Abstract
Tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of catecholamines, is activated by phosphorylation-dependent binding to 14-3-3 proteins. The N-terminal domain of TH is also involved in interaction with lipid membranes. We investigated the binding of the N-terminal domain to its different partners, both in the unphosphorylated (TH-(1–43)) and Ser19-phosphorylated (THp-(1–43)) states by surface plasmon resonance. THp-(1–43) showed high affinity for 14-3-3 proteins (Kd ∼ 0.5 μm for 14-3-3γ and -ζ and 7 μm for 14-3-3η). The domains also bind to negatively charged membranes with intermediate affinity (concentration at half-maximal binding S0.5 = 25–58 μm (TH-(1–43)) and S0.5 = 135–475 μm (THp-(1–43)), depending on phospholipid composition) and concomitant formation of helical structure. 14-3-3γ showed a preferential binding to membranes, compared with 14-3-3ζ, both in chromaffin granules and with liposomes at neutral pH. The affinity of 14-3-3γ for negatively charged membranes (S0.5 = 1–9 μm) is much higher than the affinity of TH for the same membranes, compatible with the formation of a ternary complex between Ser19-phosphorylated TH, 14-3-3γ, and membranes. Our results shed light on interaction mechanisms that might be relevant for the modulation of the distribution of TH in the cytoplasm and membrane fractions and regulation of l-DOPA and dopamine synthesis.
Introduction
The 14-3-3 proteins are a family of highly conserved, abundant acidic proteins of about 30 kDa, found in all eukaryotic organisms (1–3). These proteins have been shown to be important in cell cycle regulation, cell signaling, regulation of gene expression, DNA damage response, apoptosis, and protein trafficking. Eliminating 14-3-3 function sensitizes malignant cells toward apoptosis (4–6), and several 14-3-3-partner protein interactions are attractive targets for cancer therapy. The 14-3-3 proteins are expressed in all tissues but at particular high levels in brain. In addition, different expression levels of 14-3-3 isoforms (seven in humans) (7) have been reported (e.g. the 14-3-3σ isoform is nearly absent in many tissues, including brain, but expressed at the level of actin in keratinocytes) (8). The 14-3-3 proteins exert their function as homo- or heterodimers (1, 2, 9), often by binding one or two partners, which are phosphorylated at Ser/Thr residues within specific sequence motifs (10–12).
Tyrosine hydroxylase (TH)3 and the homologous tryptophan hydroxylase (TPH) were the first identified phosphorylated binding partners of 14-3-3 proteins (13). TH is the enzyme that catalyzes the first and rate-limiting reaction in the biosynthesis of catecholamines, the latter being essential neurotransmitters and hormones. Four residues at the regulatory N-terminal domain of mammalian TH (i.e. Ser/Thr8, Ser19, Ser31, and Ser40) are phosphorylated by a number of protein kinases. For a review, see Ref. 14. Phosphorylation at Ser19 by either Ca2+/calmodulin-dependent kinase II, mitogen-activated protein kinase-activated protein kinase 2 (15, 16), or p38-regulated/activated kinase (17) promotes the high affinity binding to 14-3-3 proteins, which increases TH activity severalfold (17, 18). TPH2 also binds to 14-3-3 proteins through phosphorylated Ser19 (19), but little is known about the structural details of the interaction between TH (or TPH2) and 14-3-3, although crystal structures of all 14-3-3 isoforms with bound peptides or serotonin N-acetyltransferase have been determined (20, 21). However, the structure of the regulatory N-terminal domain of TH and TPH2 has not been solved yet, and there is little sequence homology between the 14-3-3-interaction motifs in these hydroxylases and other partners. The phosphorylated 14-3-3-interacting proteins present three main binding motifs, RSXpSXP (mode I), RX(Y/F)XpSXP (mode II) (12), and a carboxyl-terminal (pS/pT)X1–2-COOH motif (mode III), where X is not Pro (1). The hydroxylases bind through sequence motifs that do not conform to the I–III consensus binding modes (Fig. 1), but this is not unusual among physiological substrates (1).
FIGURE 1.
Sequence alignment of the N terminus of the human aromatic amino acid hydroxylases, tyrosine hydroxylase isoform 1 (isoform b; NCBI accession code NP_000351.2; hTH1), phenylalanine hydroxylase (hPAH), tryptophan hydroxylase 2 (hTPH2), and tryptophan hydroxylase 1 (hTPH1). The main determinant for phosphorylation-dependent interaction with 14-3-3 (Ser19) is also present in neuronal hTPH2. The brackets comprise the sequence of the peptide used in this study (TH-(1–43)).
TH is essentially soluble and cytoplasmic (22), but a fraction is also found as membrane-bound both in brain, notably at nerve endings and synaptic vesicles (23–25), and in adrenal chromaffin cells, where it is associated with catecholamine secretory granules (23, 26, 27). It has further been shown that TH has affinity for isolated chromaffin granule membranes and negatively charged phospholipid layers (28, 29). The binding of TH to membranes involves the N-terminal region of the enzyme as seen with recombinant human TH isoform 1 (hTH1), where truncated forms of the enzyme lacking up to residues 33–49 do not bind to membranes (29). Thus, both the phosphorylation-dependent TH binding to 14-3-3 and the interaction of TH with membranes occur through the same N-terminal region around Ser19 (Fig. 1), and both binding processes might be physically excluding.
Also in the case of 14-3-3 proteins, which are considered to be mainly cytosolic, there are reports on their membrane association in chromaffin cells and brain, with a higher membrane binding propensity for the γ and ϵ isoforms (30–32). Moreover, 14-3-3γ is colocalized with the muscle-specific receptor tyrosine kinase at neuromuscular postsynaptic membranes (33). On the other hand, binding to 14-3-3 proteins has been reported to promote the cytoplasmic localization of specific partner proteins or to antagonize the membrane recruitment of others (34, 35). Hence, the regulation of the membrane association of proteins by their interaction with 14-3-3 appears to be a complex and probably isoform-specific process.
In this work, we have investigated the determinants for the interaction of TH with both membranes and 14-3-3 using unphosphorylated and Ser19-phosphorylated 43-residue-long polypeptides corresponding to the N-terminal region of hTH1 (i.e. TH-(1–43) and THp-(1–43), respectively) (Fig. 1). Using surface plasmon resonance (SPR) measurements (36), we have further characterized the membrane binding of the 14-3-3 isoforms abundant in brain and adrenal medulla (γ, ζ, and η) (30, 32, 37). Furthermore, we have investigated how protein-membrane interactions were influenced by protein-protein interaction between the 14-3-3s and nonphospho- and phospho-TH N-terminal polypeptides. The solution structure of TH-(1–43) and THp-(1–43) and the effect of membrane binding on their structure was studied by CD. Our results recognize the N-terminal region of TH as a 14-3-3-membrane interaction domain. We also corroborated that 14-3-3γ has a high affinity for negatively charged membranes, probably interacting through isoform-specific basic residues in helix F. A tri-partite complex may be formed between Ser19-phosphorylated TH, 14-3-3γ, and phospholipid membranes.
EXPERIMENTAL PROCEDURES
Peptides and Phospholipids
The peptides TH-(1–43) (MPTPDATTPQAKGFRRAVSELDAKQAEAIMSPRFIGRRQSLIE) and THp-(1–43) (MPTPDATTPQAKGFRRAVS(PO3)ELDAKQAEAIMSPRFIGRRQSLIE) were synthesized by CPC Scientific (San Jose, CA) at >90% purity, as seen by mass spectroscopy, and used without further purification. Lipids (i.e. phosphatidylcholine (PC) from egg yolk lecithin (95% PC), 1,2-dioleoylphosphatidylglycerol (DOPG), porcine brain phosphatidylserine (PBPS), 1-stearoyl-2-docosahexaenoylphosphatidylserine (SDPS), 1,2-dipalmitoylphosphatidylcholine (DPPC), 1,2-dimyristoylphosphatidylcholine (DMPC), 1,2-dihexanoylphosphatidylcholine (DHPC), and 1,2-dimyristoylphosphatidylserine (DMPS) were purchased from Avanti Polar Lipids, Inc., except egg yolk PC, which was from Sigma. The absence of fatty acid oxidation both in natural extracts and pure synthetic species was kindly verified by Sonnic Meier as reported (38).
Expression and Purification of Proteins
Recombinant human TH, isoform 1 (isoform b; NCBI accession code NP_000351.2; hTH1) was expressed in Escherichia coli and purified to homogeneity by heparin-Sepharose (Amersham Biosciences) chromatography as described (39). Phosphorylation of hTH1 at Ser19 by p38 regulated/activated protein kinase (PRAK) was performed as previously described (17), obtaining a stoichiometry of 0.8 phosphate incorporated/subunit of hTH1.
