Abstract
The mechanisms of how, following exocytosis, the approximately nine types of synaptic vesicle (SV) transmembrane proteins are accurately resorted to form SVs are poorly understood. The time course of SV endocytosis is very sensitive to perturbations in clathrin and dynamin, supporting the model that SV endocytosis occurs through a clathrin-mediated pathway. We recently demonstrated that removal of the clathrin adaptor protein AP-2, the key protein thought to coordinate cargo selection into clathrin-coated pits, results in a significant impairment in endocytosis kinetics. Endocytosis, however, still proceeds in the absence of AP-2, bringing into question the role of AP-2 in cargo sorting in this process. Using quantitative endocytosis assays at nerve terminals, we examined how endocytosis depends on the integrity of μ2 function. Our experiments indicate that no single perturbation in μ2 prevents restoration of endocytic function when mutated μ2 replaces native μ2, whereas introduction of multiple distributed mutations significantly impairs endocytosis. We also examined whether the presence of AP-2 is important for the functionality of the previously identified endocytic motif in an SV cargo protein, the dileucine motif in vGlut-1. These data show that while mutations in the dileucine motif slow the retrieval of vGlut-1, they only do so in the presence of AP-2. These data thus indicate that AP-2 plays a role in cargo selection but that no single aspect of μ2 function is critical, implying that a more distributed network of interactions supports AP-2 function in SV endocytosis.
Introduction
After neurotransmitter release, synaptic vesicle retrieval is important to sustain future rounds of exocytosis. Although several pathways of synaptic vesicle recycling have been proposed (1), the clathrin-mediated endocytic pathway has been the most intensively examined at nerve terminals (2). Among proteins that function in this pathway, AP-2 is thought to function as a major adaptor between plasma membrane, cargo proteins, and the clathrin coat (3, 4). AP-2 is composed of a heterotetrameric protein complex: two ∼100-kDa subunits α and β2, an ∼50-kDa subunit μ2, and a small ∼17-kDa subunit σ2. Each subunit interacts through a molecular network with numerous other proteins and membrane lipids. The μ2 subunit is mainly thought to have a role in cargo recognition (4, 5) as a major sorting adaptor. A number of important structural aspects of μ2 function have been elucidated in non-neuronal systems such as the tyrosine-based (YXXϕ) cargo recognition site (6, 7) and its phospho-regulation (6–10), membrane association sites, and a synaptotagmin binding site (11). At synapses, however, the role of these distinct sites in μ2 has not been explored. Here we investigated the function of the μ2 subunit by employing RNA interference and its rescue with mutants in primary cultured neurons. Previously, we made use of shRNA2 targeting the μ2 subunit (12), which resulted in efficient loss of AP-2, and showed that endocytosis kinetics are impaired in the absence of AP-2. Here we took two approaches to examine the functionality of μ2 with respect to the kinetics of endocytosis at nerve terminals. We rescued AP-2 knockdown with shRNA-resistant μ2, which harbored up to four different sets of mutations known to impair different functional regions of μ2, and we examined whether the impact of mutating a putative endocytic sorting motif in vGlut-1, a dileucine motif, depended on the presence of AP-2. Although mutations in the dileucine motif of vGlut-1 slow endocytosis, they did so only in the presence of AP-2, indicating that AP-2 is acting as a sorting adaptor at the synapse. However, mutational analysis of μ2 indicated that no one functional domain in this subunit plays a critical role in SV endocytosis, but when four different regions are mutated simultaneously, endocytosis is impaired.
EXPERIMENTAL PROCEDURES
Cell Culture and Optical Setup
1–3-day-old postnatal hippocampal CA3–CA1 regions were prepared as described previously (12). Briefly, 8 days after plating, neurons were transfected using calcium-phosphate-mediated gene transfer with up to three different plasmids, vGlut-1-pHluorin, shRNA targeting μ2, and an shRNA-resistant μ2 harboring up to seven mutations as well as an HA tag. In the majority of experiments, cell imaging was performed 14–21 days after plating (6–13 days after transfection). Images were acquired using a custom-built laser-illuminated epifluorescence microscope and an Andor iXon+ (model number DU-897E-BV) back-illuminated electron-multiplying CCD camera. Imaging conditions and solutions were as described previously (12).
Plasmids for shRNA-resistant μ2 and Cargo/Membrane Binding Mutants
shRNA against the μ2 subunit containing rat μ2 subunit cDNA target sequences of AP-2 was constructed as described previously (12). The HA-μ2 constructs resistant to shRNA μ2 or cargo/membrane binding mutants were generated using QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA). For live cell imaging, neurons are triple-transfected with pHluorin tagging of vGlut-1 (vG-pH), shRNA-μ2, and shRNA-resistant plasmid encoding μ2 or cargo/membrane mutated μ2 with 2:3:5 molar ratio.
