoresis and immunoblotting using an antibody against Pma1p. Additionally, a logarithmically growing S. cerevisiae culture transformed with a plasmid containing GFP-tagged Pma1p was incubated with either 0 or 10 μg/ml miconazole or 50 μg/ml edelfosine for 3 h. In vivo localization of GFP-tagged Pma1p was performed by fluorescence microscopy using a Zeiss Axioplan 2 (Carl Zeiss, Oberkochen, Germany) equipped with an AxioCam charge-coupled device camera and AxioVision 3.1 software. At least 100 cells were monitored for each condition. Experiments were repeated at least three times, and data are means of duplicate measurements.
Influence of Membrane Raft-disturbing Agents on Miconazole Activity
A S. cerevisiae overnight culture in YPD was diluted to a final concentration of 106 cells/ml in phosphate-buffered saline (PBS), followed by the addition of various concentrations of miconazole and edelfosine or MβCD. After 4.5 h of incubation at 30 °C, viability of the yeast culture was assessed by counting the number of colony-forming units on YPD-agar plates after 24 h of incubation. Percentage survival was calculated as the ratio of the number of colony-forming units after treatment to the number of colony-forming units after the Me2SO (control) treatment. Experiments were repeated at least three times, and data are means of duplicate measurements.
Fluorescence Microscopy for Visualization of the Actin Cytoskeleton
Rhodamine-phalloidin staining was performed as described previously for F-actin (14, 15).
Influence of Membrane Raft-disturbing Agents on ROS Accumulation Induced by Miconazole
A logarithmically growing S. cerevisiae culture in YPD (A600 = 2.0) was washed and resuspended in PBS in the presence of 0 or 10 μg/ml miconazole in combination with various concentrations of edelfosine or MβCD. After 1 h of incubation at 30 °C, 10 μm 2′,7′-dichlorofluorescin diacetate (Molecular Probes, Eugene, OR) was added (2). The number of fluorescent yeast cells was determined by fluorescence microscopy (Nikon Optiphot microscope; excitation at 485 nm and emission at 525 nm). Experiments were repeated at least three times, and data are means of duplicate measurements.
Quantitative Analysis of Intracellular Accumulation of Miconazole in Yeast Cells
An overnight S. cerevisiae WT culture in YPD (∼108 cells/ml) was washed and resuspended in PBS (pH 7.4). 100 μg/ml miconazole with or without 500 μg/ml edelfosine or 20 mg/ml MβCD was added to 500 μl of the above culture. To analyze the intracellular miconazole accumulation in ipt1 deletion mutant cells, S. cerevisiae WT and ipt1 deletion mutant cells were treated with miconazole but without the addition of edelfosine. After 2.5 h of incubation at 30 °C with shaking, the supernatant of the yeast cultures was collected. The cell pellet was washed three times with PBS, followed by the addition of 300 μl of 70% acetonitrile and 30% PBS. The cells were lysed using a Phastprep reciprocal shaker (Bio 101, Inc.), and the lysate was clarified by centrifugation (5 min at 3000 rpm). The miconazole concentration in both the supernatant and cell lysates was determined using HPLC based on a miconazole standard series ranging from 10 to 100 μg/ml. The HPLC system consisted of a LaChrom® L-7100 HPLC pump, a Model L-7420 UV detector set at 260 nm, an L-7200 programmable autosampler, and a D-7000 interface (Hitachi, Tokyo, Japan). 20-μl samples were injected twice. UV signals were monitored, and peaks were integrated using the D-7000 HSM software (Hitachi). The separation of miconazole was performed on a SunFire C18 3.5-μm column (4.6 × 100 mm; Waters) equilibrated with 70:30 (v/v) acetonitrile/water. The column was eluted in an isocratic way at 1.0 ml/min. Experiments were repeated at least three times, and data are means of duplicate measurements.
Statistical Analysis
Statistical analysis was performed using the unpaired t test.
RESULTS
Identification of Miconazole-resistant Yeast Deletion Mutants
To obtain more mechanistic insight in the antifungal mode of action of miconazole, we started our study by screening a S. cerevisiae deletion mutant library for resistance to miconazole by replica plating on miconazole-containing YPD-agar plates. This deletion mutant library consists of single-gene knock-outs in the S. cerevisiae BY4741 parental strain (WT) and covers all 4835 open reading frames encoding nonessential proteins.
