Abstract
Foam cell formation is a hallmark event during atherosclerosis. The current paradigm is that lipid uptake by scavenger receptor in macrophages initiates the chronic proinflammatory cascade and necrosis core formation that characterize atherosclerosis. We report here that a cytokine considered to be anti-atherogenic, interleukin-10 (IL10), promotes cholesterol uptake from modified lipoproteins in macrophages and its transformation into foam cells by increasing the expression of scavenger receptor CD36 and scavenger receptor A. Although uptake of modified lipoproteins is considered proatherogenic, we found that IL10 also increases cholesterol efflux from macrophages to protect against toxicity of free cholesterol accumulation in the cell. This process was PPARγ-dependent and was mediated through up-regulation of ABCA1 (ATP-binding cassette transporter A1) protein expression. Importantly, expression of inflammatory molecules, such as tumor necrosis factor-α, intercellular adhesion molecule-1, and MMP9 as well as apoptosis were dramatically suppressed in lipid-laden foam cells treated with IL10. The notion that IL10 can mediate both the uptake of cholesterol from modified lipoproteins and the efflux of stored cholesterol suggests that the process of foam cell formation is not necessarily detrimental as long as mechanisms of cholesterol efflux and transfer to an exogenous acceptor are functioning robustly. Our results present a comprehensive antiatherogenic role of IL10 in macrophages, including enhanced disposal of harmful lipoproteins, inhibition of inflammatory molecules, and reduced apoptosis.
Introduction
One of the key events in atherosclerosis is the formation of foam cells. Macrophages in the arterial intima take up modified LDL2 and become cholesteryl ester-laden foam cells. These foam cells are the predominant cell types (∼80%) in fatty streak at the early stage of atherosclerosis and play a pivotal role throughout lesion progression and plaque vulnerability (1, 2). Macrophage foam cell formation in atherosclerotic blood vessel intima is mediated by scavenger receptor (SR)-dependent internalization of modified LDL, which has long been considered one of the requisite initiating events in atherogenesis (2–4). The receptors that play the most important role in atherosclerosis include SR class A, types I and II, as well as the SR class B, CD36. These principal receptors are responsible for the uptake of modified LDL that leads to lipid loading in macrophages (5–7).
Interleukin-10 (IL10), an anti-inflammatory cytokine, is present within human atherosclerotic plaques (8), primarily in macrophage-rich regions. Data from IL10-deficient mice or IL10-overexpressing mice have implicated a protective role of IL10 during atherosclerosis (9–12). One of the antiatherogenic properties of IL10 may be its ability to regulate lipid metabolism in macrophages. There is, however, considerable controversy in the literature regarding the exact role that IL10 plays in this process (13–15). Whether IL10 inhibits (13, 15) or enhances foam cell formation in macrophages (14) has not been clearly defined.
Although the uptake of modified lipoproteins by SRs is thought to be central to foam cell formation, the detailed mechanism of how the uptake of modified lipoproteins by the macrophages is regulated by IL10 remains unclear. Traditionally, macrophage foam cell formation has been regarded as proatherogenic because of its role in the initiation of chronic inflammatory cascades and the induction of apoptosis. It is tempting to hypothesize that the athero-protective role of IL10 might be mediated through inhibition of foam cell formation. We show here, however, that IL10 not only stimulates scavenger receptor expression on cholesterol-laden macrophages and promotes foam cell formation but also enhances cholesterol efflux in a PPARγ-dependent manner. Further indication of its anti-atherogenic properties was evidenced by decreased expression of proinflammatory immune molecules and suppressed apoptosis in cholesterol-laden macrophages.
EXPERIMENTAL PROCEDURES
For further details on antibodies and regents, binding and uptake of AcLDL, analysis of cellular cholesteryl ester contents and lipid accumulation, flow cytometry, accumulation of lipid droplets in macrophages and foam cell formation, sequence-specific PCR primer, Western blot analysis, cholesterol efflux, and TUNEL assay see the supplemental material.
Antibodies and Reagents
Antibodies and reagents are described in the supplemental material.
Cell Culture
The mouse macrophage cell line RAW264.7 (American Type Culture Collection, Manassas, VA) was cultured in growth DMEM (DMEM containing penicillin (50 units/ml), streptomycin (50 μg/ml), and 2 mm l-glutamine) with 10% fetal calf serum. To obtain bone marrow-derived macrophages (BMDMs), bone marrow was flushed from the femur and tibia, purified through Ficoll-Paque gradient (Amersham Biosciences), and cultured in DMEM containing 20% fetal bovine serum and 30% L929 conditioned medium for 6 days. Differentiated macrophages were counted and replated in DMEM with 10% fetal calf serum for various experiments. Experiments were carried out in 6-well trays (1.5 × 106 cells/ml; Costar, Cambridge, MA) with and without AcLDL, IL10, or a combination thereof in growth medium. Control cells were given growth medium alone (vehicle). Prior to all experiments, the RAW cells were seeded by incubation for 24 h in growth medium with 10% fetal calf serum, and then the cells were refreshed with growth medium with 0.2% albumin from bovine serum (fatty acid-free) by incubation overnight. In foam cell model 1, resting macrophages were treated with growth medium containing AcLDL (50 μg/ml) in the presence or absence of 10 ng/ml IL10 for 8 or 24 h. In foam cell model 2, foam cells were obtained by loading macrophages with AcLDL (50 μg/ml) for 36 h followed by IL10 (10 ng/ml) treatment for 8 or 24 h. All experiments involving quantitation of fluorescently labeled cells were done by observers blinded to the experimental conditions.
Analysis of Cellular Cholesteryl Ester Contents and Lipid Accumulation
Macrophages were treated with or without 10 ng/ml IL10 for 8 or 24 h as mentioned above. The cellular cholesterol content was determined by the enzymatic, fluorometric method, using the cholesterol assay kit (catalog number 10007640) from Cayman Chemical (described in the supplemental material).
Flow Cytometry
To detect the role of IL10 in lipid accumulation and foam cell formation, immunofluorescence flow cytometric analysis was performed using BODIPY 493/503 (catalog number D-3922) as mentioned previously (16) (described in the supplemental material).