Immunofluorescence and Quantification
Following live cell imaging, neurons were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100, blocked with 5% bovine serum albumin, and subsequently incubated with the appropriate primary antibodies (anti-green fluorescent protein (Invitrogen), anti-α adaptin (Affinity BioReagents, Golden, CO), or anti-HA tag (Covance, Emeryville, CA) antibodies). Alexa Fluor 488- or Alexa Fluor 546-conjugated secondary antibodies were applied in primary antibody incubated samples with different color combinations as needed. Immunofluorescence images of fixed cells were acquired using an epifluorescence microscope with an electron-multiplying CCD camera or a custom-built laser-scanning microscope with custom-written control software for laser-scanning microscope. In the case of the laser-scanning microscope, images were acquired with z-sectioning mode with 12 slices and 0.4-μm spacing and merged using maximum intensity. Expression levels of AP-2 were measured at cell bodies to avoid possible spatial overlap with other cells. This was compared with the fluorescence intensity in non-transfected (green fluorescent protein-negative) cell bodies.
Image and Data Analysis
Images were analyzed in ImageJ (National Institutes of Health) using the Time Series Analyzer custom-written plug-in (available from the National Institutes of Health web site) as described previously (12).
RESULTS
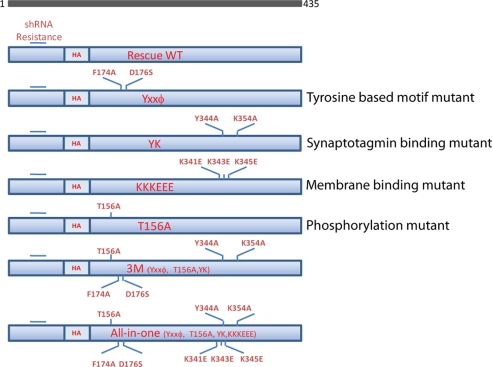
shRNA-based knockdown of AP-2 μ2 in primary cultured rat hippocampal neuron has previously allowed us to investigate the role of AP-2 in synaptic vesicle endocytosis at central nervous system synapses. Expression of shRNA targeting μ2 resulted in an ∼96% reduction in AP-2, as determined by quantitative immunocytochemistry of the α subunit (a non-shRNA targeted subunit of AP-2) that could be fully rescued by expression of shRNA-resistant μ2 subunit (12). With that in mind, we constructed cargo or membrane binding mutants of μ2 that were shRNA-resistant. As shown in Fig. 1, for the tyrosine-based motif mutant (YXXϕ), phenylalanine 174 and aspartate 176 were changed to alanine and serine, respectively. The tyrosine-based cargo recognition motif of μ2 has been shown to be controlled by a phosphorylation-dependent switch that allows for a conformational change in μ2 that is permissive for cargo recognition. Previous studies in non-neuronal cells (13, 14) have shown that mutating threonine 156 to alanine (T156A) effectively prevents cargo recognition. Synaptotagmin, one of the approximately nine SV membrane proteins, has been shown to interact with μ2 directly through two specific residues in μ2. Mutating these sites to alanine (Y334A,K335A) prevents binding (15). Finally, the interaction of μ2 with the plasma membrane is thought to depend on the presence of 3 charged lysine residues (Lys-341, Lys-343, Lys-345), which are significantly weakened when mutated to glutamate (K341E/K343E/K345E) (16). In addition to examining the effects of each of these mutations alone, we also combined them either as a triple domain mutant (3M: YXXϕ, Y334A,K335A, T156A) or as a combination of all of the above in a single construct (All-in-one). Expression of all mutants were verified by staining with anti-HA antibody (data not shown) through an engineered internal HA tag.
FIGURE 1.
Schematic diagram of μ2 cargo/membrane binding mutants. Six different shRNA-resistant μ2 variants were constructed using site-directed mutagenesis as follows. For the tyrosine recognition motif (YXXϕ) mutant (33), phenylalanine 174 and aspartate 176 were changed to alanine and serine, respectively. For the synaptotagmin binding mutant (Y334A,K335A (YK)) (15), tyrosine 344 and lysine 354 were changed to alanine. For the membrane binding mutant (K341E/K343E/K345E (KKKEEE)) (16), lysines 341, 343, and 345 were changed to glutamate. For the phosphorylation mutant (T156A) (10), threonine 156 was changed to alanine. For the collective cargo binding mutant (3M), YXXϕ, Y334A,K335A, and T156A were combined in one construct. Finally, these four single mutants were all combined in one construct (All-in-one).
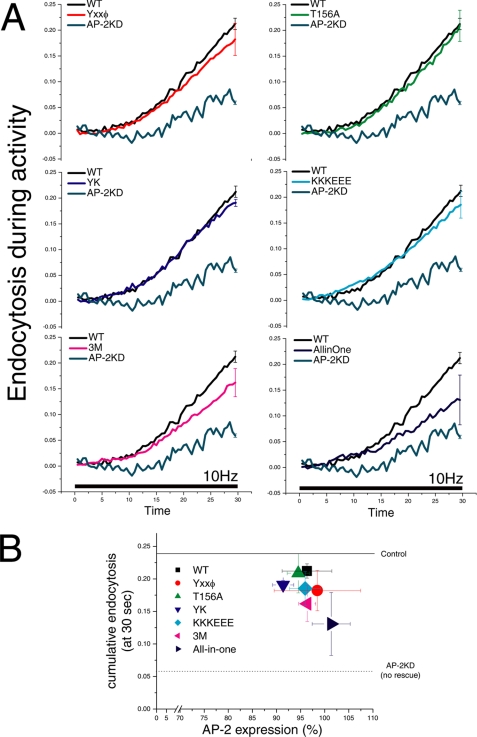
To determine how these different combinations of mutations impact endocytic function at nerve terminals, we transfected primary hippocampal neurons simultaneously with shRNA-targeting μ2, shRNA-resistant μ2 harboring the mutations mentioned above, and a pHluorin-tagged SV protein to serve as a reporter of endocytosis kinetics. We previously showed that four different SV cargo proteins examined in this fashion (vGlut-1, Synaptotagmin I, VAMP-2, and Synaptophysin) displayed identical endocytosis kinetics under normal conditions and were equally impacted by the removal of AP-2. We chose to use vGlut-1 tagged with pHluorin as it provides the highest signal-to-noise readout of the four SV proteins tested. We then examined six different quantitative aspects of function using this functional optical readout for these different mutants in the absence of native μ2: steady-state surface accumulation, activity-dependent surface accumulation, the kinetics of exocytosis, the kinetics of endocytosis during activity, the kinetics of endocytosis following activity, and the relative size of the recycling vesicle pool. All mutants restored expression of AP-2 to similar levels as native μ2, as judged by comparing quantitative α-adaptin immunostaining of transfected versus non-transfected cells (see Fig. 4B).
