Abstract
Val75 of HIV-1 reverse transcriptase (RT) plays a role in positioning the template nucleotide +1 during the formation of the ternary complex. Mutations, such as V75M and V75A, emerge in patients infected with HIV-1 group M subtype B and group O variants, after failing treatment with stavudine (d4T) and other nucleoside RT inhibitors. V75I is an accessory mutation of the Q151M multidrug resistance complex of HIV-1 RT and is rarely associated with thymidine analogue resistance mutations (TAMs). In vitro, it confers resistance to acyclovir. TAMs confer resistance to zidovudine (AZT) and d4T by increasing the rate of ATP-mediated excision of the terminal nucleotide monophosphate (primer unblocking). In a wild-type HIV-1 group O RT sequence context, V75A and V75M conferred increased excision activity on d4T-terminated primers, in the presence of PPi. In contrast, V75I decreased the PPi-mediated unblocking efficiency on AZT and d4T-terminated primers, in different sequence contexts (i.e. wild-type group M subtype B or group O RTs). Interestingly, in the sequence context of an excision-proficient RT (i.e. M41L/A62V/T69SSS/K70R/T215Y), the introduction of V75I led to a significant decrease of its ATP-dependent excision activity on AZT-, d4T-, and acyclovir-terminated primers. The excision rate of d4T-monophosphate in the presence of ATP (3.2 mm) was about 10 times higher for M41L/A62V/T69SSS/K70R/T215Y than for the mutant M41L/A62V/T69SSS/K70R/V75I/T215Y RT. The antagonistic effect of V75I with TAMs was further demonstrated in phenotypic assays. Recombinant HIV-1 containing the M41L/A62V/T69SSS/K70R/V75I/T215Y RT showed 18.3- and 1.5-fold increased susceptibility to AZT and d4T, respectively, in comparison with virus containing the M41L/A62V/T69SSS/K70R/T215Y RT.
Introduction
The human immunodeficiency virus, type 1 (HIV-1)2 reverse transcriptase (RT) plays a pivotal role in the viral life cycle; therefore, it is an important target for antiretroviral drugs, such as nucleoside and nucleotide RT inhibitors (NRTIs) (1, 2). Once phosphorylated by cellular kinases to their active triphosphate (TP) forms, NRTIs compete with the natural dNTPs for incorporation by RT into the nascent reverse transcript. Since NRTIs lack a 3′-OH group, their incorporation results in chain termination (3, 4). The development of resistance remains a major problem in the treatment of HIV-1 infections. HIV-1 becomes resistant to NRTIs by (i) improving discrimination against the RT inhibitors (5–7) or (ii) by increasing the ability of the RT to remove 3′-terminal chain terminator inhibitors from blocked DNA primers, through phosphorolysis mediated by ATP or PPi (8, 9).
Both resistance mechanisms can be relevant in the acquisition of multidrug resistance (for recent reviews, see Refs. 10 and 11). Thus, viral RTs bearing mutations A62V, V75I, F77L, F116Y, and Q151M showed decreased discrimination efficiencies against the triphosphorylated forms of thymidine analogues, such as zidovudine (β-d-(+)-3′-azido-3′-deoxythymidine (AZT)) or stavudine (β-d-(+)-2′,3′-didehydro-2′,3′-dideoxythymidine (d4T)), as well as didanosine, zalcitabine, and abacavir (6, 12, 13). These observations were in agreement with the high level resistance to those inhibitors shown in phenotypic assays by HIV variants carrying those mutations in their RTs (14, 15). On the other hand, resistance to all approved NRTIs may be increased by the accumulation of thymidine analogue resistance mutations (TAMs) (i.e. M41L, D67N, K70R, L210W, T215F or T215Y, and K219E or K219Q) in the absence or in the presence of dipeptide insertions between residues 69 and 70.
Biochemical studies have shown that TAMs confer resistance to AZT and other NRTIs by accelerating the rate at which the blocking AZT monophosphate (MP) (or other nucleoside analogue monophosphate) is removed through phosphorolytic cleavage. RTs containing mutations M41L, A62V, T69S, K70R, and T215Y plus a Ser-Ser insertion between codons 69 and 70 showed very high levels of excision activity with primers terminated with AZTMP (16). Resistance mechanisms are not mutually exclusive. Thus, A62V, an accessory mutation of the Q151M complex, was reported to increase the ATP-dependent phosphorolytic activity on thymidine analogue-terminated primers of RTs bearing dipeptide insertions (16), whereas the excision activity promoted by the presence of TAMs can be modulated by mutations that influence nucleotide discrimination (e.g. K65R, L74V, and M184V) (17–21).
V75I is an accessory mutation of the Q151M multidrug resistance complex of HIV-1 RT and contributes to increase zidovudine, stavudine, didanosine, and zalcitabine resistance in the presence of F77L, F116Y, and Q151M (15, 22, 23). In addition, V75I has been recently identified as a major acyclovir resistance mutation in cells co-infected with HIV-1 and herpes simplex virus (24, 25). Structural analysis of HIV-1 RT showed that Val75 plays a role in positioning the template nucleotide +1, during the formation of the ternary complex (26). The side chain of Val75 interacts with the side chain of Phe77 to maintain the structure of the fingers subdomain. Mutations, such as V75M, V75T, and V75A, have been reported to occur in about 4% of patients infected with HIV-1 group M subtype B and receiving NRTIs, particularly d4T (see the Stanford HIV Drug Resistance Database web site) (27, 28). In vitro, V75T confers low level resistance to stavudine and other NRTIs (29). Mutations at codon 75 have been also detected in treated patients infected with other clades of HIV-1. Thus, V75M has been found in CRF01_AE-infected patients (30), whereas V75A and V75M have been selected in the absence and in the presence of TAMs, respectively, in stavudine-treated patients infected with HIV-1 group O (31).
In this study, we have examined the effects on stavudine resistance of the amino acid substitutions V75A, V75M, and V75I in the sequence context of a wild-type HIV-1 group O RT. Our data show that V75I reduces ATP- and PPi-mediated excision of thymidine analogues in different sequence contexts, including wild-type variants of group M subtype B and group O HIV-1 RTs, as well as multidrug-resistant enzymes containing either the Q151M complex or the combination M41L/A62V/T69SSS/K70R/T215Y. These results provide evidence of the antagonism between V75I and TAMs.
EXPERIMENTAL PROCEDURES
Reverse Transcriptases
Expression and purification of BH10_ WT RT (wild-type HIV-1BH10 RT), BH10_V75I RT (HIV-1BH10 RT containing the mutation V75I), and Q151Mc RT (HIV-1NL4-3 RT containing mutations A62V/V75I/F77L/F116Y/Q151M) was performed with modified versions of plasmid p66RTB, as described previously (12, 32–34). For high level expression of wild-type HIV-1 group O (O_WT) RT, an insert derived from cleavage with EcoRI and XhoI of plasmid pRT6 and containing the RT-coding sequence of the ESP49 variant (35) was cloned at the corresponding sites of P66RTB(SS) (36). The vector used for high level expression of the p66 subunit of the MAK_SSSY RT, containing a Ser-Ser insertion between codons 69 and 70 and mutations M41L, A62V, T69S, K70R, and T215Y (16), was obtained after cloning the SalI-MscI insert purified after cleavage of pRT6-derived plasmid at the appropriate sites of p66RTB(Q151Mc) (12). An XhoI site was introduced nine nucleotides upstream of the sequence encoding for the His6 tag in order to eliminate the stop codon generated during the cloning process. Site-directed mutagenesis was carried out with the QuikChangeTM site-directed mutagenesis kit (Stratagene) by following the manufacturer's instructions and using the following mutagenic primers: 5′-GGAATCAGGAAAATACTCGAGTCGACTCACCACC-3′ and 5′-GGTGGTGAGTCGACTCGAGTATTTTCCTGATTCC-3′. The introduced mutations were confirmed by DNA sequencing. RT p66 subunits carrying a His6 tag at their C terminus were co-expressed with HIV-1 protease in Escherichia coli XL1 Blue to obtain p66/p51 heterodimers, which were later purified by ionic exchange followed by affinity chromatography (32, 33). RT concentrations were determined by active site titration as described previously (37).
