Abstract
Mycobacterium tuberculosis utilizes many mechanisms to establish itself within the macrophage, and bacterially derived cAMP is important in modulating the host cellular response. Although the genome of M. tuberculosis is endowed with a number of mammalian-like adenylyl cyclases, only a single cAMP phosphodiesterase has been identified that can decrease levels of cAMP produced by the bacterium. We present the crystal structure of the full-length and sole cAMP phosphodiesterase, Rv0805, found in M. tuberculosis, whose orthologs are present only in the genomes of slow growing and pathogenic mycobacteria. The dimeric core catalytic domain of Rv0805 adopts a metallophosphoesterase-fold, and the C-terminal region builds the active site and contributes to multiple substrate utilization. Localization of Rv0805 to the cell wall is dependent on its C terminus, and expression of either wild type or mutationally inactivated Rv0805 in M. smegmatis alters cell permeability to hydrophobic cytotoxic compounds. Rv0805 may therefore play a key role in the pathogenicity of mycobacteria, not only by hydrolyzing bacterial cAMP, but also by moonlighting as a protein that can alter cell wall functioning.
Introduction
Mycobacterium tuberculosis is probably one of the most successful human pathogens known so far, being singly responsible for the largest number of deaths worldwide due to an infectious disease. M. tuberculosis is phagocytosed by the macrophage and is able to subvert the defenses of the host by a number of mechanisms. These include the presence of a complex cell wall that prevents free passage of potentially toxic material, the ability of the bacteria to withstand the acidic environment of the phagolysosome, and to neutralize reactive oxygen and nitrogen species produced by the activated macrophage (1). An increased understanding of mechanisms utilized by this pathogen to evade the host immune system and continue to reside in the hostile environment of the macrophage, would no doubt provide avenues for the development of drugs to novel targets in the bacterium.
Cross-communication between the pathogen and host could involve the utilization of signaling molecules that are conserved evolutionarily. Cyclic AMP is found in all kingdoms of life, and proteins that synthesize and degrade the cyclic nucleotide have been well characterized. The genome of M. tuberculosis H37Rv encodes 16 mammalian-like nucleotide cyclase-like genes (2), and intracellular and extracellular levels of cAMP are very high in both pathogenic and non-pathogenic (e.g. Mycobacterium smegmatis) mycobacteria (2, 3). Recent evidence has highlighted the importance of cAMP in modulating the host macrophage response to M. tuberculosis infection (4). A single adenylyl cyclase was shown to be responsible for the burst of cAMP that is seen in the macrophage following phagocytosis of M. tuberculosis, and this bacterially derived increase in cAMP was essential to attenuate the response of the macrophage to the pathogen.
The regulated degradation of cAMP is as important as its synthesis, and mammalian cyclic nucleotide phosphodiesterases, classified as Class I enzymes, are the targets for a number of drugs (5). Class I-like enzymes could not be detected in mycobacterial genomes. Instead, we identified a Class III phosphodiesterase, the product of the rv0805 gene (accession NP_215320), that was capable of degrading mycobacterial cAMP (6). Indeed, overexpression of Rv0805 was used to reduce intracellular levels of cAMP in M. tuberculosis to show that bacterially derived cAMP was essential to attenuate macrophage killing (4). Rv0805 is a member of the superfamily of metallophosphoesterases (MPEs)6 that has been well characterized biochemically and structurally (7–9). MPEs contain five blocks of residues (D-(X)n-GD-(X)n-GNH(E/D)-(X)n-H-(X)n-GHXH) (10) with a conserved structural-fold, containing two metal ions in the active site. These enzymes cleave a variety of substrates including phosphomonoesters, phosphodiesters, and phosphotriesters (11), and a single enzyme can cleave multiple substrates in vitro. In some cases an unequivocal catalytic function in vivo for a particular MPE has been demonstrated. However, Vps29, a protein found in the retromer cargo-recognition complex, has a MPE-fold but no catalytic activity, and instead is used as a scaffolding protein (11–13). By and large, however, the roles of a large number of MPEs remain elusive.
Biochemical and mutational analysis followed by structural studies of the catalytic core of Rv0805 identified residues important for MPE activity and also revealed the close structural similarity between Rv0805 and other MPEs including Ser-Thr phosphatases, Mre11 nuclease, and purple acid phosphatases (14). Our more recent studies have shown that distant orthologs of Rv0805 can also be found in mammalian genomes (15). Intriguingly, the rv0805 gene is found only in pathogenic mycobacteria (15) and an ortholog is absent in M. smegmatis, a non-pathogenic soil mycobacterium, which nonetheless, shows modulation in intracellular cAMP levels. We therefore asked if cAMP was the sole substrate for Rv0805 in mycobacterial cells, and wished to explore the possibility that Rv0805 could be a multifunctional protein, necessitating its presence only in pathogenic mycobacteria. We have now determined the structure of the Rv0805 enzyme, including the C-terminal domain and investigated the functional role of conserved residues by using in vivo and in vitro assays. We demonstrate that Rv0805 can also utilize a number of phosphodiesters as substrates, and identify the crucial role of the C terminus in building the active site and aiding in localizing the enzyme to the cell envelope in mycobacteria. The structure of Rv0805 in complex with 5′-AMP and docking of substrates into the active site permitted a mutational approach to distinguish residues that are critical for cyclic nucleotide and linear substrate utilization. Finally, overexpression of wild type and mutant Rv0805 in M. smegmatis demonstrated that Rv0805 could have a role to play in altering the properties of the cell wall of mycobacteria, independent of its catalytic ability to hydrolyze cAMP, providing a novel line of investigation into hitherto unexplored mechanisms of cell wall construction in mycobacteria.
EXPERIMENTAL PROCEDURES
Protein Purification and Enzymatic Assays
Constructs to express Rv08051–318 and Rv08051–278 have been described earlier (6, 14). The H98A, H140A, H209A, and Y229A mutations were generated on the pPRO-Rv08051–318 plasmid (16). Sequences of mutagenic oligonucleotides are provided in supplemental Table 1.
Wild type and mutant proteins were prepared following addition of MnCl2 and FeCl3 (10 μm) to the bacterial culture medium (14). Assays with bis-(p-nitrophenyl)phosphate (bis-pNPP), p-nitrophenyl phenylphosphonate (pNPPP), and p-nitrophenyl phosphorylcholine (Sigma) were performed in a triple buffer system (MES, HEPES, diethanolamine, 50 mm, pH 8.5) containing 5 mm 2-mercaptoethanol and 10 mm NaCl in the presence of the specified substrate (10 mm) and 100 μm MnCl2 as metal cofactor (15). Kinetic parameters indicated in the text were calculated by fitting data using GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA). The heat stability of the proteins was measured by incubating Rv08051–318 and Rv08051–278 in assay buffer at 80 °C for the indicated times. Samples were cooled and activity measured following addition of substrate and incubation at 37 °C for 15 min. For circular dichroism experiments, proteins (30 mg/ml) were diluted in 20 mm Tris, pH 8.0, to a final concentration of 0.2 mg/ml and incubated at 80 °C. Aliquots of 200 μl were removed and CD spectra were collected at 37 °C (Chirascan CD Spectrometer, Applied Photophysics, UK).
Crystallization and Structure Determination
Rv08051–318 was expressed in the presence of MnSO4 and FeCl3 (10 μm) in the culture medium and purified as described earlier (14). Samples used for crystallization contained 15–30 mg/ml of Rv0805 protein in 20 mm Tris-HCl, pH 8.0, 50 mm NaCl, and 5 mm 2-mercaptoethanol. Crystals were prepared at 20 °C by hanging drop vapor diffusion, growing from a reservoir solution of 100 mm bis-Tris, pH 6.5 or 7.0, 0.2–0.4 m ammonium acetate, and 50–60% (v/v) 2-methyl-2,4-pentandiol. The crystallization drop size was 2 μl with equal volumes of protein:reservoir solution.