The plasmids for protein expression of the human 14-3-3γ, -ζ, and -η isoforms were generously provided by Prof. A. Aitken (Edinburgh, UK) as pGEX-expression vectors, and the 14-3-3 proteins (γ, ζ, and η isoforms) were expressed in E. coli (BL-21, codon+) as glutathione S-transferase fusion proteins and purified on glutathione-Sepharose 4B (GE Healthcare) essentially as reported (40). The fusion protein was cleaved by thrombin (4 units/ml) while attached to the column, at 4 °C for 1 h. The dimeric 14-3-3 proteins (about 56-kDa dimer) were further purified by gel filtration in HiLoad 16/60 Superdex 200 (Amersham Biosciences) in 50 mm sodium phosphate (pH 7.4), 150 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, giving >95% pure protein as evaluated by SDS-PAGE. The γ and ζ 14-3-3 isoform-specific antibodies were obtained from IBL (Gunma, Japan) (product code 18647) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) (C-16, sc-1019), respectively.
Preparation of Chromaffin Granule Membranes
Chromaffin granules and chromaffin granule ghosts (CGGs) from the bovine adrenal medulla were purified essentially as described (41). Briefly, the granules were isolated by differential centrifugation in 0.25 m sucrose, followed by discontinuous density gradient sucrose centrifugation. Hypoosmotic lysis of the granule preparations and additional centrifugation on a discontinuous density gradient leads to highly pure preparations of CGG, essentially free from mitochondrial and microsomal contamination (41) (supplemental Figs. S1 and S2). The cytosolic fraction was prepared by centrifugation of the cell homogenates for 1 h at 100,000 × g.
Immunoblotting
The cytosolic and CGG fractions of bovine adrenal medulla were analyzed for distribution of 14-3-3γ and -ζ. Prior to analysis, the CGGs were washed for 30 min in a physiological (intracellular) buffer (15 mm Hepes, pH 7.2, 130 mm KCl, 1 mm EDTA, 0.3 mm EGTA, 1 mm NaHPO4), incubated on ice, and subsequently centrifuged at 100,000 × g for 30 min. The protein contents of the CGG and cytosolic fractions were determined by the Bradford method (Bio-Rad), and appropriate samples were denatured, separated by SDS-PAGE, and blotted to a polyvinylidene difluoride membrane (HybondTM-P, GE Healthcare). The 14-3-3 isoform-specific antibodies were used at 0.25 μg/ml and a 1:1500 dilution for 14-3-3γ and ζ, respectively. We used alkaline phosphatase-linked secondary antibodies with the CDP-Star substrate (Sigma) for detection. Standard curves using known amounts of recombinant 14-3-3 were prepared for both isoforms in the concentration range 0–60 ng.
Preparation of Liposomes and Bicelles
Lipids solvated in chloroform were added to Kimble glass tubes in the prerequisite amounts. Due attention was given to handling and keeping the lipids and liposomes out of light and reactive atmosphere by operation in hoods and using glass containers wrapped in aluminum foil and layered with argon. The chloroform solutions where dried to lipid films under dry N2 pressure, and trace amounts of chloroform were removed by subjecting the samples to vacuum for at least 2 h. Buffer was then added, and samples were vortexed and allowed to hydrate overnight at room temperature. For liposome preparation, the solutions were subjected to seven freeze-thaw cycles using liquid N2 and a water bath (42). Finally, the hydrated multilamellar structures were extruded using a Mini-Extruder (Avanti Polar Lipids, Inc.) which was assembled using two Millipore filters of 100-nm pore size. The samples were forced through the filters 10 times using Hamilton syringes, and the resulting solution was transferred to clean, foil-wrapped Kimble tubes for immediate usage. By this procedure, large unilamellar vesicles with a size distribution around the filter pore size (i.e. 105 ± 25 nm) were produced (supplemental Fig. S3), very similar to the results of Mayer et al. (43). Liposomes were made of neutral PC and of negatively charged PC:PBPS (1:1) and PC:DOPG (1:1) and of a fatty acyl composition aiming to better approach that of synaptic membranes (i.e. 30% PC, 20% PBPS, 30% SDPS, 20% DPPC, referred to as PC:PBPS:SDPS:DPPC. The liposomes produced were used within a day.
Bicelles were prepared in stock solutions with a total lipid concentration of 16% (w/v) in 5 mm Na-Hepes, pH 7. Briefly, a lipid mix of 2:1:1 molar fractions of DHPC, DMPC, and DMPS was mixed in filtered buffer, vortexed vigorously for 10 min at 4 °C, and kept to equilibrate for 15 min at this temperature. The sample was then transferred to room temperature, vortexed, and subsequently warmed to 38 °C on a water bath for 30 min and transferred back to 4 °C for 15 min of equilibration. This cold vortex/warm vortex cycle was repeated several times until a clear, viscous solution with a blue tint occurred at 38 °C (44). The chosen composition, with 2 mol of lipid with relatively short hexanoyl acyl chains per mol of each lipid with myristoyl fatty acids, produces disc-like bicelles with ratio q = ([DMPC]+ [DMPS])/[DHPC] = 1 (44, 45) and 50 ± 15-nm diameter (supplemental Fig. S4). All buffers were filtered through 0.22-μm membrane filters (Millipore and Pall Corp.).
SPR Measurements
The SPR analyses were carried out using the Biacore 3000 biosensor (Biacore AB), normally at 25 °C. The interaction of TH-(1–43) and THp-(1–43) with 14-3-3 proteins was studied with the sensor chip CM5. Purified 14-3-3γ, -η, and -ζ were diluted in 10 mm sodium acetate, pH 5, to a final concentration of 1.6–1.8 mg/ml (∼57 μm subunit) and immobilized covalently to the hydrophilic carboxymethylated dextran matrix of CM5 (exposure time ∼10 min) by the standard primary amine coupling reaction, as described by the manufacturer. A reference surface was subjected to the same procedure but with no protein. A stable base line was obtained in the cell with immobilized protein by a continuous flow (20 μl/min) of running buffer (HBS-P buffer: 10 mm Hepes, pH 7.4, 150 mm NaCl, 0.005% surfactant P20) for about 1 h. The peptides (0.1–0.3 μg/μl; 21–63 μm) were injected in a volume of 60 μl for 3 min at a flow rate of 20 μl/min. The dissociation constant (Kd) for the interaction of each 14-3-3 protein with TH-(1–43) or THp-(1–43) was calculated from the equation Kd = kd/ka, where the apparent association (ka) and dissociation (kd) rate constants were evaluated from the differential binding curves, assuming an A + B = AB association type. The BIAevaluation program, version 3.2 (Biacore AS) was used for these calculations and also for analysis of the sensorgrams.
To monitor the interaction of TH-(1–43), THp-(1–43), 14-3-3γ, 14-3-3ζ, and 14-3-3η with membranes, an L1 sensor chip was used. The surface of the chip was first cleaned by a 2-min injection of isopropyl alcohol, 50 mm NaOH (1:1) at a flow rate of 20 μl/min, followed by washing for 30 min with running buffer (HBS-N buffer: 10 mm Hepes, pH 7.4, 150 mm NaCl). Liposome solutions that were diluted to 1 mm phospholipid concentration with running buffer were then injected at a flow rate of 10 μl/min, which resulted in a deposition of 4000–6000 response units (RU). Then the polypeptides and proteins (typically in the concentration range 0–150 μm) were applied to the captured liposomes at 10 μl/min. At the end of a binding assay, the sensor chip surface was regenerated by injecting isopropyl alcohol, 50 mm NaOH (40:60, v/v) and running buffer. The values for nonspecific binding measured in the reference cell were subtracted. The binding isotherms for maximal RU versus concentration of protein were found to be hyperbolic and processed by nonlinear least-squares analysis. The concentration of protein providing half-maximal binding (S0.5) is thus a measure of the inverse of the apparent association or partition constant (1/Ka(app)). The S0.5 values provide comparative and operational measurements of affinity.
Tyrosine Hydroxylase Activity
The TH activity in the presence of 14-3-3 and liposomes, both independently and in combination, was investigated essentially as described (46). hTH1 (0.05 μm) was preincubated for 4 min at 30 °C in 40 mm Na-Hepes, pH 7.0, with 25 μm l-Tyr, 0.05 mg/ml catalase, 30 μm Fe(II)SO4, and, when indicated, 1.8 μm 14-3-3 (γ or ζ). The samples were then further incubated 4 min at 30 °C in the absence or presence of liposomes made of PC:PBPS:SDPS:DPPC (20 μm phospholipid concentration). The reaction was started by the addition of 250 μm tetrahydrobiopterin and 5 mm dithiothreitol. After 5 min (linear conditions), the reaction was stopped by 4% acetic acid in ethanol, and l-DOPA formed was analyzed by high pressure liquid chromatography with fluorimetric detection (46).
