Abstract
We expressed full-length Na+-Ca2+ exchangers (NCXs) with mutations in two Ca2+-binding domains (CBD1 and CBD2) to determine the roles of the CBDs in Ca2+-dependent regulation of NCX. CBD1 has four Ca2+-binding sites, and mutation of residues Asp421 and Glu451, which primarily coordinate Ca2+ at sites 1 and 2, had little effect on regulation of NCX by Ca2+. In contrast, mutations at residues Glu385, Asp446, Asp447, and Asp500, which coordinate Ca2+ at sites 3 and 4 of CBD1, resulted in a drastic decrease in the apparent affinity of peak exchange current for regulatory Ca2+. Another mutant, M7, with 7 key residues of CBD1 replaced, showed a further decrease in apparent Ca2+ affinity but retained regulation, confirming a contribution of CBD2 to Ca2+ regulation. Addition of the mutation K585E (located in CBD2) into the M7 background induced a marked increase in Ca2+ affinity for both steady-state and peak currents. Also, we have shown previously that the CBD2 mutations E516L and E683V have no Ca2+-dependent regulation. We now demonstrate that introduction of a positive charge at these locations rescues Ca2+-dependent regulation. Finally, our data demonstrate that deletion of the unstructured loops between β-strands F and G of both CBDs does not alter the regulation of the exchanger by Ca2+, indicating that these segments are not important in regulation. Thus, CBD1 and CBD2 have distinct roles in Ca2+-dependent regulation of NCX. CBD1 determines the affinity of NCX for regulatory Ca2+, although CBD2 is also necessary for Ca2+-dependent regulation.
Introduction
The Na+-Ca2+ exchanger (NCX)2 is a plasma membrane protein that uses the electrochemical gradient of Na+ to extrude Ca2+ from cells (1). NCX is particularly abundant in cardiac myocytes and helps restore intracellular Ca2+ levels following excitation-contraction coupling (1). In addition to being transported substrates, cytoplasmic Na+ and Ca2+ regulate NCX activity. Intracellular Na+ decreases exchanger activity by inactivating NCX (Na+-dependent inactivation or I1), whereas cytoplasmic Ca2+ both stimulates activity and relieves the exchanger from the Na+-dependent inactivation (2, 3). By tuning exchanger activity, regulation by Na+ and Ca2+ has fundamental roles in Ca2+ homeostasis.
Regulatory Ca2+ binds to two cytoplasmic Ca2+-binding domains (CBD1 and CBD2) located within the large intracellular loop of NCX, between transmembrane segments 5 and 6 (4–9). Each CBD comprises a β-sandwich containing seven antiparallel β-strands with Ca2+-binding sites at one end of the β-sandwich (4, 5, 8, 9) and an unstructured loop connecting β-strands F and G at the opposite end of the sandwich. In CBD1, there are sites for coordinating four Ca2+ ions (Ca1–Ca4). Previous studies suggest that residues coordinating Ca2+ ions at sites 3 and 4 of CBD1 (10) set the affinity of the exchanger for cytoplasmic Ca2+, whereas recent crystal and electrophysiological data show that binding of Ca1 is not required for exchanger regulation (11). No data are available on the role of Ca2+ bound at site 2.
Although the structure of CBD2 is similar to that of CBD1, it only contains sites for two Ca2+ ions (Ca1 and Ca2). Replacement of the residues responsible for coordinating Ca2+ at its primary site (Ca1) completely abolished Ca2+ regulation (4), indicating an important role for CBD2 in exchanger regulation. Ca2+ bound at the secondary site (Ca2) appears to have no role in exchanger regulation (4).
Despite recent progress, an understanding of the mechanisms leading to activation of NCX by cytoplasmic Ca2+ is still unresolved. It is well established that Ca2+ activates the exchanger by decreasing the extent of the Na+-dependent inactivation and also by directly increasing NCX activity (2, 3). The relative contributions of CBD1 and CBD2 in the control of these mechanisms are unclear. To advance our understanding of Ca2+ regulation of the NCX, we mutated residues that coordinate Ca2+ in both domains and examined the effects on Ca2+-dependent regulation. Our findings indicate that only Ca2+ sites 3 and 4 of CBD1 are important for Ca2+ regulation and that CBD2 also contributes to this process.
