Abstract
E47 is a basic helix-loop-helix transcription factor involved in neuronal differentiation and survival. We had previously shown that the basic helix-loop-helix protein E47 binds to E-box sequences within the promoter of the TrkB gene and activates its transcription. Proper expression of the TrkB receptor plays a key role in development and function of the vertebrate nervous system, and altered levels of TrkB have been associated with important human diseases. Here we show that E47 interacts with MLK2, a mixed lineage kinase (MLK) involved in JNK-mediated activation of programmed cell death. MLK2 enhances phosphorylation of the AD2 activation domain of E47 in vivo in a JNK-independent manner and phosphorylates in vitro defined serine and threonine residues within a loop-helix structure of AD2 that also contains a putative MLK docking site. Although these residues are essential for MLK2-mediated inactivation of E47, inhibition of MLKs by CEP11004 causes up-regulation of TrkB at a transcriptional level in cerebellar granule neurons and differentiating neuroblastoma cells. These findings allow us to propose a novel mechanism by which MLK regulates TrkB expression through phosphorylation of an activation domain of E47. This molecular link would explain why MLK inhibitors not only prevent activation of cell death processes but also enhance cell survival signaling as a key aspect of their neuroprotective potential.
Introduction
Basic helix-loop-helix (bHLH)3 proteins are transcription factors that regulate gene expression to promote cell differentiation and tissue-specific cellular functions (1). For instance, NeuroD and MyoD are tissue-specific bHLH proteins involved in neurogenesis and myogenesis, respectively (2, 3). These tissue-specific proteins form dimers with other ubiquitously expressed bHLH transcription factors called E proteins, which bind to the canonical E-box sequence CANNTG and include HEB, E2–2, and the E2A gene products E12 and E47 (1). The formation of active heterodimers can be inhibited by overexpression of Id family members, which bind to and sequester E2A proteins into transcriptionally nonfunctional complexes (4). There are two activation domains within E12 and E47 proteins: AD1, which is found between amino acids 1 and 99 (5, 6), and AD2, which is between amino acids 325 and 432 (7). The AD2 domain contains a region between amino acids 345 and 408 consisting of a loop adjacent to an amphipathic α-helix, the loop-helix (LH) motif, which is conserved in yeast, Drosophila, and mammalian cells (7). c-Jun specifically represses the activity of the LH domain, and the selective recognition by c-Jun of this activation domain in pancreatic β cells suggests an important function of E2A proteins in regulating insulin control element-mediated expression (8). In addition, E2A transcriptional activity can be modulated by phosphorylation at different levels. It has been reported that phosphorylation status of E47 alters the DNA binding ability of E47 as homodimers or heterodimers in different cellular contexts (9–11). Thus, p38 MAPK has been described to phosphorylate E47 at Ser140 and promote MyoD/E47 association and muscle-specific gene transcription (12). On the other hand, it has also been observed that MEKK1 (MAPK/ERK kinase kinase 1) signaling through p38 leads to transcriptional inactivation of E47 (13). In addition, Ser/Thr kinases 3pK and MAPK-activated protein kinase 2 have been shown to interact with E47 and repress its transcriptional activity (14). Finally, E47 proteasomal degradation is associated with increased ERK MAPK activity in aged B cell precursors (15, 16).