First, we determined the minimal inhibitory concentration of miconazole for the WT strain in YPD-agar plates as 1 μg/ml. Second, screening for miconazole-resistant deletion mutants was performed on YPD-agar plates containing 10 times the minimal inhibitory concentration, i.e. 10 μg/ml miconazole. Using this genome-wide approach, 12 deletion mutants with at least 10-fold increased resistance to miconazole were identified (Table 1). Two major functional gene groups could be identified: genes involved in (i) sphingolipid and ergosterol biosynthesis and (ii) mitochondrial function. Additionally, SIP3 and ADH1 were identified, encoding a transcription factor and an alcohol dehydrogenase, respectively. Moreover, open reading frames encoding hypothetical proteins were also identified as miconazole sensitivity genes. Resistance of the individual mutants was confirmed and quantified using yeast dilutions on agar with and without miconazole (Fig. 1).
TABLE 1.
Genes that result in miconazole resistance upon deletion in S. cerevisiae
| Gene | ORFa | Description of gene product |
|---|---|---|
| Ergosterol and sphingolipid biosynthesis | ||
| ERG3 | YLR056W | C-5 sterol desaturase, catalyzes the introduction of a C(5)-C(6) double bond into episterol, a precursor in ergosterol biosynthesis |
| SKN1 | YGR143W | Protein involved in sphingolipid biosynthesis |
| IPT1 | YDR072C | Inositol phosphotransferase 1, involved in synthesis of mannosyldiinositol phosphorylceramide |
| SUR1 | YPL057C | Probable catalytic subunit of mannosylinositol phosphorylceramide synthase |
| Mitochondrial function | ||
| PTH1 | YHR189W | One of two mitochondrially localized peptidyl-tRNA hydrolases |
| MRPL23 | YOR150W | Mitochondrial ribosomal protein of the large subunit |
| YDR114C | Hypothetical protein; deletion mutant is respiratory-deficient | |
| Gene expression | ||
| SIP3 | YNL257C | Protein that activates transcription through interaction with DNA-bound Snf1p, potential Cdc28p substrate |
| Varia | ||
| ADH1 | YOL086C | Alcohol dehydrogenase |
| YOR292C | Hypothetical protein | |
| YDR068W | Hypothetical protein | |
| YPL056C | Hypothetical protein | |
a Open reading frame.
FIGURE 1.
S. cerevisiae deletion mutants that are miconazole-resistant. 5-μl samples of 5-fold serial dilutions of each yeast culture (rows) were spotted on YPD plates containing 0 μg/ml miconazole (left panel) and 10 μg/ml miconazole (right panel). Plates were incubated at 30 °C for 48 h.
Because miconazole induces ROS accumulation in susceptible fungi (2–4), it is not surprising that we identified yeast deletion mutants affected in mitochondrial function to be resistant to miconazole. Hence, in this study, we focused on the class of miconazole sensitivity genes involved in sphingolipid and ergosterol biosynthesis, i.e. IPT1, SKN1, SUR1, and ERG3. Only yeast mutants displaying at least 10-fold increased miconazole resistance in agar were selected. Other mutants in genes involved in ergosterol or sphingolipid biosynthesis seem to be characterized by less pronounced miconazole resistance.