RNA Extraction and Analysis
After incubation with or without IL10 (10 ng/ml) in the two foam cell models as described above, total cellular RNA was extracted from macrophages using RNABee (Tel-Test Inc., Friendswood, TX). RNA samples were then subjected to RNA purification using the RNeasy minikit (Qiagen). Real-time quantitative RT-PCR was performed using the ABI Prism 7700 (Applied Biosystems, Foster City, CA) Cybrgreen analysis. The sequence-specified PCR primers for CD36, SR-AI, SR-AII, ABCA1 (ATP-binding cassette protein A1), TNFα, MMP9, and ICAM-1 are described in the supplemental material. The gene products were quantified using SYBR Green assays (Applied Biosystems). mRNA signal from glyceraldehyde-3-phosphate dehydrogenase or β-actin was used for normalization.
Cholesterol Efflux
RAW cells were seeded in 24-well plates for incubation overnight and then were labeled with loading medium (DMEM, glutamine, penicillin, streptomycin, 0.2% fatty acid-free bovine serum albumin, AcLDL (50 μg/ml), 1 μCi/μl [3H]cholesterol) with or without IL10 (10 ng/ml, with either PGJ2 (5 μm) or GW9662 (10 μm)) for 36 h. The cells were washed twice with PBS, and then DMEM, glutamine, 0.2% fatty acid-free bovine serum albumin with or without IL10 (10 ng/ml) with either PGJ2 (5 μm) or GW9662 (10 μm) was added into each well and incubated for 1–2 h. The medium was then aspirated. ApoAI-independent and apoAI-dependent cholesterol efflux methods are described in detail in the supplemental material.
Targeted Deletion of PPARγ in Macrophages
PPARγflox/flox macrophage cell line was kindly provided by Dr. C. H. Lee. In these cells, loxP sites were introduced on either side of exons 1 and 2 of PPARγ as described before (17). Cre-mediated deletion of these exons was achieved by infection with adenovirus-Cre, which was generated by using the AdEasy XL adenoviral system (Stratagene) (18). PPARγflox/flox macrophages infected with adenovirus-GFP were used as control. Two days after infection, virus was washed out, and cells were cultured 1 day without virus before treatment.
Immunocytochemistry for Cleaved Caspase-3
Foam cells were obtained as described above. To induce apoptosis, the cells were deprived of serum for 24 h in the presence or absence of IL10 (10 ng/ml). In brief, cells were fixed and permeabilized with 0.1% Triton X-100 solution. Expression of cleaved caspase-3 was determined using a specific antibody (1:200 dilution; Cell Signaling Technology) followed by a secondary antibody conjugated with Alexa Fluor 546 (1:200 dilution; Invitrogen). Cells were counterstained with Hoechst 33258 for detection of nuclei, and the expression of cleaved caspase-3 was observed using a fluorescence microscope.
Statistics
Statistical comparisons between groups were performed with PRISM software (GraphPad). Data are presented as mean ± S.E. The statistical significance of differences between groups was analyzed by Student's t test. Differences were considered significant at a p value of <0.05.
RESULTS
In the current study, two distinct macrophage foam cell models were applied. In one model, the macrophages were treated with AcLDL in the presence or absence of IL10; in another model, foam cells were generated by pretreatment with AcLDL for 36–48 h and then treated with or without IL10. The first model is especially useful to study the role of IL10 in foam cell formation, whereas the latter was adopted to assess the role of IL10 in modulating a variety of functions in foam cells relevant to atherosclerosis.
IL10 Increases Dil-AcLDL Uptake in Macrophages through Up-regulation of Scavenger Receptor
To determine the contribution of IL10 to modified lipid uptake, we performed Dil-AcLDL uptake studies on macrophages. After incubation with IL10 for the indicated time, the fluorescence intensity of internalized Dil-Ac-LDL in IL10-treated cells was markedly higher than that of control cells (Fig. 1, A and B), suggesting that Dil-Ac-LDL uptake by the cells increased substantially.
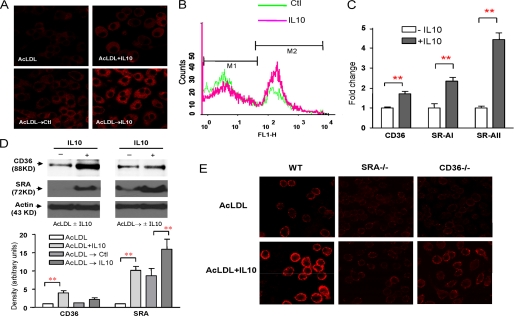
FIGURE 1.
IL10 increases Dil-AcLDL uptake through up-regulation of expression of scavenger receptors. A, resting macrophages (top) and lipid-laden macrophages (bottom) were treated either with AcLDL, with AcLDL plus IL10, with medium alone (control), or with IL10 for 24 h followed by an assay to determine the Dil-AcLDL uptake. Dil-AC-LDL uptake was determined by confocal microscopy. B, histograms of Dil-Ac-LDL uptake between control (green line) and IL10 (red line) were overlaid. The lipid-laden Raw cells were treated with either medium alone (control) or IL10 for 24 h, followed by flow cytometry analysis. C, transcripts for scavenger receptors (CD36, SR-AI, and SR-AII) were detected by real-time PCR in lipid-laden macrophages treated with medium (blank column) or with IL10 (gray column) for 24 h. The results are representative of at least three independent experiments, and values are expressed as the -fold change in abundance ± S.E. D, IL10 up-regulates CD36 and SR-A protein expression. Resting Raw cells or lipid-laden Raw cells were treated as indicated for 24 h. Expression of CD36 and SR-A protein was examined by immunoblot analysis using rabbit anti-CD36 antibody and rat anti-mouse CD204 antibody. Representative results from three experiments are shown (top). The densities of bands corresponding to CD36 and SR-A were quantitated using an image densitometer. The data shown are band densities in arbitrary optical density units from three repeated experiments (bottom). E, IL10 enhances the Dil-AcLDL uptake through scavenger receptors. Bone marrow-derived macrophages from either SR-A- or CD36-knock-out mice or C57BL/6 mice (control) were treated with either AcLDL or AcLDL plus IL10 for 24 h. Dil-AC-LDL uptake was determined by confocal microscopy. WT, wild type.