MLKs are a family of serine/threonine kinases, all of which act as mitogen-activated protein kinase kinase kinases (17). The name “mixed lineage kinases” derives from the fact that of the 11 conserved subdomains found in all protein kinases, domains 1–8 of the MLK proteins resemble serine/threonine kinases, whereas regions 9–11 share sequence similarity with those of tyrosine kinases, such as the fibroblast growth factor receptor and Src (17–19). Eight mammalian MLKs have been identified and categorized into three subfamilies on the basis of domain organization and sequence similarity: MLKs, dual leucine zipper-bearing kinases (DLKs), and zipper sterile α-motif kinases (ZAKs). However, MLK2, MLK3, DLK, and leucine zipper kinase (LZK) are the only MLKs that have been studied in any detail at a biochemical level (20–22). Although MLK3 and LZK are expressed widely, MLK2 and DLK are restricted to brain. MLK1–4 share a high degree of homology in the N-terminal 500 amino acids, which incorporate several functional domains: an SH3 domain, a kinase catalytic domain, two leucine zippers, a proline-rich region, and the Cdc42/Rac interactive binding (CRIB) domain, which mediates binding to GTP-bound Cdc42 and Rac. In contrast to the N-terminal portion of these proteins, the C termini share little homology and vary greatly in size, possibly to mediate specificity and orchestrate interactions with various other proteins. MLKs are considered primarily as kinases that act upstream of JNKs. Nonetheless, mammalian MLK3 is localized to the centrosome, and its activity is enhanced during G2/M phase transition, when the JNK pathway remains inactive (23). It has been reported that silencing MLK3 can block mitogen-stimulated B-Raf activation and prevent serum- or Ki-Ras-stimulated cell proliferation (24). In addition, MLK2 has been involved in modulation of NeuroD and Alien transcription factors (25, 26). Thus, MLKs may be involved in very different processes depending on the particular molecular context.
Little is known about the molecular effectors of MLK activity in response to external or internal inputs, but the available evidence suggests that the activity of MLK proteins is controlled by phosphorylation (27). The most striking feature of all MLK proteins is their ability to dimerize, and MLK3 autophosphorylates following homodimerization (27) via a mechanism analogous to that of receptor tyrosine kinases (with which MLKs share some sequence homology). Activation of MLK3 by Cdc42 and Rac has been shown to be a pathway for JNK activation by these G-proteins (28). Two Cdc42-inducible autophosphorylation sites in MLK3 have been identified, but it is unclear how Cdc42 induces MLK3 phosphorylation. One speculation was that the association of the CRIB domain with a GTP-bound G-protein would displace an intramolecular SH3-mediated interaction and permit homodimerization via the leucine zipper and autophosphorylation.
The activation of the JNK pathway is critical for the naturally occurring neuronal cell death in development and may be important in the pathological neuronal cell death of neurodegenerative diseases. The small molecule MLK inhibitors CEP1347 and CEP11004 prevent the activation of the JNK pathway and consequently reduce neuronal cell death in many cell culture and animal models (29). Thus, the cell death program induced by nerve growth factor deprivation in sympathetic neurons is inhibited by MLK inhibitors as a direct consequence of inhibiting downstream kinases of the JNK pathway such as MKK4 and JNK and JNK-activated transcription factors such as c-Jun (30, 31). Intriguingly, it has been reported that MLK inhibition by CEP11004 increases TrkB protein levels in cultures of cerebellar granule neurons (32). Because TrkB is a key tyrosine receptor for brain-derived neurotrophic factor involved in differentiation and survival in the nervous system (33), MLK inhibitors would also up-regulate cell survival circuits. Previous work in our laboratory has demonstrated that E47 and NeuroD bHLH proteins bind to p21CIP1 and TrkB promoters linking differentiation and cell cycle arrest in SH-SY5Y neuroblastoma cells (34). In this study we show that MLK2 binds to and phosphorylates E47 at the AD2 transactivation domain and, as a consequence, represses TrkB promoter activity. Our results provide a molecular link that explains why MLK inhibition not only prevents activation of cell death processes but also enhances cell survival signaling.