FIGURE 4.
Endocytosis during stimulation in single μ2 domain mutants shows only subtle defects that are more severe when combined. Endocytosis during stimulation was determined by comparing the fluorescence signal with and without bafilomycin during stimulation. The difference between these curves is a measure of the ongoing endocytosis during the stimulus period. A, the cumulative endocytosis at the end of the stimulus expressed as the fraction of the recycling pool for the different constructs were as follows: WT 21 ± 1% (n = 5); YXXϕ 18 ± 3% (n = 12); T156A 20 ± 3%, (n = 3); Y334A,K335A (YK) 19 ± 1% (n = 5); K341E/K343E/K345E (KKKEEE) 18 ± 3% (n = 11), 3M 16 ± 3% (n = 7), All-in-one 13 ± 4% (n = 7). Error bars indicate S.E. B, comparison of the level of AP-2 expression with endocytosis during stimulation for each of the mutants. After live cell imaging, neurons with μ2 mutants transfected were fixed and retrospectively stained with anti-α-adaptin antibody. The degree of AP-2 quantified is with respect to that of non-transfected cells. The solid line represents endocytosis during stimulation in cells without shRNA treatment (control ∼24%), and the dotted line indicates that in an shRNA-treated cell without rescue AP-2KD neuron (∼6%) (12).
Surface Accumulation of vG-pH at Rest and during Action Potential Firing
pHluorin, a variant of green fluorescent protein whose fluorescence is quenched upon proton binding (pKa ∼7.1), can be targeted to the lumen of synaptic vesicles by fusion to different SV proteins. At rest, the acidic pH of SVs results in very low fluorescence, and following exocytosis, each pHluorin undergoing a pH change to ∼7.4 increases its fluorescence ∼20-fold. After endocytosis, SVs are reacidified in ∼3–4 s (17). We and others previously exploited pHluorin-tagged synaptic vesicles to trace synaptic vesicle recycling in primary cultured hippocampal neurons (18–22) as the fluorescence reflects the balance of exocytosis and endocytosis. vG-pH proved to be advantageous as it has the lowest steady-state surface fraction of all the proteins tested when expressed in neurons (22). Previously, we showed that in the absence of AP-2, the resting surface fraction of vG-pH becomes elevated, and this accumulation was successfully rescued by expressing shRNA-resistant μ2 (12). The resting surface fraction is determined by examining the -fold change in fluorescence upon rapid alkalization by NH4Cl treatment (23). These experiments showed that the resting surface fraction is not significantly different in rescue experiments when domain mutants are compared with WT (rescue WT, 4.6 ± 1.2%, n = 9; YXXϕ, 5.3 ± 0.9%, n = 15; T156A, 5.0 ± 2.4%, n = 6; Y334A,K335A, 4.5 ± 0.4%, n = 9; K341E/K343E/K345E, 4.8 ± 1.3%, n = 6). However, when multiple mutations are introduced, the ability to rescue the phenotype is reduced (3M, 6.4 ± 2.2%, n = 7; All-in-one, 8.3 ± 0.8%, n = 6) (Fig. 2A). Similar results were obtained when examining the balance of endocytosis and exocytosis during periods of action potential firing. Four different stimulations were applied (25, 50, 100, and 300 action potentials at 10 Hz), and the fluorescence change resulting from stimulation, normalized to the maximum possible fluorescence change obtained from stimulation in the presence of bafilomycin (see below), is reported. These experiments showed that each single mutant was barely different from WT (Fig. 2B), but the All-in-one mutant has higher accumulation than WT (Fig. 2B) under all stimulus conditions.
FIGURE 2.