Other mutant RTs were also generated with the QuikChangeTM site-directed mutagenesis kit but using plasmids p66RTB(O_WT), p66RTB(MAK_SSSY), or p66RTB(Q151Mc) as templates. The mutagenic primers used were 5′-AAGTGGAGAAAGCTGGCAGACTTTAGGGAATTAAAC-3′ and 5′-GTTTAATTCCCTAAAGTCTGCCAGCTTTCTCCACTT- 3′ for O_V75A; 5′-AAGTGGAGAAAGCTGATGGACTTTAGGGAATTAAAC-3′ and 5′-GTTTAATTCCCTAAAGTCCATCAGCTTTCTCCACTT-3′ for O_V75M; 5′-AAGTGGAGAAAGCTGATAGACTTTAGGGAATTAAAC-3′ and 5′-GTTTAATTCCCTAAAGTCTATCAGCTTTCTCCACTT-3′ for O_V75I; 5′-GTTCGAGATGGAGAAAATTAATAGATTTCAGAGA-3′ and 5′-TCTCTGAAATCTATTAATTTTCTCCATCTCGAAC-3′ for MAK_SSSY_V75I; and 5′-CAGTACTAAATGGAGAAAATTAGTAGATCTCAGAGAACTTAATAAGAG-3′ and 5′-CTCTTATTAAGTTCTCTGAGATCTACTAATTTTCTCCATTTAGTACTG-3′ for Q151MC_I75V (relevant codons underlined). After mutagenesis, the entire RT coding regions were sequenced and, if correct, used for RT expression and purification.
Nucleotides and Template-Primers
Stock solutions (100 mm) of dNTPs and ATP were obtained from GE Healthcare. AZTTP was purchased from Moravek Biochemicals (Brea, CA). Acyclovir-TP and d4TTP were obtained from Sierra Bioresearch (Tucson, AZ). The α-boranophosphate derivative of d4TTP (α-borano-d4TTP) was synthesized as described previously (38, 39), and its Rp and Sp diastereoisomers were purified by reverse-phase high pressure liquid chromatography (40). Before use, nucleoside TPs were treated with inorganic pyrophosphatase (Roche Applied Science) to remove traces of PPi, as described (41). DNA oligonucleotides 21P (5′-ATACTTTAACCATATGTATCC-3′), 25PGA (5′-TGGTAGGGCTATACATTCTTGCAGG-3′), 31T (5′-TTTTTTTTTAGGATACATATGGTTAAAGTAT-3′), D38 (5′-GGGTCCTTTCTTACCTGCAAGAATGTATAGCCCTACCA-3′), and D38C (5′-GGGTCCTTTATTCCCTGCAAGAATGTATAGCCCTACCA-3′) were obtained from Invitrogen. Oligonucleotides 21P and 25PGA were labeled at their 5′ termini with [γ-32P]ATP (PerkinElmer Life Sciences) and T4 polynucleotide kinase (Promega) and then annealed to their corresponding templates (31T and D38, respectively). Template-primer 31T-21P was used in rapid quench incorporation experiments, whereas D38-25PGA was used in chain terminator excision assays and for the determination of kinetic parameters of ATP-dependent phosphorolysis.
Chain Terminator Excision Assays
RT-catalyzed DNA rescue reactions were performed with D38-25PGA DNA duplexes, as previously described (42, 43). Briefly, the phosphorylated template-primer (30 nm) was preincubated at 37 °C for 10 min in the presence of the corresponding RT at 12–24 nm active enzyme concentration in 50 mm Hepes buffer (pH 7.0), containing 15 mm NaCl, 15 mm magnesium acetate, 130 mm potassium acetate, 1 mm dithiothreitol, and 5% (w/v) polyethylene glycol 6000. Reactions were initiated by adding an equal amount of preincubation buffer containing the nucleoside RT inhibitor in its triphosphorylated form at a final concentration of 25 μm. After incubating the samples at 37 °C for 30 min, rescue reactions were initiated by adding a mixture of all dNTPs at a final concentration of 100 μm, in the presence of sodium PPi (200 μm) or ATP (3.2 mm), depending on the assay. Since the next complementary dNTP (dATP in our assay conditions) has an inhibitory effect on the rescue reaction, time courses of the unblocking and extension reactions were carried out in the presence of 1 μm dATP. In experiments designed to assess the inhibitory effect of dATP, extension reactions were incubated for 5–20 min in the presence of different concentrations of dATP. In all of those determinations, the amount of rescued primer was below 30%. Reactions were stopped by adding an equal amount of sample loading buffer (10 mm EDTA in 90% formamide containing 3 mg/ml xylene cyanol FF and 3 mg/ml bromphenol blue). Products were resolved on a denaturing 20% (w/v) polyacrylamide, 8 m urea gel, and primer rescue was quantified by phosphorimaging with a BAS 1500 scanner (Fuji) using the program Tina version 2.09 (Raytest Isotopenmessgerate Gmbh, Staubenhardt, Germany).
Pre-steady-state Kinetics of Single Nucleotide Incorporation Experiments
Pre-steady-state kinetic parameters for the incorporation of dTTP and d4TTP by O_WT, O_V75A, O_V75M, and O_V75I RTs were determined with a rapid quench instrument (model QFM-400, Bio-Logic Science Instruments, Claix, France) with reaction times ranging from 10 ms to 6 s. Reactions were performed under single turnover conditions by mixing a solution containing 50–100 nm (active sites) HIV-1 RT and a 100 nm concentration of the template-primer 31T-21P in RT buffer (50 mm Tris-HCl (pH 8.0), 50 mm KCl) and a variable concentration of nucleotide (i.e. dTTP or d4TTP) in 12 mm MgCl2 and then stopped by adding EDTA (0.3 m final concentration). The reaction products were separated on a 20% polyacrylamide/urea gel and quantified by phosphorimaging. The formation of product [P] over time was fitted with the following equation, [P] = A × [1 − exp(−kobs × t)], where A is the amplitude of the burst, and kobs is the apparent kinetic constant of formation of the phosphodiester bond. Data from single-turnover experiments were fitted to a single-exponential equation that measures the rate of dNTP incorporation (kobs) per given dNTP concentration ([dNTP]). The dependence of kobs on [dNTP] is described by the following hyperbolic equation, kobs = kpol × [dNTP]/(Kd + [dNTP]), where Kd and kpol are the equilibrium constant and the catalytic rate constant of the dNTP for RT, respectively. Kd and kpol were determined from curve-fitting using SigmaPlot.
Pre-steady-state Kinetics of the ATP-dependent Excision Reaction
For these experiments, primer 25PGA was blocked at its 3′-end with d4TTP, using terminal deoxynucleotidyltransferase, as described previously (41). Then the free d4TTP was eliminated with a mini Quick SpinTM column (Roche Applied Science). Inhibitor-terminated primer was labeled at its 5′ terminus with [γ-32P]ATP and T4 polynucleotide kinase and annealed to template D38 as described above. The 38-mer DNA template primed with 5′-32P-labeled d4T-terminated 25-mer (D38-25PGAd4T) was prepared at a final concentration of 1.1 μm. Single turnover conditions were used to study the excision of d4TMP. A solution containing 290 nm RT and 45 nm D38-25PGAd4T in RT buffer (50 mm Tris-HCl (pH 8.0), 50 mm KCl) was mixed with the same volume of RT buffer containing 3.2 mm ATP in the presence of 18 mm MgCl2. The reactions were carried out for 0–30 min at 37 °C. Aliquots were removed after defined incubation periods, quenched with sample loading buffer, and analyzed by denaturing polyacrylamide gel electrophoresis, as described above. The formation of product [P] over time was fitted to a single exponential decay, [P] = A × (exp (− kobs × t)). The kobs that equals the slope at time 0 of the [P] versus time plot was determined from curve fitting using SigmaPlot.