EXPERIMENTAL PROCEDURES
Plasmid Constructions
Plasmids pCMV-E47 (35) and SRD-MLK2 (36) contain the full-length cDNAs of human E47 and MLK2, respectively. MLK2 was tagged at its N terminus with a triple FLAG epitope and subcloned under the cytomegalovirus promoter to produce pCYC573, where several derivatives containing different fragments from MLK2 were obtained: pCYC833 (1–364 aa), pCYC843 (1–496 aa), pCYC844 (1–757 aa), and pCYC716 (327–954 aa). A K125A mutation was introduced to obtain a kinase-dead MLK2 mutant in pCYC742 using XL site-directed mutagenesis kit (Stratagene). A tandem affinity purification (TAP)-E47 fusion under the cytomegalovirus promoter (pCYC304) was also used to obtain derivatives with different fragments of E47: pCYC306 (1–220 aa), pCYC831 (221–330 aa), pCYC830 (331–400 aa), pCYC925 (331–460 aa), pCYC305 (1–540 aa), pCYC 307 (100–540 aa), and pCYC308 (100–490 aa). Point mutations within full-length E47 produced plasmids pCYC805 (S379A), pCYC899 (S352A/T355A/S359A/S379A, referred to as S/T4A), and pCYC920 (S341A/S352A/T355A/S359A/S379A, referred to as S/T5A). Point mutations within TAP-tagged E47 peptides produced plasmids pCYC836 (S341A), pCYC837 (S352A, T355A, S359A), pCYC838 (S379A), and pCYC926 (S/T5A) pGEX-KG (GE Life Sciences) derivatives containing GST fusions to E47 peptides were pCYC907 (331–460 aa) and pCYC903 (361–460 aa), and corresponding S/T5A (S341A/S352A/T355A/S359A/S379A) and S379A derivatives were pCYC921 and pCYC905, respectively. N-terminal (1–496 aa) wild-type and kinase-dead (K125A) MLK2 fusions to GST produced pCYC846 and pCYC848, respectively. The TrkB luciferase reporter construct (pCYC219) carries a 1.9-kb fragment from positions −1888 to +30 nucleotides relative to the main transcription start site of human TrkB promoter inserted into pGL3b (Promega).
Cell Culture
SH-SY5Y neuroblastoma cells, mouse embryonic fibroblasts (MEFs), HEK293T cells, and GP2–293T cells were grown at 37 °C in a humidified atmosphere of 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 20 units/ml penicillin, 20 μg/ml streptomycin, and 10% fetal bovine serum (Invitrogen). To induce cell cycle arrest and differentiation in SH-SY5Y cells, all-trans-retinoic acid (RA; Sigma) was added to a final concentration of 10 μm for 5 days. The medium was changed every 3 days. MEFs and SH-SY5Y cells were transfected with Lipofectamine 2000 (Invitrogen), and HEK293T and GP2–293T were transfected with polyethylenimine (Sigma-Aldrich), following standard protocols. Primary cultures of cerebellar granule neurons were prepared from postnatal day 7 Sprague-Dawley rat pups as described previously (32). The cells were dissociated by 1 mg/ml trypsin for 15 min prior to mechanical trituration, plated in poly-l-lysine-coated dishes at a density of 3 × 105 cells/ml and maintained in basal Eagle's medium with 25 mm KCl, 10% dialyzed fetal bovine serum, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Aphidicolin (3.3 μg/ml) was added 1 day later to reduce the number of non-neuronal cells. The MLK inhibitor CEP-11004 was kindly provided by Cephalon, Inc. (West Chester, PA). Stocks solutions of CEP-11004 (4 mm) were prepared in dimethyl sulfoxide, and a working 40 μm solution was prepared in 1% bovine serum albumin/basal Eagle's medium on the day of the experiment.