To determine if IL10 influences modified LDL uptake in lipid-laden foam cells through up-regulation of scavenger receptor expression, we studied the effects of IL10 on the expression of scavenger receptor A (SR-A) and CD36, the receptors that control the uptake of modified LDL, such as AcLDL and oxidized LDL. Real-time PCR data showed that IL10 up-regulated SR-A (type I and II) and CD36 mRNA levels (Fig. 1C). Consistent with this, SR-A and CD36 protein levels were significantly increased after IL10 treatment (Fig. 1D). To examine if SR-A and CD36 accounted for the increase in the uptake of modified LDL in response to IL10, we utilized bone marrow-derived macrophages from either SR-A- or CD36-knock-out mice. The IL10-induced increase in the uptake of modified LDL was greatly suppressed in macrophages from both SR-A- and CD36-knock-out mice (Fig. 1E), although the suppression was more pronounced in the SR-A−/− macrophages. Our results suggested that IL10 promotes uptake of modified LDL through up-regulation of expression of scavenger receptor SR-A and CD36 in macrophages.
IL10 Increases Cholesteryl Ester Content and Promotes Macrophage Foam Cell Formation
Cholesteryl esters are highly nonpolar lipids that are the major core lipid components of low and high density lipoproteins. To assess the role of IL10 in cholesteryl ester accumulation in foam cells, three distinct techniques were employed: flow cytometry analysis of BODIPY-stained cells, observation of BODIPY fluorescence by confocal microscopy, and direct chemical measurement of cellular free cholesterol and cholesteryl ester content.
BODIPY specifically binds cellular lipid droplets and can be quantitated by flow cytometry (16). Accordingly, the cholesteryl ester content of cells can be monitored by the intensity of fluorescence emitted by BODIPY. As shown in Fig. 2, A and B, IL10 (10 ng/ml) significantly promoted the accumulation of neutral lipids in IL10-treated macrophages. Changes in BODIPY fluorescence emissions as measured by flow cytometry showed that BODIPY intensity increased by 35% following IL10 treatment, as shown in Fig. 2B. To confirm the role of IL10 in cholesteryl ester accumulation, we investigated the effects of IL10 on the cellular free cholesterol and cholesteryl ester content in macrophages. Treatment of macrophages with IL10 (10 ng/ml) for 24 h significantly increased cholesteryl ester content by 1.5-fold compared with untreated controls (Fig. 2C). There was no significant difference in the content of intracellular free cholesterol between IL10-treated and untreated cells (Fig. 2C). Similar experiments using primary macrophages (BMDMs) showed that IL10 promoted cholesteryl ester accumulation in these cells as well (Fig. 2, D and E). These results indicate that IL10 can increase the cholesteryl ester accumulation and promote the macrophage-to-foam cell transformation.
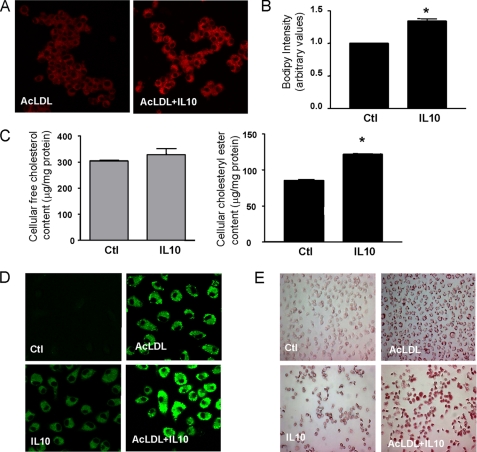
FIGURE 2.
IL10 increases cholesteryl ester accumulation. A and B, BODIPY fluorescence staining of RAW 264.7 cells. Cells were incubated for 24 h with AcLDL in the presence or absence of IL10 followed by BODIPY staining. Cholesteryl ester deposition was determined by either confocal microscopy (A) or flow cytometry (B). The data in B represent the mean ± S.E. of three separate experiments. C, IL10 enhances cholesteryl ester accumulation of RAW264.7 cells. Cells were incubated for 24 h with AcLDL in the presence or absence of IL10. Total free cholesterol and cholesteryl ester content were determined. Data are presented as mean ± S.E. of three separate experiments. *, p < 0.05 versus AcLDL alone. D and E, IL10 promotes foam cell formation. BMDMs were incubated for 24 h with either medium alone, IL10, AcLDL, or ACLDL plus IL10. The lipid accumulation was determined by either confocal microscopy (for BODIPY) (D) or optical microscopy (for Oil red O staining) (E). Ctl, control.
IL10 Induces Cholesterol Efflux through the PPARγ-ABCA1 Pathway
We next asked if IL10 plays a role in reverse cholesterol transport, especially in the presence of a cholesterol acceptor, such as lipid-free apoA1. Because PPARγ is known to play a role in reverse cholesterol transport, we sought to determine if the effect of IL10 on cholesterol efflux, if any, would depend on PPARγ. To induce cholesteryl ester accumulation, we incubated the cells for 36 h with AcLDL and then treated them with endogenous PPARγ agonist, PGJ2 (5 μm), or specific PPARγ antagonist, GW9662 (10 μm). We subsequently exposed the cells to lipid-free apoAI to induce cholesterol efflux. After 24 h, apoAI treatment induced cholesterol efflux from cholesterol-loaded macrophages, as measured by the changes in cellular cholesterol levels (Fig. 3A). Treatment with both IL10 and PPARγ agonists (PGJ2 and rosiglitazone) markedly stimulated apoAI-mediated cholesterol efflux (Fig. 3, A and B, supplemental Fig. 1), whereas GW9662 completely blocked IL10-induced cholesterol efflux (Fig. 3B). These data demonstrated that IL10 can induce cholesterol efflux from macrophage-derived foam cells and that stimulation of this efflux by IL10 is probably mediated, at least in part, by the PPARγ signaling pathway. Furthermore, targeted deletion of PPARγ in macrophages resulted in total suppression of cholesterol efflux (Fig. 3C), indicating that cholesterol efflux in macrophages after IL10 treatment is dependent on PPARγ activation.