Site-specific Footprinting
Site-specific footprints with Fe2+ were monitored on 5′-end-labeled DNA template. Hybridization of the polyacrylamide gel-purified template MG48 (5′-GAATCAGCCCTTCCAGTCCCCCCTTTTCTTTTAAAAAGTGGCTAAGAA-3′) with complementary primer P19 (5′-TTAAAAGAAAAGGGGGGAC-3′) was conducted in a buffer containing 20 mm sodium cacodylate (pH 7.0) and 20 mm NaCl, by keeping the template-primer at a final concentration of 2 μm (44). MG48 is a 5′-end-truncated version of a previously characterized 58-nt template mimicking the polypurine tract binding site (45). Template-primer (100 nm) was incubated with HIV-1 RT (225 nm) in a buffer containing 120 mm sodium cacodylate, pH 7.0, 20 mm NaCl, and 0.2 mm MgCl2 in a final volume of 20 μl. Prior to the Fe2+ treatment, complexes were preincubated for 30 min with 25 μm d4TTP or AZTTP (final concentration), depending on the assay, and then further incubated for 20 min, with different concentrations of the next nucleotide (dGTP) or phosphonoformic acid, depending on the experiments. Treatment with Fe2+ was performed essentially as described (44). Briefly, preformed complexes in a volume of 20 μl were incubated with a mixture of 2 μl of 500 μm Fe(NH4)2SO4·6H2O and 2 μl of 500 μm dithiothreitol. Reactions were allowed to proceed for 5 min and then stopped by adding 100 μl of a solution containing 0.1 m thiourea, 2 ng/μl tRNA, 10 mm EDTA, and 0.6 m sodium acetate. Samples were subsequently precipitated with ethanol and loaded on a 20% denaturing polyacrylamide gel, as described above.
Recombinant Virus and Drug Susceptibility Tests
These assays were performed as described (16, 42). Briefly, full-length RT coding sequence DNA was amplified by PCR from the different BH10 expression plasmids carrying the appropriate RT using the IN5 (5′-AATTTTCCCATTAGTCCTATTGAAACTGTACCA-3′) and IN3 (5′-TCTATTCCATCYAAAAATAGTACTTTCCTGATTCC-3′) primers. The PCR products were then co-transfected in MT-4 cells with an RT-deleted HXB2-D previously linearized with BstEII (46). Culture supernatants were harvested when the HIV-1 p24 antigen concentration surpassed 20 ng/ml. Progeny virus was propagated and titrated in MT-4 cells. The nucleotide sequence of the RT-coding region of the progeny virus was PCR-amplified with the above mentioned primers and checked for possible reversions or undesired mutations. The MT-4 cells and the deleted HXB2-D clone were obtained from the AIDS Reagent Program (Medical Research Council). Recombinant viruses were tested in triplicate with AZT, d4T, and ritonavir. The former three drugs were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. HIV-1 drug susceptibility data were obtained after infecting 30,000 MT-4 cells with 100 50% tissue culture infective doses of virus, at a multiplicity of infection of 0.003, by exposing the HIV-1-infected cultures to various concentrations of each drug (5-fold dilutions). After the MT-4 cells were allowed to proliferate for 5 days, the number of viable cells was quantified by a tetrazolium-based colorimetric method (MTT method) as described elsewhere (47).
RESULTS
Discrimination between 2′-Deoxythymidine 5′-Triphosphate and d4TTP by Wild-type and Mutant HIV-1 Group O RTs
Transient kinetic analysis was used to investigate the role in stavudine resistance of amino acid substitutions affecting Val75 in HIV-1 group O RT. Mutations V75A and V75I had a small effect on the catalytic efficiency of dTTP incorporation. In contrast, the relative value of kpol/Kd for d4TTP incorporation versus dTTP incorporation was significantly reduced for mutant V75I, in comparison with the wild-type RT (Table 1). These results suggested that V75I could confer some resistance, although differences with the wild-type enzyme were relatively small or non-significant.
TABLE 1.
Pre-steady-state kinetic constants for the incorporation of dTTP and d4TTP on a heteropolymeric DNA-DNA template-primer by wild-type and mutant HIV-1 group O RTs
The template-primer 31T-21P was used as the substrate. Data shown are the mean values ± S.D. Each of the assays was performed independently at least twice. Interassay variability was below 20%. All determinations were carried out under single turnover conditions. Previously reported kinetic parameters for dTTP incorporation obtained with O_WT and O_V75I RTs, using burst equations ([P] = A × (1 − exp(−kobs × t)) + kss × t) were similar to those shown below (36), due to the relatively low value of the steady-state kinetic constant (kss ∼ 0.05 s−1).
| Enzyme | Nucleotide | kpol | Kd | kpol/Kd | Selectivitya |
|---|---|---|---|---|---|
| s−1 | μm | μm−1 s−1 | |||
| O_WT | dTTP | 13.2 ± 1.6 | 17.3 ± 5.6 | 0.76 ± 0.26 | |
| d4TTP | 9.8 ± 0.8 | 16.6 ± 4.5 | 0.59 ± 0.17 | 1.29 ± 0.57 | |
| O_V75A | dTTP | 16.6 ± 0.8 | 17.9 ± 2.7 | 0.92 ± 0.15 | |
| d4TTP | 15.7 ± 0.9 | 14.6 ± 3.3 | 1.08 ± 0.25 | 0.85 ± 0.24 (0.66) | |
| O_V75M | dTTP | 10.0 ± 0.5 | 11.6 ± 3.0 | 0.86 ± 0.23 | |
| d4TTP | 15.2 ± 0.7 | 19.3 ± 3.0 | 0.78 ± 0.13 | 1.10 ± 0.34 (0.85) | |
| O_V75I | dTTP | 11.1 ± 1.0 | 17.1 ± 4.9 | 0.65 ± 0.19 | |
| d4TTP | 18.9 ± 2.0 | 62.2 ± 8.5 | 0.30 ± 0.05 | 2.14 ± 0.72 (1.66) |
a The selectivity is the ratio of (kpol/Kd(dTTP))/(kpol/Kd(d4TTP)). Resistance (which is shown within parenthesis) was calculated as the selectivity value obtained with each mutant enzyme divided by the selectivity obtained with the wild-type RT.
Effects of Substituting Val75 in Wild-type HIV-1 RTs on the Excision of Thymidine Analogues from Blocked DNA Primers
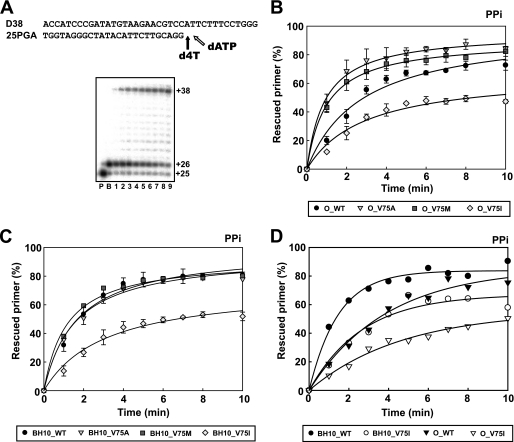
The ability of RTs to unblock d4TMP-terminated primers was assessed by using the template-primer shown in Fig. 1A. These experiments were carried out in two steps. First, the enzyme and the template-primer were incubated in the presence of d4TTP to generate a terminated 26-nucleotide primer. Then unblocking and extension reactions were carried out by adding a mixture of dNTPs and PPi (200 μm) or ATP (3.2 mm). These assays revealed that in comparison with the wild-type HIV-1 group O RT, mutants V75A and V75M had increased excision activity on d4TMP-terminated primers, thereby promoting stavudine resistance (Fig. 1B). Interestingly, the mutation V75I produced a significant decrease in the PPi-mediated excision activity. This effect was also observed when the V75I mutation was introduced in the HIV-1 group M subtype B sequence context (i.e. in the RT of the BH10 strain), but the differences between mutants V75A, V75M, and the wild-type enzyme were not significant (Fig. 1C). The reduced phosphorolytic activity of RTs bearing the V75I mutation was also observed in ATP-mediated excision assays, although the efficiency of the unblocking reaction was relatively low. After incubating for 30 min in the presence of 3.2 mm ATP, in the conditions described in the legend to Fig. 1, O_WT, O_V75I, BH10_WT, and BH10_V75I RTs were able to unblock and extend 9.5, 6.0, 12.2, and 8.0% of the d4TMP-terminated primer, respectively. The diminished excision activity associated with the V75I mutation was also observed with primers blocked with AZTMP and was independent of the RT sequence context, as shown in Fig. 1D.
FIGURE 1.