Role of Membrane Rafts in Miconazole Antifungal Activity
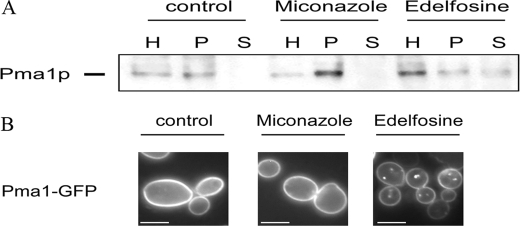
In fungal membranes, sphingolipids and ergosterol are preferentially located in specific domains termed membrane rafts. Membrane rafts are thought to compartmentalize the plasma membrane and to have an important role in cell signaling (6). Because we found mutants affected in both sphingolipid and ergosterol biosynthesis to be miconazole-resistant, we hypothesized that membrane rafts play an important role in miconazole antifungal action. To test this hypothesis, we treated S. cerevisiae WT cells with membrane raft-disturbing agents, namely edelfosine and MβCD to phenocopy mutants affected in proper membrane raft composition and analyzed whether these agents can modulate miconazole antifungal activity. Edelfosine (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine) is an anticancer lysophospholipid that interferes with sphingolipid metabolism and alters the organization and composition of lipid rafts (16). MβCD is a sterol-sequestering agent that is commonly used to disturb membrane rafts (17, 18). Administration of 10 μg/ml miconazole to a 1:100 diluted overnight culture of S. cerevisiae WT cells resulted in <0.1% survival, whereas simultaneous addition of 50 or 100 μg/ml edelfosine and miconazole resulted in increased survival of the yeast culture (72.6 ± 9.5 or 99.0 ± 4.5% survival, respectively; p < 0.01). Similar results were obtained with MβCD. Simultaneous addition of 1.2 or 2.5 mg/ml MβCD and miconazole resulted in increased survival of the yeast culture (10.9 ± 2.5 or 99.0 ± 4.5% survival, respectively; p < 0.025). Apparently, disruption of membrane rafts leads to a decrease in miconazole antifungal activity and hence antagonizes miconazole action. To analyze whether miconazole itself disrupts membrane rafts, we used Pma1p as a marker to monitor lipid rafts (8). Fractionation revealed normal enrichment of Pma1p in the raft fractions of control and miconazole-treated WT cells (Fig. 2A), indicating that miconazole does not disrupt membrane rafts. In cells treated with edelfosine, Pma1p was present in the soluble fraction (Fig. 2A), indicating that under these conditions edelfosine indeed disrupts the association of Pma1p with membrane rafts. This was corroborated by fluorescence microscopy analysis, which revealed normal localization of Pma1p in the plasma membrane upon miconazole treatment and mislocalization of Pma1p in punctuate structures upon edelfosine treatment (Fig. 2B and Table 2). Non-raft-associated Pma1p is known to be endocytosed from the plasma membrane and degraded by targeting to the vacuole (8–11). These results show that miconazole itself does not disrupt membrane rafts.
FIGURE 2.
Effect of edelfosine and miconazole on membrane rafts in S. cerevisiae WT cells. A logarithmically growing S. cerevisiae WT culture in YPD was incubated with either 0 or 10 μg/ml miconazole or 50 μg/ml edelfosine for 3 h at 30 °C. A, raft association of Pma1p was examined by detergent extraction in the following fractions: homogenate (H), detergent-resistant pellet (P), and soluble fraction (S). Proteins were precipitated by trichloroacetic acid and analyzed by gel electrophoresis and immunoblotting using an antibody against Pma1p. B, Pma1p-GFP localization was analyzed by fluorescence microscopy. Scale bars = 5 μm.
TABLE 2.
Percentage of cells with Pma1p-GFP localized intracellularly or at the cell perimeter
| Treatment | Cellsa |
|
|---|---|---|
| Pma1p-GFP internalized | Pma1p-GFP not internalized | |
| % | ||
| Me2SO | 2.7 ± 0.5 | 97.3 ± 0.5 |
| Miconazole | 2.4 ± 0.3 | 97.6 ± 0.3 |
| Edelfosine | 70.5 ± 3.5 | 29.5 ± 3.5 |
a Percentage of cells with the specified phenotype was determined as the ratio of cells with the specified phenotype (as visualized by fluorescence microscopy) to the total number of cells (n > 100).
Role of Membrane Rafts in Miconazole-induced Phenotypes
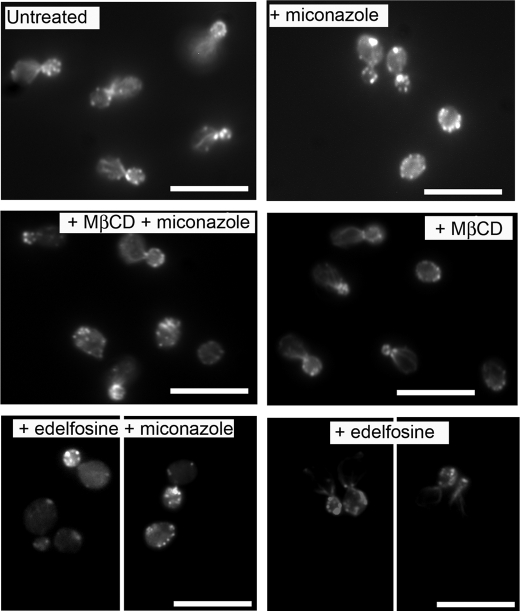
Because miconazole induces stabilization of the actin cytoskeleton prior to induction of ROS in yeast cells (4), we analyzed whether disruption of membrane rafts affects these phenotypes. To this end, we treated a logarithmically growing S. cerevisiae culture with 10 μg/ml miconazole in the presence of membrane raft-disturbing agents and analyzed induction of actin cytoskeleton stabilization and ROS accumulation. First, miconazole (0 or 10 μg/ml) with or without edelfosine (100 μg/ml) or MβCD (2.5 mg/ml) was added to S. cerevisiae cells. Samples were taken after 4 h of incubation at 30 °C to determine the effect of membrane raft disruption on actin cytoskeleton stabilization induced by miconazole. A normal organization of cortical actin patches and polarized actin cables was clearly observed in untreated cells or in cells treated with edelfosine or MβCD (Fig. 3). The addition of 10 μg/ml miconazole resulted in aggregation of F-actin as described previously (4). Combined treatment of the yeast cells with miconazole and edelfosine or MβCD resulted in alleviation of the miconazole-induced F-actin aggregation. These results demonstrate that disruption of membrane rafts antagonizes the aggregation of F-actin induced by miconazole.