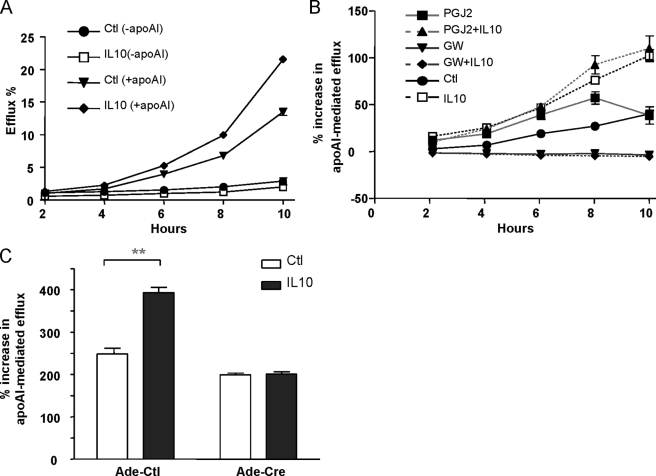
FIGURE 3.
IL10 increases apoAI-mediated cholesterol transport through the PPARγ pathway. A, RAW264.7 cells were labeled with [3H]cholesterol for 36 h in the presence or absence of 10 ng/ml IL10. ApoAI-dependent or -independent cholesterol efflux was measured by incubating [3H]cholesterol-labeled RAW264.7 cells with (+apoAI) or without apoAI (−apoAI) for the indicated times. Radioactivity in the medium was determined as a percentage of total radioactivity in the cells and medium (n = 3, mean ± S.E.). B, to determine if IL10-induced cholesterol efflux was PPARγ-dependent, RAW cells were labeled with loading medium with either medium alone, IL10 (10 ng/ml), PGJ2 (5 μm), GW9662 (10 μm), IL10 plus PGJ2, or IL10 plus GW9662 for 36 h. ApoAI-induced cholesterol efflux was detected by incubating the [3H]cholesterol-laden cells in the efflux medium in the presence or absence of apoAI for the indicated times as described in the supplemental material. C, IL10 fails to induce cholesterol efflux in PPARγ-deficient macrophages. The PPARγflox/flox macrophages were infected with either Ade-Cre or Ade-GFP (control (Ctl)). Two days after infection, virus was washed away, and cells were cultured 1 day without virus, followed by labeling with [3H]cholesterol for 36 h in the presence or absence of 10 ng/ml IL10. ApoAI-induced cholesterol efflux was detected by incubating the [3H]cholesterol-laden cells in the efflux medium in the presence or absence of apoAI for 8 h as described in the supplemental material.
Because PPARγ plays a pivotal role in mediating active free cholesterol efflux and ABCA1 expression is induced by PPARγ activation (19, 20), we wondered if PPARγ- induced ABCA1 is involved in IL10-stimulated reverse cholesterol transport. Real-time PCR results demonstrated that IL10 slightly up-regulated ABCA1 mRNA expression in macrophage-derived foam cells (Fig. 4A), although this was not significant. IL10 treatment resulted in modest increase of ABCA1 expression (Fig. 4, B and C). Furthermore, ABCA1 expression was significantly inhibited by GW9662 (Fig. 4, D and E), leading to the conclusion that the increase in cholesterol efflux induced by IL10 is via up-regulation of ABCA1 expression through PPARγ activation. Based on our findings that lipid accumulation leads to ABCA1 expression in lipid-laden foam cells and that both IL10 and PPARγ agonists (PGJ2 and rosiglitazone) stimulate CD36 expression (supplemental Fig. 2, A and B), it can be stated that PPARγ-mediated cholesterol efflux by IL10 treatment occurs through at least two mechanisms: 1) increase in CD36 expression, which facilitates the uptake of modified LDL and leads to lipid accumulation within cells, and 2) up-regulation of ABCA1 expression, which promotes cholesterol efflux.
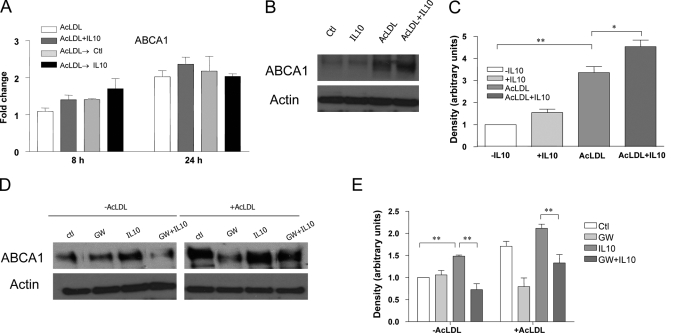
FIGURE 4.
PPARγ-regulated ABCA1 mediates IL10-indcued cholesterol efflux in macrophages. A, transcripts for ABCA1 were detected by real-time PCR either in resting macrophages treated with AcLDL or with AcLDL plus IL10, or in lipid-laden macrophages treated with medium or with IL10 for 8 or 24 h, as indicated. The results are representative of three independent experiments, and values are expressed as the -fold change in abundance ± S.E. B–E, BMDMs were incubated for 24 h with the reagents indicated. Expression of ABCA1 protein was examined by immunoblot analysis using rabbit anti-ABCA1 antibody. Representative results from three experiments are shown in B and D. The densities of bands corresponding to ABCA1 were quantitated using an image densitometer. The data shown are band densities in arbitrary optical density units from three repeated experiments (C and E). Ctl, control.