Rv08051–318 was co-crystallized with 5′-AMP by adding 5′-AMP (5 mm) to the reservoir solution. X-ray diffraction experiments were undertaken at EMBL/DESY, Hamburg, beamline BW7A, at 100 K, and wavelengths 1.008 or 0.986 Å, for apo-Rv08051–318 and 5′-AMP-Rv08051–318, respectively. Diffraction data were integrated, scaled, and merged using the HKL2000 package (17) (Table 1). The structure of full-length Rv08051–318 was solved by a combination of molecular replacement (PHASER) (18) with the structure of the catalytic core of Rv08051–278 (Protein data bank 2HY1) as a model (resolution range 32.4-2.5 Å; one clear solution with rotation function Z-score 20.9, translation function Z-score 64.2, and the log (likelihood) gain 2272), and ArpWarp (18) to build the new structural elements in Rv08051–318. In the case of a complex of Rv08051–318 with 5′-AMP, the structure of the ligand-free Rv08051–318 was used as a model by PHASER (resolution range 31.8-2.5 Å; one clear solution with rotation function Z-score 31.4, translation function Z-score 61.4, and the log(likelihood) gain 4468). Refinement was undertaken using the REFMAC suite (18) (Table 1) and manual rebuilding of the model in O (19) iteratively. Water molecules were found using ArpWarp and their positions assessed. PyMol (20) was used to prepare the figures. Over 90% of residues were within the allowed regions of the Ramachandran plot. In both structures residues Asp-125 and His-207 are in the disallowed regions, as seen in the structure of the catalytic core Rv08051–278 (14). Moreover, several mostly polar side chains, distant from the active site, have alternative conformations. Values for occupancies for these residues were estimated by varying them during the refinement. In the deposited PDB files, only the conformation with a higher occupancy for each of these residues is included. Occupancy of acetate ion or 5′-AMP bound to the active site metal ions was estimated to be 0.8.
TABLE 1.
Data collection and refinement statistics
Each structure was determined using diffraction data by one crystal.
| Rv08051–318 | 5′-AMP-Rv08051–318 | |
|---|---|---|
| Data collection | ||
| Space group | P41212 | P41212 |
| Cell dimensions | ||
| a, b, c (Å) | 100.18, 100.18, 80.25 | 100.40, 100.40, 80.80 |
| Resolution (Å) | 50-1.60 (1.64-1.60)a | 50-1.80 (1.84-1.80) |
| Rmerge | 5.7 (52.8) | 6.7 (61.9) |
| I/σI | 34.9 (2.3) | 29.5 (3.7) |
| Completeness (%) | 99.9 (99.6) | 99.9 (100.0) |
| Redundancy | 9.4 (5.0) | 8.0 (7.7) |
| Refinement | ||
| Resolution (Å) | 32.4-1.6 (1.64-1.60)a | 31.8-1.8 (1.85-1.80) |
| No. reflections (F > 0σ) | 54,293 | 38,804 |
| Rwork/Rfree | 15.9/17.7 (31.1/33.1) | 15.0/16.8 (20.4/25.7) |
| No. atomsb | ||
| Protein | 2,229 | 2,229 |
| Ligand/ion | ||
| Iron | 1 | 1 |
| Manganese | 1 | 1 |
| Bis-Tris | 14 | 14 |
| 5′-AMP (active site) | 23 | |
| Acetate (active site) | 4 | |
| MPD | 16 (2 molecules) | 24 (3 molecules) |
| Acetate | 4 | 4 |
| Water | 292 | 247 |
| B-factors | ||
| Protein | 24.3 | 26.5 |
| Ligand/ion | ||
| Iron | 14.3 | 17.0 |
| Manganese | 14.4 | 16.7 |
| Bis-Tris | 20.7 | 23.9 |
| 5′-AMP (active site) | 29.2 | |
| Acetate (active site) | 16.1 | |
| MPD | 42.1 | 55.9 |
| Acetate | 54.9 | 64.7 |
| Water | 33.9 | 34.8 |
| Wilson B-factor | 26.6 | 29.1 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.008 | 0.009 |
| Bond angles (°) | 1.16 | 1.24 |
a Values in parentheses are for highest-resolution shell.
b Values for asymmetric unit, containing one monomer of the dimer. The dimer is generated using symmetry operation Y, X, −Z.
Docking of Substrates
The two-dimensional sketches of bis-pNPP, 3′,5′-cAMP, and 2′,3′-cAMP were created using ISIS draw (MDL Information Systems, Inc.), and imported into VIDA (OpenEye Scientific Software) to create SMILES records (21), which were the starting inputs for the LigPrep workflow. The three-dimensional coordinates of 10 conformers for each ligand were generated and the structure of the Rv08051–318 dimer was subjected to the “protein preparation” wizard of the Schrödinger Maestro (version 8.5, Schrödinger, LLC) with default values. All crystallographically observed solvent molecules were removed keeping only Mn2+ and Fe3+. The Receptor-Grid generation module of Glide was invoked to create the docking grid corresponding to the vicinity of 5′-AMP coordinates, incorporating residue information from both Rv0805 protomers. Flexible docking within the grid of all the conformers of the ligands was carried out using the SP option of Glide module. During energy minimization, the ligand atoms were allowed to move, whereas the protein atoms were constrained to their prepared geometry. At least 10 energy optimized poses along with their Glide score were written for each of the ligands.
Expression of Rv08051–318 and Rv08051–278 in M. smegmatis
The Rv08051–318 and Rv08051–278 coding sequences were amplified as described earlier (6), and cloned into pMV10–25 (22), to allow expression of Rv0805 under the mycobacterial hsp60 gene promoter. A control vector (pMV vector control) was generated by digesting pMV10–25 with NheI and XbaI and religating the vector backbone. Plasmids expressing mutant Rv08051–318 proteins were generated by digesting the respective pPRO-Rv08051–318 plasmids with NcoI and XbaI, isolating the ∼900-bp fragment containing the mutation, and replacing the fragment released from similarly digested pMV-Rv0805 1–318 with the fragment containing the mutation. Plasmids were electroporated in M. smegmatis and cultured as described earlier (23). Mid-log phase cells of M. smegmatis were harvested by centrifugation, washed with 20 mm Tris-HCl, pH 8.5, containing 0.1% Tween 20, and lysed by sonication in buffer containing 200 mm Tris-HCl, pH 8.2, containing 100 mm NaCl, 5 mm β-mercaptoethanol, 4 mm NaH2PO4, 10% glycerol, 2 mm phenylmethylsulfonyl fluoride, and 1 mm benzamidine. Subcellular fractions were prepared as described (24). Cell wall and cell membrane fractions were resuspended in lysis buffer and protein content was measured by a modified Bradford procedure (25).
Levels of intracellular and extracellular cAMP were measured as described earlier (23). Phosphodiesterases assays were performed with different subcellular fractions of M. smegmatis (50 μg of total protein in the presence of bis-pNPP (2 mm) or 2′,3′-cAMP (5 mm)).
Western Blot Analysis
Subcellular fractions prepared from M. tuberculosis H37Rv were obtained from the Colorado State University (26). Protein (50 μg) from various subcellular fractions was subjected to 12% SDS-PAGE and blotted onto a polyvinylidene difluoride membrane (Millipore). Monoclonal antibodies against Rv08051–318 were raised and culture supernatants were used at a dilution of 1:100 for blotting with chemiluminescent detection (ECL Plus, GE Healthcare).