EXPERIMENTAL PROCEDURES
Mutagenesis and RNA synthesis were performed as described previously (12). RNAs encoding for NCXs were injected into Xenopus laevis oocytes. Oocytes were kept at 18 °C for 4–7 days. Inside-out giant patch recordings of outward NCX currents were performed as described previously (12, 13). Borosilicate glass pipettes of about 20–30 μm were utilized. Intracellular solutions were rapidly changed using a computer-controlled 20-channel solution switcher. Measurements were obtained using pipette solution (100 mm N-methylglucamine, 10 mm HEPES, 20 mm tetraethylammonium hydroxide, 0.2 mm niflumic acid, 0.2 mm ouabain, 8 mm Ca(OH)2 (pH 7, adjusted with methanesulfonic acid)) and bath solution (100 mm CsOH or 100 mm NaOH, 20 mm tetraethylammonium hydroxide, 10 mm HEPES, 10 mm EGTA or HEDTA, and different Ca(OH)2 concentrations to obtain the desired final free Ca2+ concentrations (pH 7, using methanesulfonic acid)). Free Ca2+ concentrations were calculated according to the WEBMAXC program (14) and confirmed with a Ca2+ electrode.
Ca2+ activation curves were obtained by perfusing solutions with different ion concentrations. Data were normalized to the maximum values and fitted to a Hill function. Each point is the average of between three and six experiments. Values are mean ± S.E. PCLAMP (Axon Instruments, Burlingame, CA) software was used for acquisition and analysis. Data were acquired on line at 4 ms/point and filtered at 50 Hz using an 8-pole Bessel filter. Experiments were performed at 35 °C and at a holding potential of 0 mV.
RESULTS
The crystal structures of the CBDs of the NCX have been resolved, and the regions involved in coordinating Ca2+ are shown in Fig. 1. Our goal was to determine the regulatory roles of specific Ca2+ ions that bind in CBD1 and CBD2. Residues mutated in this work are underlined. We utilized the giant patch technique (13) to characterize the response of mutant exchangers to cytoplasmic Ca2+. Outward exchange currents were elicited by rapidly applying Na+ (100 mm) into the bath at the intracellular surface of the patch. Ca2+ (8 mm) was continuously present within the pipette at the extracellular surface. As can be seen in the traces recorded from the wild type exchanger (WT) (Fig. 2A), exchange current peaks and then decays to a steady-state value when Na+ is applied to the intracellular surface. The decay is triggered by the high intracellular Na+ (Na+-dependent inactivation or I1). Increasing cytoplasmic Ca2+ concentrations increases the peak currents and antagonizes I1 (3).
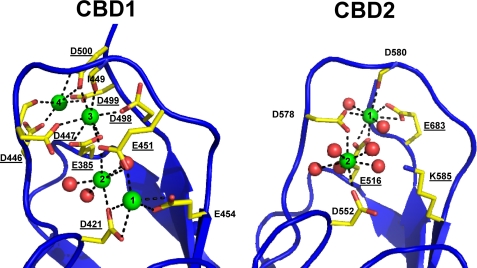
FIGURE 1.
Structures of the Ca2+-binding sites of CBD1 and CBD2. Shown are the organization of residues involved in coordinating Ca2+ ions in CBD1 (left) and CBD2 (right) (Protein Data Bank codes 2PDK and 2QVM). Residues mutated in this study are underlined.
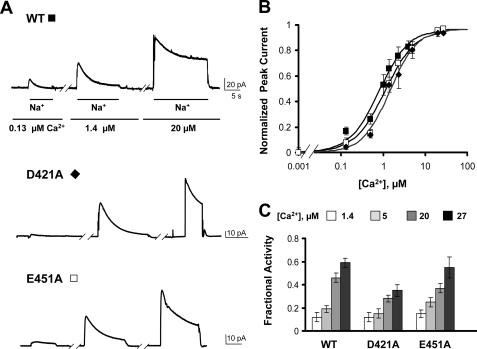
FIGURE 2.
Ca2+ ions coordinated by sites 1 and 2 of CBD1 are not essential for Ca2+ regulation. A, examples of giant patch recordings from oocytes expressing the indicated construct. Outward currents were generated by rapidly applying 100 mm Na+ into the bath (intracellular surface) with 8 mm Ca2+ in the pipette. Representative traces in the presence of three different intracellular Ca2+ concentrations are shown (0.13, 1.4, and 20 μm). Neither Na+-dependent inactivation nor Ca2+ regulation is much affected by mutations at positions 421 or 451. B, Ca2+ dependence of outward exchanger current. Values were measured at the peak of the current. Residual current recorded in the absence of regulatory Ca2+ has been subtracted. Each point is the average of between two and six experiments. C, summary of the fractional activity values for the WT and the exchanger mutants measured in the presence of the indicated Ca2+ concentrations. Fractional activity was measured as the ratio between steady-state and peak currents. Each point is the average of between three and six experiments.