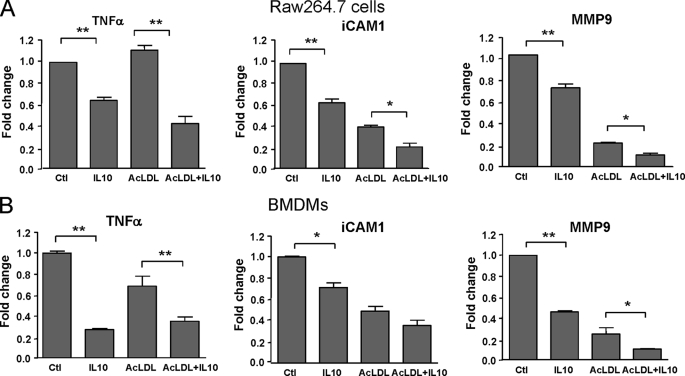
IL10 Inhibits Transcription of Proinflammatory Molecules in Foam Cells
To investigate whether the protective role of IL10 involves regulation of proinflammatory molecules, the expression patterns of TNFα, ICAM-1, and MMP9 in the presence or absence of IL10 were examined using real-time PCR (Fig. 5). The results indicated that there was a dramatic suppression of all three inflammatory molecules by IL10 in both RAW 264.7 cells and BMDMs (Fig. 5). This suggests that IL10, despite its role in promoting cholesteryl ester accumulation and foam cell formation, has a powerful inhibitory effect on the transcription of proinflammatory molecules in foam cells.
FIGURE 5.
IL10 suppresses proinflammatory gene transcription. Transcripts for TNFα, ICAM-1, and MMP9 were detected by real-time PCR in either Raw264.7 macrophages or BMDMs treated with medium alone, AcLDL, IL10, or ACLDL plus IL10 for 24 h. The results are representative of at least three independent experiments, and values are expressed as the -fold change in abundance ± S.E.
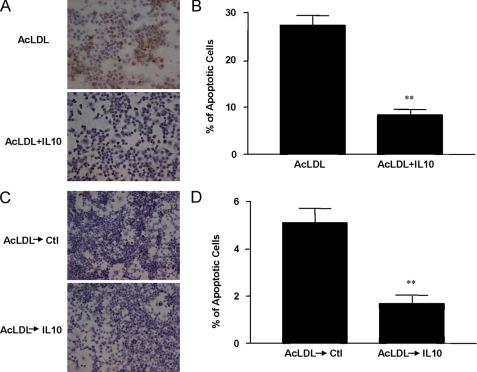
IL10 Protects against Apoptosis of AcLDL-induced Foam Cells
To determine whether IL10 could promote foam cell survival, we measured the expression of cleaved (activated) caspase-3, a marker of apoptotic cells, in lipid-laden foam cells. Apoptosis in the IL10-treated foam cells was substantially attenuated, as exhibited by decreased expression of cleaved caspase 3 (supplemental Fig. 3, A and B). Using TUNEL staining as another indicator of apoptotic cells, we found much fewer TUNEL-positive foam cells in the IL10-treated group compared with the untreated group (Fig. 6, A and C), suggesting that IL10 protected DNA against AcLDL-induced DNA fragmentation (8.3 ± 1.0 and 27.3 ± 2.1% in AcLDL-stimulated cells with and without IL10, respectively; p < 0.05; 1.7 ± 0.3 and 5.1 ± 0.6% in AcLDL-pretreated foam cells in the presence or in the absence or IL10, respectively) (Fig. 6, B and D). These results indicate that IL10 can prevent apoptotic cell death of lipid-laden foam cells.
FIGURE 6.
IL10 inhibits apoptosis in lipid-laden cells. Resting macrophages (Raw264.7 cells) were treated with or without IL10 for 24 h in the presence of AcLDL before TUNEL assay (A). TUNEL-positive macrophages from A were counted and compared (B). In addition, lipid-laden cells obtained by incubation with AcLDL for 36 h were treated with medium for 24 h in the presence or absence of IL10 before testing for apoptosis (C) and counted for TUNEL-positive cells (D). DNA fragmentation in apoptotic cells was assessed by TUNEL assay. Cells were photographed with a bright field microscope. Apoptotic cells were stained specifically in brown. *, p < 0.05 versus untreated group (mean ± S.E., n = 6). Ctl, control.
DISCUSSION
The current established paradigm suggests that macrophages play a critical role in the genesis and progression of atherosclerosis. More specifically, macrophages that have taken up modified lipoproteins and have been transformed into foam cells are thought to be highly atherogenic because of their ability to promote a proinflammatory milieu within the vessel wall. There are two distinct processes that may affect the atherogenicity of macrophage foam cells. First is the uptake of cholesterol from modified lipoproteins and the resultant cholesterol accumulation. The second is the efflux of cholesterol out of the cell through the reverse cholesterol transport system. Traditionally, molecules or agents that promote cholesterol uptake and/or inhibit cholesterol efflux have been considered proatherogenic, whereas molecules or agents that inhibit uptake and/or promote efflux have been considered antiatherogenic.
Our study provides strong evidence that IL10, the prototypic anti-inflammatory cytokine of the Th2 type, can enhance cholesterol uptake as well as cholesterol efflux in macrophages. In contrast to the established paradigm, our data support the hypothesis that enhancement of cholesterol uptake may be athero-protective because this process actively removes the highly atherogenic lipoproteins that have diffused into the vessel wall and have become oxidatively modified. The modified lipoproteins themselves can trigger many proatherogenic responses by enhancing the recruitment of inflammatory cells, such as monocytes and T lymphocytes, and by activating these cells once they are in the subendothelial space (2, 4, 21). Thus, it would be highly beneficial to rid the vessel wall environment of the modified lipoprotein molecules. Macrophages have evolved to express a series of scavenger receptors to recognize and take up these inflammatory lipoprotein molecules. However, the accumulated cholesterol as a result of taking up these molecules itself triggers proatherogenic responses within the vessel wall. The macrophage is also capable of disposing the accumulated cholesterol via a reverse cholesterol transport mechanism that involves shuttling cholesterol via several cholesterol transporters, the main ones being ABCA1 and ABCG1.
