Abstract
When methyl-substituted aromatic compounds are degraded via ortho (intradiol)-cleavage of 4-methylcatechol, the dead-end metabolite 4-methylmuconolactone (4-ML) is formed. Degradation of 4-ML has only been described in few bacterial species, including Pseudomonas reinekei MT1. The isomerization of 4-ML to 3-methylmuconolactone (3-ML) is the first step required for the mineralization of 4-ML and is catalyzed by an enzyme termed 4-methylmuconolactone methylisomerase (MLMI). We identified the gene encoding MLMI in P. reinekei MT1 and solved the crystal structures of MLMI in complex with 3-ML at 1.4-Å resolution, with 4-ML at 1.9-Å resolution and with a MES buffer molecule at 1.45-Å resolution. MLMI exhibits a ferredoxin-like fold and assembles as a tight functional homodimeric complex. We were able to assign the active site clefts of MLMI from P. reinekei MT1 and of the homologous MLMI from Cupriavidus necator JMP134, which has previously been crystallized in a structural genomics project. Kinetic and structural analysis of wild-type MLMI and variants created by site-directed mutagenesis indicate Tyr-39 and His-26 to be the most probable catalytic residues. The previously proposed involvement of Cys-67 in covalent catalysis can now be excluded. Residue His-52 was found to be important for substrate affinity, with only marginal effect on catalytic activity. Based on these results, a novel catalytic mechanism for the isomerization of 4-ML to 3-ML by MLMI, involving a bislactonic intermediate, is proposed. This broadens the knowledge about the diverse group of proteins exhibiting a ferredoxin-like fold.
Introduction
Aromatic compounds are among the most widely distributed organic substances in nature. Microorganisms have developed the ability to use these abundant compounds as carbon and energy sources. One example is Pseudomonas reinekei MT1, which can use both methyl- and chloro-substituted aromatics as its only carbon source.
P. reinekei MT1 has been described to use uncommon routes to degrade these substances (1, 2). Although most other bacteria degrade methyl-substituted aromatics via meta (extradiol)-cleavage of methylcatechols, this strain specializes in the degradation of such compounds via ortho (intradiol)-cleavage (1). Ortho-cleavage of 4-methylcatechol typically results in the formation of 4-methylmuconolactone (4-ML)2 as a dead-end product (1, 3, 4).
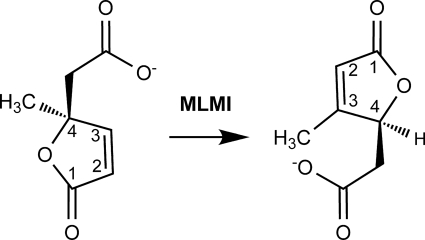
This molecule cannot be mineralized by downstream enzymes of the ortho-cleavage pathway (3-oxoadipate pathway) as the subsequent step requires the abstraction of a proton from the C4 carbon atom (5). Transformation of 4-ML to 3-ML generates such a proton at the C4 carbon atom. This isomerization step is performed by an enzyme termed 4-methylmuconolactone methylisomerase (MLMI) (Fig. 1), which is not part of the 3-oxoadipate pathway. MLMI activity has previously only been described in Cupriavidus necator JMP134 and Rhodococcus rhodochrous N75 (6, 7). The encoding gene has been localized in the genome of C. necator JMP134 (GenBank™ accession numbers: AAZ60870 and CAA67958) (8), and a putative MLMI encoding gene has been localized on plasmid pHG1 of C. necator H16 (GenBank™ accession number: AAP86134). P. reinekei MT1 also contains MLMI-like activity for the isomerization of 4-ML, as recently shown by Camara et al. (1).
FIGURE 1.
Schematic representation of the reaction catalyzed by MLMI. The numbering system is shown for substrate (left) and product (right).
An analysis of the catalytic activity of MLMI from C. necator JMP134 and R. rhodochrous N75 has shown that, besides 4-ML, both enzymes are able to transform 1-methylbislactone to 3-ML. This indicates that the enzymes catalyze lactone ring formation and opening, rather than a migration of the methyl substituent. Based on its rapid transformation, the bislactone has also been discussed as a possible intermediate of the isomerization of 4-ML to 3-ML (6, 7). The occurrence of an enzyme-bound intermediate has been addressed as well, supported by the finding that MLMI activity is sensitive to heavy metals and thiol reagents (6, 7). To reveal the details of the catalytic mechanism, information regarding the active site architecture in the context of substrate/product binding is crucial. The crystal structure of JMP134 MLMI is available from a structure genome project (Protein Data Bank (PDB) ID: 2ifx). However, no active site was assigned, and no catalytic mechanism has been deduced from the data.
In this work we present the identification, crystallization, and characterization of MLMI from P. reinekei MT1. We solved crystal structures of different MLMI variants in complex with different ligands to elucidate the enzymatic mechanism of this enzyme. Considering these structures, we were able to assign the active site cleft of MLMI and pinpoint the residues important for substrate binding and catalysis. Based upon these data, we propose a mechanism for the isomerization catalyzed by MLMI, which is crucial for bacterial growth on methyl-substituted aromatics via ortho-cleavage of 4-methylcatechol.
EXPERIMENTAL PROCEDURES
Materials
(4S)-4-Methylmuconolactone, (4S)-3-methylmuconolactone, 1-methyl-3,7-dioxo-2,6-dioxabicyclo[3.3.0]octane (1-methylbislactone), and 3-methyl-cis,cis-muconate were prepared as previously described (1, 4, 6).