Mutations within CBD1
First, the effects of mutations within CBD1 were analyzed. Fig. 2 shows examples of WT, D421A, and E451A exchanger currents recorded at different intracellular Ca2+ concentrations. Asp421 and Glu451 are primarily involved in coordinating Ca2+ at Ca1 and Ca2 of CBD1 (Fig. 1). Similar to WT, currents recorded from oocytes expressing these mutants peaked rapidly and then decayed over several seconds because of the Na+-dependent inactivation. Peak currents from both WT and mutant exchangers were enhanced by raising the intracellular concentration of regulatory Ca2+. Because the onset of the Na+-dependent inactivation is slow (3), the peak current reflects mainly the effects of Ca2+ on exchanger activation. Peak currents as a function of Ca2+ concentration for WT and mutant exchangers were fitted to a Hill function (Fig. 2B). The extrapolated values of the apparent Ca2+ affinities were 0.86 μm for the WT exchanger and 1.46 and 1.12 μm for D421A and E451A, respectively, indicating that mutations at these sites slightly reduce the sensitivity of NCX for cytoplasmic Ca2+. In addition to activating the exchanger, increasing regulatory Ca2+ releases NCX from Na+-dependent inactivation (2, 10). The effects of Ca2+ on Na+-dependent inactivation are analyzed by measuring fractional currents calculated as the ratio of the steady-state current to the peak current (fractional activity). Both WT and D421A and E451A mutant exchangers show a similar reduction in the extent of Na+-dependent inactivation with increasing Ca2+ (Fig. 2C), indicating that the mutations did not alter the effects of Ca2+ on Na+ regulation. Overall, our data indicate that Ca1 and Ca2 of CBD1 contribute minimally to regulation of the exchanger by Ca2+.
The roles of residues coordinating Ca2+ to sites 3 and 4 of CBD1 were also investigated. Residues Glu385, Asp447, Ile449, Glu451, Asp498, and Asp500 coordinate Ca2+ at site 3, whereas Asp446, Asp447, Asp499, and Asp500 comprise site 4. Single mutants D447V, D498I, and D500V were previously shown to alter markedly the apparent affinity of the exchanger for cytoplasmic Ca2+ (10). We now characterize the biophysical properties of two mutants E385A and double mutant D446A/D447A and further investigate the effects of replacing Asp500 with valine. Glu385 exclusively coordinates Ca2+ at site 3, whereas mutants D446A/D447A and D500V will perturb Ca2+ binding at both sites 3 and 4. Fig. 3 shows representative outward current traces recorded at the indicated regulatory Ca2+ concentrations from oocytes expressing the mutant exchangers. Activation of currents from E385A, double mutant D446A/D447A, and D500V required higher intracellular Ca2+ than did the WT exchanger. The dependence of the peak current on intracellular Ca2+ for WT and exchanger mutants is shown in Fig. 3B. Mutations at these sites decreased the apparent Ca2+ affinity of the exchanger by about 5-fold (K1/2 micromolar values are 0.86, 3.51, 4.80, and 4.93 for WT, E385A, D446A/D447A, and D500V, respectively), indicating an important role for Ca2+ at CBD1 sites Ca3 and Ca4 in exchanger regulation.
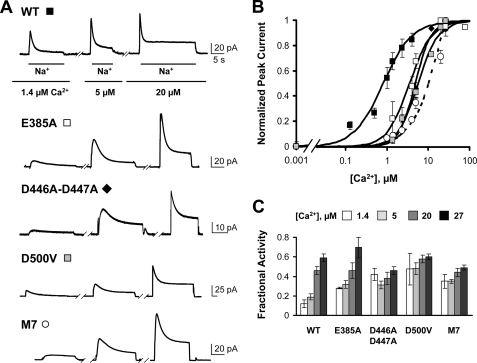
FIGURE 3.
Mutations of residues coordinating Ca2+ at sites 3 and 4 of CBD1 decrease the exchanger Ca2+ affinity. A, representative outward currents recorded from oocytes expressing the indicated mutant. Residues Glu385, Asp421, Asp446, Asp447, Asp498, Asp499, and Asp500 of CBD1 were mutated to Ala in mutant M7. Notice that higher Ca2+ concentrations are required to activate the mutant exchangers. Ca2+ concentrations are shown below the traces. B, dose-response curves for cytoplasmic Ca2+ for WT and the indicated exchanger mutants. Current amplitudes were measured at peak currents. Residual current recorded in the absence of Ca2+ has been subtracted. Each point is the average of between two and four experiments. The M7 peak current was normalized at the highest concentration of Ca2+ examined because saturation was not obtained. C, fractional activity values for each exchanger. Measurements were done in the presence of the indicated Ca2+ concentrations.
Next, we constructed and characterized an exchanger with mutations in 7 of the 10 acidic amino acids (designated M7, with the following residues mutated to alanine: 385, 421, 446, 447, 498, 499, and 500) that are directly involved in the coordination of Ca2+ to CBD1. CBD1 of M7 should be incapable of binding Ca2+. Although it was not possible to quantify the apparent Ca2+ affinity of peak currents because of lack of saturation, M7 retained Ca2+ regulation (Fig. 3, A and B), unmasking a role for CBD2 in exchanger regulation.