Effect of amino acid substitutions affecting Val75 in phylogenetically diverse HIV-1 RTs, on rescue DNA polymerization reactions carried out in the presence of PPi. A, reactions were carried out with 38/25-mer DNA-DNA heteropolymeric complexes (sequences shown above). First, the inhibitor was incorporated at position +1 of the 25-nucleotide primer (lane P) to generate a 26-nucleotide product (lane B). Excision of d4TMP and further primer extension in the presence of 3.2 mm ATP or 200 μm PPi and a mixture of dNTPs leads to the formation of a fully extended 38-nucleotide product. The next complementary dNTP (dATP, in the assay conditions) can inhibit the rescue reaction. Time courses of primer rescue reactions are shown in lanes 1–9, which correspond to aliquots removed 1, 2, 3, 4, 5, 6, 8, and 10 min after the addition of 200 μm PPi. The time courses of primer rescue reactions initiated from d4T-terminated primers using wild-type and mutant HIV-1 group O RTs and wild-type and mutant HIV-1 group M subtype B RTs are shown in B and C, respectively. D, effect of V75I on rescue reactions initiated from AZT-terminated primers. All dNTPs in the assays were supplied at 100 μm, except for dATP, whose concentration was 1 μm. Active enzyme concentration in these assays was 12 nm. Represented values were obtained from two or three independent experiments.
Effects of V75I on the Excision Activity of Multidrug-resistant RTs
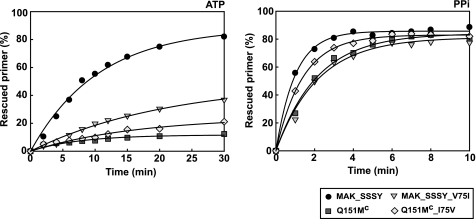
The effects of substituting Ile for Val75 were also analyzed in the sequence context of multidrug-resistant RTs, such as the one containing the Q151M mutation complex (Q151Mc RT) or an excision-proficient RT carrying a Ser-Ser insertion between codons 69 and 70 and mutations M41L, A62V, T69S, K70R, and T215Y (MAK_SSSY RT). As shown in Fig. 2, a reduction in the ATP- or PPi-mediated excision activity on primers terminated with d4TMP was obtained by introducing the V75I mutation in the MAK_SSSY RT. In addition, eliminating the V75I mutation from the Q151Mc RT sequence rendered an enzyme with slightly increased excision activity. The largest effects of substituting Val or Ile at position 75 were observed with the MAK_SSSY RT mutant in ATP-dependent phosphorolytic reactions.
FIGURE 2.
Effect of substituting Val for Ile75 or Ile for Val75 in multidrug-resistant RTs on rescue DNA polymerization reactions initiated from d4T-terminated primers. Time courses of excision reactions were carried out in the presence of 3.2 mm ATP (left) or 200 μm PPi (right). Assays were done with the template-primer shown in Fig. 1A. All dNTPs in the assays were supplied at 100 μm, except for dATP, whose concentration was 1 μm. Active enzyme concentration in these assays was 12 nm. Represented values were obtained from two or three independent experiments. Interassay variability was below 12% in these experiments.
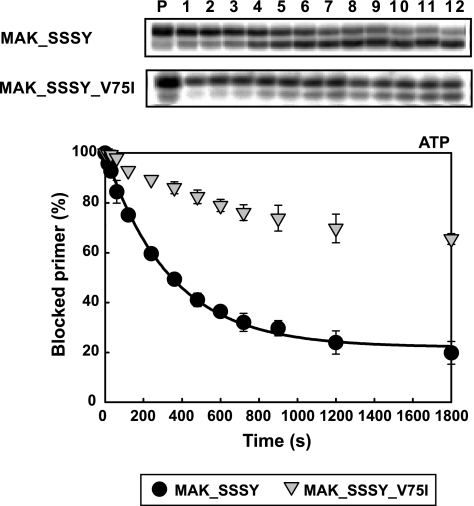
The d4TMP excision rates for MAK_SSSY RT and its derivative containing V75I were determined in the presence of 3.2 mm ATP, under single-turnover conditions and using a d4TMP-terminated primer, which was previously annealed to a 38-nucleotide template (D38) (Fig. 3). MAK_SSSY RT showed an excision rate (kobs) of 0.178 ± 0.007 min−1, and the introduction of V75I in the sequence context of MAK_SSSY RT led to a 10-fold decrease of the excision rate (kobs value was 0.0167 ± 0.0014 min−1).
FIGURE 3.
Effects of V75I on the kinetics of ATP-dependent excision activity of MAK_SSSY RT. Time courses for the excision reaction of a d4T-terminated primer (26-mer) annealed to a 38-mer DNA template (45 nm), catalyzed by MAK_SSSY and MAK_SSSY_V75I RTs (290 nm), were determined in the presence of 3.2 mm ATP. Lane P shows the d4T-terminated primer before treatment with ATP. The time courses of the reactions are shown in lanes 1–12, where samples correspond to aliquots removed 15, 30, 60, 120, 240, 360, 480, 600, 720, 900, 1200, and 1800 s, respectively, after the addition of ATP.
Excision reactions can be inhibited by the next complementary nucleotide. We and others have shown that the ATP-mediated excision of d4TMP from blocked primers can be inhibited in vitro at concentrations of the next complementary dNTP between 0.5 and 25 μm (41, 48). In the presence of 3.2 mm ATP, the MAK_SSSY RT showed an IC50 for the next complementary dNTP of 6.37 ± 0.62 μm, whereas its mutant derivative carrying the V75I mutation was about 3 times less susceptible to dNTP inhibition (IC50 = 17.73 ± 4.01 μm). These results suggested that the V75I mutation could interfere with the excision activity by affecting the translocation state of the blocked template-primer.
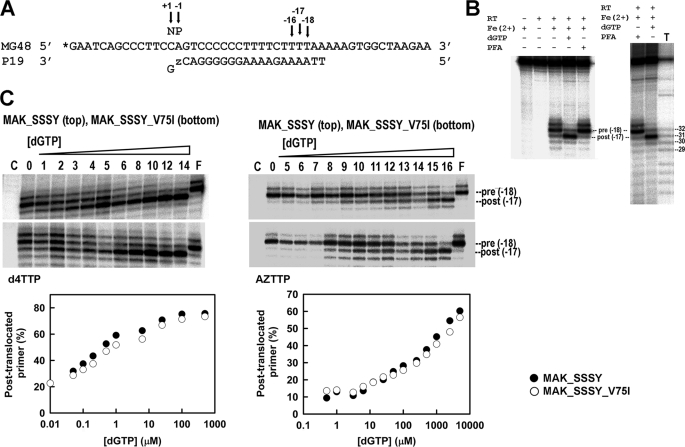
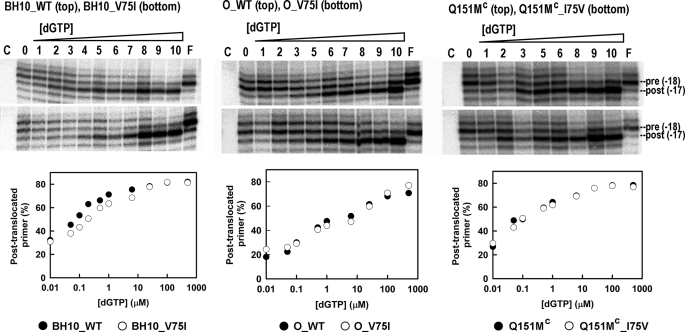
Site-specific footprinting experiments using the classical method with [Fe(EDTA)]2− that yields hydroxyl radicals via Fenton-like chemistry (44) were carried out in order to determine the impact of V75I on the translocation status of HIV-1 RT. DNA-DNA duplexes containing the MG48 template and the primer P19 terminated with d4TTP or with AZTTP were used as substrates (Fig. 4). In the presence of MG48-P19, treatment with Fe2+ caused site-specific cleavage at positions −17 and −18, which correspond to post-translocational (P-site complex) and pretranslocational (N-site complex) configurations of the template-primer in the HIV-1 RT. Fe2+-mediated cuts were assigned by means of a T-ladder (Fig. 4B).
FIGURE 4.