Localization and Identification of the mmlI Gene
To identify the mmlI gene in P. reinekei MT1, the mmlJ gene encoding a methylmuconolactone isomerase (MMLI) was first identified. mmlJ is located in C. necator JMP134 (GenBank™ accession number AAZ60869) and C. necator H16 (GenBank™ accession number AAP86133) downstream of mmlI (8). The mmlJ gene was amplified by PCR using degenerate primers NH3MMLIF1 (5′-AUGYTNATHTGYGTNGAAATGAC-3′) and NH3MMLIR1 (5′-GCYTTDATNGKYTCRTCYTCGT-3′), which were designed based on the N-terminal protein sequence of a partially purified MMLI from P. reinekei MT1 (data not shown). The 75-bp-generated fragment was cloned into the pGEM-T Easy vector (Promega, Madison, WI), transformed into Escherichia coli Max Efficiency DH5α-competent cells (Invitrogen), and sequenced. A specific primer, NH3MMLIF3 (5′-CGTCAGTATTCCCCGTGACAT-3′), was designed based on that sequence and was used in a second PCR round with a reverse degenerate primer, NH3MMLIR4 (5′- TRSYGYSYSRCSRSRCGCYA-3′), based on a sequence alignment of methylmuconolactone and muconolactone isomerases. The newly generated 125 bp fragment was cloned in the pGEM-T Easy vector, transformed into E. coli JM109 (Stratagene) and sequenced. A fosmid library (1) was screened by PCR using specific primers for mmlJ NH3MMLIF3 and NH3MMLIR7 (5′-AGGTGGGGCCACTTGCCCGAC-3′). A positive fosmid clone was purified with the FosmidMAX DNA purification kit (Epicenter). To identify the mmlI gene, direct sequencing upstream of the gene mmlJ was performed. The nucleotide sequence of the mmlI and part of the mmlJ gene has been deposited in the GenBank™ data base under accession number GQ141876.
Cloning, Expression, and Purification
The P. reinekei MT1 mmlI gene was PCR-amplified with BsaI-tailed primers mmlIBsaIF (5′-TACGTCGGTCTCAGCGCATGATTCGTATCCTTTATCTGT-3′) and mmlIBsaIR (5′-TACGTCGGTCTCATATCATTAAACCAGCTCTTCAGTGACGA-3′) and inserted into the expression vector pASKIBA7plus (IBA) via the BsaI restriction site, resulting in vector pASKIBAmmlI. The N-terminal Strep-tagged protein was expressed in E. coli JM109 by adding anhydrotetracyclin (200 μg/liter). Following a 3-h incubation at 30 °C, cells were centrifuged, and the pellet was resuspended in Tris/HCl (100 mm, pH 8.0, supplemented with 150 mm NaCl). Cells were disrupted with a French press (Aminco, Silver Spring, MD), and cell debris was removed by ultracentrifugation for 30 min at 30,000 × g and 4 °C. MLMI was purified in a single step by affinity chromatography using a gravity flow Strep-Tactin-Sepharose column (IBA) and Tris/HCl (100 mm, pH 8.0, supplemented with 2.5 mm desthiobiotin and 150 mm NaCl) as eluant. Fractions containing the protein were pooled and concentrated to a final concentration of 7 mg/ml for subsequent crystallization.
Site-directed Mutagenesis
Site-directed mutations in gene mmlI were generated using the QuikChange site-directed mutagenesis kit (Stratagene) with the following primers MLMI_H26AF (5′-AAAGAGTGCGTGGTGGCTTTCCAGATGTCCG-3′) and MLMI_H26AR (5′-CGGACATCTGGAAAGCCACCACGCACTCTTT-3′) for the H26A variant, MLMI_H52AF (5′-AATCCTACGGATACCGCTGTGCCGTATCTGGACG-3′) and MLMI_H52AR (5′-CGTCCAGATACGGCACAGCGGTATCCGTAGGATT-3′) for the H52A variant, MLMI_Y39FF (5′-CCGGGGCTGCACAAATTCGAAGTGCGACTGGTTGC-3′) and MLMI_Y39FR (5′-GCAACCAGTCGCACTTCGAATTTGTGCAGCCCCGG-3′) for the Y39F variant, and MLMI_C67SF (5′-TGCGATCGGTGAATCCTGGTTTGCCAGTGAGG-3′) and MLMI_C67SR (5′-CCTCACTGGCAAACCAGGATTCACCGATCGCA-3′) for the C67S variant. The pASKIBAmmlI plasmid was used as template in a thermal cycling reaction of 30 s at 95 °C (denaturation), 16 cycles of 30 s at 95 °C (denaturation), 60 s at 55 °C (annealing), and 225 s at 68 °C (extension). Template DNA was removed by incubation with the DpnI restriction enzyme. Mutagenesis was verified by sequencing and proteins were expressed in E. coli XL1-Blue and purified as described above for the wild-type protein.