The effects of cytoplasmic Ca2+ on the Na+-dependent inactivation of WT and exchanger mutants were then investigated. First, the fraction of inactivated mutant exchangers was quantified by measuring fractional activity. As shown in Fig. 3C, the fractional activity values measured for E385A, D446A/D447A, D500V, and M7 were significantly higher than that of WT (values are 0.12 ± 0.04 for WT, 0.28 ± 0.01 for E385A, 0.42 ± 0.06 for D446A/D447A, 0.47 ± 0.16 for D500V, and 0.35 ± 0.07 for M7, measured in the presence of 1.4 μm regulatory Ca2+), indicating that the Na+-dependent inactivation was less pronounced in the mutant exchangers.
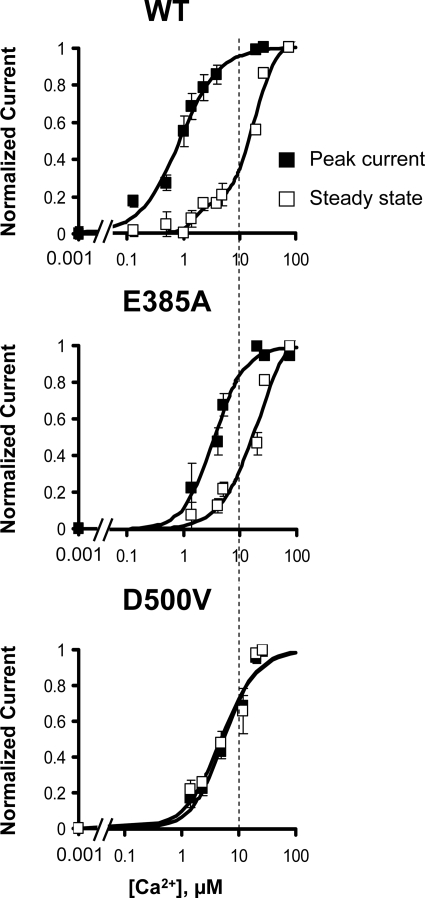
To investigate Ca2+ regulation further, we examined the Ca2+ dependence of steady-state current. Ca2+ influences steady-state current by both increasing exchanger activity and relieving NCX from the Na+-dependent inactivation. For the WT exchanger, the dependence of the steady-state current on Ca2+ is shifted to a much higher Ca2+ than the peak current (Fig. 4) (3). Fig. 4 also shows normalized peak and steady-state Ca2+ activation curves for mutants E385A and D500V. The gap between the concentration dependences of peak and steady-state currents for regulatory Ca2+ was significantly reduced in E385A mutant because of a decrease in Ca2+ sensitivity of the peak current. Interestingly, for mutant D500V, the steady-state current is saturable and shows a nearly identical affinity as the peak current. In this mutant, the affinity of the peak current is simultaneously reduced, and the sensitivity of the steady-state current to cytoplasmic Ca2+is increased. The results emphasize the important role that Ca2+ ions coordinated by sites 3 and 4 of CBD1 play in exchanger regulation.
FIGURE 4.
Ca2+ sensitivities of peak and steady-state NCX currents. Ca2+ dependences of outward current for peak (■) and steady-state (□) currents generated by WT and the indicated NCX mutants are shown. Note that, in contrast to WT, exchanger D500V shows the same affinity for Ca2+ for both peak and steady-state currents.
Mutations within CBD2
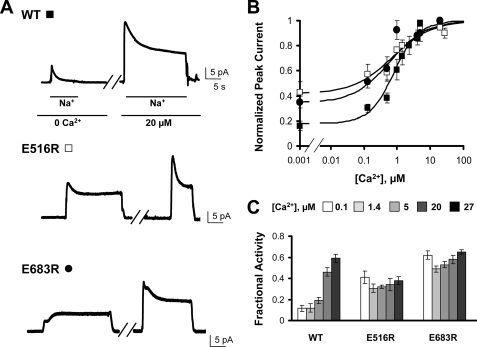
We next examined the effects of mutations within CBD2. Previously, we showed that three mutants within CBD2 (E516L, D578V, and E683V) lack Ca2+ regulation (4). These three anionic residues all contribute directly to the binding of the primary Ca2+ (Ca1) to CBD2. We refer to Ca1 of CBD2 as the primary Ca2+ because Ca2 is bound loosely and appears to have no function (4). We hypothesized that introducing a positive charge at these sites might partially mimic a Ca2+ ion and perhaps rescue Ca2+ regulation. Fig. 5 shows representative outward NCX currents for WT and mutants E516R and E683R in the absence and presence of Ca2+. (Mutant D578R has been previously characterized and shown to be regulated by Ca2+ (15).) In the absence of regulatory Ca2+, a substantial component of NCX current was present that was augmented by raising intracellular Ca2+. Thus, the E516R and E683R mutants partially retained Ca2+ regulation of peak current in contrast to mutants in which neutral amino acids were used as replacements. Fig. 5B summarizes the effects of Ca2+ on peak currents, indicating that the Ca2+-sensitive components of these mutants have apparent affinities for Ca2+ similar to those of WT (K1/2 micromolar values are 0.86, 0.72, and 0.6 for WT, E516R, and E683R, respectively).