CD
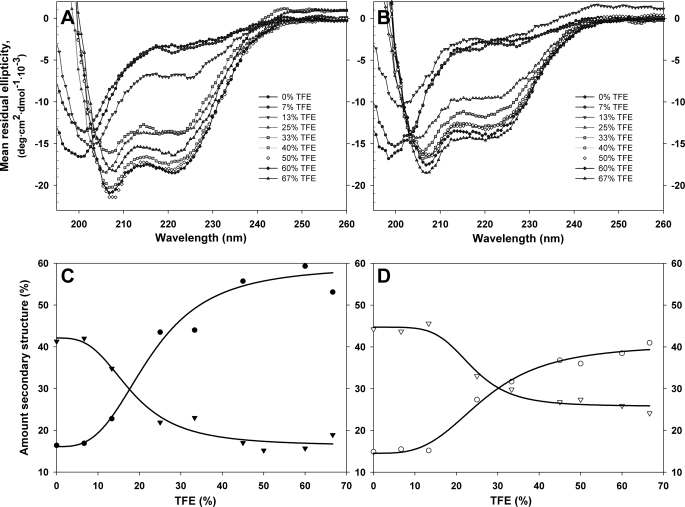
Far-UV CD measurements were performed with a Jasco J-810 spectropolarimeter equipped with a PTC-348WI Peltier element for temperature control at 25 °C using a quartz cell with a path length of 1 mm. CD spectra of TH-(1–43) and THp-(1–43) (20 μm) in the presence of bicelles, q = 1, 4% (w/v), were performed in Na-Hepes, pH 7.0. Trifluoroethanol (TFE) and liposome titrations were performed in 20 mm citric acid, phosphate, pH 7.4, with constant concentration (16–21 μm) of TH-(1–43) and THp-(1–43) in a final volume of 300–350 μl. Buffer blanks were subtracted using accompanying software from Jasco, and the data were imported into Sigma Plot version 9.0. (SPSS Inc.) and smoothed using running average with a sampling proportion of 0.05. Mean residual ellipiticity (θ) was calculated as follows, θ = ϵ/(10·C·n·l), where C represents sample concentration in mol/liter, n is number of amino acids in the peptide, and l is path length of the cuvette in cm. The fraction of random coil and α-helical structure was estimated by the CDNN software (47) using CD data from 200 to 260 nm.
RESULTS
Binding of TH-derived N-terminal Polypeptides to 14-3-3 Proteins
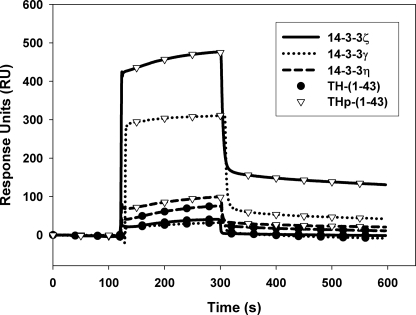
We applied SPR to investigate the interactions of the N-terminal domain of hTH1 with the three 14-3-3 isoforms, γ, ζ, and η, by using specific peptides corresponding to the sequence 1–43 of hTH1 (Fig. 1) in its unphosphorylated (TH-(1–43)) and Ser19-phosphorylated (THp-(1–43)) states. As shown in Fig. 2, the SPR sensorgrams revealed a very low binding of unphosphorylated TH-(1–43) to the 14-3-3 proteins. In contrast, phosphorylated THp-(1–43) binds very effectively, notably to 14-3-3ζ and 14-3-3γ (Fig. 2), and with much higher affinity than for the unphosphorylated peptide (Table 1). The Kd for the interaction of 14-3-3ζ with Ser19-phosphorylated full-length hTH1 was found to be 0.01–0.40 μm (17, 48) and about 0.42 μm for the phosphorylated N-terminal and regulatory domains of hTH1 (residues 1–157) (49). These values are very similar to those obtained here for the interaction with the ζ and γ isoforms (Table 1), thus showing that THp-(1–43) includes the structural determinants for the interaction with 14-3-3. The interaction of THp-(1–43) with the η isoform provides a very low sensorgram response (Fig. 2) and affinity (Table 1) compared with the two other 14-3-3 isoforms. Moreover, the difference in affinities between TH-(1–43) and THp-(1–43) is small, and the affinity of the former for 14-3-3η is high (Table 1), possibly indicating that binding to this isoform is less dependent on phosphorylation.
FIGURE 2.
Association between different 14-3-3 isoforms and TH-(1–43) and THp-(1–43) peptides derived from tyrosine hydroxylase. 14-3-3γ, -η, and -ζ proteins were immobilized on the sensor chip CM5 at ∼10,763, 10,985, and 10,623 RU, respectively, and TH-(1–43) and THp-(1–43) (0.1 μg/μl) were then injected at 25 °C and pH 7.4. The figure shows representative sensorgrams for the binding of TH-(1–43) (●) or THp-(1–43) (▿) to 14-3-3γ (dotted line), 14-3-3η (dashed line) and 14-3-3ζ (solid line).
TABLE 1.
The interaction of TH-(1–43) and THp-(1–43) with 14-3-3 proteins measured by SPR
Kd values were obtained as Kd = kd/ka, where the apparent association rate (ka) and dissociation rate (kd) constants were evaluated from the analysis of the sensorgrams (Fig. 2).
| Ligand |
Kd for the binding to |
||
|---|---|---|---|
| 14-3-3γ | 14-3-3ζ | 14-3-3η | |
| μm | |||
| TH-(1–43) | 1554 ± 70 | 3969 ± 55 | 41.3 ± 12.5 |
| THp-(1–43) | 0.55 ± 0.13 | 0.44 ± 0.15 | 6.8 ± 0.7 |
Binding of TH-derived N-terminal Peptides and of 14-3-3 Isoforms to Phospholipid Bilayers
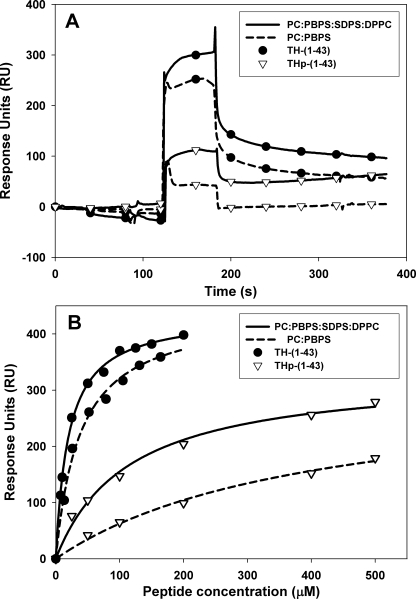
A previous study indicated that the interaction of hTH1 with phospholipid bilayers at neutral pH involved motifs included in the N-terminal sequence (within the first 49 N-terminal residues (29)). We investigated the membrane interaction of TH-(1–43) and THp-(1–43) using SPR. Liposomes made of PC (neutral bilayer), PC:PBPS (1:1), PC:DOPG (1:1), and PC:PBPS:SDPS:DPPC (negatively charged bilayers) were injected and immobilized to the four flow cells on an L1 sensor chip, an ideal substrate for liposome adsorption (50, 51). Liposomes were deposed on the chip at a low flow (<10 μl/min) which has been found to provide a stable lipid surface (52, 53).
Initially, a flow cell without liposomes was used as reference, but the association of the TH-(1–43) and THp-(1–43) peptides with the uncovered L1 sensor chip was quite high due to nonspecific binding, as is often the case for peripheral proteins (51). Because the association of the peptides with PC liposomes was low (e.g. 142 RU for 150 μm THp-(1–43) interacting with PC), these liposomes were used in the reference channel. The data from the binding at the reference (PC) channel was then subtracted from the other channels to compensate for bulk refractive index effects and nonspecific binding (54). Both TH-(1–43) and THp-(1–43) interact with the negatively charged vesicles (Fig. 3A). The concentration for half-maximal interaction (S0.5) is obtained by fitting the concentration-dependent RU values for peptide interaction with liposomes to a single-rectangular, two-parameter curve (Fig. 3B). These S0.5 values are summarized in Table 2. The interaction of unphosphorylated TH-(1–43) with phospholipid bilayers was stronger than that of THp-(1–43), and the highest binding affinity was measured for TH-(1–43) and liposomes made of PC:PBPS:SDPS:DPPC (S0.5 = 24.5 ± 1.5 μm). The affinity is, in fact, higher than measured for the binding of full-length hTH1 to other membrane-mimicking surfaces, such as coated beds (29), and the TH peptides also appear appropriate to reveal the specificities for phosphorylation state and phospholipid composition in the membrane interaction of hTH1.