Activity Assay
The enzymatic activity was measured in 50 mm (sodium/potassium) phosphate buffer, pH 7.3, by monitoring the increase in absorbance at 225 nm resulting from the conversion of 4-ML or 1-methylbislactone into 3-ML as previously described (6). Protein concentrations were determined by the Bradford method using the Bio-Rad protein assay (9). A unit of activity is the amount of protein needed to transform 1 μmol of substrate per minute into product at 25 °C. To test the inhibition of MLMI activity by pCMB, enzyme with a concentration of 0.7 mg/liter was incubated with 20 μm pCMB in 50 mm (sodium/potassium) phosphate buffer (pH 7.3) for 30 min. Activities were assayed as stated above and compared with the activity of untreated enzyme solution incubated under the same conditions. The effect of pH on enzyme activity was assayed at 25 °C in 50 mm sodium phosphate buffer (pH 5.75–8) or 50 mm Tris-HCl buffer (pH 7.5–9). Vmax, kcat, and apparent Km values of MLMI and derivative enzymes were determined using 6–450 μm of 4-ML. Kinetic data were calculated from the initial velocities using the Michaelis-Menten equation by non-linear regression (KaleidaGraph, Synergy Software). For the standard test, a concentration of 110 μm of substrate was used. Turnover numbers (kcat values) were calculated assuming a subunit molecular mass of 14,325 Da.
Crystallization and Data Collection
Crystals of MLMI were grown during an experiment at 19 °C using a sitting drop, vapor-diffusion experiment. A 2-μl drop containing a mixture of 1 μl of 7 mg/ml MLMI in elution buffer mixed with 1 μl of crystallization buffer (25% w/v polyethylene glycol 1500, 0.1 m MMT buffer, pH 5 (dl-malic acid, MES, and Tris base in a 1:2:2 molar ratio)) yielded tetragonal bipyramidal crystals of MLMI within a few days that diffracted x-ray radiation up to a resolution of 1.4 Å. The crystal of MLMI in complex with 3-ML (product) was obtained by soaking apo-state crystals in a soaking solution (25% w/v polyethylene glycol 1500, 20 mm MMT buffer, pH 5) containing 50 mm (4S)-4-ML (substrate). Following the same approach, the crystal in complex with the substrate was obtained after soaking the derivative enzyme H52A with 50 mm (4S)-4-ML in the same soaking solution. In all cases, either the crystallization buffer or the soaking solution was used as the cryoprotection solution.
Data sets used for the determination of the crystal structures reported in this work were collected at the European Synchrotron Radiation Facility (Grenoble, France) (beamlines ID14-4, ID23-1 and ID23-2). Data indexing and processing were done with the XDS program package (10). Data statistics are summarized in Table 1.
TABLE 1.
Crystallographic data and refinement statistics
| MLMI (MES) | MLMI (3-ML) | MLMI_H52A (4-ML) | |
|---|---|---|---|
| Data collection statistics | |||
| Beamline | ESRF (ID23-2) | ESRF (ID23-1) | ESRF (ID14-4) |
| Space group | C2221 | C2221 | C2221 |
| Unit cell dimensions | |||
| a (Å) | 81.9 | 81.8 | 82.1 |
| b (Å) | 84.2 | 84 | 84.4 |
| c (Å) | 150.2 | 150.1 | 150.3 |
| Wavelength (Å) | 0.873 | 1.105 | 0.979 |
| Resolution (Å)a | 42.10-1.45 (1.50-1.45) | 42.0-1.40 (1.50-1.40) | 75.24-1.90 (2.00-1.90) |
| Unique reflectionsa | 91,817 (8,808) | 101,311 (18,705) | 41,197 (5,743) |
| Multiplicitya | 8.0 (8.3) | 9.9 (9.9) | 7.23 (7.2) |
| Completeness (%)a | 100 (100) | 99.8 (99.5) | 99.4 (99) |
| I/σIa | 14.5 (2.8) | 15.9 (3.5) | 14.7 (3.5) |
| Rmerge (%)ab | 8.4 (71.6) | 8.9 (68.2) | 11.3 (56.4) |
| Wilson B-factor (Å2) | 21.3 | 20.2 | 23.2 |
| Solvent content (%) | 38.5 | 42.8 | 47.1 |
| Refinement statistics | |||
| Rcrys (%)c | 18.2 | 17.6 | 18.2 |
| Rfree (%)d | 20.9 | 20.8 | 23.1 |
| Number of atoms | |||
| Protein | 4,089 | 4,191 | 3,894 |
| Ligand | 52 | 44 | 77 |
| Solvent | 441 | 550 | 524 |
| Room mean square deviation from ideality | |||
| Bond lengths (Å) | 0.015 | 0.019 | 0.018 |
| Bond angle (°) | 1.619 | 1.908 | 1.577 |
| Average B-factor | |||
| Protein (Å2) | 16.1 | 15.6 | 16.4 |
| Ligand (Å2) | 25.1 | 15.5 | 23.5 |
| Solvent (Å2) | 31.1 | 29.2 | 28.7 |
| Ramachandran plot (%) (most favored regions/allowed/generously allowed/disallowed) | 97.5/2.5/0/0 | 95.9/3.6/0/0.5 | 97.2/2.8/0/0 |
a Values in parentheses refer to statistics in the highest resolution shell.
b Rmerge = Σ|Iobs − 〈I〉|/ΣIobs.
c Rcrys = (Σ|Fo − Fc|/ΣFo) × 100, where Fo and Fc are the observed and calculated structure-factor amplitudes, respectively.
d Rfree was computed using 5% of the data assigned randomly.
Structure Determination and Refinement
The structures were solved by molecular replacement using MOLREP from the CCP4 program package (11). For this, the crystal structure of homologous MLMI from C. necator JMP134 (PDB entry code 2ifx) was used as the search model. Automatic model building was carried out using ARP/wARP (12). Further manual model building was performed using Coot (13), and refinement was done with REFMAC5 (14). Initial coordinates and crystallographic restraints for the different ligands (MES, 3-ML, and 4-ML) were generated using the PRODRG server (15).
PDB Accession Number
The coordinates and the structure factors were deposited in the Protein Data Bank (PDB) under the following accession numbers 3HDS, 3HF5, and 3HFK.