There is emerging evidence that foam cell formation mediated by uptake of cholesterol from modified lipoproteins via scavenger receptor may not play a causal role in the atherosclerotic pathogenesis. An intriguing recent finding showed that an increase in atherosclerotic lesion area in male SR-A−/− mice corresponded with a profound reduction in foam cell formation in vivo (22), as measured by cellular cholesterol and cholesteryl ester content, whereas overexpression of SR-A in either the LDLR−/− or ApoE−/− mouse model can attenuate atherosclerosis (23, 24). This suggests that lipid uptake by SRs in these mice may in fact protect against atherosclerosis. Indeed, engagement of SR-A responsible for the uptake of chemically modified lipoprotein can protect cultured macrophages from apoptosis induced by oxysterols and oxidized LDL (25). Additionally, comparisons of atherosclerosis-prone and -resistant rabbit models have shown that SR-A is increased in macrophages of rabbits with low atherosclerotic response (26). Together with our findings, these observations suggest that the increase in the lipid uptake itself does not necessarily instigate a proinflammatory setting and apoptotic cell death.
A distinct feature of early atherosclerotic lesion is the presence of cholesteryl ester-laden macrophages, or foam cells (1, 2). Indeed, cholesterol esterification is a protective response to the accumulation of excess free cholesterol in conditions in which cholesterol efflux pathways become saturated or in the absence of an acceptor, such as apoAI or high density lipoprotein (27). In advanced atherosclerosis, however, there is a progressive increase in the accumulation of unesterified or “free” cholesterol in macrophages as lesions progress (28–30). This event contributes to foam cell death, lesion progression, and lesional necrosis (29, 30), leading eventually to vascular occlusion and acute thrombosis (31–33). Promoting cellular cholesterol efflux during free cholesterol loading strongly protects macrophages against free cholesterol-induced toxicity (29, 30). Important in this process is ABCA1, which mediates cholesterol efflux from these cells in the presence of cholesterol acceptors, such as lipid-free apoAI (34, 35). Analysis of ABCA1-deficient Tangier disease patients has identified ABCA1 as a rate-limiting step in the efflux of cholesterol (36). Tangier disease patients are at an increased risk for cardiovascular disease, including coronary heart disease (35) and atherosclerosis (37, 38). It has been well documented that ABCA1-mediated free cholesterol efflux constitutes a protective mechanism against macrophage lipid overloading (39, 40). Thus, the increase in ABCA1-dependent cholesterol efflux by IL10 is a crucial factor in the prevention of excessive free cholesterol accumulation in macrophages and free cholesterol-induced toxicity.
Cholesterol loading of macrophages leads to the induction of ABCA1 (34, 35, 41). Our current findings demonstrate that ABCA1-mediated cholesterol efflux to apoAI in response to IL10 is PPARγ-dependent. This, along with our uptake data, shows that IL10 triggers pathways for both cholesterol uptake and efflux by up-regulating both CD36 and ABCA1. Previous studies using conditional disruption of the PPARγ gene in mice also showed a reduction in basal and PPARγ ligand-induced expression of CD36, LXRα, ABCA1, and ABCG1 in macrophages and reduced cholesterol efflux (42). Thus, the net result of the action of IL10 in macrophages may be the removal of modified lipoproteins from the artery wall by facilitating the breakdown of these harmful lipoproteins by the lesion macrophages and efficient disposal of cytotoxic cholesterol through reverse cholesterol transport.
Although some results on the mechanism of IL10 action on macrophage have been previously reported, the data have been controversial (13–15). Although IL10 can promote lipid accumulation and foam cell formation (14), IL10 can also prevent macrophage cholesterol overloading and stimulate cholesterol efflux (13, 15). As such, the role of IL10 in modulating cholesterol homeostasis in macrophages remained enigmatic. Our findings help to clarify this confusion by showing that IL10 promotes not only the uptake of modified LDL via CD36 and SR-A but also the cholesterol efflux via the PPARγ-ABCA1 pathway. It is important to note that unlike the previous studies, which utilized resting macrophages to assess the role of IL10 in macrophage lipid metabolism (13–15), our studies utilized a more physiologically relevant model of lipoprotein-induced foam cells to assess not only how IL10 affects foam cell formation itself but how it alters the function of macrophages after they have been transformed into foam cells.
Excessive inflammation within the arterial wall is a risk factor for cardiovascular disease and promotes atherogenesis (2, 3). Foam cells in lesion can exacerbate the inflammation by secreting proinflammatory cytokines that can affect virtually every cell type in the lesion (2, 4, 27). IL10 has potent anti-inflammatory properties, including inhibition of proinflammatory cytokines and chemokines as well as the expression of endothelial adhesion molecules (11, 43, 44). Consistent with these findings, we showed that IL10 markedly inhibited the expression of ICAM-1, MMP9, and TNFα, all of which are considered proatherogenic, in lipid-laden macrophages (Fig. 5). Because IL10 suppresses NFκB activity (45), which induces expression of a variety of proinflammatory molecules, such as TNFα, MMP9, and ICAM-1, it is likely that IL10 suppressed expression of these proinflammatory molecules by inhibiting the NF-κB signaling pathway.
Apoptosis of macrophages plays an important role in the formation of the necrotic core during the progression of atherosclerosis. In the present study, IL10 markedly inhibited apoptosis in lipid-loaded foam cells despite its role in lipid accumulation and foam cell formation (Fig. 6 and supplemental Fig. 2). We have previously demonstrated that overexpressing IL10 in T-lymphocytes markedly attenuates macrophage apoptosis in atherosclerotic lesions of mice (46). Supporting our results are previous studies of human atherosclerotic plaques, which revealed that increased local levels of IL10 are associated with decreased apoptotic activity within diseased human arteries (8).