Monitoring Cell Wall Permeability
Approximately 107 cells (based on optical density measurements) from mid-log phase cultures of each strain were spotted onto 7H10 agar plates containing 5 μg/ml of malachite green, 10 μg/ml crystal violet (MP Biomedicals), or 0.01% SDS. The plates were incubated at 37 °C for 2–3 days and photographed.
RESULTS
Promiscuity of Rv0805 in Substrate Utilization
Earlier preparations of Rv0805 resulted in mixtures of monomeric and dimeric forms, with dimerization dependent on the occupancy of the active site with the metal ions (14). Inclusion of Mn2+ and Fe3+ in the medium in which expression of recombinant Rv0805 was performed now resulted in >95% of dimeric purified Rv0805, which was enzymatically active without the addition of extra Mn2+ during the assay (data not shown). Rv0805 hydrolyzed multiple substrates (Table 2), and could cleave p-nitrophenyl phosphorylcholine efficiently (Vmax 41.7 ± 0.15 μmoles/min/mg of protein; Km 3.3 ± 0.18 mm), and thymidine 5′-monophosphate-p-nitrophenyl ester to low extents (∼5–10% of the activity seen with bis-pNPP). Cleavage of pNPPP (Table 2) with the formation of p-nitrophenol (pNP) demonstrates that a phosphorus center with only three oxygen atoms suffices for phosphoester hydrolysis. 2′,3′-cAMP was a better cyclic nucleotide substrate than 3′,5′-cAMP, with the products of hydrolysis of 3′,5′-cAMP being 5′- and 3′-AMP in a ratio of 2:1, whereas 2′,3′-cAMP was hydrolyzed to 3′- and 2′-AMP in a ratio of 4:1 (supplemental Fig. S1). A recent report has shown that 2′,3′-cGMP is also hydrolyzed by Rv0805 as efficiently as 2′,3′-cAMP (9), indicating that there may be no specific interaction of the protein with the base of the cyclic nucleotide. To appreciate the features in Rv0805 that allow this wide substrate utilization, we decided to understand the structural properties of the full-length enzyme and identify residues that are important for substrate binding.
TABLE 2.
Promiscuous substrate utilization of Rv08051–318 and Rv08051–278
Assays were performed three times in duplicate and values shown represent the mean ± S.E.
| Bis-pNPP |
pNPPP |
3′,5′-cAMP |
2′,3′-cAMP |
|||||
|---|---|---|---|---|---|---|---|---|
| Km | Vmax | Km | Vmax | Km | Vmax | Km | Vmax | |
| mm | μmol/min/mg | mm | μmol/min/mg | mm | μmol/min/mg | mm | μmol/min/mg | |
| Rv08051–318 | 0.9 ± 0.08 | 74 ± 0.8 | 0.9 ± 0.1 | 112.7 ± 5 | 5.5 ± 1 | 1.1 ± 0.1 | 1 ± 0.03 | 14.7 ± 0.25 |
| Rv08051–278 | 0.3 ± 0.04 | 10.2 ± 1 | 1.06 ± 0.14 | 27.8 ± 2.75 | 7.5 ± 1.26 | 1.1 ± 0.09 | 2.8 ± 0.36 | 14.2 ± 0.17 |
Structure of Rv08051–318
Newly purified full-length Rv0805 protein (residues 1–318; Rv08051–318) readily and rapidly formed proteolytically stable crystals. Mass spectrometric analysis and N-terminal amino acid sequencing of the new crystals demonstrated that the protein was of the size predicted for the Rv08051–318 construct (supplemental Fig. S2). The protein that was crystallized earlier as the Rv0805 catalytic core (14) contained residues 1–278, which we refer to here as Rv08051–278. The structure of Rv08051–278 was defined only for residues Pro10–Pro265, excluding loops Gly26–Val35 and Tyr229–Gln247 (supplemental Fig. S2).
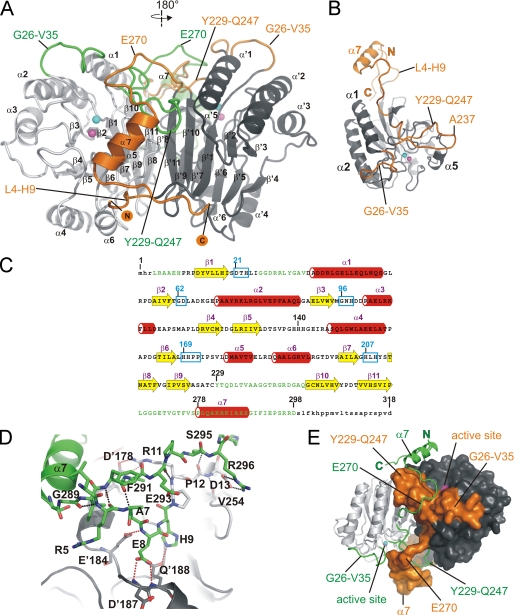
Rv08051–318 forms a homodimer, with the protomers related by a 2-fold crystallographic axis (Fig. 1A). Electron density was well defined for residues Leu4–Asp298, which now reveal several new structural features not previously seen in the structure of the catalytic core. These include a short stretch of residues (Leu4–His9) at the N terminus as well as loops Gly26–Val35 and Tyr229–Gln247 (Fig. 1, A–C). The solvent-exposed stretch of residues Leu4–Asp13 runs almost parallel and in the same direction as the C-terminal residues of the same protomer (Gly289–Asp298) with several hydrogen bonds between the residues of these two regions (Fig. 1D). The N terminus is also held in place by polar interactions to the residues of the other protomer (Fig. 1D). Two additional loops that are now defined in Rv08051–318 are stabilized by the presence of C-terminal residues Leu266–Asp298 of the other protomer (Fig. 1, A and E). The lack of ordered structure for residues beyond Asp298 in Rv08051–318 is either due to the absence of stabilization of these residues by other parts of the dimer (Fig. 1, A and D) or the absence of contacts with symmetry related molecules in the crystal.
FIGURE 1.
Crystal structure of the Rv08051–318 homodimer. A, ribbon representation of the Rv08051–318 dimer. The MPE catalytic core is colored light gray (left-side protomer) or dark gray (right-side protomer). New structural elements are colored orange (attached to the dark gray catalytic core) or green (attached to the light gray catalytic core). N and C termini of the dark gray/orange protomer are marked. α-Helices and β-sheets are indicated, dark gray/orange protomer with a prime. Active site metals ions are shown as spheres: Fe3+, cyan; Mn2+, magenta. B, Rv08051–318 monomer: catalytic core, dark gray; new structural elements, orange. Active site metals are shown as in A. C, secondary structure elements of Rv08051–318 monomer superimposed onto the amino acid sequence (β-strands, yellow arrows; α-helices, red cylinders). Newly defined parts of the Rv08051–318 structure are in green. Highly conserved MPE family regions are highlighted by cyan boxes and several residues indicated in the text are numbered. Small letters represent parts of the structure that are not defined by electron density. D, polar interactions: between N-terminal and C-terminal residues of the same protomer (black dashed lines); between N-terminal peptide of one protomer and residues of the other protomer (red dashed line). Residues of the protomer in dark gray/orange combination are marked with a prime (i.e. D′187) and the light gray/green protomer as normal (i.e. D13). E, swapped structural elements between the protomers in the Rv08051–318 dimer. Color code is the same as in A. Protomer colored dark gray/orange is shown as a surface and the light gray/green protomer as ribbon.
Intra- and inter-protomer interactions lead to swapping of structural elements between the two protomers (Fig. 1, A and D). The lasso-shaped loop Tyr229–Gln247 is sandwiched between the Gly26–Val35 loop and the hydrophobic region Val176–Val181 including helix α5 of the same protomer. Furthermore, it leans on the C-terminal part of the helix α1 and is capped by the residues of the C-terminal peptide (Leu266–Pro278), both belonging to the other protomer (Fig. 1, A and B). The loops Gly26–Val35 and Tyr229–Gln247 from one protomer form a channel, filled by the capping stretch of the C-terminal domain of the other protomer (Fig. 1E). These three structural elements in Rv08051–318 are interconnected via hydrogen bonds as well as hydrophobic interactions, and build a wall beyond the active site (Fig. 1, A, B, and E).