Tandem Affinity Purification
TAP-tagged N-terminal (1–220 aa) or C-terminal (100–651 aa) human E47 fragments or TAP control cDNAs were cloned into a retroviral expression vector (pBABE-puro) to obtain pCYC313, pCYC315 and pCYC316, respectively. Retroviruses were produced in GP2–293T packaging cells by transfection of pBABE-puro derivatives and helper pVSV-G. The cells were maintained at 32 °C in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Supernatant containing viruses was recovered after 48, 72, and 96 h, filtered (0.45 μm), and used for SH-SY5Y infection with the addition of 5 μg/ml polybrene. After 2 days, the medium was removed, and infected cells were selected with 0.25 μg/ml puromycin. A pBABE-puro derivative expressing green fluorescent protein (pCYC186) was used to estimate infection efficiencies. Ten 150-mm plates (8 × 106 cells seeded), and twenty 150-mm plates (5 × 106 cells seeded) were used for TAP experiments with proliferating or RA-differentiated SH-SY5Y cells, respectively. The cells were lysed in TNT buffer (10 mm Tris-HCl, pH 8, 150 mm NaCl, 0.1% Triton X-100, 1 mm DTT) with 2 mm MgCl2 and protease and phosphatase inhibitors and centrifuged for 3 min at 200 × g. Triton X-100 was raised to 1% whenever the large C-terminal fragment of E47 was used. Obtained supernatants were first used for immunoprecipitation with 100 μl of IgG Sepharose 6 Fast Flow beads (Amersham Biosciences) for 2 h. After five washes, 300 units/ml AcTEV protease (Invitrogen) was added to the column, which was incubated for 2 h at room temperature to elute E47 in TNT buffer with 0.5 mm EDTA. The second immunoprecipitation was carried out with calmodulin beads (Stratagene) in TNT buffer with 1 mm magnesium acetate, 1 mm imidazole, and 2 mm CaCl2. After five washes, calmodulin beads were suspended in 50 mm NH4CO3H, pH 8, and proteins were analyzed by nano-liquid chromatography coupled to nano-electrospray ionization and tandem mass spectrometry analysis.
Protein Phosphatase Treatment
HEK293T cells were co-transfected with E47 and MLK2 expression plasmids in 60-mm plates. One day after transfection, the cells were lysed in 200 μl of 20 mm HEPES-KOH, pH 7.9, 125 mm NaCl, 0.1% Nonidet P-40, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, and protease inhibitors; sonicated on ice; and spun for 10 min at 12,000 × g. Supernatant was divided into 50-μl aliquots and treated with 4 units of SAP (shrimp alkaline phosphatase; Roche Applied Science) or 400 units of λ protein phosphatase (NEB) as directed by the supplier. Phosphatase inhibitors (1 mm sodium fluoride, 1 mm β-glycerophosphate, 5 mm sodium pyrophosphate, 1 mm EGTA) were added when indicated. The samples were incubated 90 min at 37 °C (SAP) or 30 °C (λ phosphatase). To stop the reaction, 2× SDS-PAGE loading buffer was added, and the samples were analyzed by Western blot.
Immunoprecipitation and Western Blot Analysis
For immunoprecipitation experiments, HEK293T cells were harvested 24 h after transfection. The cells were resuspended in lysis buffer (20 mm HEPES-KOH, pH 7.9, 125 mm NaCl, 0.1% Nonidet P-40, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, protease, and phosphatase inhibitors), sonicated on ice, and spun for 10 min at 12,000 × g. The supernatants were incubated with 50 μl of αFLAG M2-agarose beads (Sigma). The samples were rocked for 2 h at 4 °C, and the beads were collected by centrifugation (1 min at 2000 × g), washed three times with 1 ml of cold lysis buffer, and finally resuspended in loading buffer, boiled, and loaded onto SDS-PAGE gels. Western blot analysis was performed as previously described (34) with αE47 (SC-763), αFLAG (Sigma), horseradish peroxidase-α-horseradish peroxidase (Sigma), αpS63-c-Jun (Cell Signaling), and αJNK (Cell Signaling) antibodies used as recommended by the suppliers.