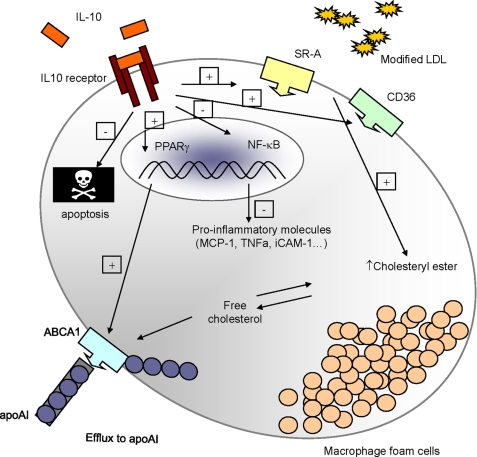
In conclusion, although IL10 treatment of macrophages resulted in increased uptake of AcLDL through up-regulation of scavenger receptors (Fig. 1), the inflammatory response was inhibited (Fig. 5), as was apoptosis of the foam cells (Fig. 6 and supplemental Fig. 3). Thus, the functional role of scavenger receptors in foam cells and the role of foam cells in atherosclerosis may be more complex than previously envisioned and need to be extensively studied in a variety of experimental models. The results from the present study show that the uptake of modified LDL and foam cells themselves are perhaps not necessarily detrimental, as long as mechanisms for cholesterol effluxing and for transferring cholesterol to an exogenous acceptor are functioning robustly. Our results present a comprehensive antiatherogenic role of IL10 in macrophages, including enhancing disposal of harmful lipoproteins, inhibition of inflammatory molecules, and reduced apoptosis, as shown schematically in Fig. 7. Together with earlier studies, this work demonstrated the feasibility of involving IL10 in a therapeutic strategy for an antiatherogenic treatment.
FIGURE 7.
Schematic overview of the protective role of IL10 during atherosclerosis involving regulation of lipid metabolism in macrophages. Upon binding to its receptor, IL10 up-regulates scavenger receptors, such as SR-A (SR-AI and SR-AII) and CD36, which account for a dramatic increase in modified LDL uptake by macrophages, promoting cholesteryl ester accumulation and foam cell formation. Moreover, IL10 promotes ABCA1-mediated free cholesterol efflux to apoAI in a PPARγ-dependent manner. Despite its effect on promoting foam cell formation, IL10 markedly represses the expression of proinflammatory molecules, such as TNFα, MCP-1, and ICAM-1, presumably through an inhibition of NF-κB activity, as documented before (45), and diminishes apoptosis in the lipid-laden foam cells.
Supplementary Material
Acknowledgment
We thank Hongwei Wang for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant HL075677 (to W. A. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- LDL
- low density lipoprotein
- AcLDL
- acetylated LDL
- IL10
- interleukin-10
- SR
- scavenger receptor
- apoA1
- apolipoprotein A1
- TUNEL
- terminal dUTP nick-end labeling
- BMDM
- bone marrow-derived macrophage
- DMEM
- Dulbecco's modified Eagle's medium
- TNF
- tumor necrosis factor
- ICAM-1
- intercellular adhesion molecule 1.
REFERENCES
- 1.Ross R. (1995) Annu. Rev. Physiol. 57, 791–804 [DOI] [PubMed] [Google Scholar]
- 2.Glass C. K., Witztum J. L. (2001) Cell 104, 503–516 [DOI] [PubMed] [Google Scholar]
- 3.Lusis A. J. (2000) Nature 407, 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P. (2002) Nature 420, 868–874 [DOI] [PubMed] [Google Scholar]
- 5.de Winther M. P., Hofker M. H. (2000) J. Clin. Invest. 105, 1039–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunjathoor V. V., Febbraio M., Podrez E. A., Moore K. J., Andersson L., Koehn S., Rhee J. S., Silverstein R., Hoff H. F., Freeman M. W. (2002) J. Biol. Chem. 277, 49982–49988 [DOI] [PubMed] [Google Scholar]
- 7.Acton S. L., Scherer P. E., Lodish H. F., Krieger M. (1994) J. Biol. Chem. 269, 21003–21009 [PubMed] [Google Scholar]
- 8.Mallat Z., Heymes C., Ohan J., Faggin E., Lesèche G., Tedgui A. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 611–616 [DOI] [PubMed] [Google Scholar]
- 9.Pinderski Oslund L. J., Hedrick C. C., Olvera T., Hagenbaugh A., Territo M., Berliner J. A., Fyfe A. I. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2847–2853 [DOI] [PubMed] [Google Scholar]
- 10.Smith D. A., Irving S. D., Sheldon J., Cole D., Kaski J. C. (2001) Circulation 104, 746–749 [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka T., Okada T., Maeda Y., Ikeda U., Shimpo M., Nomoto T., Takeuchi K., Nonaka-Sarukawa M., Ito T., Takahashi M., Matsushita T., Mizukami H., Hanazono Y., Kume A., Ookawara S., Kawano M., Ishibashi S., Shimada K., Ozawa K. (2004) Gene Ther. 11, 1772–1779 [DOI] [PubMed] [Google Scholar]
- 12.Anguera I., Miranda-Guardiola F., Bosch X., Filella X., Sitges M., Marín J. L., Betriu A., Sanz G. (2002) Am. Heart J. 144, 811–817 [DOI] [PubMed] [Google Scholar]
- 13.Rubic T., Lorenz R. L. (2006) Cardiovasc. Res. 69, 527–535 [DOI] [PubMed] [Google Scholar]
- 14.Halvorsen B., Waehre T., Scholz H., Clausen O. P., von der Thüsen J. H., Müller F., Heimli H., Tonstad S., Hall C., Frøland S. S., Biessen E. A., Damås J. K., Aukrust P. (2005) J. Lipid Res. 46, 211–219 [DOI] [PubMed] [Google Scholar]
- 15.Mei C. L., Chen Z. J., Liao Y. H., Wang Y. F., Peng H. Y., Chen Y. (2007) Cell Biol. Int. 31, 1456–1461 [DOI] [PubMed] [Google Scholar]
- 16.Gocze P. M., Freeman D. A. (1994) Cytometry 17, 151–158 [DOI] [PubMed] [Google Scholar]
- 17.He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J. M., Evans R. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C. H., Chawla A., Urbiztondo N., Liao D., Boisvert W. A., Evans R. M., Curtiss L. K. (2003) Science 302, 453–457 [DOI] [PubMed] [Google Scholar]