The C-terminal peptide is mainly built of a coil, with residues Pro278–Ser288 forming the only α helix in this domain, α7 (Fig. 1). α7 helix stacks against the hydrophobic α5 helix of the other protomer in a parallel manner, pointing in the same direction. The amino acid sequence of the C-terminal domain of Rv0805 is unique to this protein and therefore results in a fold that is distinct from that seen in other MPEs such as Mre11 (7) or GpdQ (11).
The core MPE domain of Rv08051–318 superimposes closely to Rv08051–278 (r.m.s. deviation value for Cα atoms of residues Arg40–Pro220 is 0.48 Å), but with significant shifts in particular parts of the structure. In Rv08051–318, a hydrophobic stretch of residues at the N-terminal part of the α5 helix (Ile173–Val183) is pressed down toward the active site by the α7 helix of the other protomer (supplemental Fig. S3). This new feature helps to engage Tyr210 of the same protomer in one fully occupied conformation via a hydrogen bond between the OH group of Tyr210 and OG atom of Ser175 and amide N of Val176. In the Rv08051–278 structure, the absence of interaction with Tyr210 caused it to flip between two equally occupied conformations (14). Flipping is additionally prevented in Rv08051–318 by the presence of the Tyr229–Gln247 loop of the same protomer placed above Tyr210 (supplemental Fig. S3).
Another significant difference is located in loop Val136–Gly142, at the His139 and His140 positions (supplemental Fig. S3). Although one of the reasons for this difference may be the presence of the new structural elements in Rv08051–318, this could also be caused by the molecule of bis-Tris from the crystallization solution, which binds close to these residues in Rv08051–318 (supplemental Fig. S3).
Swapped Structural Elements in Rv0805
Domain swapping in proteins occurs at positions where the protein tends to locally unfold prior to complete unfolding (27). The two structural elements that are directly involved in domain swapping in Rv08051–318 are loop Tyr229–Gln247 of one protomer and Leu266–Ile290 in the C-terminal peptide of the other protomer, including the α7 helix (Fig. 1). The N-terminal part of the α7 helix at Pro278 is positioned above the tip (residues 236AAGG239) of loop Tyr229–Gln247 of the other protomer.
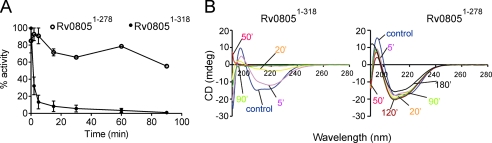
To test if additional structural elements in Rv08051–318 affect the stability of the protein in comparison to the catalytic core, we monitored the activity of both Rv0805 constructs following incubation at 80 °C. The activity of Rv08051–318 was dramatically reduced following 5 min of incubation and completely lost after 30 min (Fig. 2A). On the other hand, elevated temperatures did not significantly affect the activity of Rv08051–278. Circular dichroism showed severe changes in the secondary structure of Rv08051–318 after short incubation times, whereas the spectra of Rv08051–278 showed structural changes only after 2 h of incubation at 80 °C (Fig. 2B). Therefore, it is likely that the C terminus might play the role of a local unfolding center because the absence of this sequence in Rv08051–278 renders the protein significantly more stable than the full-length enzyme. This feature may also account for the proteolytic cleavage of Rv08051–318 at Pro278 into Rv08051–278 seen in our earlier preparations of recombinant protein (14).
FIGURE 2.
Thermal stability of Rv0805. A, purified Rv08051–318 and Rv08051–278 proteins (1 μg each) were incubated at 80 °C in assay buffer, in the absence of substrate. At the indicated times, an aliquot was removed and chilled on ice. Assays were performed on all samples at the end of incubation by addition of bis-pNPP as the substrate. B, circular dichroism spectra were collected at 37 °C after incubation of proteins at 80 °C at the indicated times. Control, spectrum at 37 °C with no preincubation at 80 °C.
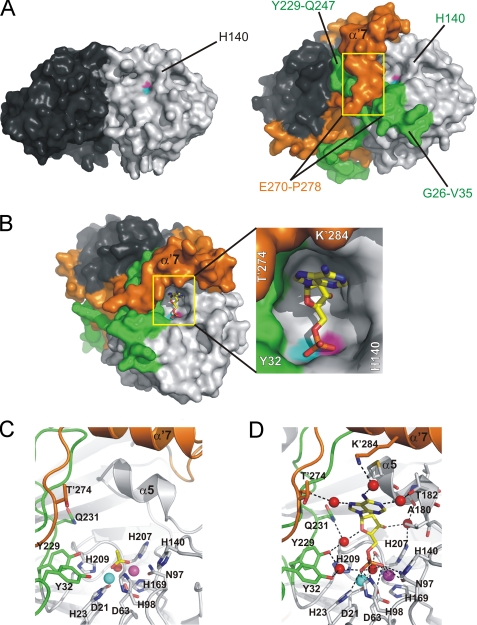
Active Site of Rv08051–318 and a Complex with 5′-AMP
The structure of Rv08051–318 reveals new structural elements that significantly contribute to the architecture of the active site. Whereas the active site appeared broad and open to the solvent in Rv08051–278, loops Gly26–Val35 and Tyr229–Gln247 in the Rv08051–318 frame the active site by forming a rim (Fig. 3A). Residues Tyr32, Tyr229, and Gln231 constitute a network of hydrogen bonds between each other and the active site metals, either directly or through water molecules, thereby keeping the active site in place (Fig. 3C). However, the main structural organizer of the architecture of the Rv08051–318 active site rim is the C-terminal domain (Leu266–Asp298) of the other protomer which, as the main domain-swapped structural element, folds on top of the active site (Figs. 1A and 3). The active site of Rv08051–318 contains an acetate ion bound to the metal ions in the similar bidentate manner (Fig. 3C and supplemental Fig. S3) as the phosphate ion seen earlier in Rv08051–278 (14), resulting from a relatively high concentration of acetate in the crystallization solution (0.2–0.4 m).
FIGURE 3.
The active site of Rv0805. A, comparison of Rv08051–278 (left) and Rv08051–318 (right) dimer shows a significant closure of the active site in Rv08051–318. In this view, only one of the two equal active sites is seen (light gray/green protomer). Yellow rectangles cover the regions of residues Glu′270-Pro′278 that run into α′7 helix from the bottom up. B, Rv08051–318 active site with bound 5′-AMP (left panel). 5′-AMP is shown as sticks (carbon, yellow; oxygen, red; nitrogen, blue; phosphorus, orange). Middle panel, zoom of the active site. C, zoom of the active site showing acetate in sticks bound to Rv08051–318 (carbon, yellow; oxygen, red; spheres are Fe3+, cyan; Mn2+, magenta; planar active site water, red). D, 5′-AMP-Rv08051–318 complex. For the 5′-AMP complex, polar interactions with the active site residues, metals, and water molecules are shown. Color code is the same as described in the legend to Fig. 1, residues of the protomer in dark gray/orange combination are marked with a prime.
To appreciate the role of this C-terminal domain in regulating the activity of Rv0805, we purified Rv08051–278 in a manner similar to Rv08051–318, and monitored the activity of both proteins to a variety of substrates (Table 2). Rv08051–278 showed similar activity as the full-length enzyme for substrates containing the cyclic phosphodiester bond, such as 2′,3′-cAMP and 3′,5′-cAMP. However, Rv08051–278 had a lower activity than Rv08051–318 with the larger colorimetric substrates containing a linear phosphodiester bond, emphasizing the important structural contribution made by the C terminus of Rv0805 to the active site.