Site-specific footprinting of RT-DNA-DNA complexes. A, sequence of the DNA-DNA template-primer indicating the hyperreactive cleavage sites (at positions −16, −17, and −18). The 32P-labeled 5′-end of the template is marked with an asterisk. B, cleavage patterns obtained after treatment with Fe2+, using MAK_SSSY RT and the DNA duplex containing a d4TMP-terminated primer in the absence and in the presence of the next complementary dNTP (i.e. dGTP). Lane T shows a T-ladder obtained with KMnO4-piperidine (49). C, cleavage patterns obtained after Fe2+ treatment of RT-DNA-DNA complexes in the absence of nucleotide (lane 0) or in the presence of different concentrations of dGTP: 0.01 μm (lane 1), 0.05 μm (lane 2), 0.1 μm (lane 3), 0.2 μm (lane 4), 0.5 μm (lane 5), 1 μm (lane 6), 3.125 μm (lane 7), 6.25 μm (lane 8), 12.5 μm (lane 9), 25 μm (lane 10), 50 μm (lane 11), 100 μm (lane 12), 250 μm (lane 13), 500 μm (lane 14), 1 mm (lane 15), and 5 mm (lane 16) or in the presence of 500 μm foscarnet (lane F). C, a control carried out in the absence of Fe2+. The graphs below show the nucleotide-dependent changes of the translocation status with MAK_SSSY and MAK_SSSY_V75I RTs in the presence of d4TMP-terminated primers (left) or AZTMP-terminated primers (right).
As expected, the addition of the next complementary dNTP (i.e. dGTP under our assay conditions) promoted the formation of the “dead end” complex and the inhibition of the excision reaction while leading to a significant increase of the intensity of the band at position −17, corresponding to the post-translocational configuration of the viral RT (Fig. 4B). On the other hand, foscarnet shifts the translocational equilibrium toward a pretranslocated (and sometimes hypertranslocated) position (50, 51) (Fig. 4B).
Fig. 4C shows the distribution of pre- and post-translocated complexes in the presence of increasing concentrations of dGTP for the multidrug-resistant mutant M41L/A62V/T69SSS/K70R/T215Y and its derivative containing V75I. The dGTP concentration required to obtain 50% of complexes in the post-translocational stage was >1000 times lower for dd4TMP- than for AZTMP-terminated primers. Differences between M41L/A62V/T69SSS/K70R/T215Y and M41L/A62V/T69SSS/K70R/V75I/T215Y were non-significant for AZTMP-terminated primers. However, in the presence of d4TMP-terminated primers, the MAK_SSSY RT showed a higher preference for the post-translocational configuration. Estimates of the concentrations of dGTP required to obtain 50% of the complexes in the post-translocational stage were 0.29 ± 0.06 μm for MAK_SSSY RT and 0.62 ± 0.15 for MAK_SSSY_V75I RT.
Other RTs (i.e. wild-type BH10 RT, wild-type group O RT, and the multidrug-resistant mutant A62V/F77L/F116Y/Q151M as well as their derivatives with V75I) showed similar cleavage patterns, with small differences in the band intensities at positions −17 and −18 (Fig. 5). However, higher dGTP concentrations were required to shift the equilibrium toward the post-translocational stage for O_WT and O_V75I RTs than for the other enzymes. Interestingly, in comparison with the mutant BH10_V75I, the BH10_WT RT showed a higher preference for the post-translocational stage within the range of 0.05–0.5 μm dGTP. However, the effects of V75I on the translocational equilibrium of O_WT and A62V/V75I/F77L/F116Y/Q151M RTs were not significant.
FIGURE 5.
Site-specific footprinting of RT-DNA-DNA complexes containing d4TMP-terminated primers. Cleavage patterns obtained after Fe2+ treatment of RT-DNA-DNA complexes in the absence of nucleotide (lane 0) or in the presence of different concentrations of dGTP (0.01 μm (lane 1), 0.05 μm (lane 2), 0.1 μm (lane 3), 0.2 μm (lane 4), 0.5 μm (lane 5), 1 μm (lane 6), 6.25 μm (lane 7), 25 μm (lane 8), 100 μm (lane 9), and 500 μm (lane 10)) or in the presence of 500 μm foscarnet (lane F). C, a control carried out in the absence of Fe2+. The graphs below show the nucleotide-dependent changes of the translocation status with BH10_WT and BH10_V75I RTs (left), O_WT and O_V75I RTs (center), and Q151Mc and Q151Mc_I75V RTs (right). Estimates of the dGTP concentrations required to obtain 50% of the complexes in the post-translocational stage were 0.079 ± 0.012 μm for BH10_WT RT, 0.22 ± 0.04 μm for BH10_V75I, 0.50 ± 0.15 μm for O_WT RT, 0.45 ± 0.21 for O_V75I RT, 0.098 ± 0.027 μm for Q151Mc RT, and 0.112 ± 0.029 μm for Q151Mc_I75V RT.
These results were also broadly in agreement with site-specific footprinting experiments carried out with the template-primer used in the dNTP inhibition experiments (i.e. D38-25PGA) that showed that V75I had an impact on the formation of the dead end complex both in the presence of wild-type BH10 RT and in the presence of the mutant M41L/A62V/T69SSS/K70R/T215Y (data not shown).
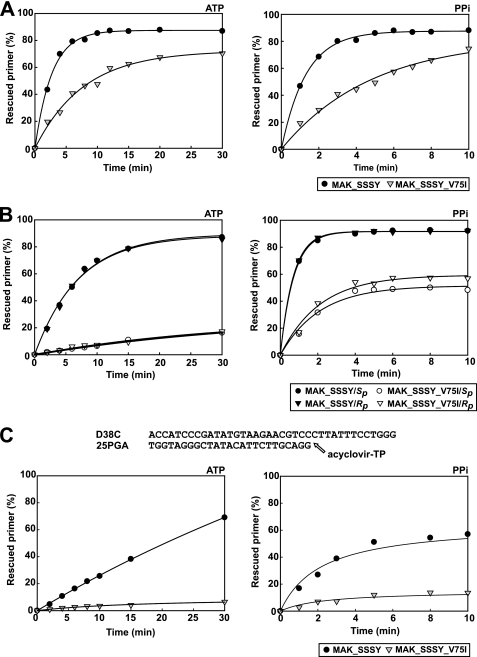
Further excision experiments with d4TMP-terminated primers showed that the V75I mutation impaired both the ATP- and the PPi-dependent phosphorolytic activity of the multidrug-resistant RT containing the insertion and the accompanying TAMs (i.e. M41L/A62V/T69SSS/K70R/T215Y) in reactions carried out with DNA duplexes containing an AZTMP-terminated primer (Fig. 6A). The peptide backbone of Val75 interacts with the 5′ template overhang in the ternary complex of HIV-1 RT, although an indirect effect on Gln151 and other residues in the dNTP binding site could also occur and affect the enzyme's activity (26, 34). The geometry of the phosphodiester bond at the 3′-end of the primer could interfere with excision. However, the time courses of the excision reactions carried out with primers terminated with the Rp and Sp diastereoisomers of α-borano derivatives of d4TMP (i.e. α-(Rp)-BH3-d4TMP and α-(Sp)-BH3-d4TMP) were almost identical (Fig. 6B). However, in all cases, the V75I mutation produced a significant decrease in the excision rate, which was larger in ATP-dependent phosphorolytic reactions.
FIGURE 6.
Effects of V75I on rescue DNA polymerization reactions initiated from primers terminated with AZTMP (A), α-BH3-d4TMP diastereoisomers (B), or acyclovir-MP (C). Panels on the left correspond to reactions carried out in the presence of 3.2 mm ATP, whereas those on the right were obtained with 200 μm PPi. The assays in A and B were performed with the template-primer shown in Fig. 1A, whereas the template-primer D38C-25PGA was used in rescue reactions carried out with primers terminated with acyclovir-MP. All dNTPs were supplied at 100 μm, except for dATP, whose concentration was 1 μm. Active RT concentration in these assays was 12 nm. Represented values were obtained from two or three independent experiments. Interassay variability was below 12% in these experiments.
Interestingly, V75I also impaired the excision activity on primers terminated with acyclovir (Fig. 6C), suggesting a broad impact on ATP-dependent phosphorolytic activity on primers blocked with several chain-terminating inhibitors. However, it should be noted that V75I impairs the incorporation of acyclovir-TP (25), and therefore, acyclovir excision is not expected to be relevant in vivo.
Phenotypic Drug Susceptibility of Recombinant HIV-1
Recombinant HIV-1 clones containing the excision-proficient MAK_SSSY RT and its derivative containing V75I (i.e. M41L/A62V/T69SSS/K70R/V75I/T215Y) were assayed to establish the level of resistance to thymidine analogues. As reported in Table 2, the MAK_SSSY RT conferred >80-fold increased AZT resistance and 5.8-fold increased d4T resistance in comparison with wild-type HIV-1 containing the BH10 RT. The V75I mutation decreased resistance to both thymidine analogues, although the effects were much more significant with AZT.