Surface fraction of vG-pH in the presence of μ2 mutations. A, the -fold change in vG-pH fluorescence at presynaptic boutons in the presence of different μ2 variants was measured during NH4Cl application to determine the resting surface fraction. The surface fractions at rest were not significantly different in individual domain mutants when compared with WT (rescue WT, 4.6 ± 1.2%, n = 9; YXXϕ, 5.3 ± 0.9%, n = 15; T156A, 5.0 ± 2.4%, n = 6; Y334A,K335A (YK), 4.5 ± 0.4%, n = 9; K341E/K343E/K345E (KKKEEE), 4.8 ± 1.3%, n = 6). Combined mutants (3M and All-in-one) revealed a gradual increase in surface fraction (3M, 6.4 ± 2.2%, n = 7; All-in-one, 8.3 ± 0.8%, n = 6) *, p < 0.05. The solid line indicates the surface fraction of cells without shRNA treatment (control 3.4%), and the dotted line indicates the surface fraction of AP-2KD cell without rescue (13.0%) (12). Error bars indicate S.E. B, surface accumulation of vG-pH was measured at the end of four different stimulation protocols (25, 50, 100, and 300 action potentials) in the presence of the μ2 mutants. Neurons expressing μ2 with single domain mutants showed similar activity-dependent accumulation of vG-pH to WT. However, neurons expressing the All-in-one mutant showed higher accumulation (300 action potentials, rescue WT, 36.3 ± 4.5%, n = 8; YXXϕ, 37.7 ± 4.8%, n = 8; T156A, 37.23 ± 4.4%, n = 15; Y334A,K335A, 43.7 ± 6.1%, n = 11; K341E/K343E/K345E, 39.4 ± 4.3%, n = 5; 3M, 45.43 ± 7.3%, n = 4; All-in-one, 46.43 ± 6.9%, n = 10).
The Rate of Exocytosis and Vesicle Recycling Pool Size in the Presence of μ2 Mutations
We next examined whether mutants of μ2 for cargo/membrane recognition affect the kinetics of exocytosis or size of vesicle recycling pool. To test this hypothesis, we measured the kinetics of exocytosis of the recycling pool using vG-pH in response to action potential firing in the presence of the proton pump blocker bafilomycin. In the presence of bafilomycin, SV reacidification is blocked, and vG-pH fluorescence reports the kinetics of exocytosis. The maximum absolute fluorescence change is taken as a relative measure of the total recycling pool size. As shown in Fig. 3, A and B, the kinetics of exocytosis were not significantly different when comparing WT and each of the different mutants (WT, 42.3 ± 4.5 s, n = 12; YXXϕ, 45.5 ± 7.5 s, n = 14; T156A, 37.6 ± 5.0 s, n = 6; Y334A,K335A, 43.4 ± 7.2 s, n = 13; K341E/K343E/K345E, 45.3 ± 6.9 s, n = 12; 3M, 45.4 ± 11.2 s, n = 7; All-in-one, 42.0 ± 7.0 s, n = 5). The apparent recycling vesicle pool size was also insensitive to mutations in μ2 (Fig. 3C; YXXϕ, 92.2 ± 9.7%, n = 12; T156A, 94.1 ± 16.4%, n = 12; Y334A,K335A, 90.2 ± 8.2%, n = 12; K341E/K343E/K345E, 94.7 ± 9.9%, n = 11) with the exception of the fully mutated form, which showed a modest reduction in pool size (3M, 81.9 ± 15.5%, n = 6; All-in-one, 71.9 ± 11.1%, n = 8).
FIGURE 3.
The rate of exocytosis and the size of recycling vesicle pool in μ2 mutant-expressing neurons. A, representative trace of exocytosis of vG-pH in μ2 mutant-expressing neurons. Neurons were triple-transfected with vG-pH, shRNA μ2, and rescue of each mutant and stimulated (1200 action potentials at 10 Hz) to trigger exocytosis of the entire recycling vesicle in the presence of bafilomycin (1 μm). YK, Y334A,K335A; KKKEEE, K341E/K343E/K345E. Error bars indicate S.E. B, the rate of exocytosis was determined from a single exponential fit. Time constants for exocytosis are not significantly different across the different mutants (WT, 42.3 ± 4.5 s, n = 12; YXXϕ, 45.5 ± 7.5 s, n = 14; T156A, 37.6 ± 5.0 s, n = 6; Y334A,K335A, 43.4 ± 7.2 s, n = 13; K341E/K343E/K345E, 45.3 ± 6.9 s, n = 12; 3M, 45.4 ± 11.2 s, n = 7; All-in-one, 42.0 ± 7.0 s, n = 5). C, the relative size of the recycling pool in transfected neurons was determined by measuring the maximum intensity of the change in vG-pH fluorescence during prolonged stimulation in the presence of bafilomycin. All cells were triple-transfected with same DNA mixture condition. Relative pool size to WT was determined as follows: YXXϕ, 92.2 ± 9.7%, n = 12; T156A, 94.1 ± 16.4 s, n = 12; Y334A,K335A, 90.2 ± 8.2 s, n = 12; K341E/K343E/K345E, 94.7 ± 9.9 s, n = 11; 3M, 81.9 ± 15.5 s, n = 6; All-in-one, 71.9 ± 11.1 s, n = 8) *, p <0.05. The solid line indicates the SV recycling pool of AP-2KD (12).