We were able to co-crystallize Rv0805 in the presence of 5′-AMP, thus providing a visualization of product in the active site of a cyclic nucleotide MPE. 5′-AMP is buried deeply in the active site pocket, with the purine base in a syn-conformation and the ribose ring in the C3-endo-conformation (Fig. 3, B and D, and supplemental Fig. S4). Its main anchor to the active site is through its phosphate group that is bound to the active site in a similar manner to that of free phosphate in Rv08051–278 (14). The phosphate moiety of 5′-AMP makes contacts to several water molecules in the active site, including the planar active site water, to both metals ions, and directly to side chains of His23, Asp63, Asn97, and His98 (Fig. 3D). There is no direct interaction with any of the side chains of Rv0805 with the purine base or with the ribose ring, in agreement with the earlier observation that cGMP was utilized as efficiently as a substrate by Rv0805 (6, 9). The purine ring is placed parallel to the short hydrophobic helix α5 of the protomer in which the active site is located. The ribose and the purine rings are, however, involved in indirect hydrogen bonding via water molecules to both protomers (Fig. 3D). It is likely that the sum of all these interactions is substantial and thus responsible for the poor release of 5′-AMP from the active site and therefore relatively low rate of hydrolysis of 3′,5′-cAMP (Table 2 and supplemental Fig. S1B). Binding of 5′-AMP does not result in any major changes of the active site architecture. The mode of binding of 5′-AMP is very similar to binding of dAMP, a product of an exonucleolitically cleaved DNA end in Mre11 (7), which supports a common phosphoesterase mechanism between members of the MPE family.
We attempted to prepare crystals of Rv0805 in the presence of either 2′,3′-cAMP or 3′,5′-cAMP. Binding of these ligands in any of these crystal forms was not well defined, but due to the tetrahedral shape of the electron density above the metal ions in these structures, we assume that the cyclic nucleotides were hydrolyzed to their corresponding monophosphates during complex formation in crystals. However, the shape of the electron density in the place of a ribose and purine did not allow unambiguous modeling of the nucleotides. Preparation of crystals in the presence of 2′-AMP or 3′-AMP did not result in complex formation.
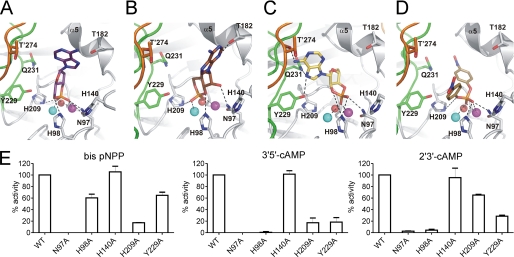
Docking of Substrates in the Active Site of Rv08051–318 and Mutational Analysis
Molecular docking results with multiple substrates in the new high resolution structure of the active site of Rv0805 (Fig. 4) differ significantly to studies reported earlier (9, 14), largely because the previous docking was performed on the crystal structure of Rv08051–278, which was incomplete in detail in the absence of the C terminus. The phosphate oxygen atoms of all substrates are at coordinating distances (2.1–3.3 Å) from both Fe3+ and Mn2+, indicating the critical positioning of the phosphorous atom in the binding site. Asn97 side chain hydrogen bonds to the phosphate moiety of all substrates, the exception being the interaction with 3′,5′-cAMP in an orientation that yields 5′-AMP as a product (Fig. 4B). Here, Asn97 is in hydrogen bonding distance with the ribose hydroxyl group (O2). Asn97 also stabilizes the phosphate moiety of the product 5′-AMP (Fig. 3D). In agreement with the critical role for this residue, the N97A mutant shows no activity with bis-pNPP or 3′,5′-cAMP, and only 2% activity of wild type with 2′,3′-cAMP (Fig. 4E).
FIGURE 4.
Docking of substrates into the active site of Rv08051–318 dimer. A, 3′,5′-cAMP in orientation that releases 3′-AMP upon hydrolysis. B, 3′,5′-cAMP in orientation that releases 5′-AMP upon hydrolysis. C, 2′,3′-cAMP in orientation that releases 3′-AMP upon hydrolysis. D, bis-pNPP in an orientation that releases pNP upon hydrolysis. In all panels, potential polar interactions are depicted as dashed black lines. Color code for the protein is the same as in Fig. 1. Substrates are shown as sticks. Oxygen, red; nitrogen, blue; phosphorus, orange; carbon, violet, 3′,5′-cAMP in A; brown, 3′,5′-cAMP in B; yellow, 2′,3′-cAMP in C; dark yellow, bis-pNPP in D. Fe3+ (cyan), Mn2+ (magenta), and planar active site water (red) are shown as spheres. Residues of the protomer in dark gray/orange combination are marked with a prime. E, wild type or mutant Rv08051–318 proteins were assayed with the substrates indicated. Values represent the mean ± S.E. of assays repeated three times in duplicate, with two different batches of proteins, and are normalized to the activity seen with the wild type protein.
Docking of 3′,5′-cAMP resulted in two equally possible positions of this substrate (Fig. 4, A and B), explaining the almost equal ratios of 3′- and 5′-AMP formed on hydrolysis by Rv08051–318 (supplemental Fig. S1B). The distance between NE2 of His98 and the oxygen from the phosphodiester bond of 3′,5′-cAMP is ∼3–3.5 Å (Fig. 4, A and B), allowing stabilization of the transition state and release of 3′-AMP or 5′-AMP as product. The phosphodiester bond in the lowest energy model of 2′,3′-cAMP (Fig. 4C) is oriented toward, but placed farther, from His98 than in both potential orientations of 3′,5′-cAMP. The phosphodiester bond in bis-pNPP points away from the His98 side chain (Fig. 4D), suggesting that His98 could have an important role in facilitating hydrolysis of 3′,5′-cAMP and 2′3′-cAMP, but not bis-pNPP. Indeed, the H98A mutant protein showed significant activity (∼60% of wild type activity) with bis-pNPP as substrate (Fig. 4E). In contrast, a dramatic reduction in activity was seen with both 3′,5′-cAMP and 2′,3′-cAMP. The importance of His98 in contributing to 2′,3′-cAMP hydrolytic activity has been alluded to earlier (9).
The purine ring in the docked 3′,5′-cAMP molecule does not make any direct contact with the protein. In contrast, the N6 of the purine ring of 2′,3′-cAMP is predicted to form hydrogen bonds to the carbonyl oxygen of Thr274 of the other protomer, and the N7 atom is also in hydrogen-bonding distance with a hydroxyl group of Tyr229 and side chain amide of Gln231 (Fig. 4C). These multiple interactions could account for the more optimal use of 2′,3′-cAMP as a substrate for Rv0805. The almost exclusive formation of 3′-AMP as the product of hydrolysis of 2′,3′-cAMP can be explained by the fact that an opposite orientation of 2′,3′-cAMP in the active site (which would yield 2′-AMP as a product), is energetically less favored due to potential clashes with the active site cleft residues, including helix α5. The Y229A mutant protein showed only a marginal reduction in the efficiency of hydrolysis of bis-pNPP, but 2′,3′-cAMP and 3′,5′-cAMP were both poorly hydrolyzed (Fig. 4E). Thus, despite the lack of direct hydrogen bonds between the protein and purine ring/ribose of 3′,5′-cAMP, the Y229A mutation may disrupt a network of water molecules between Tyr229 and the phosphodiester bond of 3′,5′-cAMP, as seen in the crystal structure of the 5′-AMP complex (Fig. 3D).