TABLE 2.
Susceptibility of HIV-1 constructs to AZT, d4T, and ritonavir
The IC50 values represent the mean ± S.D. of at least three tests, with each one performed six times. The -fold increase in IC50 relative to the wild-type HXB2 virus control carrying the RT sequence of BH10 is shown in parenthesis.
| RTs | IC50 |
||
|---|---|---|---|
| AZT | d4T | Ritonavir | |
| nm | |||
| BH10_WT | 3.6 ± 0.2 | 354.1 ± 59.3 | 53.3 ± 19.1 |
| MAK_SSSY | 297.0 ± 119.2 (82.5) | 2042.4 ± 483.5 (5.8) | 53.0 ± 15.0 (1.0) |
| MAK_SSSY_V75I | 16.4 ± 4.1 (4.5) | 1229.3 ± 453.6 (3.5) | 63.3 ± 24.4 (1.2) |
DISCUSSION
Despite the significant advances in antiretroviral therapy and the increasing knowledge about drug resistance mutations, interpretation of mutational patterns is often difficult due to poor phenotypic correlates, unexpected interactions between amino acid substitutions involved in resistance, and uncertainties about the contribution of secondary mutations in different sequence contexts. Several mutations at codon 75, such as V75A, V75T, and V75M have been related to stavudine resistance in major clades of HIV-1 (e.g. group M subtype B and group O strains) (27, 29–31, 52). Our kinetic analysis of nucleotide incorporation shows that in the sequence context of an HIV-1 group O RT, V75M has only slightly increased discrimination ability between d4TTP and dTTP in comparison with the wild-type enzyme. However, both stavudine-related mutations V75M and V75A were shown to confer increased phosphorolytic activity on d4TMP-terminated primers (Fig. 1), a result that is consistent with their selection in vivo in stavudine-treated patients who were infected with HIV-1 group O variants (31). However, these differences were not observed with a prototypic HIV-1 group M subtype B RT (i.e. BH10 RT).
Interestingly, our analysis shows that V75I has a minor influence on discrimination between d4TTP and dTTP but impairs thymidine analogue excision in different RT sequence contexts, including wild-type and drug-resistant enzymes (Figs. 1 and 2). Reversion of the excision phenotype by V75I was particularly evident with the mutant containing the dipeptide insertion and TAMs, such as M41L, K70R, and T215Y.
Val75 is located in the β3-β4 hairpin loop (residues 56–77) and plays a role in positioning the template nucleotide +1 during the formation of the ternary complex of RT, duplex DNA, and the incoming dNTP (26). The comparison with the crystal structure of the HIV-1 RT binary complex (53) revealed that in the ternary complex, the distances between the peptide backbones of Val75 and Gln151 and between Val75 and the single-stranded template were reduced (34). In addition, the side chain of Val75 interacts with residues, such as Val60, Phe77, or Tyr146, that are important to maintain the structure of the fingers subdomain while influencing the architecture of the dNTP binding site (52). It has been shown that in a subtype B HIV-1 RT sequence context, V75T increases d4T excision (52), whereas V75I has the opposite effect (this work). Subtle differences affecting the accessibility of the PPi donor to the terminating nucleotide at the 3′-end of the primer could be invoked to explain the observed differences, although further studies will be necessary to provide a reliable mechanistic interpretation.
In agreement with the results obtained with purified RTs, phenotypic assays revealed that the recombinant HIV-1 containing mutations M41L/A62V/T69SSS/K70R/V75I/T215Y was about 18 times more susceptible to AZT than its homologues lacking the V75I change. These results were also consistent with the predicted AZT -fold change values of 3.3 and 22.3, obtained for M41L/A62V/T69SSS/K70R/V75I/T215Y and M41L/A62V/T69SSS/K70R/T215Y, respectively, using the VirtualPhenotypeTM-LM bioinformatics tool (Virco BVBA, Mechelen, Belgium) (data not shown). However, as anticipated by the VirtualPhenotypeTM-LM predictions, V75I had a relatively small impact on d4T resistance in phenotypic assays, as a consequence of the inhibition of the excision reaction by the relatively high dNTP levels found in the MT-4 cells used in the drug susceptibility assays. Phenotypic assays with recombinant HIV-1 clones also showed that the addition of V75I to an RT sequence background containing M41L, A62V, T69S, K70R, V75I, T215Y, and the Ser-Ser insertion between positions 69 and 70 had an antagonistic effect on d4T resistance.
Our results demonstrate that V75I shares a common biochemical mechanism with RT mutations, such as K65R (21, 54), L74V (19, 20), Y181C (55), and M184V (17, 18), as well as with a number of foscarnet-related resistance mutations (i.e. W88G, E89K, A114S, S117T, Q161L, and M164I) (56, 57) and with the deletion of Thr69, which is often associated with the Q151M complex (58–60). All of those mutations increase HIV-1 susceptibility to AZT by reducing the phosphorolytic activity of the enzyme. Phenotypic drug susceptibility assays have demonstrated that, as in the case of V75I, other amino acid changes (e.g. K65R, L74V, W88G, E89K, L92I, A114S, S117T, S156A, F160Y, Q161L, M164I, Y181C, and M184V) are able to enhance AZT susceptibility in the presence of TAMs (17, 20, 21, 55–57, 61, 62).
V75I impairs excision by reducing the rate of chain terminator removal. Moreover, in the presence of TAMs, V75I contributes to increase the IC50 for the next complementary nucleotide by facilitating the pretranslocation configuration of the d4TMP-terminated primer. However, the effects of V75I on the formation of a dead end complex with primers terminated with d4T could be of little significance due to the relatively small differences between the IC50 values obtained with excision-proficient RTs with or without V75I. On the other hand, as in the case of L74V or M184V (19), V75I does not affect the translocational equilibrium in the presence of the next complementary dNTP when the primer is blocked with AZTMP.
The relevance of the ATP-dependent phosphorolytic mechanism in vivo depends on the concentration of ATP and the dNTP levels inside the cell. Estimates of dNTP concentrations ranged from 0.2 μm to 24.5 μm in resting human lymphocytes, whereas concentrations can be 2–10-fold higher in activated human lymphocytes, depending on the assay conditions and dNTP analyzed. The highest dNTP levels have been reported for established cell lines, such as H-9, U937 promonocytes, CEM lymphoblasts, or SupT1 cells, where nucleotide concentrations range from 50 to 300 μm (for reviews, see Refs. 63 and 64). Under these conditions, it is clear that rescue of inhibitor-terminated primers could be more relevant for the acquisition of resistance to AZT than for d4T (41, 48).
Despite the extensively reported evidence of cross-resistance between AZT and d4T in clinical settings (65–67), the relatively high dNTP levels could explain the large differences between AZT and d4T susceptibilities obtained in phenotypic assays (65, 68, 69). For example, M41L/L210W/T215Y confers >100-fold increased resistance to AZT in phenotypic assays but only a 2.1-fold increase in the IC50 for d4T relative to the wild-type HIV-1 (70). In contrast, in the presence of M184V, M41L/L210W/T215Y conferred 15-fold increased resistance to AZT but a mere 1.8-fold increased resistance to d4T. It is widely accepted that M184V impairs excision of thymidine analogues (17, 18), but in phenotypic assays these effects, which are clearly detectable with AZT, are only marginal with d4T, due to the high dNTP levels present in SupT1 and MT-4 cells. In this context, our phenotypic data showing a 2-fold reduction of the IC50 for d4T when V75I is present are most significant. The biochemical studies reported in this work also suggest that this reduction is a consequence of the lower d4TMP excision rate of the V75I-containing RT, rather than an effect of its decreased susceptibility to dNTP inhibition.
An extensive analysis of amino acid covariation in RT sequences from more than 7,000 people infected with HIV-1 group M subtype B virus obtained from the Stanford HIV Drug Resistance Database has revealed that V75I is strongly associated with mutations of the Q151M complex, whereas V75M is positively associated with TAMs such as M41L, L210W, or T215Y (71). The antagonistic effects between V75I and TAMs could be relevant for the acquisition of AZT resistance in HIV-2. Wild-type HIV-2 RT contains Ser instead of Thr at position 215, and only one nucleotide change is needed to obtain the substitution S215Y. However, Q151M emerges as the major AZT resistance mutation in HIV-2 RT, whereas TAMs are relatively uncommon in HIV-2-infected patients (72–74). Molecular modeling studies suggested that several amino acid differences around the putative ATP binding site of HIV-2 RT in comparison with the HIV-1 RT could account for these differences (75). The presence of Ile75 in the wild-type HIV-2 RT and our results showing the diminished excision activity conferred by V75I provide additional arguments to explain why the Q151M pathway is preferred over the accumulation of TAMs in the acquisition of thymidine analogue resistance in HIV-2.