FIGURE 5.
Introduction of a positive charge at position 516 or 683 partially rescues NCX Ca2+ regulation. Previously we have shown that NCX mutants E516L, D578V, and E683V lack Ca2+ regulation. Introduction of a positive charge at any of these positions partially restores NCX Ca2+ regulation. A, outward currents recorded from oocytes expressing the indicated construct. B, Peak current values versus cytoplasmic Ca2+ for WT (■), E516R (□), and E683R (●). C, fractional activity values measured at different Ca2+ concentrations for each exchanger.
Fig. 5C shows a summary of the effects of Ca2+ on fractional activity of the WT and mutant exchangers. For WT, the extent of Na+-dependent inactivation decreases as Ca2+ is elevated as indicated by increased fractional activity. As observed previously for mutants E516L, D578V, and E683V, introduction of a positive charge at positions 516 and 683 disrupts the Ca2+ sensitivity of fractional activity.
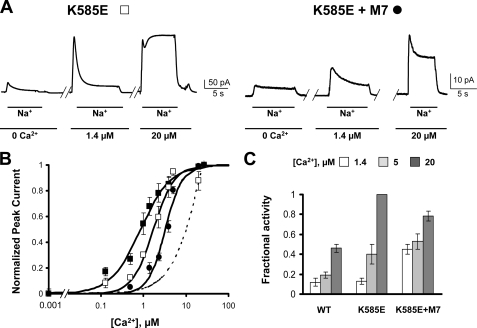
Lys585 in CBD2 forms a salt bridge with Asp552 and Glu648 in the absence of Ca2+, conferring structural integrity to the Ca2+-free form (4). (Note that Glu683 of NCX1.1 studied here is equivalent to Glu648 of the splice variant NCX1.4 used in the crystallization studies.) Introduction of a negative charge at this location (K585E) slightly decreases the Ca2+ sensitivity of the peak current (4). There is also a marked alteration in the effect of Ca2+ on Na+-dependent inactivation. The steady-state current becomes much more sensitive to intracellular Ca2+. This can be seen in Fig. 6A, where 20 μm Ca2+ completely removes the Na+-dependent inactivation of the K585E mutant, whereas this is not true of the WT exchanger (Figs. 2A and 3A; see also Fig. 6C).
FIGURE 6.
Introduction of K585E into the NCX-M7 background increases apparent Ca2+ affinity. A, outward currents recorded from excised patches of oocytes expressing the indicated construct. Bath [Ca2+] is shown below the traces. B, peak current values versus cytoplasmic Ca2+ for WT (■), K585E (□), and K585E (●) in M7 background. The apparent affinities are 0.86 μm (WT), 1.7 μm (K585E), and 3.3 μm (K585E+M7). The dashed line shows the dose-response curve for Ca2+ for mutant M7 from Fig. 3. The apparent affinity of M7 for Ca2+ cannot be quantified due to lack of saturation. C, fractional activity values at different Ca2+ concentrations for each exchanger.
We inferred above that the low affinity Ca2+ regulation of the CBD1 mutant M7 was due to CBD2. We tested that idea by introduction of the K585E mutation into the M7 background. If the K585E mutation does increase the affinity of CBD2 for Ca2+, then it might partially rescue the low apparent Ca2+ affinity of M7. Indeed, this turns out to be the case. Addition of the mutation K585E into the M7 background resulted in an increase in apparent Ca2+ affinity of the peak current (Fig. 6B).