Luciferase Assays
Luciferase were performed essentially as described (34) with a dual luciferase reporter assay system (Promega). Routinely, 1 μg of firefly luciferase reporter plasmid and 0.05 μg of Renilla luciferase pRL-TK control plasmid (Promega) were used to determine relative expression values. To assess effects caused by E47 or MLK2, 0.8 or 0.2 μg of each expression plasmid (or empty vector) were added to each transfection assay. Firefly/Renilla luciferase ratios obtained from a promoterless vector were substracted as background, and the resulting values were normalized to control conditions.
Production and Purification of Recombinant Proteins
All of the GST fusions were expressed in Escherichia coli BL21 (DE3) by adding 0.4 mm isopropyl-β-d-thiogalactopyranoside to cultures at a cell density of 0.3 A600 and subsequent incubation for 4 h at 30 °C. The proteins were purified using glutathione-Sepharose beads as directed by the supplier (Amersham Biosciences) in 500 μl of lysis buffer containing 25 mm HEPES, pH 7.9, 0.3 m KCl, 1 mm EDTA, 0.1% Nonidet P-40, 10% glycerol, 1 mm DTT, and protease inhibitors. Elution buffer contained 40 mm reduced glutathione, 20 mm NaCl, 0.5 mm DTT, and 50 mm Tris-HCl, pH 8.
In Vitro Kinase Assay
Kinase reactions were carried out in 20 μl (20 mm HEPES, pH 7.5, 15 mm MgCl2, 2 mm DTT, 0.1 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, protease, and phosphatase inhibitors) containing 25 μm nonradioactive ATP, 5 μCi of [γ-32P]ATP, 0.2 μg of GST-MLK2 (or GST-MLK2 KD), and 2 μg of the corresponding substrate proteins and were incubated for 20 min at 30 °C. Phosphorylated products were separated by SDS-PAGE, stained with Coomassie Brilliant Blue to ensure equal loading of substrate proteins and MLK2 kinase, dried, and analyzed by autoradiography.
Reverse Transcription Real Time PCR
RNA was extracted (RNeasy kit; Qiagen), treated with DNaseI (Qiagen), and reverse transcribed with Superscript II reverse transcriptase (Invitrogen). Relative mRNA levels were determined by quantitative real time PCR using TaqMan probes from Applied Biosystems, TrkB (Hs 001778811_m1), and transferrin receptor (TFRC) (4333770F) for SH-SY5Y cells, TrkB 5′(Rn 01441749), TrkB 3′ (Rn 00820626), and glyceraldehyde-3-phosphate dehydrogenase (Rn 01749022_g1) for cerebellar granule neurons. PCRs were run and analyzed with an iCycler iQ real time detection system (Bio-Rad).
RESULTS
E47 Interacts with MLK2
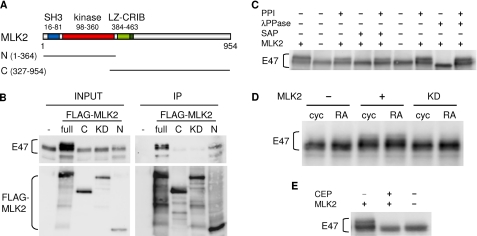
To identify neuronal proteins that interact with transcriptional complexes containing E47, we carried out TAP experiments using E47 as bait in SH-SY5Y human neuroblastoma cells either proliferating or undergoing differentiation with RA (37). SH-SY5Y cells were infected with retroviral vectors expressing TAP-tagged human N-terminal (1–220 aa) or C-terminal (100–651 aa) E47 moieties and subsequently selected by puromycin treatment. Purified E47-TAP complexes were analyzed by nano-liquid chromatography coupled to nano-electrospray ionization and tandem mass spectrometry analysis. One of the identified peptides specifically present in the N-terminal E47-TAP pulldown was matched to MLK2, one of the mixed lineage kinases that had been initially identified as signaling effectors in the nervous system (17, 38, 39). To confirm and analyze further the interaction between E47 and MLK2, HEK293T cells were transfected with full-length E47 and different FLAG-tagged MLK2 constructs (Fig. 1A), and corresponding cell extracts were used for immunoprecipitation experiments with αFLAG beads. Although the interaction of E47 with full-length FLAG-MLK2 was readily detected (Fig. 1B), the co-immunoprecipitation efficiency decreased significantly when only the C-terminal moiety of MLK2 was used, suggesting that the interacting region would lie in the N-terminal sequences of MLK2 that include the SH3 motif and the kinase domain. In agreement with this, the N-terminal region of MLK2 was sufficient to co-immunoprecipitate E47 at a similar relative efficiency compared with full-length MLK2. Finally, supporting a key role for the kinase activity of MLK2, a kinase-dead mutant of MLK2 was unable to co-immunoprecipitate E47 (Fig. 1B). Because MLKs undergo autophosphorylation to become fully active (17, 19), this result suggests that autophosphorylation might also be important to acquire a competent conformation to interact with E47. NeuroD overexpression did not affect co-immunoprecipitation of E47 and FLAG-MLK2, suggesting that NeuroD would not mediate their interaction (25). Indeed, MLK2 requires its LZ domain to interact with NeuroD (25), although it is not involved in its interaction with E47 (Fig. 1B).