RESULTS
The mmlI Gene of P. Reinekei MT1 Encodes a Functional mmlI MLMI
P. reinekei MT1 was found to transform 4-ML to 3-ML. The mmlI gene encoding this activity was identified in the genome of P. reinekei MT1. The deduced protein shows 70% of sequence identity with MLMI of C. necator JMP134 (GenBank™ accession number AAZ60870) and 69% of sequence identity with the putative MLMI of C. necator H16 (GenBank™ accession number AAP86134). Thus, MT1 MLMI is the third 4-methylmuconolactone methylisomerase, whose sequence information is available.
The mmlI gene was cloned, and the recombinant protein was purified to apparent homogeneity. Kinetic measurements indicated a specific activity of 57.3 ± 8.2 units/mg of protein toward 4-ML and 28.8 ± 1.0 units/mg of protein toward 1-methylbislactone. Activity with 3-methyl-cis,cis-muconate was below the detection limit (<0.02 unit/mg). The Michaelis-Menten parameters for 4-ML transformation (Km = 52.0 ± 5.8 μm and kcat = 24.2 ± 0.8 s−1) are slightly lower than those values previously reported for the JMP134 enzyme (Km = 176.1 ± 24.1 μm; kcat = 98.5 ± 5.5 s−1). Analysis of kcat/Km versus pH showed highest enzyme efficiency at a pH of 6.3–6.5, ∼2-fold higher than at pH 7.5. Akin to the enzymes of C. necator JMP134 and R. rhodochrous N75, MLMI of P. reinekei MT1 could be inhibited with pCMB (6, 7), with a remaining activity of 6% after incubation with 20 μm pCMB for 30 min.
Overall Structure
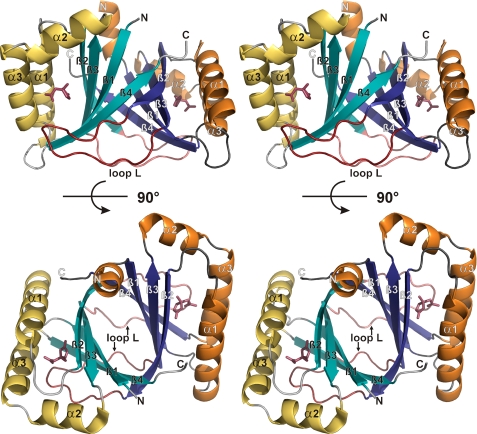
In all crystal structures presented in this work, MLMI crystallizes as a homodimer with two dimers per asymmetric unit in C2221 space group symmetry. The general fold is highly similar to the homologous enzyme of C. necator JMP134 (PDB ID: 2ifx) with an approximate root mean square deviation of 1.0 Å for the common Cα atoms. From the crystal structure of MLMI it is apparent that the recombinant enzyme is a tightly linked homodimer. Each monomer consists of three α-helices and four antiparallel β-strands, arranged in a βαββααβ topology, and exhibits a ferredoxin-like fold belonging to the split α-β sandwich group of the α+β fold class (16). Dimerization leads to the formation of an eight-stranded intermolecular β-barrel, surrounded by α-helices. The interior of this barrel is hydrophobic. The dimer has an approximately globular shape. Loop L connecting β2 and β3 of one monomer forms a prominent interaction with α3 and β4 from the second monomer (Fig. 2).
FIGURE 2.
Stereo representation of the overall MLMI structure. Two different orientations of the dimer are depicted (90° rotation in the barrel axis). Secondary structure elements are numbered and shown in yellow/orange (α-helices) and cyan/blue (β-strands). 4-ML and loop L are colored red.
The main structural difference between the two monomers is located at the amino-terminal Strep tag. In one monomer, residues −7 to 0 from the Strep-tag® form a well defined helix that contacts helices α1 and α3 of a symmetry-related molecule. These contacts are not present in the other monomer, due to the disorder of the Strep-tag®.
Comparison with Structurally Related Proteins
Structural alignments using the DALI server (17) show that MLMI is structurally similar to >50 proteins (DALI Z-scores above 7) belonging to the split α-β sandwich group. Most of these proteins, as MT1 MLMI, form dimers. However, they show similarities lower than 20% with MLMI at the sequence level and are produced by phylogenetically distant organisms belonging to all three domains of life. Most of the related proteins from bacterial origin are monooxygenases with putative or confirmed function (18, 19) or putative stress proteins of unknown function. From archaea, only a putative transcriptional regulator (PDB ID: 2dbb) shows structural similarity with MLMI. Related eukaryotic proteins, which are all from plant origin, are described as putative stress-induced proteins of unknown function (20, 21).
The Active Site Cleft
As can be expected for a small enzyme like MLMI, all secondary structure elements are involved in forming the active site cleft, which harbors either an MES buffer molecule or a muconolactone ligand in the solved crystal structures (Fig. 2). The binding pocket is predominantly apolar and is formed by residues of helices α1 (His-26), α2 (Tyr-78), and α3 (Trp-87, Phe-88, Gly-91, and Ile-95) and strands β1 (Tyr-6 and Leu-8), β2 (Tyr-39), β3 (Cys-67), and β4 (Leu-98). The pocket is solvent-exposed on one side and partially closed by loop L from the other molecule of the functional dimer, with residue His-52 contacting the bound ligand. However, no ordered water molecule with a potential catalytic function is present in the active site.