Role of the F-G Loops of CBD1 and CBD2 in Ca2+ Regulation of the Exchanger
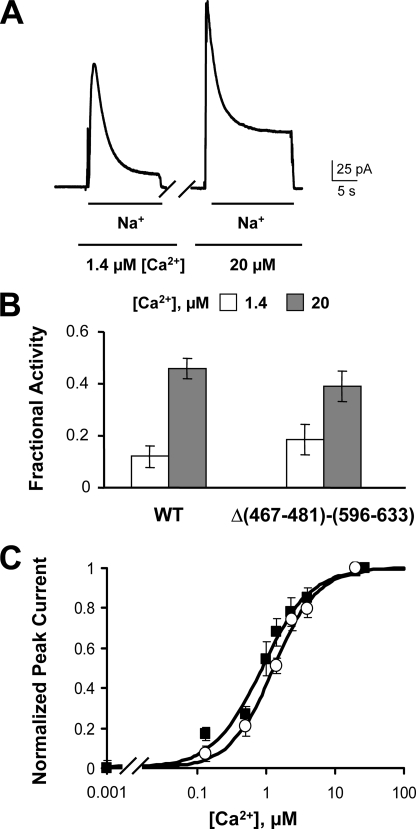
The structures of CBD1 and CBD2 have been determined by both NMR and crystal structure (4–6, 8). Exceptions, however, are the long loops between β-strands F and G of both CBDs, which are unstructured. Although this F-G loop is well conserved among the CBD1 sequences of different exchangers, the corresponding amino acids of CBD2 vary substantially due to alternative splicing, resulting in CBD2s of varying length (16). To determine the functional role of these portions of the CBDs, we deleted residues 467–481 (CBD1) and 596–633 (CBD2) of NCX1.1 (mutant Δ(467–481)-(596–633)) to determine the effects on Ca2+ regulation. Fig. 7A shows outward currents recorded from oocytes expressing the exchanger carrying the double deletion. Similar to WT, exchange current peaked upon application of intracellular Na+ and then was inactivated due to the high intracellular Na+. Raising the intracellular Ca2+ concentration further activated the exchanger and decreased the extent of the Na+-dependent inactivation. The fraction of steady-state to peak current values obtained for the WT and Δ(467–481)-(596–633) exchangers was not significantly different (Fig. 6B) (0.12 ± 0.04 and 0.46 ± 0.04 for WT and 0.18 ± 0.06 and 0.39 ± 0.06 for Δ(467–481)-(596–633), measured in the presence of 1.4 and 20 μm Ca2+, respectively), indicating that these two regions of the exchanger are not important for Na+-dependent inactivation. The effects of regulatory Ca2+ on peak outward current mediated by Δ(467–481)-(596–633) and WT exchangers are shown in Fig. 7C. Both exchangers exhibit a similar increase in the peak current as regulatory Ca2+ is raised. In summary, deletion of the F-G loops of CBD1 and CBD2 did not significantly alter the biophysical properties of the exchanger, indicating that these two unstructured regions do not play a fundamental role in secondary regulation of the exchanger by Ca2+.
FIGURE 7.
Deletion of the F-G loops of CBD1 and CBD2 does not alter exchanger properties. A, outward currents recorded from oocytes expressing NCX with residues 467–481 and 596–633 deleted. B, fractional activity values calculated from WT and Δ(467–481)-(596–633) currents recorded in the presence of 1.4 and 20 μm cytoplasmic Ca2+. C, peak values versus cytoplasmic Ca2+ for WT (■) and the deletion mutant (○). The apparent affinity values for the regulatory Ca2+ dependence of peak current are 0.86 μm for WT and 1.28 μm for Δ(467–481)-(596–633).
DISCUSSION
Regulatory Ca2+ modulates the activity of the NCX by binding to two cytoplasmic domains encompassing residues 371–501 (CBD1) and 501–678 (CBD2) (4–9, 17). CBD1 and CBD2 are both located within the large cytoplasmic loop of the exchanger between transmembrane segments 5 and 6 (7). Recently, the crystal structures of the exchanger CBDs have been resolved (4, 8, 9). CBD1 and CBD2 bind four and two Ca2+ ions, respectively. The domains have similar structures comprising seven antiparallel β-strands with the Ca2+-binding sites within the connecting loops at one end of the structure. CBD1 may undergo large conformational changes upon unbinding and binding of Ca2+ (5, 18, 19), although more constrained movements of CBD1 have also been reported (6). In contrast, CBD2 undergoes only minor structural rearrangements upon binding Ca2+ (4). CBD2 of the exchanger of Drosophila melanogaster does not appear to bind Ca2+ (9). Consistent with this observation, the Drosophila exchanger displays anomalous regulatory properties (20).
Binding of cytoplasmic Ca2+ to these domains triggers two measurable molecular processes: Ca2+ activates the exchange current and also rescues current from Na+-dependent inactivation (1, 21). Experimentally, these effects are investigated by measuring the initial peak of exchange current and the steady state current, respectively. Because Na+-dependent inactivation occurs over several seconds, the effects of Ca2+ on peak currents mainly reflect exchanger activation, whereas steady-state currents are a function of effects of Ca2+ on both exchanger activation and rescue from the Na+-dependent inactivation. The conformational changes that drive these two regulations are unclear as are the contributions of each CBD. To elucidate the roles of CBD1 and CBD2, we mutated strategic residues within these two domains, guided by the crystal structures, and we determined their effects on Ca2+ regulation using electrophysiology. We first examined the effects of mutations within CBD1. Mutations of residues Asp421 and Glu451 minimally alter the Ca2+ sensitivity of NCX. These residues primarily coordinate Ca1 and Ca2. Thus, our data indicate that the Ca1 and Ca2 sites within CBD1 are not crucial in conferring Ca2+ regulation to the exchanger. These results are supported by recent structural and electrophysiological data showing that a mutant exchanger unable to bind Ca2+ at position 1 displays a phenotype similar to the WT exchanger (11).