Endocytosis during Activity with μ2 Mutants
We previously showed that during ongoing action potential firing, endocytosis can be specifically impaired in the absence of dynamin-1 (24), suggesting that elevations in intracellular calcium may act more directly on some aspects of the endocytic machinery. We therefore examined the kinetics of endocytosis during stimulation for the different μ2 mutants. We determined endocytosis kinetics during activity by comparing the change in fluorescence during action potential stimulation with and without bafilomycin (25). These data allow one to determine what fraction of the total recycling pool undergoes endocytosis during stimulation. We previously showed that in the absence of AP-2, endocytosis during activity is profoundly suppressed (∼6% at 30 s when compared with ∼24% in WT at this time point) but could be almost fully rescued by reintroducing shRNA-resistant μ2. Here we examined the kinetics of endocytosis during 30 s of stimulation at 10 Hz for μ2 harboring different mutations (Fig. 4A). Similar to the effect seen on resting surface fraction and activity-driven accumulation, we found that no single domain mutant significantly impacted the ability of μ2 to restore function when compared with WT (WT, 21 ± 1%, n = 5; YXXϕ, 18 ± 3%, n = 12; T156A, 20 ± 3%, n = 3; Y334A,K335A, 19 ± 1%, n = 5; K341E/K343E/K345E, 18 ± 3%, n = 11); however, both the 3M and the All-in-one combined mutant were impaired (3M, 16 ± 3% n = 7; All-in-one, 13 ± 4%, n = 7). To determine whether these results were correlated with AP-2 expression level for each mutant, we measured the expression level of AP-2 complex by retrospective quantitative immunofluorescence using anti-α-adaptin. As shown in Fig. 4B, all of the mutants examined express to a similar extent and are within ∼10% of the levels obtained in non-transfected cells. Thus, the impairment of μ2 function when harboring these mutations does not result from the inability of μ2 to express or AP-2 to assemble.
Endocytosis after Stimulation with μ2 Mutants
We previously showed (12) that in the absence of AP-2, the kinetics of endocytosis after periods of activity were significantly impacted for all four SV proteins examined (vGlut-1, Synaptotagmin I, VAMP-2, and Synaptophysin). These studies also revealed an AP-1- and brefeldin A-sensitive compensation that resulted in a multiexponential decay in pHluorin fluorescence with the appearance of a faster and slower than normal component of endocytosis. Removal of AP-1 or brefeldin A treatment restored single exponential behavior in the absence of AP-2. Expression of each of the mutant μ2 constructs also resulted in single exponential decays of vG-pH. The kinetics of decay were very consistent with the results described in the previous figures. Single domain mutants of μ2 only modestly slow endocytosis when compared with WT rescue (Fig. 5A; WT, 18.1 ± 1.2 s, n = 9; YXXϕ, 20.1 ± 1.0 s, n = 12; T156A, 22.3 ± 0.8 s, n = 16; Y334A,K335A, 19.7 ± 1.0 s, n = 19; K341E/K343E/K345E, 21.4 ± 0.8 s, n = 15), whereas combined mutants (3M and All-in-one) slow endocytosis more significantly (3M, 24.7 ± 2.3 s, n = 14; All-in-one, 33.8 ± 2.6 s, n = 14). Analysis of single bouton behavior was consistent with these ensemble-average results (Fig. 5A, insets). We further examined whether different mutations might manifest different behavior under different stimulation conditions; however, endocytosis following a range of stimuli (25, 50, 100, and 300 action potentials) showed little variation in kinetics (Fig. 5B). Collectively, these results suggest that multiple connections between AP-2 and cargo/membrane are required in the process of synaptic vesicle endocytosis. It is possible that specific domains of μ2 might only be critical in mediating selection and endocytosis of specific cargo molecules. We therefore examined whether or not retrieval of Synaptotagmin I itself would be specifically sensitive to mutations in μ2, and in particular, ones that weaken Synaptotagmin I binding. Analysis of the kinetics of endocytosis of tagmin-pHluorin (pHluorin-tagged Synaptotagmin I) in the presence of the Y334A,K335A variant of μ2 showed that it has the same sensitivity as vG-pH to this mutation. The endocytosis time constants were also similar for the other single domain μ2 mutants when probed with tagmin-pHluorin when compared with vG-pH (supplemental Fig. S1).
FIGURE 5.
Endocytosis following stimulation in single μ2 domain mutants shows only subtle defects that are more severe when combined. A, representative ensemble average traces of endocytosis from neurons expressing either WT or various μ2 mutants. Post-stimulus vG-pH fluorescence decays were fit to single exponential decays. Individual domain mutants slow endocytosis only marginally (rescue WT, 18.1 ± 1.2 s, n = 9; YXXϕ, 20.1 ± 1.0 s, n = 12; T156A, 22.3 ± 0.8 s, n = 16; Y334A,K335A (YK), 19.7 ± 1.0 s, n = 19; K341E/K343E/K345E (KKKEEE), 21.4 ± 0.8 s, n = 15), whereas combined mutants slow endocytosis more significantly (3M, 24.7 ± 2.3 s, n = 14; All-in-one, 33.8 ± 2.6 s, n = 14). Inset, the distribution of single exponential time constants for individual bouton analysis from rescue WT or mutants derived from between 139 and 324 boutons. B, time constants of endocytosis after stimulation for different number of stimuli (25, 50, 100, and 300 action potentials at 10 Hz, respectively, n = 8–18 cells) in μ2 mutant-expressing neurons. Error bars indicate S.E. C, comparison of post-stimulus endocytosis time constant with degree of AP-2 expression for each rescue construct used. After live cell imaging, cells were fixed and labeled with anti-α-adaptin antibody and quantified as in Fig. 4. The solid line indicates the time constant of cells that did not receive shRNA (control) (∼15 s), and the dotted line shows the time constant of AP-2KD without rescue (∼42 s) from (12).