FIGURE 3.
SPR responses to TH-(1–43) and THp-(1–43) binding to lipsomes. A, sensograms of TH-(1–43) (•) and THp-(1–43) (▿) (50 μm peptide) bound to PC:PBPS (dashed line) and PC:PBPS:SDPS:DPPC (solid line). B, the dependence of SPR responses to liposome binding on the concentration of TH-(1–43) (●) and THp-(1–43) (▿) peptides (i.e. the binding isotherms). The curves (PC:PBPS (dashed line) and PC:PBPS:SDPS:DPPC (solid line)) were fitted using a single-rectangle, two-parameter equation in Sigmaplot. R2 values were in all cases >0.99. Extracted S0.5 values are presented in Table 2.
TABLE 2.
The interaction of TH-(1–43), THp-(1–43), and 14-3-3 proteins with liposomes of different composition as measured by SPR
| Ligand | S0.5a for the binding to liposomes made of |
||
|---|---|---|---|
| PC:PBPS (1:1) | PC:DOPG (1:1) | PC:PBPS:SDPS:DPPC | |
| μm | |||
| TH-(1–43) | 38 ± 4 | 58 ± 10 | 24.5 ± 1.5 |
| THp-(1–43) | 144 ± 19 | 474 ± 13 | 135 ± 15 |
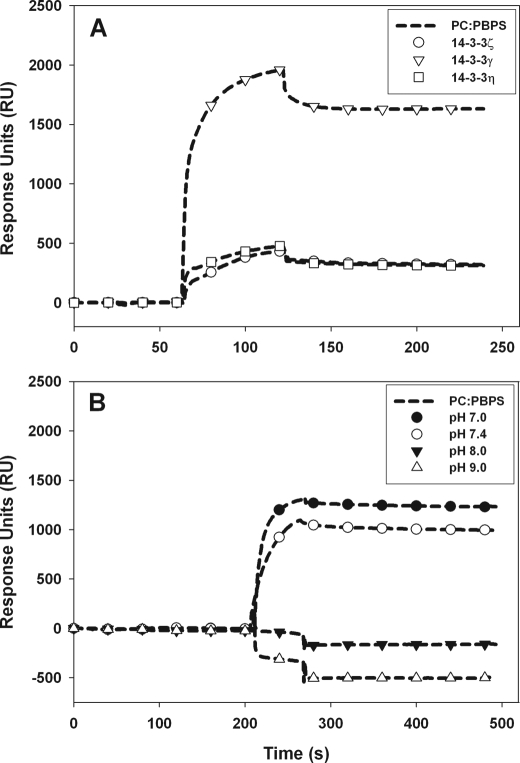
| 14-3-3ζ | 10 ± 0.5 | 21 ± 3.8 | 5 ± 0.1b |
| 14-3-3η | 13 ± 1.7 | 20 ± 2.4 | —b |
| 14-3-3γ | 1.9 ± 0.1c | 8.7 ± 0.4c | 1.3 ± 0.5c |
| 14-3-3γ·THp-(1–43) (1:2) | 1.3 ± 0.2 | —b | 0.64 ± 0.1 |
a S0.5, concentration of peptides, 14-3-3 proteins, or 14-3-3γ·THp-(1–43) complex for half-maximal binding, obtained from binding isotherms (Fig. 3).
b Not measured.
c The S0.5 values for 14-3-3γ are significantly different for the values obtained for 14-3-3ζ and -η isoforms with the three liposome compositions (analysis of variance; p < 0.005).
Further, we examined the association of 14-3-3γ, -ζ, and -η with negatively charged phospholipids by SPR. Here again the binding of the proteins to the membrane was performed using liposomes made of 100% PC covering the sensor chip in the reference channel. All three 14-3-3 proteins interacted with the liposomes, but, as seen by the sensorgrams, the binding intensity to PC:PBPS (1:1) (Fig. 4A) or to PC:PBPS:SDPS:DPPC (data not shown) increased 4-fold for 14-3-3γ compared with the ζ and η isoforms. The strongest interaction was again measured for 14-3-3γ, notably with PC:PBPS:SDPS:DPPC liposomes (S0.5 ∼ 1.3 μm at pH 7.4), which thus shows considerably higher binding affinity for negatively charged bilayers than the other 14-3-3 isoforms and the TH-derived peptides (Table 2). The binding of 14-3-3γ for negatively charged membranes appears to be pH-dependent, and at the conditions used in this study, no binding was observed at alkaline pH (8.0–9.0) by SPR (Fig. 4B).
FIGURE 4.
The binding of 14-3-3 isoforms to negatively charged phospholipid bilayers. A, representative sensorgrams. The L1 sensor chip was loaded with liposomes made of PC:PBPS (1:1); 14-3-3γ (▿), 14-3-3η (□), and 14-3-3ζ (○) proteins (17 μm subunit) were injected (at pH 7.4); and association-dissociation curves were monitored as described under “Experimental Procedures.” B, sensorgrams for the binding of 14-3-3γ to liposomes made of PC:PBPS at pH 7.0 (●), 7.4 (○), 8.0 (▾), and 9.0 (▵).
Distribution of 14-3-3γ and 14-3-3ζ in the Cytoplasmic and Membrane Fraction of Chromaffin Cells
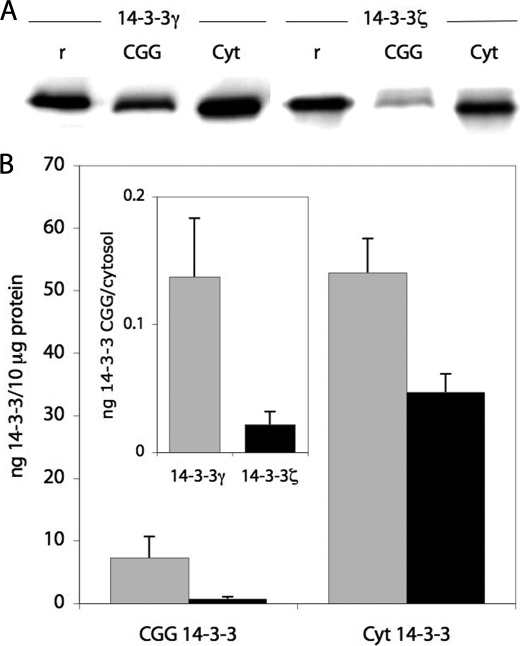
It has previously been shown that 14-3-3γ interacts with isolated chromaffin granule membranes from adrenal medulla, a binding that is not dependent on membrane-protein partners (30). This result is in agreement with the preferential binding that we have measured for the γ isoform to phospholipidic bilayers in this work (Fig. 4A and Table 2). There are reports indicating that the binding of 14-3-3γ to membranes also occurs in vivo in neuronal and neuroendochrine tissue (30, 32, 37). In chromaffin cells, 14-3-3 proteins are involved in calcium-dependent exocytosis (55), and in order to obtain further insights into the relative content of the γ isoform in the membrane and the cytosol of these neuroendochrine cells, we performed quantitative Western blot analysis. The distribution of 14-3-3γ and 14-3-3ζ between the cytosol and CGGs, taken as the soluble and membrane fractions, respectively, was estimated using isoform-specific antibodies and recombinant 14-3-3. As in the brain, 14-3-3γ and -ζ are the major isoforms in chromaffin cells (30), whereas the η isoform is not readily detected.
The relative content (per μg of total protein) of both isoforms is, as expected (30), higher in the cytoplasm than in the membranes (i.e. 45- and 7-fold higher content in cytoplasm than in membrane for ζ and γ, respectively) (Fig. 5). Moreover, 14-3-3γ was found to be more abundant than 14-3-3ζ in both fractions (Fig. 5); γ largely predominates over ζ in the membrane (1.5- and 10-fold higher content of γ versus ζ in the cytoplasm and the membrane, respectively) (Fig. 5). These relative contents of the major γ and ζ isoforms in chromaffin granules are rather similar to those found in rat hippocampus by Schindler et al. (37). In this brain preparation, γ also predominated over ζ, mainly in the microsomal fraction (mostly containing ER but also synaptosomes and plasma membrane) (i.e. the ratio γ/ζ was ∼1.5 in cytoplasm and ∼5 in the microsomal fraction). In the latter fraction, only these two isoforms are readily detected.
FIGURE 5.
Immunodetection and quantification of 14-3-3γ and -ζ in cytosolic fraction from adrenal medulla (Cyt) and membrane fraction (CGG). A, immunodetection of 14-3-3γ and -ζ in washed CGG (15 μg of total protein applied) and cytosolic fraction (5 μg of total protein applied) using isoform-specific antibodies (see “Experimental Procedures”). For comparison, 20 ng of recombinant 14-3-3ζ (r (right)) and γ (r (left)) were loaded. B, quantification of 14-3-3γ (gray bars) and 14-3-3ζ (black bars) levels in CGG and cytosol of chromaffin cells, using isoform-specific antibodies and recombinant 14-3-3 as a standard. Absolute levels ± S.D. are given relative to 10 μg of total protein. The relative distribution of 14-3-3γ and -ζ between CGG and cytosol is also calculated (inset).