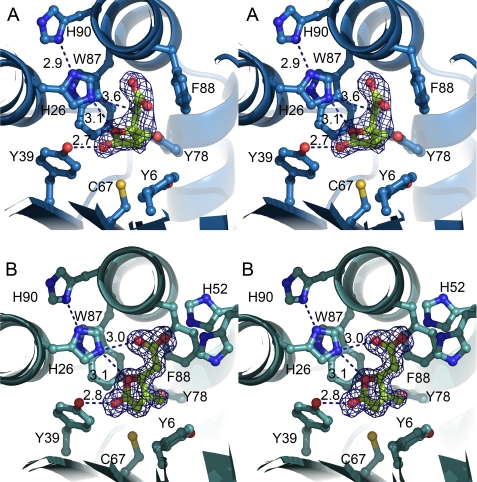
Residues forming direct hydrogen bonds with the ligand and therefore having a potential catalytic role are His-26, Tyr-39, and His-52 (Fig. 3). In the product-bound structure (Fig. 3B), the side chain of His-26 contacts the carboxyl group of 3-ML with a distance of 3.0 Å, and the oxygen atom of the lactone ring with a distance of 3.1 Å. The side chain of loop L residue His-52 can also form close interactions with this carboxyl group but does not adopt a single conformation. Tyr-39 forms a hydrogen bond between the side-chain hydroxyl and the keto group of the lactone ring, with a distance of 2.8 Å. Cys-67, which has been discussed to be potentially involved in covalent catalysis (6, 7) is located more distantly from 3-ML, with a closest distance of ∼4 Å (Fig. 3).
FIGURE 3.
Stereo representation of the active site cleft of MLMI. A, 4-ML in the active site cleft of the derivative H52A enzyme. B, 3-ML in the active site cleft of the wild-type enzyme. Residues closer than 4 Å to the ligand molecules are shown as sticks. The σA-weighted [Fo − Fc] electron density map contoured at 3 σ for 4-ML and 3-ML is colored blue. To calculate this map, the ligand molecules were removed from the model, and model bias was reduced by perturbing the atom positions by 0.3 Å and the B-values by 5 Å2, and then refining this perturbed model by performing 15 REFMAC cycles.
Kinetic Analysis of MLMI Variants
To identify the residues involved in catalysis, His-26, Tyr-39, His-52, and Cys-67 were replaced by site-directed mutagenesis. The catalytic activity of these variants was measured, and kinetic parameters were calculated. The H26A variant showed a marked decrease in catalytic activity (kcat) by two orders of magnitude, while the exchange of His-52 to alanine resulted in an increase in Km by a factor of ∼9, but only a small change in kcat. The variants C67S and Y39F exhibited less pronounced but still significant changes in the catalytic parameters (Table 2). Incubation of variant C67S with pCMB did not significantly decrease its activity (>65% of activity remaining after a 30-min incubation).
TABLE 2.
Enzymatic activity of MLMI and variants
| MLMI variant | Specific activitya | Vmax | Km | kcat | kcat/Km |
|---|---|---|---|---|---|
| Units/mg | Units/mg | μm | s−1 | s−1m−1 × 106 | |
| MLMI (wild type) | 57.3 ± 8.2 | 101.6 ± 3.2 | 52.0 ± 5.8 | 24.2 ± 0.8 | 0.5 |
| H26A | 0.04 ± 0.03 | NDb | ND | ND | ND |
| Y39F | 14.1 ± 1.8 | 24.3 ± 1.7 | 45.0 ± 11.6 | 5.8 ± 0.4 | 0.1 |
| H52A | 37.3 ± 3.6 | 197.8 ± 14.2 | 458.3 ± 54.1 | 47.2 ± 3.4 | 0.1 |
| C67S | 15.6 ± 2.2 | 30.5 ± 2.4 | 97.2 ± 22.7 | 7.3 ± 0.6 | 0.1 |
a The specific activity was determined at a concentration of 110 μm of substrate in phosphate buffer, 50 mm, pH 7.3.
b ND, not determined.
MLMI Variant H52A in Complex with Substrate 4-ML
The crystal structure of MLMI in complex with the substrate was obtained by soaking variant H52A crystals with enantiomeric pure (4S)-4-ML. Comparison between substrate- and product-bound structures shows that the lactone ring of (4S)-4-ML is rotated ∼90° relative to the ring of (4S)-3-ML. However, the residues involved in the coordination of both muconolactones are essentially the same in both structures, with slightly different interaction distances due to the rotation. His-26 interacts with the carboxyl group of (4S)-4-ML at a distance of 3.2 Å. The side chain of His-26 can interact with the oxygen atom of the lactone ring at a distance of 3.1 Å and with the C5 with a distance of 3.6 Å. Tyr-39 interacts with the keto group of the ring at a 2.7-Å distance. Cys-67 is oriented toward the methyl group on the lactone ring, with a distance of 3.7 Å. The distance of Cys-67 toward the C2 atom increases from 4 Å in the substrate-bound structure to ∼6 Å in the product-bound structure due to the different lactone ring orientation. Interestingly, a second ordered substrate molecule was found close to the active site in contact with Ala-52. This underlines a potential role of His-52 regulating the entrance to the active site.