The Dileucine Motif of vGlut-1 Requires AP-2 for Function
Recently a dileucine-based motif [DE]XXXL[LI] (5, 19) in vGlut-1 was identified (19). This sorting motif has been shown to be critical for endocytosis of specific cargo proteins in a number of non-neuronal systems (e.g. Nef and CD4) (26, 27) as has been shown to operate via AP-2 through interaction with the σ2 and α subunit of AP-2 (28). Mutating the dileucine to dialanine (vG-AA-pH) slows endocytosis (19). Given that complete removal of AP-2 leaves functional, albeit slow endocytosis, we sought to determine whether the dileucine in vGlut-1 was interacting via AP-2 in its influence on endocytosis at synapses. We compared the impact of expressing vG-AA-pH in control nerve terminals with that of expressing this mutant in nerve terminals in which AP-2 was removed by knockdown. When expressed in WT synapses, vG-AA-pH shows slowed endocytosis (τ = 24.7 ± 5.4 s) when compared with vG-pH (τ = 15.6 ± 1.2 s), consistent with previously reported results (19) (Fig. 6, B and C). When vG-AA-pH is expressed in the absence of AP-2, however, the resulting kinetics are no different from when measured with vG-pH. In the absence of AP-2, vG-AA-pH shows two components of endocytosis (Fig. 6B, middle) whose time constants are indistinguishable from those obtained with vG-pH in the absence of AP-2 (τfast = 12.1 ± 1.6 s, τslow = 89.2 ± 19.4 s,τsingle = 38.0 ± 1.6 s). Furthermore, treatment with brefeldin A also resulted in single exponential decay, similar to AP-2KD neurons probed with vG-pH. When vG-AA-pH is combined with the All-in-one mutant of μ2, endocytosis slows somewhat when compared with the same mutant probed with vG-pH (τ = 38.4 ± 3.8 s) and displays monoexponential decay (Fig. 6, B and C). Taken together, these results indicate that endocytic function conveyed by the dileucine in vGlut-1 requires the AP-2 adaptor complex.
FIGURE 6.
Functional interaction between the vGlut-1 dileucine motif and AP-2. A, resting surface fraction of vG-AA-pH derived from the fluorescence response to NH4Cl. The -fold change in fluorescence was measured in shRNA-treated and untreated vG-AA-pH-transfected neurons. Resting surface fractions in vG-AA-pH-expressing neurons were significantly different from control (vG-AA-pH 6.5 ± 1.3%, n = 3). In the absence of AP-2 (shRNA without rescue), the surface fraction of vG-AA-pH (13.7 ± 1.7%, n = 3) is similar to that in vG-pH. In the presence of the All-in-one mutant, the surface fraction becomes elevated when probed with vG-AA-pH (All-in-one; 9.6 ± 2.4%, n = 4). The solid line indicates the surface fraction of non-shRNA (control) cells (3.4%), and the dotted line indicates the surface fraction of AP-2KD cell with shRNA rescue (13.0%) (12). Error bars indicate S.E. B, representative ensemble average traces of endocytosis from vG-AA-pH in control and μ2 knockdown and All-in-one mutant μ2 rescued neurons. Insets, the trace is displayed as semi-log plot to describe the double component in the case where no μ2 is present. C, ensemble average time constant of endocytosis from vG-AA-pH experiments. The colored dashed lines represent the value of the fast (blue) and slow (red) time constants that appear in the absence of AP-2 when probed with vG-pH, and the dotted line represents the value of the time constant of the single component exponential decay that results when AP-1 compensation is eliminated in the absence of AP-2 by brefeldin A (BFA) treatment (12). The results were as follows: control, 15.6 ± 1.2 s, n = 6; vG-AA-pH only, 24.7 ± 5.4 s, n = 4; vG-AA-pH with All-in-one, 38.4 ± 3.8 s; vG-AA-pH with no rescue in AP-2KD, τsingle = 38.0 ± 1.6 s, τfast = 12.1 ± 1.6 s, τslow = 89.2 ± 19.4 s, n = 5; no rescue in AP-2KD treated with brefeldin A (10 mg/ml), 39.2 ± 2.4 s, n = 5.