The Interaction of 14-3-3γ with Membranes Depends on Structural Determinants Different from Those for Its Interaction with THp-(1–43)
The results so far indicate that 14-3-3 isoforms distribute differently between the membrane and the cytosol, which will probably also affect the localization of 14-3-3 binding partners, such as phosphorylated TH. In addition, TH also interacts with membranes without the involvement of 14-3-3γ (Fig. 3) (29). However, the low affinity of phosphorylated THp-(1–43) for phospholipid bilayers (Fig. 3 and Table 2) indicates that only the non-phosphorylated TH could have a certain interaction with membranes in vivo. Moreover, for phosphorylated TH, binding to 14-3-3 through the N-terminal domain around Ser19 would most probably exclude the interaction of the same region with the membrane. On the other hand, it was relevant to investigate if the interactions of 14-3-3γ with either the phosphorylated partner or the membrane were self-exclusive processes. We therefore investigated the binding of the 14-3-3γ·THp-(1–43) complex to membranes. First, liposomes made of PC:PBPS (1:1) were immobilized to the sensor chip L1, and then either 14-3-3γ, THp-(1–43), or 14-3-3γ in complex with THp-(1–43) (prepared at different ratios ensuring complex formation based on Kd values (Table 1)) were injected. As shown in Table 3, the 14-3-3γ·THp-(1–43) complex and 14-3-3γ alone bind to the liposomes at a similar extent (similar RU). In fact, the affinity is slightly increased for the THp-(1–43) bound 14-3-3γ compared with the unbound 14-3-3 protein (Table 2). These results indicate that the binding of THp-(1–43) to 14-3-3γ does not affect the interaction between 14-3-3γ and the membrane and that binding to these partners most probably involves different regions of the 14-3-3γ protein surface.
TABLE 3.
The interaction of the THp-(1–43)·14-3-3γ complex with negatively charged liposomes made of PC:PBPS (1:1) as measured by SPR
| Ligand | RU |
|---|---|
| 14-3-3γ (2.50 μm) | 880 |
| THp-(1–43) (1.25 μm) | 30 |
| THp-(1–43) (2.50 μm) | 32 |
| THp-(1–43) (5.00 μm) | 34 |
| 14-3-3γ·THp-(1–43) (2.5 μm, 1.25 μm) | 820 |
| 14-3-3γ·THp-(1–43) (2.5 μm, 2.5 μm) | 830 |
| 14-3-3γ·THp-(1–43) (2.5 μm, 5.0 μm) | 830 |
Effect of Liposome Binding on TH Activity
In agreement with previous findings (29), the binding of phosphorylated hTH1 (Ser(P)19-hTH1) to liposomes reduced the activity of the phosphorylated sample by 30% (Table 4). This reduction in activity is similar to that obtained for the unphosphorylated sample (data not shown). On the other hand, although the activity of hTH1 is not noticeably affected by the phosphorylation, the activity of the phosphorylated enzyme at standard conditions is increased by 10 and 45% upon preincubation with 14-3-3γ and -ζ, respectively (Table 4). Interestingly, the prior incubation with the 14-3-3 proteins at conditions that ensure total and partial liposome binding of the Ser(P)19-hTH1·14-3-3γ and Ser(P)19-hTH1·14-3-3ζ complexes, respectively (Table 2), reverses the inhibition of the activity by liposome binding, and the activity of the hydroxylase is preserved (notably for the Ser(P)19-hTH1·14-3-3γ complex compared with that of control) (Table 4).
TABLE 4.
TH activity measurements
| Proteinsa | Liposomesb | TH activityc | Percentage of control |
|---|---|---|---|
| nmol l-DOPA/min/mg | % | ||
| Control (Ser(P)19-hTH1) | No | 533 ± 52 | 100 |
| Ser(P)19-hTH1 with 14-3-3γ | No | 613 ± 60 | 115 |
| Ser(P)19-hTH1 with 14-3-3ζ | No | 778 ± 80 | 146 |
| Ser(P)19-hTH1 | Yes | 373 ± 50 | 70 |
| Ser(P)19-hTH1 with 14-3-3γ | Yes | 544 ± 10 | 102 |
| Ser(P)19-hTH1 with 14-3-3ζ | Yes | 480 ± 90 | 90 |
a The concentration of Ser(P)19-hTH1 and 14-3-3 proteins was 0.05 μm and 1.8 μm subunit, respectively.
b Large unilamellar vesicles made of PC:PBPS:SDPS:DPPC, 20 μm final lipid concentration.
c The results represent the mean ± S.D. of three measurements.
The Conformation of TH-(1–43) and THp-(1–43) in Solution and When Bound to Membranes
In order to obtain further insights into the interaction of hTH1 with 14-3-3 and with negatively charged membranes, we investigated the conformation of the TH-(1–43) and THp-(1–43) peptides. A similar CD spectrum characteristic of random coil structure, with a minimum at 198 nm, and compatible with lack of persistent, well defined structure is obtained for both peptides (Fig. 6, A and B). We then investigated the propensity of the peptides to adopt a secondary structure in response to non-aqueous conditions. TFE is often used as a membrane-mimicking solvent, and it is known to strongly induce helical conformations in proteins and peptides. As can be seen in Fig. 6, C and D, TH-(1–43) and THp-(1–43) behave quite differently. The characteristic CD spectrum of α-helix with double minima at 208 and 222 nm appears at lower concentrations of TFE for TH-(1–43) than for THp-(1–43). The phosphorylated peptide is more resistant to adopt the helical structure, and even at 60% TFE, it maintains a higher fraction of random coil structure (Fig. 6, C and D).
FIGURE 6.
Response of TH-(1–43) (A and C) and THp-(1–43) (B and D) to various amounts of TFE assessed by CD. Far-UV spectra of TH-(1–43) (A) and THp-(1–43) (B) in various amounts of TFE. Spectra were taken in 20 mm citric acid-phosphate, pH 7.4, and 25 °C. Peptide concentration was constant (16 μm) throughout both titrations. Shown is the amount (percentage) of random coil (▾ and ▿) and helix (● and ○) for TH-(1–43) (C, filled symbols) and THp-(1–43) (D, open symbols). The amount of secondary structure was estimated using the CDNN program (47), and the fittings (line) are logistic four-parameter curves that only aim to guide the eye.
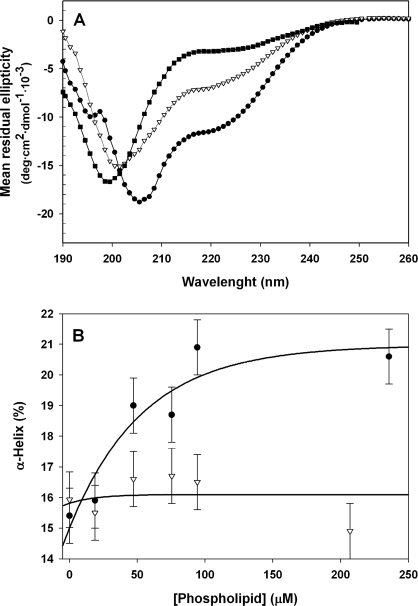
Finally, we investigated the conformation of the peptides upon binding to membranes. Two model systems where used: spherical bilayer vesicles and disc-like bicelles with a circular bilayer plane encircled by DHPC lipids in a micelle arrangement (44). The quality of far-UV CD spectra in the presence of bicelles was better than in the presence of vesicles, the latter causing decreased signal-to-noise in peptide spectra due to increased light scattering at shorter wavelengths. Thus, the bicelles allowed for a much higher effective concentration of phospholipids. The spectra of TH-(1–43) and THp-(1–43) in the presence of bicelles revealed conformational differences between both peptides when bound to membranes, notably a decreased ellipticity at 208 and 222 nm for TH-(1–43) compared with THp-(1–43) (Fig. 7A). An estimation of the amount of α-helix in the peptides obtained from titrations with increasing lipid concentrations is shown in Fig. 7B. As seen in the presence of TFE, phosphorylation appears to lower the peptide propensity for collapse to α-helical structure. This lowered propensity would, in addition to electrostatic considerations associated with the incorporation of the negative phosphate, reduce binding to negatively charged membranes because a helical membrane conformation is energetically favorable (56–58).
FIGURE 7.