FIGURE 3.
Lipid raft disruption affects miconazole-induced stabilization of the actin cytoskeleton. A logarithmically growing S. cerevisiae WT culture in YPD was diluted in PBS and treated with 0 or 10 μg/ml miconazole with or without 100 μg/ml edelfosine or 2.5 mg/ml MβCD. After 4 h of incubation at 30 °C, cells were fixed and stained with rhodamine-phalloidin to determine organization of F-actin structures. Scale bars = 5 μm.
Second, treatment of S. cerevisiae cells with 10 μg/ml miconazole resulted in 26.7 ± 2.4% ROS-positive cells, whereas combined treatment of the yeast cells with miconazole and edelfosine or MβCD resulted in a decrease in ROS-positive cells (namely 8.1 ± 1.1 or 7.2 ± 1.0% ROS-positive cells upon co-incubation with 50 or 100 μg/ml edelfosine, respectively (p < 0.01); and 8.3 ± 1.8 or 9.0 ± 2.4% ROS-positive cells upon co-incubation with 1.2 or 2.5 mg/ml MβCD, respectively (p < 0.025)). The percentage of ROS-positive cells of a yeast culture upon control treatment (i.e. Me2SO control) was 4.7 ± 1.9%. These data indicate that the addition of either edelfosine or MβCD antagonizes miconazole-induced ROS accumulation in S. cerevisiae. Hence, we demonstrated that disruption of membrane rafts via edelfosine or MβCD antagonizes both the actin cytoskeleton stabilization and endogenous ROS accumulation induced by miconazole.
Effect of Membrane Raft Disruption on Intracellular Accumulation of Miconazole in Yeast Cells
Based on all data, it is clear that membrane rafts play an important primary role in the mode of antifungal action of miconazole. Therefore, we focused on a putative involvement of membrane rafts in intracellular accumulation of miconazole and analyzed miconazole accumulation in yeast cells in the presence and absence of edelfosine or MβCD. To this end, we treated a non-diluted overnight culture of S. cerevisiae WT cells in YPD with 100 μg/ml miconazole with or without 500 μg/ml edelfosine. After 2.5 h of incubation, we determined the concentration of miconazole in the cells and in the corresponding supernatant via HPLC analysis. Treatment of the cells with miconazole resulted in intracellular accumulation of 97.4 ± 1.5% miconazole, whereas 2.6 ± 1.5% miconazole was left in the corresponding supernatant of the treated cells. Co-incubation of the culture with miconazole and edelfosine resulted in 2-fold reduced intracellular accumulation of miconazole, namely 55.1 ± 5.7% intracellular miconazole and 44.9 ± 2.3% miconazole remaining in the supernatant (p < 0.01). The corresponding survival percentages using these specific experimental conditions (increased inoculum and increased concentrations of miconazole and edelfosine) were 1.3% for miconazole-treated culture versus 40.3% for miconazole- and edelfosine-treated culture, pointing to a correlation between intracellular miconazole accumulation and its fungicidal activity. Similar results were obtained with MβCD. Combined treatment of the yeast cells with 100 μg/ml miconazole and 20 mg/ml MβCD resulted in 2-fold reduced intracellular accumulation of miconazole, namely 54.3 ± 0.7% intracellular miconazole and 45.7 ± 0.7% miconazole remaining in the supernatant (p < 0.001). In summary, these results document the essential role for membrane rafts in the intracellular accumulation and killing potential of miconazole.