His209 is another highly conserved residue in MPEs and coordinates Fe3+ in Rv0805. This residue is positioned almost opposite to His98, with the metal ion pair between them. Docked positions of 3′,5′-cAMP and bis-pNPP places them between His98 and His209 such that either residue could influence the hydrolysis of these substrates. According to the model 2′,3′-cAMP may be placed farthest (Fig. 4C), and with the least favorable orientation toward the His209 side chain. In agreement with this, mutation of His209 to Ala significantly decreased the hydrolysis of 3′,5′-cAMP and bis-pNPP (Fig. 4E), but not 2′,3′-cAMP.
Current docking analysis showed that His140 was placed at some distance from the substrates (Fig. 4). This positioning is in contrast to what was observed in the Rv08051–278 structure (14) (supplemental Fig. S3) where the different modes of binding of substrates was a result of the absence of the C terminus and, consequently, the structured loops Gly26–Val35 and Tyr229–Gln247. We re-investigated the catalytic properties of the H140A mutant protein using protein purified with metal supplementation in the media. No alteration in catalytic activity was seen with any of the substrates and the H140A mutant protein (Fig. 4E), in agreement with current docking analysis that indicates a minimal role for His140 in substrate interaction. Earlier preparations of the H140A mutant showed a marked loss of activity toward 3′,5′-cAMP (14) and we attribute the current result to the possibility that subtle conformational changes in the active site may occur if the Rv0805 is produced and folded in the presence or absence of metals.
Expression of Rv0805 in Mycobacteria Results in Perturbations of cAMP Levels and Cell Wall Permeability
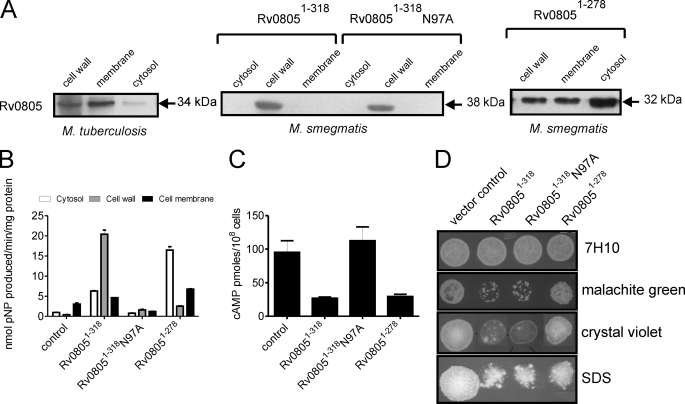
Although Rv0805 may cleave cAMP in vivo and in vitro, given its promiscuity in recognizing structurally diverse substrates, it is likely that there are additional components in mycobacterial cells that either serve as substrates for Rv0805 or modulate its activity within the cell. We monitored the expression of Rv0805 in subcellular fractions prepared from M. tuberculosis by Western blot analysis, and found that a large fraction was localized to the cell membrane and the cell wall, with a minor amount seen in the cytosol (Fig. 5A). Our results are in contrast to those reported recently (4) where FLAG-tagged Rv0805 expressed in M. tuberculosis was found to be predominantly intracellular. If one assumes that the major role for Rv0805 in the cell would be to hydrolyze intracellular cAMP, localization in the cell wall is intriguing.
FIGURE 5.
Subcellular localization and function of Rv08051–318 and Rv08051–278 in mycobacteria. A, subcellular fractions (50 μg of protein) of M. tuberculosis were subjected to Western blot analysis using a monoclonal antibody to Rv0805. His-tagged wild type or mutant Rv08051–318 or Rv08051–278 were expressed in M. smegmatis and subcellular fractions prepared. Protein (50 μg) was subjected to Western blot analysis using a monoclonal antibody to Rv0805. The molecular sizes indicated include the addition of additional residues coming from the His6 tag and vector. B, protein (50 μg) was taken for assay using bis-pNPP as a substrate. Note that expression of Rv08051–278 was higher in cells than that of Rv08051–318 (panel A), but the activity with bis-pNPP is lower for Rv08051–278 than Rv08051–318 (see Table 2). Subcellular fractions prepared from a strain of M. smegmatis transformed with the control vector (control) was used to detect background activity. C, intracellular cAMP was measured in a control strain of M. smegmatis, and in strains expressing wild type or mutant Rv08051–318 proteins or Rv08051–278. Cells were harvested at late-log phase for cAMP measurements. D, cells (107) of the indicated strains were spotted on 7H10 agar plates containing 5 μg/ml of malachite green, 10 μg/ml of crystal violet, or 0.01% SDS and incubated for 2–3 days, after which the plates were photographed.
We used the knowledge obtained from the structure and activities of Rv08051–318 and Rv08051–278, and the information obtained from structure-based mutational analyses, to delineate the roles of Rv0805 in the cell. We expressed Rv08051–318 and Rv08051–278 wild type proteins as well as the catalytically dead N97A mutant full-length protein in M. smegmatis, which has no close ortholog of Rv0805 encoded in its genome (6). No difference in growth rate or colony morphology was seen in cells overexpressing Rv0805 (data not shown). Expression of wild type Rv08051–318 or the N97A mutant in M. smegmatis resulted in its localization predominantly to the cell wall with very little seen in the membrane and cytosol fractions. Expression of Rv08051–278 was consistently higher than the full-length enzyme in cells (∼5-fold based on immunoreactivity), with the majority found in the cytosol, and some in the membrane and cell wall fractions (Fig. 5A). Therefore, the C-terminal region of Rv0805 aids in more efficient localization of the protein to the cell wall, perhaps by acting as a recognition motif for components of the secretory machinery. Moreover, the fact that the last 20 amino acids are unstructured could suggest that this stretch of amino acids contains an interaction motif assisting in localization of the protein.
Rv0805 expressed in M. smegmatis was catalytically active, because increased hydrolysis of bis-pNPP was seen in subcellular fractions over and above that seen in control cells (Fig. 5B). Increased phosphodiesterase activity seen in the cytosolic fractions of cells expressing Rv08051–278 was commensurate with its high level of expression in this cellular compartment. No significant hydrolysis of bis-pNPP over and above that seen in vector-transformed cells was seen in fractions prepared from cells expressing the N97A mutant protein, as would be expected from its low catalytic activity (Fig. 4E). We also monitored cAMP levels in cells expressing either Rv08051–318 or Rv08051–278 (Fig. 5C). A reduction in intracellular cAMP was seen in both cells to equivalent extents. As expected, expression of the catalytically inactive N97A mutant protein did not lead to a decrease in intracellular cAMP levels.
The specific and unusual localization of Rv0805 in the cell wall could suggest that hydrolysis of substrates for Rv0805 in that subcellular compartment may perturb and alter cell wall permeability. We tested the sensitivity of M. smegmatis strains overexpressing Rv08051–318 or Rv08051–278 to malachite green and crystal violet, two lipophilic inhibitors of cell growth that have been used to screen for cell wall perturbations in mycobacteria (28, 29) and SDS. As seen in Fig. 5D, cells expressing Rv08051–318 were highly sensitive to malachite green, crystal violet, and SDS. Most unexpectedly, sensitivity was still seen in cells expressing the N97A mutant protein, indicating that the increased sensitivity was brought about by Rv0805 in a catalytically independent manner. Moreover, cells expressing Rv08051–278 were as resistant as control cells to malachite green and crystal violet, and showed some resistance to SDS. It is to be noted that the absolute amount of Rv08051–278 in the cell wall fraction was similar to Rv08051–318 as monitored by Western blotting (Fig. 5A). Therefore, the perturbations induced in the cell wall on expression of Rv08051–318 may not solely be a result of its presence in the cell wall, but also critically dependent on the residues beyond Pro278. Thus, the C terminus of Rv0805 is important not only for modeling the active site to accommodate more linear substrates/regulatory molecules that may be present in the cell, but also to modulate cell wall properties in a manner independent of the catalytic activity of the enzyme.