The conformation of TH-(1–43) and THp-(1–43) bound to PBPS:PC bilayers. A, CD spectra of TH-(1–43) (similar to that of THp-(1–43)) in 20 mm citric acid-phosphate, pH 7.4 (■), or of TH-(1–43) (●) and THp-(1–43) (▿) in the same buffer in the presence of DHPC:DMPS:DMPC bicelles (4% (w/v) lipid concentration. B, estimation of α-helical structure for TH-(1–43) (●) and THp-(1–43) (▿) upon titration with increasing lipidic concentrations in the liposomes. The vertical bars indicate one S.D. value of three (based on independent experiments) extractions of α-helical data by CDNN done for the buffer condition. A four-parameter exponential decay to maximum function has been fitted to both sets of data to aid visual interpretation.
DISCUSSION
A 14-3-3-interacting N-terminal Domain in hTH1; Effect of Phosphorylation
TH and TPH2, the two neuronal aromatic amino acid hydroxylases, contain additional 30–40 residue N-terminal sequences with respect to both phenylalanine hydroxylase, largely present in liver, and TPH1, present in the periphery, pineal gland, and intestine. The additional sequences in TH and TPH2 (Fig. 1), which include the phosphorylation-targeted Ser19 (17, 19), most probably constitute functional domains in the neuronal hydroxylases, as recently suggested for the N-terminal sequence in TPH2 (59). The 43-residue N-terminal region of TH was used in this work to investigate determinants for binding of this functional domain to membranes and 14-3-3 proteins. Comparison of the Kd/S0.5 values for the interaction of full-length hTH1 with either 14-3-3 proteins (17, 48) or membranes (29) with those obtained in this work with the TH-(1–43) and THp-(1–43) peptides suggests that the main determinants for the interactions are included in this N-terminal domain. The TH-derived peptides are thus valuable to reveal the binding specificities.
The domain appears to be characterized by a disordered structure in solution and a propensity to adopt helical structure in the presence of TFE and when bound to membranes, according to the predicted helical propensity of the region (29) and the partitioning-folding coupling mechanism (56, 57). The level of maximal induction of α-helix is ∼20% lower for the Ser19-phosphorylated domain relative to its non-phosphorylated counterpart (Fig. 6, C and D), which also readily shows increased helix formation when bound to membranes (Fig. 7). All together, these results indicate a more extended conformation for the phosphorylated domain, providing information on the conformational determinants for its interaction with 14-3-3 proteins. Some partners of 14-3-3 that bind without a phosphorylation requirement (i.e. the ExoS and R18 peptides) adopt a helical structure when bound to 14-3-3 in a reversed orientation, which is primarily dependent on hydrophobic interactions (21, 60). Nevertheless, the majority of the partners require a phosphate-directed binding to the 14-3-3 proteins, and structural studies available so far show the phosphate-surrounding regions as extended coils (see Ref. 21 and references therein). Our results suggest that the phosphate group aids in providing the peptide the stretched conformation suitable for interaction with 14-3-3. This effect would thus come in addition to charge-charge interaction of the phosphate with the positively charged binding groove in 14-3-3 (3, 20, 21).
With respect to putative effects on the binding to 14-3-3 of phosphorylation at other sites in the N-terminal domain, it has been shown that phosphorylation at Ser31 by ERK1/2 does not trigger binding to 14-3-3 (14); nor does phosphorylation at Ser40 (using PKA) promote binding to 14-3-3γ or -ζ (48). Nevertheless, the simultaneous phosphorylation at both Ser19 and Ser40 (e.g. using MAPKAP-K2) increases the affinity of the interaction of 14-3-3 at Ser19 (48). It is thus conceivable that phosphorylation at sites other than Ser19 contributes to the fine tuning of the interaction of TH with 14-3-3 (and with membranes).
hTH1 and 14-3-3γ as Peripheral Proteins
As it emerges from this and previous works (26, 28, 29), both hTH1 and 14-3-3γ may be classified as peripheral proteins. Although mainly cytoplasmic, these proteins can bind to membranes. The capacity to transiently interact with membranes is also known as amphitropism (61–63). Membrane binding may regulate the function of amphitropic proteins, which are often involved in signal transduction, one of the functions of 14-3-3. It has been proposed that TH binding to membranes of secretory vesicles in adrenal medulla might regulate the activity of the enzyme and play a role in coordinating TH activity and catecholamine release (28, 64). However, membrane binding of hTH1 is accompanied by a decrease in TH activity (29), and a functional role for direct membrane binding does not seem clear for this enzyme. Moreover, the interaction of TH with membranes occurs with rather too low affinity to account for a significant binding in vivo. Thus, measurements of the amount of TH in rat nervous system tissue place the monomer concentration at around 1 μm (65). Although this concentration varies with species, tissue, and state of the organism (65, 66), it is reasonable to assume that the concentration of mammalian TH will be in the lower micromolar range in brain and adrenal medulla. Taking into account an S0.5 = 24–58/135–500 μm for the interaction of TH-(1–43) and THp-(1–43) with liposomes (Table 2), which agrees well with a Kd = 230–380 μm for the interaction of TH with beads coated with negatively charged phospholipid membranes (29), the direct membrane interaction of TH is not expected to be substantial. It is probable that the fraction of TH that is found as membrane-bound both in brain and in adrenal chromaffin cells (23, 26, 27) might be targeted to the membrane through interaction with 14-3-3γ. Moreover, the 14-3-3γ-mediated membrane interaction of TH preserves the enzymatic activity (Table 4).
Molecular Basis for 14-3-3γ Membrane Affinity
14-3-3γ shows a high affinity for negatively charged membranes (S0.5 = 1–9 μm). The lipid composition of the liposomes and bicelles used in this work, notably for PC:PBPS and PC:PBPS:SDPS:DPPC, is selected to reproduce the charge and fluidity of membranes of chromaffin granules and brain synaptic vesicles and of synaptosomal membranes (67). Different from TH (see above), the S0.5 values indicate that a significant amount of 14-3-3γ is expected to partition into available vesicular or microsomal membranes at physiological conditions, as suggested by earlier studies (30, 32, 55). The S0.5 values (Table 2) for 14-3-3γ and 14-3-3ζ are also in agreement with their preferential distribution between cytosol and membrane (Fig. 5). The binding of 14-3-3γ to membranes shows slightly higher affinity with THp-(1–43) bound (Table 2), indicating that membrane binding most probably involves sites other than the peptide binding groove.
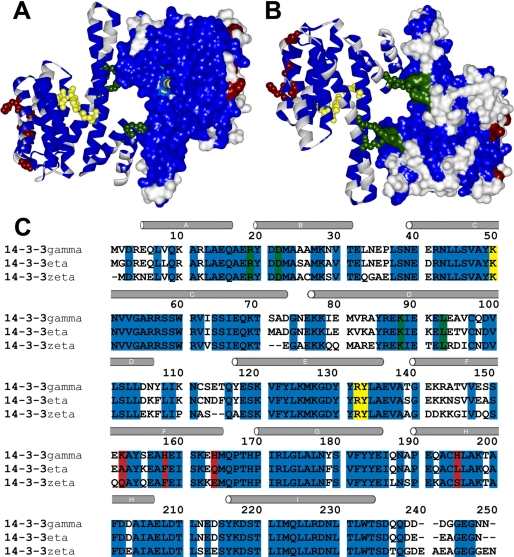
When plotting amino acid variations onto the structural scaffold of 14-3-3, it is evident that the peptide binding grove and the dimerization motif is highly conserved, both with respect to amino acids and their desolvation energies (21) (Fig. 8A). The opposite, convex side of the proteins is less conserved (Fig. 8B), and it is most probable that membrane interaction of the γ isoform occurs through motifs in this side. The most notable differences are found in α-helices A, B, and F (from N-terminal to C-terminal) as well as in the vicinity of the loop region between helices C and D (3). Thus, we surmise that the isoform specificity with respect to high membrane affinity can be found within the relatively limited selection of residues specific to γ in these regions. Fig. 8C shows conserved (blue) and non-conserved (white and red) residues within the three isoforms here studied, 14-3-3γ, -ζ, and -η; the red-highlighted non-conserved residues represent a charge change. As is the case for many proteins interacting with lipid membranes (34, 62, 68, 69), the 14-3-3γ-membrane interaction takes place at pH values where this acidic protein is clearly negatively charged. A rough estimate gives an approximate global charge of −30 for the 14-3-3γ dimer at pH 7.4, but at the same time, the protein binds to negatively charged membranes. It should be noted that both acidic and basic residues can be involved in protein binding to negatively charged membranes (61, 70) and that the actual pH experienced by a residue can be lower in the vicinity of the membrane (71, 72). Moreover, three of four of the charge-changing highlighted residues (i.e. Lys152, His158, and His164) are unique basic residues in γ among the seven 14-3-3 human isoforms (see Fig. 4 in Ref. 3). Interestingly, the fourth charge-changing residue (i.e. His195) is only a corresponding basic residue (Arg) in ϵ, the other isoform for which membrane association has been shown (30–32). All of these residues are located in helix F, and positively charged His164 would thus be excellently placed to anchor the C terminus of this helix to the membrane (Fig. 8B). Due to the symmetry of the 14-3-3 unit, this motif might anchor two far away points of the dimer, facilitating consolidation of protein-bilayer interactions across the interaction interface. The decreased binding at alkaline pH (Fig. 4B) further supports the involvement of His residues in the binding to negatively charged membranes.