Because membrane rafts are patches that are enriched in sphingolipids and ergosterol, we further analyzed whether the reduced miconazole susceptibility of the miconazole-resistant deletion mutants can be explained by reduced intracellular miconazole accumulation. To this end, we treated non-diluted overnight cultures of S. cerevisiae WT and ipt1 deletion mutant cells with 100 μg/ml miconazole. Treatment of the S. cerevisiae WT cells with miconazole resulted in 96.0 ± 1.2% intracellular miconazole accumulation. Treatment of the ipt1 deletion mutant cells with miconazole resulted in only 67.0 ± 1.8% intracellular miconazole accumulation (p < 0.01). This reduced accumulation in the ipt1 deletion mutant can explain its reduced sensitivity to miconazole treatment.
DISCUSSION
To obtain more mechanistic insight in the mode of antifungal action of miconazole, we screened the complete set of haploid deletion mutants of S. cerevisiae for increased resistance to miconazole in agar. As such, we identified 12 miconazole sensitivity genes, which, upon deletion, result in at least 10-fold increased resistance to miconazole. In this study, we focused on the functional group of miconazole sensitivity genes implicated in sphingolipid and ergosterol biosynthesis, represented by IPT1, SKN1, SUR1, and ERG3. The role of ERG3 in azole resistance was already demonstrated because treatment of yeast with azoles results in the accumulation of 14α-methylated sterols and 14α-methylergosta-8,24(28)-dein-3,6-diol (19, 20). Formation of the latter sterol metabolite is thought to be catalyzed by Δ5,6-desaturase (encoded by ERG3). Hence, inactivation of ERG3 can suppress toxicity and therefore cause azole resistance (19, 20). Additionally, we found various mutants affected in sphingolipid biosynthesis to be miconazole-resistant, suggesting a possible role for membrane rafts in miconazole antifungal action. Sphingolipids and ergosterol are enriched in membrane domains termed membrane rafts. Membrane rafts are thought to compartmentalize the plasma membrane and to have an important role in cell signaling (6). We have demonstrated that disruption of these rafts by treatment with edelfosine or MβCD interferes with miconazole antifungal action as well as with miconazole-induced stabilization of the actin cytoskeleton and ROS accumulation. These data point to an important primary role for membrane rafts in miconazole antifungal action. Using HPLC analysis, we further demonstrated that co-incubation of miconazole and either lipid raft-disturbing agent results in reduced intracellular accumulation of miconazole in yeast cells.
In conclusion, administration of agents that disturb lipid rafts in the plasma membrane, by affecting either sphingolipid biosynthesis or ergosterol sequestration (i.e. edelfosine or MβCD, respectively), abolishes the antifungal action and accumulation of miconazole. Whether the reduced intracellular accumulation of miconazole upon treatment of yeast cells with membrane-disturbing compounds is caused by a reduced uptake in yeast cells or by increased efflux remains to be determined. Moreover, the miconazole-resistant ipt1 deletion mutant showed reduced intracellular miconazole accumulation, correlating intracellular accumulation of miconazole with yeast cell death.
A general role for plasma membrane (phospho)lipid and sterol composition in azole accumulation is postulated (21, 22). In a study tracking the development of low-level fluconazole resistance in Candida albicans, a gradual increase in membrane fluidity of fluconazole-adapted strains was demonstrated, whereas the phospholipid composition of the adapted strains was not significantly altered (21). However, ergosterol content was reduced, whereas sphingolipid content was higher in resistant than in susceptible isolates. Hence, that study demonstrates that altering the ratio of ergosterol to sphingolipid content influences susceptibility to fluconazole. Moreover, Löffler et al. (22) compared the plasma membrane composition of five fluconazole-resistant C. albicans isolates with that of three fluconazole-sensitive ones. They demonstrated that one resistant C. albicans isolate had a decreased amount of ergosterol and a lower phosphatidylcholine/phosphatidylethanolamine ratio in the plasma membrane. They postulated that these changes in plasma membrane lipid and sterol composition could be responsible for an altered uptake of fluconazole and hence for reduced intracellular fluconazole accumulation. Whether membrane rafts are involved in intracellular accumulation of azoles in general remains to be determined.
To our knowledge, this is the first report describing a role for a specific membrane compartment in intracellular accumulation of miconazole. Because membrane rafts have been suggested to be involved in endocytosis (23), it remains to be determined whether miconazole is taken up in S. cerevisiae cells by endocytosis. If so, our observed reduction of miconazole accumulation in yeast with disturbed membrane rafts could be explained by a reduced uptake of the drug.