- 19.Chawla A., Boisvert W. A., Lee C. H., Laffitte B. A., Barak Y., Joseph S. B., Liao D., Nagy L., Edwards P. A., Curtiss L. K., Evans R. M., Tontonoz P. (2001) Mol. Cell 7, 161–171 [DOI] [PubMed] [Google Scholar]
- 20.Chinetti G., Lestavel S., Bocher V., Remaley A. T., Neve B., Torra I. P., Teissier E., Minnich A., Jaye M., Duverger N., Brewer H. B., Fruchart J. C., Clavey V., Staels B. (2001) Nat. Med. 7, 53–58 [DOI] [PubMed] [Google Scholar]
- 21.Ross R. (1999) N. Engl. J. Med. 340, 115–126 [DOI] [PubMed] [Google Scholar]
- 22.Moore K. J., Kunjathoor V. V., Koehn S. L., Manning J. J., Tseng A. A., Silver J. M., McKee M., Freeman M. W. (2005) J. Clin. Invest. 115, 2192–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Eck M., De Winther M. P., Herijgers N., Havekes L. M., Hofker M. H., Groot P. H., Van Berkel T. J. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2600–2606 [DOI] [PubMed] [Google Scholar]
- 24.Whitman S. C., Rateri D. L., Szilvassy S. J., Cornicelli J. A., Daugherty A. (2002) J. Lipid Res. 43, 1201–1208 [PubMed] [Google Scholar]
- 25.Liao H. S., Kodama T., Geng Y. J. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1968–1975 [DOI] [PubMed] [Google Scholar]
- 26.Teupser D., Stein O., Burkhardt R., Nebendahl K., Stein Y., Thiery J. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 1299–1305 [DOI] [PubMed] [Google Scholar]
- 27.Li A. C., Glass C. K. (2002) Nat. Med. 8, 1235–1242 [DOI] [PubMed] [Google Scholar]
- 28.Mitchinson M. J., Hardwick S. J., Bennett M. R. (1996) Curr. Opin. Lipidol. 7, 324–329 [DOI] [PubMed] [Google Scholar]
- 29.Warner G. J., Stoudt G., Bamberger M., Johnson W. J., Rothblat G. H. (1995) J. Biol. Chem. 270, 5772–5778 [DOI] [PubMed] [Google Scholar]
- 30.Tabas I., Marathe S., Keesler G. A., Beatini N., Shiratori Y. (1996) J. Biol. Chem. 271, 22773–22781 [DOI] [PubMed] [Google Scholar]
- 31.Mallat Z., Besnard S., Duriez M., Deleuze V., Emmanuel F., Bureau M. F., Soubrier F., Esposito B., Duez H., Fievet C., Staels B., Duverger N., Scherman D., Tedgui A. (1999) Circ. Res. 85, e17–e24 [DOI] [PubMed] [Google Scholar]
- 32.Fazio S., Babaev V. R., Murray A. B., Hasty A. H., Carter K. J., Gleaves L. A., Atkinson J. B., Linton M. F. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4647–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolodgie F. D., Narula J., Burke A. P., Haider N., Farb A., Hui-Liang Y., Smialek J., Virmani R. (2000) Am. J. Pathol. 157, 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tall A. R., Wang N. (2000) J. Clin. Invest. 106, 1205–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attie A. D., Kastelein J. P., Hayden M. R. (2001) J. Lipid Res. 42, 1717–1726 [PubMed] [Google Scholar]
- 36.Bodzioch M., Orsó E., Klucken J., Langmann T., Böttcher A., Diederich W., Drobnik W., Barlage S., Büchler C., Porsch-Ozcürümez M., Kaminski W. E., Hahmann H. W., Oette K., Rothe G., Aslanidis C., Lackner K. J., Schmitz G. (1999) Nat. Genet. 22, 347–351 [DOI] [PubMed] [Google Scholar]
- 37.Clee S. M., Kastelein J. J., van Dam M., Marcil M., Roomp K., Zwarts K. Y., Collins J. A., Roelants R., Tamasawa N., Stulc T., Suda T., Ceska R., Boucher B., Rondeau C., DeSouich C., Brooks-Wilson A., Molhuizen H. O., Frohlich J., Genest J., Jr., Hayden M. R. (2000) J. Clin. Invest. 106, 1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dam M. J., de Groot E., Clee S. M., Hovingh G. K., Roelants R., Brooks-Wilson A., Zwinderman A. H., Smit A. J., Smelt A. H., Groen A. K., Hayden M. R., Kastelein J. J. (2002) Lancet 359, 37–42 [DOI] [PubMed] [Google Scholar]
- 39.Cavelier L. B., Qiu Y., Bielicki J. K., Afzal V., Cheng J. F., Rubin E. M. (2001) J. Biol. Chem. 276, 18046–18051 [DOI] [PubMed] [Google Scholar]
- 40.Singaraja R. R., Fievet C., Castro G., James E. R., Hennuyer N., Clee S. M., Bissada N., Choy J. C., Fruchart J. C., McManus B. M., Staels B., Hayden M. R. (2002) J. Clin. Invest. 110, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costet P., Luo Y., Wang N., Tall A. R. (2000) J. Biol. Chem. 275, 28240–28245 [DOI] [PubMed] [Google Scholar]
- 42.Akiyama T. E., Sakai S., Lambert G., Nicol C. J., Matsusue K., Pimprale S., Lee Y. H., Ricote M., Glass C. K., Brewer H. B., Jr., Gonzalez F. J. (2002) Mol. Cell Biol. 22, 2607–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang R., Rutschman R. L., Greaves D. R., Murray P. J. (2002) J. Immunol. 168, 3402–3411 [DOI] [PubMed] [Google Scholar]
- 44.Trogan E., Choudhury R. P., Dansky H. M., Rong J. X., Breslow J. L., Fisher E. A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P., Wu P., Siegel M. I., Egan R. W., Billah M. M. (1995) J. Biol. Chem. 270, 9558–9563 [DOI] [PubMed] [Google Scholar]
- 46.Pinderski L. J., Fischbein M. P., Subbanagounder G., Fishbein M. C., Kubo N., Cheroutre H., Curtiss L. K., Berliner J. A., Boisvert W. A. (2002) Circ. Res. 90, 1064–1071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.