DISCUSSION
MLMI from P. reinekei MT1 Is Structurally Related to MLMI from C. necator
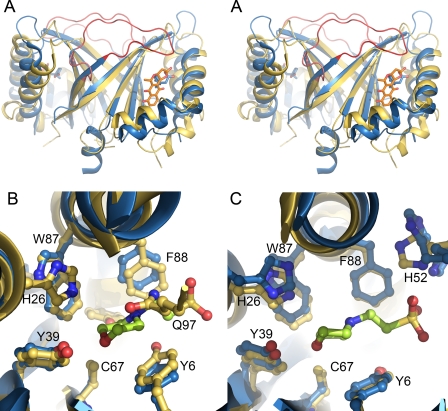
MLMI from P. reinekei MT1 is homologous to a putative MLMI from C. necator H16 and the MLMI from C. necator JMP134, the only currently known members of the MmlI protein family (PF09448). All active site residues are conserved at the sequence level among all three MLMIs sequenced so far. The crystal structure of C. necator JMP134 confirms the close structural relationship between these enzymes. However, it is interesting to note that only one of the monomers of the structure of JMP134 MLMI shows an active site fold highly similar to the active site of MT1 MLMI. In contrast, the active site of the second monomer of the JMP134 enzyme is obstructed by a well defined Gln-97 residue occupying the position where the ligand molecules are normally bound in MT1 MLMI (Fig. 4B). This obstruction is mainly caused by crystal contacts and thus does not represent a biologically relevant conformation. This is supported by the fact that loop L residues 49–59 would clash with residues of a symmetry-related monomer if present in a conformation similar to the other monomer. These residues were not modeled due to weak electron density in the crystal structure of JMP134 MLMI. An analysis of the other monomer of JMP134 MLMI (obtained from the electron density server (22)) showed unmodeled electron density, which can reasonably be explained by a MES molecule located in the same orientation as seen in MT1 MLMI. This allows the assignment of the active site cleft in JMP134, which is essentially identical to the MT1 MLMI cleft.
FIGURE 4.
Comparison between MLMI of P. reinekei MT1 and structurally related enzymes. A, stereo representation of MLMI from P. reinekei MT1 (blue, PDB ID: 3hds) superimposed with ActVA-orf6 (yellow, PDB ID: 1n5v). The ligands bound in the respective active sites are colored in blue and orange. Loop L is highlighted in dark and light red, respectively. B and C, superposition of the active sites of both monomers from MLMI from strain MT1 (blue) and strain JMP134 (yellow).
Convergent Evolution of Proteins Structurally Related to MLMI
Although MLMI enzymes show no homology (<20% identity) to any other protein with described function, a remarkable amount of structurally related proteins from all three domains of life was found in a DALI search. Many of the proteins have been crystallized in complex with different buffer components or biologically relevant ligands, and in most cases, these molecules are bound to a cleft equivalent to the MLMI active site cleft. However, the function of these proteins is quite variable.
Most of the related proteins can be classified into three groups. The first group encompasses putative stress-induced proteins from bacterial and plant origin with unknown function (e.g. PDB ID: 3bn7, 3bde, 1q53, 1tr0, and 3bgu). They belong to the dabb protein family (PF07876), however it is not clear if and how these proteins actually play a role in stress response. The second group comprises enzymes related to metabolism, predominantly putative (e.g. PDB ID: 2bf0, 1x7v, 1q8b, and 3fgv) and described (PDB ID: 1n5v and 1tuv) monooxygenases (18, 19) and proteins belonging to the EthD family (PF07110). Members of the EthD protein family are associated with ethyl-tert-butyl ether degradation. However, none of the genes encoding proteins structurally related to MLMI belongs to clusters involved in ethyl-tert-butyl ether degradation. The third group consists of putative transcriptional regulators of the AsnC protein family (PF01037). These proteins contain two domains: a carboxyl-terminal domain, which seems to be involved in ligand response and adopts a ferredoxin-like fold like MLMI, and a helix-turn-helix domain, which is involved in DNA binding.
It is known that the ferredoxin-like fold is particularly suitable for ligand binding, even though the function and ligand binding site of proteins with this fold are extremely diverse (23). The diverse function and low sequence similarity of the proteins related to MLMI indicate that they may have arisen independently and converged to the same fold (16).
Important Residues for Catalysis and Substrate Affinity
So far, both biochemically described MLMIs are inhibited by pCMB, a thiol-group inhibitor, leading to the presumption that a cysteine residue could be involved in covalent catalysis (6, 7). Cys-67 would be the most probable candidate for this function, because it is positioned in proximity to the active site cleft. However, replacement of this residue by serine decreased catalytic activity only moderately by ∼75%. The moderate decrease in activity in the C67S variant and the fact that the distance of ∼4 Å to the muconolactone ligands is too long for a covalent bond clearly excludes an involvement of Cys-67 in covalent catalysis. Instead, it indicates the inhibition of the wild-type enzyme by pCMB to be mainly due to steric interference with the substrate. Nevertheless, the moderate but significant decrease in activity of the C67S mutant indicates an indirect role of Cys-67 in catalysis.
His-26, Tyr-39, and His-52 are the only residues that are positioned favorably to be involved in transformation of 4-ML (Fig. 3). H26A is the variant exhibiting the strongest decrease in activity. Unfortunately, efforts to crystallize this variant have so far been unsuccessful, probably because His-26 has an important structural role and forms interactions with several surrounding aromatic residues (Trp-87, His-90, and Phe-94). In particular, the tight interaction with the His-90 side chain via a hydrogen bond with 2.8-Å distance may be important for both positioning and activating His-26. Tyr-39 interacts with the carbonyl oxygen atom of 4-ML at a distance of 2.75 Å, and variant Y39F presented a 75% decrease in specific activity toward 4-ML, but no significant change in the Km. This favors a possible catalytic role of Tyr-39 over a role in binding affinity. In contrast, the ∼9-fold increase in the Km of H52A with only a small increase in kcat indicates that this residue is of minor importance for catalytic activity but has a role in binding affinity. This is supported by the crystal structure of variant H52A in complex with 4-ML. The replacement of His-52 by an alanine residue allows the binding of a second substrate molecule close to the active site, which forms a hydrogen bond to the main-chain nitrogen of the introduced Ala-52. Thus, this second substrate molecule may indicate a possible intermediate step in the substrate entry pathway toward the active site, which may be normally regulated by His-52. As the side chain is truncated in the H52A variant, the exchange rate of ligands in the active site is increased, which was apparent by the higher Km value in variant H52A.