FIGURE 1.
MLK2 interacts with E47 enhancing its phosphorylation level. A, scheme showing the different domains in MLK2. SH3, Src homology 3 motif; LZ, leucine zipper. The N-terminal (N, 1–364 aa) and C-terminal (C, 327–954 aa) MLK2 moieties used in immunoprecipitation assays are depicted. B, in vivo interaction between MLK2 and E47. HEK293T cells were transfected with full E47 and the indicated FLAG-MLK2 (full, N-terminal, C-terminal, and kinase-dead (KD) mutant) constructs. Immunoprecipitation was carried out with αFLAG beads, and E47 and FLAG-MLK2 levels in input and immunoprecipitation (IP) samples were analyzed by Western blot. C, E47 phosphorylation by MLK2 in HEK293T cells. Cell extracts from HEK293T transfected with E47 and MLK2 were treated with λ (λPPase) or shrimp alkaline (SAP) phosphatases and/or phosphatase inhibitors (PPI). E47 phosphorylation was assessed as a shift in mobility by Western blot. D, E47 phosphorylation by MLK2 in SH-SY5Y cells. Western blot analysis of E47 in the presence of transfected MLK2 or its kinase-dead mutant (KD) in proliferating (cyc) or RA-differentiating (RA) SH-SY5Y cells. E, effect of the MLK2 inhibitor CEP11004 on MLK2-mediated E47 phosphorylation. HEK293T cells were transfected with MLK2 and E47 and treated with 400 nm CEP11004 for 24 h. E47 phosphorylation levels were analyzed by Western blot.
MLK2 Enhances Phosphorylation of E47
A shift in the mobility of E47 was visible by Western blot of HEK293T cell extracts when wild-type MLK2, but not the kinase-dead mutant (KD), was co-expressed (Fig. 1B). Because MLK2 has been shown to be constitutively active when overexpressed (36), our observations would suggest that MLK2 phosphorylates E47, either directly or indirectly. The retarded E47 bands caused by MLK2 co-expression collapsed into a faster migrating band after treatment of cell extracts with shrimp alkaline phosphatase (Fig. 1C), demonstrating that MLK2 enhances phosphorylation of E47. Moreover, treatment of cell extracts with λ protein phosphatase also revealed a basal phosphorylation status for E47 in HEK293T cells, which likely reflects the demonstrated participation of other kinases in E47 phosphorylation (9–16). A kinase-dependent shift in E47 mobility was also observed in extracts from SH-SY5Y cells transfected with MLK2, either cycling or undergoing differentiation with RA (Fig. 1D). Finally, phosphorylation of E47 was inhibited when MLK2-expressing cells were treated with CEP11004, a specific MLK inhibitor (Fig. 1E). In summary, our results indicate that MLK2 induces E47 phosphorylation in vivo.