Endocytosis Capacity in μ2 Mutants
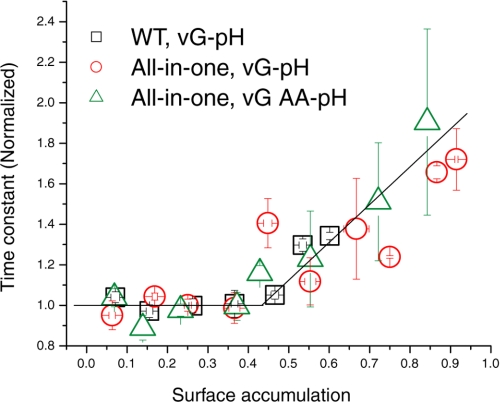
We recently showed that the time course of endocytosis is largely invariant with respect to the number of vesicles being endocytosed at each synapse provided that the total number of vesicles does not exceed some critical value (29). We termed this critical value the “endocytic capacity.” When the number of synaptic vesicles awaiting endocytosis exceeds this value, endocytosis begins to slow. When the number of synaptic vesicles needing to be endocytosed is below this value, they appear to become internalized with a fixed time constant. Presumably the ability to assemble sufficient endocytic machinery becomes limiting as the endocytic load increases above a critical value. From this previous study, a simple question that arises is whether this endocytic capacity is impacted by the presence of mutations that specifically disrupt AP-2 function. To test this hypothesis, we examined how endocytosis varied as a function of the total accumulation of the synaptic vesicle pool on the surface by varying the total number of stimuli driving exocytosis in synapses where AP-2 function has been disrupted. The net fluorescence change at the end of given stimulation reflects the accumulation of vG-pH on axonal surface. The degree of accumulation was normalized to the total recycling pool size obtained from maximal stimulation in bafilomycin. The time constants were normalized to the mean value of the invariant region (i.e. for the region where endocytosis appears invariant). As shown in Fig. 7, the endocytic capacity in μ2-depleted neurons rescued with WT is very similar to that previously reported for non-shRNA-treated neurons where the time constant remained invariant for accumulations up to ∼40% of the total recycling pool (Fig. 7) (29). Although the All-in-one mutant μ2 as well as the vG-AA-pH both allowed much higher accumulation of SV proteins during stimulation (consistent with the slowed endocytosis), the endocytic capacity remained similar to WT. Thus, we conclude that impairment of AP-2 function does not impact the endocytic capacity, only the time constant with which synaptic vesicles can be internalized when the endocytic capacity is not exceeded.
FIGURE 7.
Endocytic capacity is not impacted by mutations in μ2. Time constants for WT μ2 rescue (n = 12), All-in-one mutant μ2 rescue (n = 9), and All-in-one mutant μ2 rescue probed with vG-AA-pH (n = 5) were measured as a function of surface accumulation. Plots of decay constants were obtained using various stimulus conditions (2.5–50 s) at 10 Hz. The time constant is normalized with the average time constant in the non-saturating region. (for rescue WT, 18.6 ± 0.8 s; for All-in-one, 32.7 ± 1.6 s; for All-in-one with vG-AA-pH, 38.0 ± 2.9 s). The decay constants start to increase after ∼40% for each neuron. Error bars indicate S.E.
DISCUSSION
The presynaptic terminal of the synapse is one of the most specialized subcellular units for membrane trafficking, coupling exocytosis and endocytosis to sustain neuronal communication. A proteomic analysis of SVs identified approximately nine different types of SV membrane proteins (cargo proteins) that must be retrieved during the reformation of SV in the synaptic vesicle cycle (30). Although in general the evidence indicating that vesicle recycling is a clathrin-, dynamin-, and AP-2-dependent process is very strong, the means by which different SV cargo proteins are sorted into vesicles with precise stoichiometry is unknown. In clathrin-mediated endocytosis, AP-2 is a key adaptor protein linking coat formation (clathrin) and cargo selection at the plasma membrane and additionally appears to interact with many components of the endocytic machinery, suggesting that it acts as a “molecular hub” (31). We previously showed that removal of AP-2 through knockdown of μ2 leads to a significant slowing, but not the elimination of endocytosis following exocytosis at nerve terminals (12). Here we took advantage of the ability to replace native μ2 with variants harboring mutations to examine whether previously identified aspects of μ2 function are critical in allowing μ2 to operate at the synapse. We targeted four known functional domains in μ2: the tyrosine motif recognition site, the Synaptotagmin binding site, the phosphorylation site that coordinates cargo recognition via tyrosine-based motifs, and a set of lysines that promotes membrane association. Quantitative analysis of endocytosis kinetics in the presence of these mutations indicates that no single functional site is critical in the process of endocytosis but that each instead acts in a graded fashion when combined. It is possible that use of a tyrosine-based motif might be more important in a different SV cargo protein than the one tested here; however, if they do exist, our results imply that they do not act in a dominant fashion for controlling the endocytosis of either vGlut-1 or Synaptotagmin I. Collectively, these results suggest that AP-2, and in particular μ2, functions through many weak interactions in controlling endocytosis of SVs at nerve terminals, rendering it fairly robust to possible perturbations. The question of how the accurate sorting of SV membrane proteins is accomplished remains open. The distributed nature of μ2 functional interactions in SV endocytosis and the absence of a severe endocytic phenotype in the absence of AP-2 both suggest that SV endocytosis is likely driven through the action of additional adaptors, such as the protein stonin2 (32).
Acknowledgments
The vGlut-pHluorin and vG-AA-pHluorin constructs were kindly provided by Susan Voglmaier and Robert Edwards (University of California, San Francisco), and Synaptotagmin- pHluorin constructs were kindly provided by James Rothman (Yale University). HA-labeled μ2 was kindly provided by Alexander Sorkin (University of Colorado). We thank the members of the Ryan laboratory as well as Jeremy Dittman for useful discussions and Ricky Kwan for excellent technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant 36942 (to T. A. R.).
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- shRNA
- short hairpin RNA
- SV
- synaptic vesicle
- HA
- hemagglutinin
- WT
- wild type
- AP2-KD
- AP-2 knockdown
- vG-pH
- vGlut-1 tagged with pHluorin.