FIGURE 8.
Conserved and non-conserved residues between 14-3-3γ, -η, and -ζ. A structural representation of the human 14-3-3γ dimer (Protein Data Bank code 2b05), with one monomer shown as a surface and one as a backbone ribbon, is depicted from the ligand binding site (concave) (A) and the opposite site (convex) (B). C, sequence alignment of human 14-3-3γ, -ζ, and -η. The same color code is used in A–C. Residues conserved among the three isoforms are shown in blue and non-conserved in white and red, the latter denoting residues where differences between γ and both ζ and η lead to a change in residue ionization. In yellow and green are shown residues involved in phosphopeptide ligand binding and dimerization, respectively.
In conclusion, our results provide support, at the molecular level, for the notion that 14-3-3γ may have a role in shuttling a population of Ser19-phosphorylated TH into a membrane location. Bound to 14-3-3γ, TH makes this transfer without compromising its activity. Chen et al. (25) observed that TH associated with the membranes of synaptic vesicles was activated by phosphorylating conditions, including phosphorylation at Ser19 in TH. Although the involvement of 14-3-3γ in this process was not investigated, a modulation of the cytoplasmic/membrane distribution of TH by phosphorylation-mediated interaction with 14-3-3 proteins appears probable. The membrane interaction might be modulated through changes in membrane composition (e.g. charge) or protein modifications. Interestingly, the succeeding enzyme in the synthesis of dopamine from l-DOPA (i.e. aromatic l-amino acid decarboxylase), has also been found to be membrane-associated (73, 74). Further cellular and physiological studies are necessary to corroborate the functional implications of the interactions revealed in this work.
Supplementary Material
Acknowledgments
We thank Ali J. Sepulveda Muñoz for expert technical help, Anja Underhaug for assistance in setting up the initial SPR liposome-protein protocols, and Jaakko Saraste for valuable help with electron microscopy of the chromaffin granule ghosts and generous gifts for immunodetection. Electron microscopy was performed at the Molecular Imaging Center (Functional Genomics (FUGE) program, Norwegian Research Council), University of Bergen. We also thank Anne Mari Tveit and Sonnich Meier (Institute of Marine Research, Bergen) for expert analysis of phospholipid mixtures by gas chromatography.
This work was supported by grants from the Norwegian Cancer Society, the Research Council of Norway, and Helse-Vest.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- TH
- tyrosine hydroxylase
- TH-(1–43)
- truncated human hTH1 containing N-terminal residues 1–43
- THp-(1–43)
- 19 S-phosphorylated human TH-(1–43)
- TPH
- tryptophan hydroxylase
- hTH1
- human tyrosine hydroxylase isoform 1
- CGG
- chromaffin granule ghost
- DHPC
- 1,2-dihexanoylphosphatidylcholine
- DMPC
- 1,2-dimyristoylphosphatidylcholine
- DMPS
- 1,2-dimyristoylphosphatidylserine
- DOPG
- 1,2-dioleoylphosphatidylglycerol
- DPPC
- 1,2-dipalmitoylphosphatidylcholine
- l-DOPA
- l-3,4-dihydroxyphenylalanine
- PBPS
- porcine brain phosphatidylserine
- PC
- phosphatidylcholine
- RU
- response unit(s)
- SDPS
- 1-stearoyl-2-docosahexaenoyl-phosphatidylserine
- SPR
- surface plasmon resonance
- TFE
- trifluoroethanol.
REFERENCES
- 1.Aitken A. (2006) Semin. Cancer Biol. 16, 162–172 [DOI] [PubMed] [Google Scholar]
- 2.Fu H., Subramanian R. R., Masters S. C. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 617–647 [DOI] [PubMed] [Google Scholar]
- 3.Gardino A. K., Smerdon S. J., Yaffe M. B. (2006) Semin. Cancer Biol. 16, 173–182 [DOI] [PubMed] [Google Scholar]
- 4.Benzinger A., Muster N., Koch H. B., Yates J. R., 3rd, Hermeking H. (2005) Mol. Cell Proteomics 4, 785–795 [DOI] [PubMed] [Google Scholar]
- 5.Hermeking H. (2003) Nat. Rev. Cancer 3, 931–943 [DOI] [PubMed] [Google Scholar]
- 6.Li Z., Zhao J., Du Y., Park H. R., Sun S. Y., Bernal-Mizrachi L., Aitken A., Khuri F. R., Fu H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenquist M., Sehnke P., Ferl R. J., Sommarin M., Larsson C. (2000) J. Mol. Evol. 51, 446–458 [DOI] [PubMed] [Google Scholar]
- 8.Moreira J. M., Shen T., Ohlsson G., Gromov P., Gromova I., Celis J. E. (2008) Mol. Cell Proteomics 7, 1225–1240 [DOI] [PubMed] [Google Scholar]
- 9.Liang X., Butterworth M. B., Peters K. W., Walker W. H., Frizzell R. A. (2008) J. Biol. Chem. 283, 27418–27425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridges D., Moorhead G. B. (2005) Sci. STKE 2005, re10. [DOI] [PubMed] [Google Scholar]
- 11.Bustos D. M., Iglesias A. A. ( 2006) Proteins 63, 35– 42 [DOI] [PubMed] [Google Scholar]
- 12.Yaffe M. B., Rittinger K., Volinia S., Caron P. R., Aitken A., Leffers H., Gamblin S. J., Smerdon S. J., Cantley L. C. (1997) Cell 91, 961–971 [DOI] [PubMed] [Google Scholar]
- 13.Ichimura T., Isobe T., Okuyama T., Yamauchi T., Fujisawa H. (1987) FEBS Lett. 219, 79–82 [DOI] [PubMed] [Google Scholar]
- 14.Dunkley P. R., Bobrovskaya L., Graham M. E., von Nagy-Felsobuki E. I., Dickson P. W. (2004) J. Neurochem. 91, 1025–1043 [DOI] [PubMed] [Google Scholar]
- 15.Sutherland C., Alterio J., Campbell D. G., Le Bourdellès B., Mallet J., Haavik J., Cohen P. (1993) Eur. J. Biochem. 217, 715–722 [DOI] [PubMed] [Google Scholar]
- 16.Vulliet P. R., Woodgett J. R., Cohen P. (1984) J. Biol. Chem. 259, 13680–13683 [PubMed] [Google Scholar]
- 17.Toska K., Kleppe R., Armstrong C. G., Morrice N. A., Cohen P., Haavik J. (2002) J. Neurochem. 83, 775–783 [DOI] [PubMed] [Google Scholar]
- 18.Itagaki C., Isobe T., Taoka M., Natsume T., Nomura N., Horigome T., Omata S., Ichinose H., Nagatsu T., Greene L. A., Ichimura T. (1999) Biochemistry 38, 15673–15680 [DOI] [PubMed] [Google Scholar]
- 19.Winge I., McKinney J. A., Ying M., D'Santos C. S., Kleppe R., Knappskog P. M., Haavik J. (2008) Biochem. J. 410, 195–204 [DOI] [PubMed] [Google Scholar]
- 20.Obsil T., Ghirlando R., Klein D. C., Ganguly S., Dyda F. (2001) Cell 105, 257–267 [DOI] [PubMed] [Google Scholar]
- 21.Yang X., Lee W. H., Sobott F., Papagrigoriou E., Robinson C. V., Grossmann J. G., Sundström M., Doyle D. A., Elkins J. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17237–17242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman S. (1995) Adv. Enzymol. Relat. Areas Mol. Biol. 70, 103–220 [DOI] [PubMed] [Google Scholar]
- 23.Kuczenski R. T., Mandell A. J. (1972) J. Biol. Chem. 247, 3114–3122 [PubMed] [Google Scholar]