Catalytic Mechanism of MLMI Involves a Bislactonic Intermediate
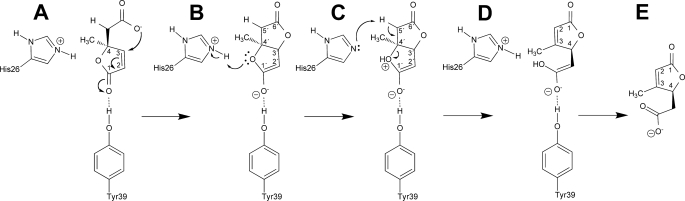
The active site cleft is mainly formed by hydrophobic residues, with only a few polar interactions to the bound ligand (Fig. 3). The cleft adopts the form of a well, which is solvent-exposed on one side and partially closed by loop L residue His-52 from a neighboring molecule. Tyr-39 is positioned at the bottom of the well and can form a hydrogen bond with the carbonyl function of a bound 4-ML substrate molecule via its hydroxyl group. In this way, a positive partial charge can be stabilized at the C3 carbon atom (Fig. 5) by delocalization of the double bond in the lactone ring. This activates C3 for an intramolecular nucleophilic attack by the carboxyl group of the substrate. The hydrophobicity of the active site cleft might further be important for directing this intramolecular reaction. The elimination of the Tyr-39 hydroxyl group in the Y39F variant decreases the activity of MLMI but does not completely eliminate it. This could be expected, because the role of activating the C3 carbon atom might also be fulfilled by a water molecule located near the position of the eliminated hydroxyl group.
FIGURE 5.
Proposed catalytic mechanism of MLMI. A, the nucleophilic attack of the carbonyl oxygen atom at the C3 carbon atom of 4-ML is facilitated by delocalization of charge toward the lactone carbonyl oxygen atom. B, His-26 catalyzes the opening of the formed bislactonic intermediate by donating a proton to the original lactone oxygen atom and C, abstracting a proton from the C5 carbon atom. D, upon keto-enol tautomerization, the product 3-ML (E) is formed.
His-90 forms a hydrogen bond with the side chain of His-26, which may be crucial to activate His-26 and to position it favorably for the second reaction step. His-26 donates a proton to the oxygen atom of the newly formed lactone ring of the ligand, which is located at a distance of 3.25 Å. The formed positively charged oxonium ion supports the proton abstraction by His-26 from position C5′, which is located at a distance of 3.77 Å. An opening of the lactone ring neutralizes the positive charge at the oxonium oxygen and drives the reaction toward the proton abstraction from position C5′. The product 3-ML is formed by a subsequent ketoenol tautomerization (Fig. 5). The proposed mechanism is in agreement with the observed highest enzyme efficiency at a pH of 6.3–6.5, indicating a histidine to act both as a catalytic base and catalytic acid. In addition, the high catalytic activity toward 1-methyl-3,7-dioxo-2,6-dioxabicyclo[3.3.0]octane (1-methylbislactone) is explained, as the enol form of this substance is proposed as an intermediate in the reaction.
Alternative mechanisms of isomerization have been discussed previously (6, 7). A direct abstraction of the C5 proton from 4-ML would result in the formation of 3-methyl-cis,cis-muconate. However, no activity was observed against this compound. Moreover, the conjugated electronic π-system in 3-methyl-cis,cis-muconate forces this molecule into a planar configuration, which might not fit into the catalytic pocket of MLMI and therefore may be excluded as a reaction intermediate.
A migration of the methyl substituent can also be excluded as the enzyme evidently catalyzes lactone ring cleavage. It is thus obvious that transformation of 4-ML to 3-ML gives rise to the product with its lactone ring pointing toward His-52. This probably causes a decreased affinity toward the product bound in that orientation and its release from the active site.
Due to the highly similar structures of substrate and product, it is reasonable, however, to assume that both 3-ML and 4-ML can bind in a similar orientation. This is supported by the crystal structure of wild-type MLMI in complex with the catalytically produced product, where the lactone ring of the product is oriented with the lactone ring pointing away from His-52, like in the structure in complex with substrate. However, this binding pattern, according to the proposed mechanism, is only achieved after product formation. The substrate entry and product release may be further regulated by the flexible His-52 residue acting as a “gating” residue.
Previous data (24) indicate that the biologically relevant lactones present a 4S configuration. We showed that the enzyme transforms (4S)-4-ML, with (4S)-3-ML as the only product. The chirality of the active site cleft allows only transformation of one substrate enantiomer, which then is directed to one defined product enantiomer.
Mechanistic Comparison with Structurally Related Enzymes
The closest homologous structures with described mechanism are ActVA-Orf6 monooxygenase (18) and YgiN (19). Although the details of catalysis are not completely comparable to MLMI, the enzymes share an important tyrosine residue as one common feature in the proposed mechanisms. In MLMI, Tyr-39 is involved in catalysis by stabilizing a resonance structure with positive partial charge at C3, which then can be attacked by the carboxyl oxygen atom for ring closure. In ActVA-Orf6 monooxygenase, a similar stabilization of the hydroxy form within a tautomeric equilibrium is established by a tyrosine residue. Comparison of both structures indicates that both the position and the length of loop L are important for the adaptation to different ligands, with His-52 in MLMI working as a gating residue. Residues of loop L therefore seem to be most important for substrate binding, but not for catalysis (Fig. 4).