Acknowledgments
We thank Prof. Christopher McMaster for the kind gift of edelfosine, Dr. Annick Breton for the kind gift of the plasmid encoding GFP-tagged Pma1p, and Dr. Hugo Vanden Bossche for critical remarks.
This work was supported by Grant 030023 from IWT-Vlaanderen and by a postdoctoral fellowship from the Industrial Research Fund (Katholieke Universiteit Leuven; to K. T.).
- ROS
- reactive oxygen species
- HPLC
- high pressure liquid chromatography
- MβCD
- methyl-β-cyclodextrin
- WT
- wild-type
- YPD
- yeast/peptone/dextrose
- GFP
- green fluorescent protein
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Kelly S. L., Lamb D. C., Baldwin B. C., Corran A. J., Kelly D. E. (1997) J. Biol. Chem. 272, 9986–9988 [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi D., Kondo K., Uehara N., Otokozawa S., Tsuji N., Yagihashi A., Watanabe N. (2002) Antimicrob. Agents Chemother. 46, 3113–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.François I. E., Cammue B. P., Borgers M., Ausma J., Dispersyn G. D., Thevissen K. (2006) Anti-Infect. Agents Med. Chem. 5, 3–13 [Google Scholar]
- 4.Thevissen K., Ayscough K. R., Aerts A. M., Du W., De Brucker K., Meert E. K., Ausma J., Borgers M., Cammue B. P., François I. E. (2007) J. Biol. Chem. 282, 21592–21597 [DOI] [PubMed] [Google Scholar]
- 5.De Nollin S., Van Belle H., Goossens F., Thone F., Borgers M. (1977) Antimicrob. Agents Chemother. 11, 500–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajendran L., Simons K. (2005) J. Cell Sci. 118, 1099–1102 [DOI] [PubMed] [Google Scholar]
- 7.Balguerie A., Bagnat M., Bonneu M., Aigle M., Breton A. M. (2002) Eukaryot. Cell 1, 1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagnat M., Chang A., Simons K. (2001) Mol. Biol. Cell 12, 4129–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong X., Chang A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9104–9109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q., Chang A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12853–12858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenkolb M., Zenzmaier C., Leitner E., Schneiter R. (2002) Mol. Biol. Cell 13, 4414–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaigg B., Timischl B., Corbino L., Schneiter R. (2005) J. Biol. Chem. 280, 22515–22522 [DOI] [PubMed] [Google Scholar]
- 13.Gaigg B., Toulmay A., Schneiter R. (2006) J. Biol. Chem. 281, 34135–34145 [DOI] [PubMed] [Google Scholar]
- 14.Gourlay C. W., Ayscough K. R. (2005) Biochem. Soc. Trans. 33, 1260–1264 [DOI] [PubMed] [Google Scholar]
- 15.Gourlay C. W., Carpp L. N., Timpson P., Winder S. J., Ayscough K. R. (2004) J. Cell Biol. 164, 803–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaremberg V., Gajate C., Cacharro L. M., Mollinedo F., McMaster C. R. (2005) J. Biol. Chem. 280, 38047–38058 [DOI] [PubMed] [Google Scholar]
- 17.Foster L. J., de Hoog C. L., Mann M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siafakas A. R., Wright L. C., Sorrell T. C., Djordjevic J. T. (2006) Eukaryot. Cell 5, 488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupetti A., Danesi R., Campa M., Del Tacca M., Kelly S. (2002) Trends Mol. Med. 8, 76–81 [DOI] [PubMed] [Google Scholar]
- 20.Watson P. F., Rose M. E., Ellis S. W., England H., Kelly S. L. (1989) Biochem. Biophys. Res. Commun. 164, 1170–1175 [DOI] [PubMed] [Google Scholar]
- 21.Kohli A., Smriti N. F., Mukhopadhyay K., Rattan A., Prasad R. (2002) Antimicrob. Agents Chemother. 46, 1046–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löffler J., Einsele H., Hebart H., Schumacher U., Hrastnik C., Daum G. (2000) FEMS Microbiol. Lett. 185, 59–63 [DOI] [PubMed] [Google Scholar]
- 23.Kirkham M., Parton R. G. (2005) Biochim. Biophys. Acta 1745, 273–286 [DOI] [PubMed] [Google Scholar]