- 24.McGeer E. G., McGeer P. L., Wada J. A. (1971) J. Neurochem. 18, 1647–1658 [DOI] [PubMed] [Google Scholar]
- 25.Chen R., Wei J., Fowler S. C., Wu J. Y. (2003) J. Biomed. Sci. 10, 774–781 [DOI] [PubMed] [Google Scholar]
- 26.Kuhn D. M., Arthur R., Jr., Yoon H., Sankaran K. (1990) J. Biol. Chem. 265, 5780–5786 [PubMed] [Google Scholar]
- 27.Treiman M., Weber W., Gratzl M. (1983) J. Neurochem. 40, 661–669 [DOI] [PubMed] [Google Scholar]
- 28.Morita K., Teraoka K., Oka M. (1987) J. Biol. Chem. 262, 5654–5658 [PubMed] [Google Scholar]
- 29.Thórólfsson M., Døskeland A. P., Muga A., Martínez A. (2002) FEBS Lett. 519, 221–226 [DOI] [PubMed] [Google Scholar]
- 30.Roth D., Morgan A., Martin H., Jones D., Martens G. J., Aitken A., Burgoyne R. D. (1994) Biochem. J. 301, 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones D. H., Martin H., Madrazo J., Robinson K. A., Nielsen P., Roseboom P. H., Patel Y., Howell S. A., Aitken A. (1995) J. Mol. Biol. 245, 375–384 [DOI] [PubMed] [Google Scholar]
- 32.Martin H., Rostas J., Patel Y., Aitken A. (1994) J. Neurochem. 63, 2259–2265 [DOI] [PubMed] [Google Scholar]
- 33.Strochlic L., Cartaud A., Mejat A., Grailhe R., Schaeffer L., Changeux J. P., Cartaud J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 18189–18194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hekman M., Albert S., Galmiche A., Rennefahrt U. E., Fueller J., Fischer A., Puehringer D., Wiese S., Rapp U. R. (2006) J. Biol. Chem. 281, 17321–17336 [DOI] [PubMed] [Google Scholar]
- 35.Muslin A. J., Xing H. (2000) Cell. Signal. 12, 703–709 [DOI] [PubMed] [Google Scholar]
- 36.Besenicar M., Macek P., Lakey J. H., Anderluh G. (2006) Chem. Phys. Lipids 141, 169–178 [DOI] [PubMed] [Google Scholar]
- 37.Schindler C. K., Heverin M., Henshall D. C. (2006) J. Neurochem. 99, 561–569 [DOI] [PubMed] [Google Scholar]
- 38.Meier S., Mjøs S. A., Joensen H., Grahl-Nielsen O. (2006) J. Chromatogr. A 1104, 291–298 [DOI] [PubMed] [Google Scholar]
- 39.Haavik J., Le Bourdelles B., Martinez A., Flatmark T., Mallet J. (1991) Eur. J. Biochem. 199, 371–378 [DOI] [PubMed] [Google Scholar]
- 40.Clokie S. J., Cheung K. Y., Mackie S., Marquez R., Peden A. H., Aitken A. (2005) FEBS J. 272, 3767–3776 [DOI] [PubMed] [Google Scholar]
- 41.Terland O., Flatmark T. (1980) Biochim. Biophys. Acta 597, 318–330 [DOI] [PubMed] [Google Scholar]
- 42.Mayer L. D., Hope M. J., Cullis P. R., Janoff A. S. (1985) Biochim. Biophys. Acta 817, 193–196 [DOI] [PubMed] [Google Scholar]
- 43.Mayer L. D., Hope M. J., Cullis P. R. (1986) Biochim. Biophys. Acta 858, 161–168 [DOI] [PubMed] [Google Scholar]
- 44.Glover K. J., Whiles J. A., Wu G., Yu N., Deems R., Struppe J. O., Stark R. E., Komives E. A., Vold R. R. (2001) Biophys. J. 81, 2163–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vold R. R., Prosser R. S., Deese A. J. (1997) J. Biomol. NMR 9, 329–335 [DOI] [PubMed] [Google Scholar]
- 46.Haavik J., Flatmark T. (1980) J. Chromatogr. 198, 511–515 [DOI] [PubMed] [Google Scholar]
- 47.Böhm G., Muhr R., Jaenicke R. (1992) Protein Eng. 5, 191–195 [DOI] [PubMed] [Google Scholar]
- 48.Kleppe R., Toska K., Haavik J. (2001) J. Neurochem. 77, 1097–1107 [DOI] [PubMed] [Google Scholar]
- 49.Obsilova V., Nedbalkova E., Silhan J., Boura E., Herman P., Vecer J., Sulc M., Teisinger J., Dyda F., Obsil T. (2008) Biochemistry 47, 1768–1777 [DOI] [PubMed] [Google Scholar]
- 50.Höning S., Ricotta D., Krauss M., Späte K., Spolaore B., Motley A., Robinson M., Robinson C., Haucke V., Owen D. J. (2005) Mol. Cell 18, 519–531 [DOI] [PubMed] [Google Scholar]
- 51.Cho W., Bittova L., Stahelin R. V. (2001) Anal. Biochem. 296, 153–161 [DOI] [PubMed] [Google Scholar]
- 52.Cooper M. A., Hansson A., Löfås S., Williams D. H. (2000) Anal. Biochem. 277, 196–205 [DOI] [PubMed] [Google Scholar]
- 53.Erb E. M., Chen X., Allen S., Roberts C. J., Tendler S. J., Davies M. C., Forsén S. (2000) Anal. Biochem. 280, 29–35 [DOI] [PubMed] [Google Scholar]
- 54.Ober R. J., Ward E. S. (1999) Anal. Biochem. 271, 70–80 [DOI] [PubMed] [Google Scholar]
- 55.Morgan A., Burgoyne R. D. (1992) Nature 355, 833–836 [DOI] [PubMed] [Google Scholar]
- 56.Wimley W. C., White S. H. (1996) Nat. Struct. Biol. 3, 842–848 [DOI] [PubMed] [Google Scholar]
- 57.White S. H., Wimley W. C. (1999) Annu. Rev. Biophys. Biomol. Struct. 28, 319–365 [DOI] [PubMed] [Google Scholar]
- 58.Hurley J. H. (2006) Biochim. Biophys. Acta 1761, 805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy K. L., Zhang X., Gainetdinov R. R., Beaulieu J. M., Caron M. G. (2008) J. Biol. Chem. 283, 13216–13224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ottmann C., Yasmin L., Weyand M., Veesenmeyer J. L., Diaz M. H., Palmer R. H., Francis M. S., Hauser A. R., Wittinghofer A., Hallberg B. (2007) EMBO J. 26, 902–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Halskau Ø., Frøystein N. A., Muga A., Martínez A. (2002) J. Mol. Biol. 321, 99–110 [DOI] [PubMed] [Google Scholar]
- 62.Johnson J. E., Cornell R. B. (1999) Mol. Membr. Biol. 16, 217–235 [DOI] [PubMed] [Google Scholar]
- 63.Burn P. (1988) Trends Biochem. Sci. 13, 79–83 [DOI] [PubMed] [Google Scholar]
- 64.Morita K., Hamano S., Oka M. (1994) Neurochem. Int. 25, 403–411 [DOI] [PubMed] [Google Scholar]
- 65.Weissmann D., Labatut R., Richard F., Rousset C., Pujol J. F. (1989) J. Neurochem. 53, 793–799 [DOI] [PubMed] [Google Scholar]
- 66.Ginovart N., Marcel D., Bezin L., Gagne C., Pujol J. F., Weissmann D. (1996) Brain Res. 719, 45–55 [DOI] [PubMed] [Google Scholar]
- 67.Westhead E. W. (1987) Ann. N.Y. Acad. Sci. 493, 92–100 [DOI] [PubMed] [Google Scholar]
- 68.Agasøster A. V., Halskau Ø., Fuglebakk E., Frøystein N. A., Muga A., Holmsen H., Martínez A. (2003) J. Biol. Chem. 278, 21790–21797 [DOI] [PubMed] [Google Scholar]
- 69.Glomm W. R., Halskau Ø., Jr., Hanneseth A. M., Volden S. (2007) J. Phys. Chem. B 111, 14329–14345 [DOI] [PubMed] [Google Scholar]
- 70.Johnson J. E., Xie M., Singh L. M., Edge R., Cornell R. B. (2003) J. Biol. Chem. 278, 514–522 [DOI] [PubMed] [Google Scholar]
- 71.Vaz W. L., Nisksch A., Jähnig F. (1978) Eur. J. Biochem. 83, 299–305 [DOI] [PubMed] [Google Scholar]
- 72.van der Goot F. G., González-Mañas J. M., Lakey J. H., Pattus F. (1991) Nature 354, 408–410 [DOI] [PubMed] [Google Scholar]
- 73.Poulikakos P., Vassilacopoulou D., Fragoulis E. G. (2001) Neurochem. Res. 26, 479–485 [DOI] [PubMed] [Google Scholar]
- 74.Kokkinou I., Nikolouzou E., Hatzimanolis A., Fragoulis E. G., Vassilacopoulou D. (2009) Blood Cells Mol. Dis. 42, 92–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.