Recent studies investigating the kinetics and equilibrium properties of Ca2+ binding to CBD1 and CBD2 detected two low affinity Ca2+ sites in CBD1 (22). Our data would suggest that these sites are Ca1 and Ca2. Because the Ca2+-binding affinities for these sites are higher than 30 μm (22), they may not be occupied under the conditions in which our electrophysiological experiments were performed.
In sharp contrast to the mutations that disrupt the Ca1 and Ca2 sites of CBD1, mutations of residues involved in forming the binding sites for Ca3 and Ca4 (E385A, D446V–D447V, and D500V) drastically alter the Ca2+ sensitivity of NCX. The decrease of the apparent affinity for cytoplasmic Ca2+ observed in these mutants emphasizes their important role in Ca2+ regulation of the NCX.
A mutant of particular interest is D500V, which disrupts the binding of Ca2+ to sites 3 and 4 of CBD1. As shown in Fig. 4, the Ca2+ dose-response curves of the D500V peak and steady-state currents overlap. This observation reflects the fact that this mutation abolishes the sensitivity of the Na+-dependent inactivation for Ca2+ as shown in Fig. 3C. Only the activation of the exchanger transport by Ca2+ is observed. D500V is the first single mutation within CBD1 known to alter the effects of Ca2+ on Na+-dependent inactivation therefore revealing an influence of CBD1 in this process.
The recognition of a second CBD in NCX is recent, and the role of this domain is less studied. To gain insight into the function of CBD2 in controlling activity, we inactivated CBD1 by mutating 7 of the 10 amino acids that coordinate Ca2+ binding to CBD1. This M7 exchanger was still Ca2+-regulated, though with decreased Ca2+ affinity. As we predict CBD1 of M7 cannot bind Ca2+, the remaining Ca2+ sensitivity must reflect the binding of Ca2+ to CBD2. Because of competition between Ca2+ and Na+ at the transport site, it was not possible to increase the intracellular Ca2+ concentration sufficiently to determine the apparent affinity of CBD2 for Ca2+ accurately. However, the apparent Ca2+ affinity of CBD2 was increased and saturation was achieved when the mutation K585E of CBD2 was introduced into the M7 background. Lys585 is near the Ca2+-binding sites of CBD2, and its replacement with a negative charge increases the apparent affinity for Ca2+ to relieve Na+-dependent inactivation (4) (Fig. 5). Introduction of the K585E mutation into the M7 background appears to increase the Ca2+ affinity of CBD2, therefore conferring increased apparent Ca2+ affinity to M7. The result confirms that the Ca2+ regulation of M7 is due to CBD2. Alternatively, interactions between CBD1 and CBD2 may exist, and mutations in one CBD could affect the affinity of the other domain for Ca2+.
To investigate further the role of CBD2 in controlling the Ca2+ regulation of the exchanger, two of the anionic amino acids (Glu516 and Glu683) that coordinate the primary Ca2+ of CBD2 were replaced with positively charged lysines. Previously, we had shown that NCX mutants E516L, D578V, and E683V lack Ca2+ regulation (4). Introduction of a positive charge at any of these positions may mimic the presence of Ca2+ and therefore rescue Ca2+ regulation. Electrophysiological characterization of NCX mutant exchangers E516R and E683R revealed that indeed these mutants were regulated by Ca2+ with affinities similar to that of the WT exchanger. The mutant D578R has previously been shown to also be regulated by Ca2+ (15). Thus, it appears that a specific conformation of CBD2 is required to permit Ca2+ regulation to occur. This permissive conformation is provided by a positive charge at position 516 or 683 but not by substitution with a neutral amino acid. A functional CBD2 allows the Ca2+ affinity for regulation to be set by CBD1.
The structures of the domains of the exchanger that bind Ca2+ within the intracellular loop are known, with the exception of residues 467–481 and 596–633 within the F-G loops of CBD1 and CBD2, respectively. Because of high flexibility, the structure of these regions in the mammalian NCX remains undetermined. Likewise, their functional roles, if any, are unknown. Of particular interest are amino acid residues 596–633 (encoding exons C to F of NCX1.1) of CBD2 as this region varies greatly among exchanger isoforms due to alternative splicing (16). The use of alternative exons suggests a potential role of this region in exchanger regulation. For example, in the Drosophila NCX, which has anomalous inhibition by regulatory Ca2+, the amino acid sequence of the F-G loop is quite different from NCX1, and the region is structured forming two helices in close proximity to the β-barrel structure of CBD2 (9). This region may influence either the binding of Ca2+ to CBD1 or the transduction of the Ca2+ regulatory signal (9). We explored the contribution of the F-G loops to regulation of NCX1.1 by deletion and by characterizing the resultant mutant with respect to activation by cytoplasmic Ca2+. Our data indicate that these portions of the CBD domains do not play any significant role in Ca2+ regulation.