Finally, it should be noted that our study also reveals that accessory mutations in the Q151M complex could have different effects on excision, since we have previously demonstrated that A62V contributes to increase ATP-mediated excision of thymidine analogues in the presence of one, two, or three TAMs (16). From a mechanistic point of view, antagonistic mutations are likely to affect the optimal alignment of the PPi donor with the phosphodiester group located at the 3′-end of the blocked primer. A more complete understanding of the precise mechanisms underlying these interactions may come from the determination of the crystal structures of ternary complexes containing RTs carrying the relevant combinations of mutations.
This work was supported by Spanish Ministry of Science and Innovation Grant BIO2007/60319, Fundación para la Investigación y Prevención del SIDA en España (FIPSE) Grant 36771/08, the Fondo de Investigación Sanitaria (through “Red Temática de Investigación Cooperativa en SIDA” Grant RD06/0006), and an institutional grant from the Fundación Ramón Areces. This work was also supported by Spanish Ministry of Science and Innovation Grants BFU2006/01066 and PI07/0098 (to M. A. M.).
- HIV-1 and -2
- human immunodeficiency virus type 1 and 2, respectively
- RT
- reverse transcriptase
- NRTI
- nucleoside RT inhibitor
- TP
- triphosphate
- AZT
- 3′-azido-3′-deoxythymidine
- d4T
- 2′,3′-didehydro-2′,3′-dideoxythymidine
- TAM
- thymidine analogue resistance mutation
- MP
- monophosphate
- WT
- wild-type
- d4TTP
- 2′,3′-didehydro-2′,3′-dideoxythymidine triphosphate
- d4TMP
- 2′,3′-didehydro-2′,3′-dideoxythymidine monophosphate
- AZTTP
- 3′-azido-3′-deoxythymidine triphosphate.
REFERENCES
- 1.Basavapathruni A., Anderson K. S. (2007) FASEB J. 21, 3795–3808 [DOI] [PubMed] [Google Scholar]
- 2.Sarafianos S. G., Marchand B., Das K., Himmel D. M., Parniak M. A., Hughes S. H., Arnold E. (2009) J. Mol. Biol. 385, 693–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schinazi R. F., Hernandez-Santiago B. I., Hurwitz S. J. (2006) Antivir. Res. 71, 322–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Clercq E., Neyts J. (2009) Handb. Exp. Pharmacol. 189, 53–84 [DOI] [PubMed] [Google Scholar]
- 5.Sarafianos S. G., Das K., Clark A. D., Jr., Ding J., Boyer P. L., Hughes S. H., Arnold E. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 10027–10032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray A. S., Basavapathruni A., Anderson K. S. (2002) J. Biol. Chem. 277, 40479–40490 [DOI] [PubMed] [Google Scholar]
- 7.Deval J., Navarro J. M., Selmi B., Courcambeck J., Boretto J., Halfon P., Garrido-Urbani S., Sire J., Canard B. (2004) J. Biol. Chem. 279, 25489–25496 [DOI] [PubMed] [Google Scholar]
- 8.Arion D., Kaushik N., McCormick S., Borkow G., Parniak M. A. (1998) Biochemistry 37, 15908–15917 [DOI] [PubMed] [Google Scholar]
- 9.Meyer P. R., Matsuura S. E., Mian A. M., So A. G., Scott W. A. (1999) Mol. Cell. 4, 35–43 [DOI] [PubMed] [Google Scholar]
- 10.Vivet-Boudou V., Didierjean J., Isel C., Marquet R. (2006) Cell. Mol. Life. Sci. 63, 163–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menéndez-Arias L. (2008) Virus Res. 134, 124–146 [DOI] [PubMed] [Google Scholar]
- 12.Deval J., Selmi B., Boretto J., Egloff M. P., Guerreiro C., Sarfati S., Canard B. (2002) J. Biol. Chem. 277, 42097–42104 [DOI] [PubMed] [Google Scholar]
- 13.Deval J., Alvarez K., Selmi B., Bermond M., Boretto J., Guerreiro C., Mulard L., Canard B. (2005) J. Biol. Chem. 280, 3838–3846 [DOI] [PubMed] [Google Scholar]
- 14.Shirasaka T., Kavlick M. F., Ueno T., Gao W. Y., Kojima E., Alcaide M. L., Chokekijchai S., Roy B. M., Arnold E., Yarchoan R., Mitsuya H. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iversen A. K., Shafer R. W., Wehrly K., Winters M. A., Mullins J. I., Chesebro B., Merigan T. C. (1996) J. Virol. 70, 1086–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cases-González C. E., Franco S., Martínez M. A., Menéndez-Arias L. (2007) J. Mol. Biol. 365, 298–309 [DOI] [PubMed] [Google Scholar]
- 17.Götte M., Arion D., Parniak M. A., Wainberg M. A. (2000) J. Virol. 74, 3579–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer P. L., Sarafianos S. G., Arnold E., Hughes S. H. (2002) J. Virol. 76, 3248–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel F. A., Marchand B., Turner D., Götte M., Wainberg M. A. (2005) Antimicrob. Agents Chemother. 49, 2657–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miranda L. R., Götte M., Liang F., Kuritzkes D. R. (2005) Antimicrob. Agents Chemother. 49, 2648–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White K. L., Chen J. M., Feng J. Y., Margot N. A., Ly J. K., Ray A. S., Macarthur H. L., McDermott M. J., Swaminathan S., Miller M. D. (2006) Antivir. Ther. 11, 155–163 [DOI] [PubMed] [Google Scholar]
- 22.Maeda Y., Venzon D. J., Mitsuya H. (1998) J. Infect. Dis. 177, 1207–1213 [DOI] [PubMed] [Google Scholar]
- 23.Kosalaraksa P., Kavlick M. F., Maroun V., Le R., Mitsuya H. (1999) J. Virol. 73, 5356–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon M. A., Siliciano J. D., Lai J., Liu J. O., Stivers J. T., Siliciano R. F., Kohli R. M. (2008) J. Biol. Chem. 283, 31289–31293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tchesnokov E. P., Obikhod A., Massud I., Lisco A., Vanpouille C., Brichacek B., Balzarini J., McGuigan C., Derudas M., Margolis L., Schinazi R. F., Götte M. (2009) J. Biol. Chem. 284, 21496–21504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H., Chopra R., Verdine G. L., Harrison S. C. (1998) Science 282, 1669–1675 [DOI] [PubMed] [Google Scholar]
- 27.Fitzgibbon J. E., DiCola B., Arnold E., Das K., Sha B. E., Pottage J. C., Jr., Nahass R., Gaur S., John J. F., Jr. (2001) Antivir. Ther. 6, 231–238 [PubMed] [Google Scholar]
- 28.McColl D. J., Chappey C., Parkin N. T., Miller M. D. (2008) Antivir. Ther. 13, 189–197 [PubMed] [Google Scholar]
- 29.Lacey S. F., Larder B. A. (1994) Antimicrob. Agents Chemother. 38, 1428–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ariyoshi K., Matsuda M., Miura H., Tateishi S., Yamada K., Sugiura W. (2003) J. Acquir. Immune Defic. Syndr. 33, 336–342 [DOI] [PubMed] [Google Scholar]
- 31.Rodes B., de Mendoza C., Rodgers M., Newell A., Jimenez V., Lopez-Brugada R. M., Soriano V. (2005) AIDS Res. Hum. Retroviruses 21, 602–607 [DOI] [PubMed] [Google Scholar]
- 32.Boretto J., Longhi S., Navarro J. M., Selmi B., Sire J., Canard B. (2001) Anal. Biochem. 292, 139–147 [DOI] [PubMed] [Google Scholar]