Conclusions
The high resolution structure of MLMI in complex with its substrate and product allowed the assignment and a detailed analysis of the active site of this enzyme, from which a catalytic mechanism for MLMI of P. reinekei MT1 could be proposed. This new mechanism broadens the knowledge about the functions performed by the ferredoxin-like fold and may give valuable information about the unknown functions of other members of this fold.
Acknowledgments
We greatly acknowledge the support of Dr. Birgit Hofmann (Helmholtz Center for Infection Research, Braunschweig, Germany) at the beginning of this work. The technical assistance from the staff of beamlines ID14-4, ID23-1 and ID23-2 of the European Synchrotron Radiation Facility, Grenoble, and beamline Proxima 1 from Synchrotron Soleil, during data collection is gratefully appreciated. We thank D. Würdemann, M. Wos-Oxley, and T. Woo for critically reading the manuscript.
This work was funded by Deutsche Forschungsgemeinschaft-International Research Training Group 653.
The atomic coordinates and structure factors (codes 3HDS, 3HF5, and 3HFK) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) GQ141876.
- 4-ML
- 4-methylmuconolactone
- 3-ML
- 3-methylmuconolactone
- MLMI
- 4-methylmuconolactone methylisomerase
- MMLI
- methylmuconolactone isomerase
- 1MES
- 2-(N-morpholino)ethane sulfonate
- pCMB
- p-chloromercuribenzoate
- 1-methylbislactone
- 1-methyl-3,7-dioxo-2,6-dioxabicyclo[3.3.0]octane.
REFERENCES
- 1.Cámara B., Bielecki P., Kaminski F., dos Santos V. M., Plumeier I., Nikodem P., Pieper D. H. (2007) J. Bacteriol. 189, 1664–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikodem P., Hecht V., Schlömann M., Pieper D. H. (2003) J. Bacteriol. 185, 6790–6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catelani D., Fiecchi A., Galli E. (1971) Biochem. J. 121, 89–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knackmuss H. J., Hellwig M., Lackner H., Otting W. (1976) Eur. J. Appl. Microbiol. 2, 267–276 [Google Scholar]
- 5.Chari R., Whitman C., Kozarich J., Ngai K., Ornston L. (1987) J. Am. Chem. Soc. 109, 5520–5521 [Google Scholar]
- 6.Pieper D. H., Stadler-Fritzsche K., Knackmuss H. J., Engesser K. H., Bruce N. C., Cain R. B. (1990) Biochem. J. 271, 529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce N. C., Cain R. B., Pieper D. H., Engesser K. H. (1989) Biochem. J. 262, 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erb R. W., Timmis K. N., Pieper D. H. (1998) Gene 206, 53–62 [DOI] [PubMed] [Google Scholar]
- 9.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 10.Kabsch W. (1988) J. Appl. Crystallogr. 21, 67–72 [Google Scholar]
- 11.Vagin A., Teplyakov A. (1997) J. Appl. Crystallogr. 30, 1022–1025 [Google Scholar]
- 12.Perrakis A., Morris R., Lamzin V. S. (1999) Nat. Struct. Biol. 6, 458–463 [DOI] [PubMed] [Google Scholar]
- 13.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 14.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. Sect. D. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 15.Schüttelkopf A. W., van Aalten D. M. (2004) Acta Crystallogr. D. Biol. Crystallogr. 60, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 16.Orengo C. A., Jones D. T., Thornton J. M. (1994) Nature 372, 631–634 [DOI] [PubMed] [Google Scholar]
- 17.Sander C., Schneider R. (1991) Proteins 9, 56–68 [DOI] [PubMed] [Google Scholar]
- 18.Sciara G., Kendrew S. G., Miele A. E., Marsh N. G., Federici L., Malatesta F., Schimperna G., Savino C., Vallone B. (2003) EMBO J. 22, 205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams M. A., Jia Z. (2005) J. Biol. Chem. 280, 8358–8363 [DOI] [PubMed] [Google Scholar]
- 20.Dgany O., Gonzalez A., Sofer O., Wang W., Zolotnitsky G., Wolf A., Shoham Y., Altman A., Wolf S. G., Shoseyov O., Almog O. (2004) J. Biol. Chem. 279, 51516–51523 [DOI] [PubMed] [Google Scholar]
- 21.Lytle B. L., Peterson F. C., Kjer K. L., Frederick R. O., Zhao Q., Thao S., Bingman C., Johnson K. A., Phillips G. N., Jr., Volkman B. F. (2004) J. Biomol. NMR 28, 397–400 [DOI] [PubMed] [Google Scholar]
- 22.Kleywegt G. J., Harris M. R., Zou J. Y., Taylor T. C., Wählby A., Jones T. A. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2240–2249 [DOI] [PubMed] [Google Scholar]
- 23.Russell R. B., Sasieni P. D., Sternberg M. J. (1998) J. Mol. Biol. 282, 903–918 [DOI] [PubMed] [Google Scholar]
- 24.Cain R. B., Freer A. A., Kirby G. W., Rao G. V. (1989) J. Chem Soc. Perkin Trans. I. 202–203 [Google Scholar]