In conclusion, whereas cAMP production by mycobacteria has been shown to be important for the virulence of M. tuberculosis (4), we suggest that an additional role of Rv0805 in mycobacteria is independent of its ability to hydrolyze cAMP. Because the gene encoding Rv0805-like protein is found only in pathogenic mycobacteria, and is predicted to be a functional protein in M. leprae (which has undergone a large degree of pseudogenization), Rv0805 may play a critical role in the pathogenicity of mycobacteria, not only by hydrolyzing cAMP in the cell, but also “moonlighting” as a cell wall remodeling protein.
DISCUSSION
In the current study we report structures of the Rv0805 protein alone and in complex with 5′-AMP, revealing unique features that could account for its promiscuous substrate selection and putative role in modeling the cell wall. Because Rv0805 does not seem to possess a classical signal sequence, its localization to the cell membrane and cell wall could be via a specialized secretion system operative in mycobacteria. It appears that the C-terminal domain of Rv0805 is used to better adjust the substrates into the active site as well as facilitate subcellular localization of the protein. Because the last 20 residues of Rv08051–318 remain disordered in the crystal, we propose that the floppy nature of this peptide may serve as an anchor for Rv0805 to interact with either the mycobacterial membrane or cell wall, or to other proteins. Further studies directed toward this aspect are ongoing in the laboratory.
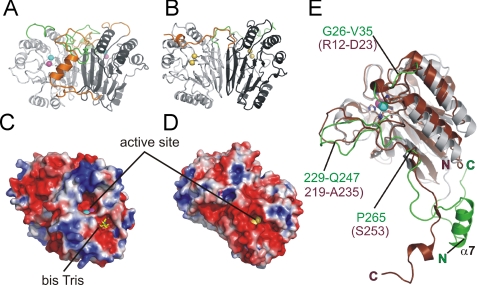
The structure of Rv0805 is strikingly similar to another MPE family member, glycerophosphodiesterase (GpdQ) (11, 30) from Enterobacter aerogenes, which cleaves mono-, di- and triphosphoesters, with glycerophosphoethanolamine proposed to be its natural substrate. Using the Protein Structure Comparison Service (SSM) at the European Bioinformatics Institute (31), the r.m.s. deviation values were calculated for the core regions of both structures, using the CA atoms of residues Val15–Pro265 of Rv0805, and Leu2–Ser253 of GpdQ (PDB 3D03). The r.m.s. deviation value between the monomer structures of Rv0805 and GpdQ (chain A) was 1.8 Å and between the dimers (using chains A and B of 3D03) was 2.1 Å. Especially impressive is the similarity of the homodimer interface in both proteins (Fig. 6), seen so far in only these two proteins. The highly conserved residues of the MPE active site are well superposed between the two proteins (Fig. 6E), but major structural differences in Rv08051–318 and GpdQ are in the region of loops Tyr229–Gln247 and Val219–Ala235, respectively, the C termini beyond residue Pro265 in Rv0805 and Ser253 in GpdQ, as well as at the extreme N terminus, which is significantly shorter in the GpdQ structure (Fig. 6E). GpdQ forms a trimer of dimers that may further help regulate its substrate specificity. Both Rv0805 and GpdQ have negatively charged active sites but the overall distribution of the electrostatic charge in Rv08051–318 is significantly different to GpdQ (Fig. 6, C and D). Adjacent to the active site, Rv08051–318 contains another negatively charged cleft, observed as the bis-Tris binding region (Fig. 6C), encircled by a rim of positively charged residues that build a plateau-shaped solvent-exposed surface, which may have significance in interaction with cell wall components of mycobacteria.
FIGURE 6.
Comparison of the Rv08051–318-fold with GpdQ. A, Rv08051–318 dimer as a ribbon representation (colored as described in the legend to Fig. 1). B, GpdQ (3D03) dimer, ribbon representation. The MPE domains are in light and dark gray, as in Rv0805. The GpdQ cap domain Ser253–Arg274 is colored orange or green. Cobalt ions in the GpdQ active site are in yellow. C, electrostatic surface of Rv08051–318; and D, electrostatic surface of GpdQ dimer (PDB 3D03). Calculations were done using the Poisson-Boltzmann equation (APBS) (33). Cut off −2.5 kT/e was used for negative potential (red) and 2.5 kT/e for positive potential (blue). Active site metals are shown as spheres. Rv0805 and GpdQ are symmetric homodimers related by 180° axis, therefore in the present view, only one active site of the two can be seen. Bis-Tris bound to Rv08051–318 is shown in sticks (carbon, yellow; oxygen, red; nitrogen, blue). E, superposition of Rv08051–318 (gray green) and GpdQ (3d03; chain A) (brown) monomers. Active site metal ions of the two structures superimpose very closely, therefore for clarity, only Fe3+ (cyan) and Mn2+ (magenta) of Rv08051–318 are shown as spheres. Residues in Rv0805 are marked in green, and in brown for GpdQ. The structural alignment in E was done according to Ref. 31.
Crystal structures of Rv08051–318 alone and in complex with 5′-AMP, together with molecular docking and accompanying biochemical analysis, now provide a possible explanation for the observed substrate promiscuity of the Rv0805. The Rv0805 active site is broad enough to accept small molecules with a cyclic or linear phosphodiester bond, but which side of the phosphodiester bond is cleaved depends on the geometry of the substrate and its binding orientation (see Table 2 and Fig. 4). Interestingly, in vitro, linear phosphodiesters seemed to be kinetically more favored by Rv08051–318 than cyclic substrates. Docking experiments show that bis-pNPP is anchored to the active site only via its phosphate moiety, with no significant interactions with the rest of the protein. It seems likely that the shape of the Rv08051–318 active site, rather than specific residues, allow linear substrates to bind with an orientation that enables more effective hydrolysis than the cyclic phosphodiester substrates. The mechanism of hydrolysis of cyclic phosphodiesters may follow that proposed for Mre11 (7, 32), with His98 as a crucial residue coordinating the transition state complex. On the other hand, His209 may take this role in the case of linear substrates (Fig. 4).
The role of Rv0805 in the biology of pathogenic bacteria is not known. A recent report has utilized the overexpression of Rv0805 in M. tuberculosis as a means of regulating cAMP levels in the bacterial cell and thereby transcription of tumor necrosis factor α (4). In these studies, N-terminal FLAG-tagged Rv0805 was predominantly localized to the cytosol with some seen in the particulate fraction of cells. This is in contrast to our results, where Rv0805 in M. tuberculosis and M. smegmatis was preferentially localized to the cell envelope. Because we now show that Rv0805 can alter the properties of the cell wall, the low virulence seen of M. tuberculosis overexpressing Rv0805 in earlier studies (4) could also perhaps be attributed to modifications in the cell wall of the bacteria, in addition to lowering intracellular cAMP levels.
There is additional, albeit circumstantial evidence, for a role of Rv0805 in regulating cell wall processes in M. tuberculosis. In a recent study to identify new analogs of trichlosan that could act as inhibitors of the enol reductase InhA (33), genes were identified that were up-regulated in the presence of these inhibitors. Most of these genes were those involved in fatty acid and cell wall biosynthesis and Rv0805 was also up-regulated 1.5-fold. With the background of the data presented here, the association of Rv0805 with a gene regulatory network associated with cell wall function may not be coincidental.
A recent study to identify proteins in M. tuberculosis that allowed the bacterium to withstand acidic conditions present in the phagosome identified 21 transposon insertion mutants that were sensitive to medium acidification (34). More than 70% of these mutants were in genes that are annotated as being involved in cell wall functions, such as peptidoglycan and lipoarabinomanan biosynthesis. One transposon mutant contained an insertion within the rv0805 gene. Once again, there is suggestive evidence that the major role of Rv0805 could be related to cell wall function, in assisting the bacterium to respond to varied stress conditions.