In summary, our results indicate that both CBD1 and CBD2 contribute to the Ca2+ regulation of the NCX, although the exact roles of each domain are not completely resolved. Residues coordinating Ca2+ sites 3 and 4 in CBD1 and the primary Ca2+-binding site (Ca1) in CBD2 are key in Ca2+ regulation and are involved in controlling both I1 and I2 processes. Further effort is needed to determine how CBD1 and CBD2 communicate with one another. Likewise, further studies are required to understand the transduction of the binding of regulatory Ca2+ to activation of the NCX.
Acknowledgment
We thank Dr. Vincent Chaptal for providing Fig. 1.
This work was supported, in whole or in part, by National Institutes of Health Grant HL49101.
- NCX
- Na+-Ca2+ exchanger
- CBD
- Ca2+-binding domain
- HEDTA
- N-(2-hydroxyethyl)ethylenediamine-N,N′,N′-triacetic acid
- I1
- Na+-dependent inactivation
- WT
- wild type.
REFERENCES
- 1.Philipson K. D., Nicoll D. A., Ottolia M., Quednau B. D., Reuter H., John S., Qiu Z. (2002) Ann. N. Y. Acad. Sci. 976, 1–10 [DOI] [PubMed] [Google Scholar]
- 2.Hilgemann D. W., Collins A., Matsuoka S. (1992) J. Gen. Physiol. 100, 933–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilgemann D. W., Matsuoka S., Nagel G. A., Collins A. (1992) J. Gen. Physiol. 100, 905–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besserer G. M., Ottolia M., Nicoll D. A., Chaptal V., Cascio D., Philipson K. D., Abramson J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18467–18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilge M., Aelen J., Vuister G. W. (2006) Mol. Cell 22, 15–25 [DOI] [PubMed] [Google Scholar]
- 6.Johnson E., Bruschweiler-Li L., Showalter S. A., Vuister G. W., Zhang F., Brüschweiler R. (2008) J. Mol. Biol. 377, 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levitsky D. O., Nicoll D. A., Philipson K. D. (1994) J. Biol. Chem. 269, 22847–22852 [PubMed] [Google Scholar]
- 8.Nicoll D. A., Sawaya M. R., Kwon S., Cascio D., Philipson K. D., Abramson J. (2006) J. Biol. Chem. 281, 21577–21581 [DOI] [PubMed] [Google Scholar]
- 9.Wu M., Wang M., Nix J., Hryshko L. V., Zheng L. (2009) J. Mol. Biol. 387, 104–112 [DOI] [PubMed] [Google Scholar]
- 10.Matsuoka S., Nicoll D. A., Hryshko L. V., Levitsky D. O., Weiss J. N., Philipson K. D. (1995) J. Gen. Physiol. 105, 403–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaptal V., Ottolia M., Mercado-Besserer G., Nicoll D. A., Philipson K. D., Abramson J. (2009) J. Biol. Chem. 284, 14688–14692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottolia M., Nicoll D. A., Philipson K. D. (2005) J. Biol. Chem. 280, 1061–1069 [DOI] [PubMed] [Google Scholar]
- 13.Hilgemann D. W., Lu C. C. (1998) Methods Enzymol. 293, 267–280 [DOI] [PubMed] [Google Scholar]
- 14.Patton C., Thompson S., Epel D. (2004) Cell Calcium 35, 427–431 [DOI] [PubMed] [Google Scholar]
- 15.Dunn J., Elias C. L., Le H. D., Omelchenko A., Hryshko L. V., Lytton J. (2002) J. Biol. Chem. 277, 33957–33962 [DOI] [PubMed] [Google Scholar]
- 16.Quednau B. D., Nicoll D. A., Philipson K. D. (1997) Am. J. Physiol. Cell Physiol. 272, C1250–C1261 [DOI] [PubMed] [Google Scholar]
- 17.Nicoll D. A., Ottolia M., Philipson K. D. (2002) Ann. N. Y. Acad. Sci. 976, 11–18 [DOI] [PubMed] [Google Scholar]
- 18.Xie Y., Ottolia M., John S. A., Chen J. N., Philipson K. D. (2008) Am. J. Physiol. Cell Physiol. 295, C388–C393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottolia M., Philipson K. D., John S. (2004) Biophys. J. 87, 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hryshko L. V., Matsuoka S., Nicoll D. A., Weiss J. N., Schwarz E. M., Benzer S., Philipson K. D. (1996) J. Gen. Physiol. 108, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M., Niggli E. (1999) J. Membr. Biol. 168, 107–130 [DOI] [PubMed] [Google Scholar]
- 22.Boyman L., Mikhasenko H., Hiller R., Khananshvili D. (2009) J. Biol. Chem. 284, 6185–6193 [DOI] [PubMed] [Google Scholar]