- 33.Matamoros T., Deval J., Guerreiro C., Mulard L., Canard B., Menéndez-Arias L. (2005) J. Mol. Biol. 349, 451–463 [DOI] [PubMed] [Google Scholar]
- 34.Matamoros T., Kim B., Menéndez-Arias L. (2008) J. Mol. Biol. 375, 1234–1248 [DOI] [PubMed] [Google Scholar]
- 35.Menéndez-Arias L., Abraha A., Quiñones-Mateu M. E., Mas A., Camarasa M. J., Arts E. J. (2001) J. Biol. Chem. 276, 27470–27479 [DOI] [PubMed] [Google Scholar]
- 36.Alvarez M., Matamoros T., Menéndez-Arias L. (2009) J. Mol. Biol. 392, 872–884 [DOI] [PubMed] [Google Scholar]
- 37.Kati W. M., Johnson K. A., Jerva L. F., Anderson K. S. (1992) J. Biol. Chem. 267, 25988–25997 [PubMed] [Google Scholar]
- 38.He K., Hasan A., Krzyzanowska B., Shaw B. R. (1998) J. Org. Chem. 63, 5769–5773 [DOI] [PubMed] [Google Scholar]
- 39.Shaw B. R., Sergueev D., He K., Porter K., Summers J., Sergueeva Z., Rait V. (2000) Methods Enzymol. 313, 226–257 [DOI] [PubMed] [Google Scholar]
- 40.Meyer P., Schneider B., Sarfati S., Deville-Bonne D., Guerreiro C., Boretto J., Janin J., Véron M., Canard B. (2000) EMBO J. 19, 3520–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mas A., Vázquez-Alvarez B. M., Domingo E., Menéndez-Arias L. (2002) J. Mol. Biol. 323, 181–197 [DOI] [PubMed] [Google Scholar]
- 42.Mas A., Parera M., Briones C., Soriano V., Martínez M. A., Domingo E., Menéndez-Arias L. (2000) EMBO J. 19, 5752–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matamoros T., Franco S., Vázquez-Alvarez B. M., Mas A., Martínez M. A., Menéndez-Arias L. (2004) J. Biol. Chem. 279, 24569–24577 [DOI] [PubMed] [Google Scholar]
- 44.Götte M., Maier G., Gross H. J., Heumann H. (1998) J. Biol. Chem. 273, 10139–10146 [DOI] [PubMed] [Google Scholar]
- 45.Marchand B., Götte M. (2003) J. Biol. Chem. 278, 35362–35372 [DOI] [PubMed] [Google Scholar]
- 46.Kellam P., Larder B. A. (1994) Antimicrob. Agents. Chemother. 38, 23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. (1988) J. Virol. Methods 20, 309–321 [DOI] [PubMed] [Google Scholar]
- 48.Meyer P. R., Matsuura S. E., Schinazi R. F., So A. G., Scott W. A. (2000) Antimicrob. Agents Chemother. 44, 3465–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarthy J. G., Williams L. D., Rich A. (1990) Biochemistry 29, 6071–6081 [DOI] [PubMed] [Google Scholar]
- 50.Marchand B., Tchesnokov E. P., Götte M. (2007) J. Biol. Chem. 282, 3337–3346 [DOI] [PubMed] [Google Scholar]
- 51.Meyer P. R., Rutvisuttinunt W., Matsuura S. E., So A. G., Scott W. A. (2007) J. Mol. Biol. 369, 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selmi B., Boretto J., Navarro J. M., Sire J., Longhi S., Guerreiro C., Mulard L., Sarfati S., Canard B. (2001) J. Biol. Chem. 276, 13965–13974 [DOI] [PubMed] [Google Scholar]
- 53.Ding J., Das K., Hsiou Y., Sarafianos S. G., Clark A. D., Jr., Jacobo-Molina A., Tantillo C., Hughes S. H., Arnold E. (1998) J. Mol. Biol. 284, 1095–1111 [DOI] [PubMed] [Google Scholar]
- 54.White K. L., Margot N. A., Ly J. K., Chen J. M., Ray A. S., Pavelko M., Wang R., McDermott M., Swaminathan S., Miller M. D. (2005) AIDS 19, 1751–1760 [DOI] [PubMed] [Google Scholar]
- 55.Selmi B., Deval J., Alvarez K., Boretto J., Sarfati S., Guerreiro C., Canard B. (2003) J. Biol. Chem. 278, 40464–40472 [DOI] [PubMed] [Google Scholar]
- 56.Arion D., Sluis-Cremer N., Parniak M. A. (2000) J. Biol. Chem. 275, 9251–9255 [DOI] [PubMed] [Google Scholar]
- 57.Meyer P. R., Matsuura S. E., Zonarich D., Chopra R. R., Pendarvis E., Bazmi H. Z., Mellors J. W., Scott W. A. (2003) J. Virol. 77, 6127–6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menéndez-Arias L., Matamoros T., Cases-González C. E. (2006) Curr. Pharm. Des. 12, 1811–1825 [DOI] [PubMed] [Google Scholar]
- 59.Villena C., Prado J. G., Puertas M. C., Martínez M. A., Clotet B., Ruiz L., Parkin N. T., Menéndez-Arias L., Martinez-Picado J. (2007) J. Virol. 81, 4713–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kisic M., Mendieta J., Puertas M. C., Parera M., Martínez M. A., Martinez-Picado J., Menéndez-Arias L. (2008) J. Mol. Biol. 382, 327–341 [DOI] [PubMed] [Google Scholar]
- 61.Tachedjian G., Mellors J., Bazmi H., Birch C., Mills J. (1996) J. Virol. 70, 7171–7181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammond J. L., Koontz D. L., Bazmi H. Z., Beadle J. R., Hostetler S. E., Kini G. D., Aldern K. A., Richman D. D., Hostetler K. Y., Mellors J. W. (2001) Antimicrob. Agents Chemother. 45, 1621–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Traut T. W. (1994) Mol. Cell. Biochem. 140, 1–22 [DOI] [PubMed] [Google Scholar]
- 64.Smith A. J., Scott W. A. (2006) Curr. Pharm. Des. 12, 1827–1841 [DOI] [PubMed] [Google Scholar]
- 65.Lin P. F., Samanta H., Rose R. E., Patick A. K., Trimble J., Bechtold C. M., Revie D. R., Khan N. C., Federici M. E., Li H., Lee A., Anderson R. E., Colonno R. J. (1994) J. Infect. Dis. 170, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 66.Pellegrin I., Izopet J., Reynes J., Denayrolles M., Montes B., Pellegrin J. L., Massip P., Puel J., Fleury H., Segondy M. (1999) AIDS 13, 1705–1709 [DOI] [PubMed] [Google Scholar]
- 67.Coakley E. P., Gillis J. M., Hammer S. M. (2000) AIDS 14, F9–F15 [DOI] [PubMed] [Google Scholar]
- 68.Larder B. A., Chesebro B., Richman D. D. (1990) Antimicrob. Agents Chemother. 34, 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petropoulos C. J., Parkin N. T., Limoli K. L., Lie Y. S., Wrin T., Huang W., Tian H., Smith D., Winslow G. A., Capon D. J., Whitcomb J. M. (2000) Antimicrob. Agents Chemother. 44, 920–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhee S. Y., Liu T., Ravela J., Gonzales M. J., Shafer R. W. (2004) Antimicrob. Agents Chemother. 48, 3122–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhee S. Y., Liu T. F., Holmes S. P., Shafer R. W. (2007) PLoS Comput. Biol. 3, e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandin E., Lindborg L., Gyllensten K., Broström C., Hagberg L., Gisslen M., Tuvesson B., Blaxhult A., Albert J. ( 2003) AIDS Res. Hum. Retroviruses 19, 543– 550 [DOI] [PubMed] [Google Scholar]
- 73.van der Ende M. E., Prins J. M., Brinkman K., Keuter M., Veenstra J., Danner S. A., Niesters H. G., Osterhaus A. D., Schutten M. (2003) AIDS 17, Suppl. 3, S55– S61 [DOI] [PubMed] [Google Scholar]
- 74.Descamps D., Damond F., Matheron S., Collin G., Campa P., Delarue S., Pueyo S., Chêne G., Brun-Vézinet F. and the French ANRS HIV-2 Cohort Study Group (2004) J. Med. Virol. 74, 197–201 [DOI] [PubMed] [Google Scholar]
- 75.Boyer P. L., Sarafianos S. G., Clark P. K., Arnold E., Hughes S. H. (2006) PLoS Pathog. 2, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]