Although the precise role of Rv0805 in mycobacteria is still not known, the structural and biochemical details that we have provided here strongly suggests that whereas one of its substrates in the cell could indeed be cAMP, surface electrostatics and binding sites for other molecules (i.e. bis-Tris (Fig. 6C and supplemental Fig. S3)), indicate that there could be other substrates for Rv0805. More intriguingly, Rv0805 may alter properties of the cell wall of mycobacteria in a catalytically independent manner, perhaps by acting as a scaffold as seen for Vps29 (13). Because the mycobacterial cell wall is unique in its composition and believed to play an important role in cellular physiology and pathophysiology, further studies on the role of Rv0805 in mycobacteria are warranted. The availability of information in atomic detail on the active site of Rv0805 should allow the development of novel inhibitors of Rv0805. These can be used to dissect the catalytic roles for Rv0805 in the cell, independently of its other non-catalytic functions that result from unique structural elements present in the protein.
Supplementary Material
Acknowledgments
We acknowledge the help of Maja Capuder and Petra DraškoviČ at various stages of this study. We thank the staff at the synchrotron facilities at EMBL/DASY, Hamburg, Germany, and XRD beamline, Elettra, Trieste, Italy, for support with the data collection of various forms of Rv0805 crystals. We also thank Dr. Avinash R. Shenoy for critical reading of the manuscript.
This work was supported in part by grants from the Slovenian Research Agency (to M. P. and U. D.), the Department of Biotechnology, Government of India, and the Department of Science and Technology, Government of India for an Indo-Slovenian joint project.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S4.
The atomic coordinates and structure factors (codes 3IB7 and 3IB8) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- MPE
- metallophosphoesterase
- bis-pNPP
- bis-(p-nitrophenyl)phosphate
- pNPPP
- p-nitrophenyl phenylphosphonate
- r.m.s.
- root mean square
- PDB
- Protein Data Bank
- MES
- 4-morpholineethanesulfonic acid
- bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2- (hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1.Ehrt S., Schnappinger D. (2009) Cell. Microbiol. 11, 1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenoy A. R., Visweswariah S. S. (2006) FEBS Lett. 580, 3344–3352 [DOI] [PubMed] [Google Scholar]
- 3.Shenoy A. R., Visweswariah S. S. (2006) Trends Microbiol. 14, 543–550 [DOI] [PubMed] [Google Scholar]
- 4.Agarwal N., Lamichhane G., Gupta R., Nolan S., Bishai W. R. (2009) Nature 460, 98–102 [DOI] [PubMed] [Google Scholar]
- 5.Conti M., Beavo J. (2007) Annu. Rev. Biochem. 76, 481–511 [DOI] [PubMed] [Google Scholar]
- 6.Shenoy A. R., Sreenath N., Podobnik M., Kovacevic M., Visweswariah S. S. (2005) Biochemistry 44, 15695–15704 [DOI] [PubMed] [Google Scholar]
- 7.Hopfner K. P., Karcher A., Craig L., Woo T. T., Carney J. P., Tainer J. A. (2001) Cell 105, 473–485 [DOI] [PubMed] [Google Scholar]
- 8.Voegtli W. C., White D. J., Reiter N. J., Rusnak F., Rosenzweig A. C. (2000) Biochemistry 39, 15365–15374 [DOI] [PubMed] [Google Scholar]
- 9.Keppetipola N., Shuman S. (2008) J. Biol. Chem. 283, 30942–30949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter W. (2002) Proteins 46, 278–286 [DOI] [PubMed] [Google Scholar]
- 11.Jackson C. J., Carr P. D., Liu J. W., Watt S. J., Beck J. L., Ollis D. L. (2007) J. Mol. Biol. 367, 1047–1062 [DOI] [PubMed] [Google Scholar]
- 12.Hierro A., Rojas A. L., Rojas R., Murthy N., Effantin G., Kajava A. V., Steven A. C., Bonifacino J. S., Hurley J. H. (2007) Nature 449, 1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins B. M., Skinner C. F., Watson P. J., Seaman M. N., Owen D. J. (2005) Nat. Struct. Mol. Biol. 12, 594–602 [DOI] [PubMed] [Google Scholar]
- 14.Shenoy A. R., Capuder M., Draskovic P., Lamba D., Visweswariah S. S., Podobnik M. (2007) J. Mol. Biol. 365, 211–225 [DOI] [PubMed] [Google Scholar]
- 15.Tyagi R., Shenoy A. R., Visweswariah S. S. (2009) J. Biol. Chem. 284, 5217–5228 [DOI] [PubMed] [Google Scholar]
- 16.Shenoy A. R., Visweswariah S. S. (2003) Anal. Biochem. 319, 335–336 [DOI] [PubMed] [Google Scholar]
- 17.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 18.Collaborative Computational Project, Number 4 ( 1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760– 76315299374 [Google Scholar]
- 19.Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. (1991) Acta Crystallogr. A 47, 110–119 [DOI] [PubMed] [Google Scholar]
- 20.DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, Palo Alto, CA [Google Scholar]
- 21.Weininger D. ( 1988) J. Chem. Inf. Comput. Sci. 28, 31– 36 [DOI] [PubMed] [Google Scholar]
- 22.Delogu G., Bua A., Pusceddu C., Parra M., Fadda G., Brennan M. J., Zanetti S. (2004) FEMS Microbiol. Lett. 239, 33–39 [DOI] [PubMed] [Google Scholar]
- 23.Dass B. K., Sharma R., Shenoy A. R., Mattoo R., Visweswariah S. S. (2008) J. Bacteriol. 190, 3824–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezwan M., Lanéelle M. A., Sander P., Daffé M. (2007) J. Microbiol. Methods 68, 32–39 [DOI] [PubMed] [Google Scholar]
- 25.Zor T., Selinger Z. (1996) Anal. Biochem. 236, 302–308 [DOI] [PubMed] [Google Scholar]
- 26.Shenoy A. R., Sreenath N. P., Mahalingam M., Visweswariah S. S. (2005) Biochem. J. 387, 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding F., Prutzman K. C., Campbell S. L., Dokholyan N. V. (2006) Structure 14, 5–14 [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Nikaido H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 4011–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kana B. D., Gordhan B. G., Downing K. J., Sung N., Vostroktunova G., Machowski E. E., Tsenova L., Young M., Kaprelyants A., Kaplan G., Mizrahi V. (2008) Mol. Microbiol. 67, 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadler K. S., Tanifum E. A., Yip S. H., Mitić N., Guddat L. W., Jackson C. J., Gahan L. R., Nguyen K., Carr P. D., Ollis D. L., Hengge A. C., Larrabee J. A., Schenk G. (2008) J. Am. Chem. Soc. 130, 14129–14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krissinel E., Henrick K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 [DOI] [PubMed] [Google Scholar]
- 32.Williams R. S., Moncalian G., Williams J. S., Yamada Y., Limbo O., Shin D. S., Groocock L. M., Cahill D., Hitomi C., Guenther G., Moiani D., Carney J. P., Russell P., Tainer J. A. (2008) Cell 135, 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyne M. E., Sullivan T. J., amEnde C. W., Lu H., Gruppo V., Heaslip D., Amin A. G., Chatterjee D., Lenaerts A., Tonge P. J., Slayden R. A. (2007) Antimicrob. Agents Chemother. 51, 3562–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandal O. H., Pierini L. M., Schnappinger D., Nathan C. F., Ehrt S. (2008) Nat. Med. 14, 849–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.