REFERENCES
- 1.Wu L. G., Ryan T. A., Lagnado L. (2007) J. Neurosci. 27, 11793–11802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slepnev V. I., De Camilli P. (2000) Nat. Rev. Neurosci. 1, 161–172 [DOI] [PubMed] [Google Scholar]
- 3.Robinson M. S. (2004) Trends Cell Biol. 14, 167–174 [DOI] [PubMed] [Google Scholar]
- 4.Schmid S. L. (1997) Annu. Rev. Biochem. 66, 511–548 [DOI] [PubMed] [Google Scholar]
- 5.Bonifacino J. S., Traub L. M. (2003) Annu. Rev. Biochem. 72, 395–447 [DOI] [PubMed] [Google Scholar]
- 6.Rapoport I., Miyazaki M., Boll W., Duckworth B., Cantley L. C., Shoelson S., Kirchhausen T. (1997) EMBO J. 16, 2240–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen D. J., Evans P. R. (1998) Science 282, 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conner S. D., Schmid S. L. (2003) J. Cell Biol. 162, 773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conner S. D., Schmid S. L. (2002) J. Cell Biol. 156, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricotta D., Conner S. D., Schmid S. L., von Figura K., Honing S. (2002) J. Cell Biol. 156, 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haucke V., De Camilli P. (1999) Science 285, 1268–1271 [DOI] [PubMed] [Google Scholar]
- 12.Kim S. H., Ryan T. A. (2009) J. Neurosci. 29, 3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olusanya O., Andrews P. D., Swedlow J. R., Smythe E. (2001) Curr. Biol. 11, 896–900 [DOI] [PubMed] [Google Scholar]
- 14.Motley A. M., Berg N., Taylor M. J., Sahlender D. A., Hirst J., Owen D. J., Robinson M. S. (2006) Mol. Biol. Cell 17, 5298–5308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haucke V., Wenk M. R., Chapman E. R., Farsad K., De Camilli P. (2000) EMBO J. 19, 6011–6019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höning S., Ricotta D., Krauss M., Späte K., Spolaore B., Motley A., Robinson M., Robinson C., Haucke V., Owen D. J. (2005) Mol. Cell 18, 519–531 [DOI] [PubMed] [Google Scholar]
- 17.Atluri P. P., Ryan T. A. (2006) J. Neurosci. 26, 2313–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granseth B., Odermatt B., Royle S. J., Lagnado L. (2006) Neuron 51, 773–786 [DOI] [PubMed] [Google Scholar]
- 19.Voglmaier S. M., Kam K., Yang H., Fortin D. L., Hua Z., Nicoll R. A., Edwards R. H. (2006) Neuron 51, 71–84 [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Alfonso T., Kwan R., Ryan T. A. (2006) Neuron 51, 179–186 [DOI] [PubMed] [Google Scholar]
- 21.Sankaranarayanan S., Ryan T. A. (2000) Nat. Cell Biol. 2, 197–204 [DOI] [PubMed] [Google Scholar]
- 22.Balaji J., Ryan T. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 20576–20581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sankaranarayanan S., De Angelis D., Rothman J. E., Ryan T. A. (2000) Biophys. J. 79, 2199–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson S. M., Brasnjo G., Hayashi M., Wölfel M., Collesi C., Giovedi S., Raimondi A., Gong L. W., Ariel P., Paradise S., O'Toole E., Flavell R., Cremona O., Miesenböck G., Ryan T. A., De Camilli P. (2007) Science 316, 570–574 [DOI] [PubMed] [Google Scholar]
- 25.Schweizer F. E., Ryan T. A. (2006) Curr. Opin. Neurobiol. 16, 298–304 [DOI] [PubMed] [Google Scholar]
- 26.Aiken C., Konner J., Landau N. R., Lenburg M. E., Trono D. (1994) Cell 76, 853–864 [DOI] [PubMed] [Google Scholar]
- 27.Byland R., Vance P. J., Hoxie J. A., Marsh M. (2007) Mol. Biol. Cell 18, 414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly B. T., McCoy A. J., Späte K., Miller S. E., Evans P. R., Höning S., Owen D. J. (2008) Nature 456, 976–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balaji J., Armbruster M., Ryan T. A. (2008) J. Neurosci. 28, 6742–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takamori S., Holt M., Stenius K., Lemke E. A., Grønborg M., Riedel D., Urlaub H., Schenck S., Brügger B., Ringler P., Müller S. A., Rammner B., Gräter F., Hub J. S., De Groot B. L., Mieskes G., Moriyama Y., Klingauf J., Grubmüller H., Heuser J., Wieland F., Jahn R. (2006) Cell 127, 831–846 [DOI] [PubMed] [Google Scholar]
- 31.Schmid E. M., McMahon H. T. (2007) Nature 448, 883–888 [DOI] [PubMed] [Google Scholar]
- 32.Jung N., Wienisch M., Gu M., Rand J. B., Müller S. L., Krause G., Jorgensen E. M., Klingauf J., Haucke V. (2007) J. Cell Biol. 179, 1497–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins B. M., McCoy A. J., Kent H. M., Evans P. R., Owen D. J. (2002) Cell 109, 523–535 [DOI] [PubMed] [Google Scholar]