MLK2 Inhibits E47 Transactivation Activity on the TrkB Promoter
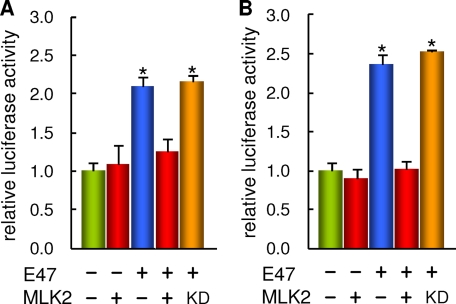
Because MLK2 had been implicated in NeuroD modulation (25) and nuclear receptor co-repression (26), we wanted to study the effects of MLK2 overexpression on E47 transcriptional activity. We had shown that E47 is able to activate the TrkB promoter through E-box sequences important for achieving full transcriptional activity during SH-SY5Y differentiation (34). Thus, we used our previously established luciferase-reporter assay to analyze the role of MLK2 on E47 transcriptional activity. MLK2 was able to inhibit most effects caused by E47 co-expression on TrkB-promoter reporter expression in both proliferating and RA-treated cells (Fig. 2), whereas it had no significant effect on corresponding reporter basal levels. This inhibition was due neither to lower E47 protein levels in the presence of MLK2 (Fig. 1D) nor to a change in E47 intracellular localization, which was mostly nuclear under these conditions (data not shown). Moreover, the MLK2 kinase-dead mutant was unable to exert this inhibition (Fig. 2), which reinforced the notion that MLK2 regulates E47 through phosphorylation. Interestingly, MLK2 was not able to revert E47-mediated activation of the p21CIP1 promoter (data not shown), suggesting that MLK2 would exert specific roles to regulate differentiation and survival, but not cell cycle events.
FIGURE 2.
MLK2 inhibits E47 transactivation activity on the TrkB promoter in SH-SY5Y cells. Effects of MLK2 on E47-driven TrkB promoter activity in proliferating (A) or RA-differentiating (B) SH-SY5Y cells. One day after seeding (A) or 3–4 days after seeding in the presence of RA (B), cells were transfected with a TrkB-promoter luciferase construct and E47 and either wild-type MLK2 or the kinase-dead (KD) mutant, and harvested 1 day after transfection to determine luciferase activities. The mean values and standard errors of three independent determinations of a representative experiment are plotted. Significant differences (α = 0.05) with control are indicated with an asterisk.
MLK2 Phosphorylates Ser/Thr Residues in the LH Motif of E47
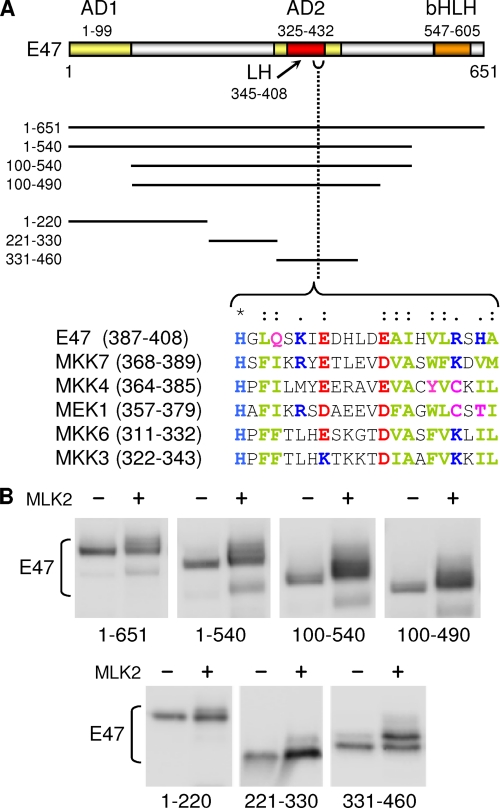
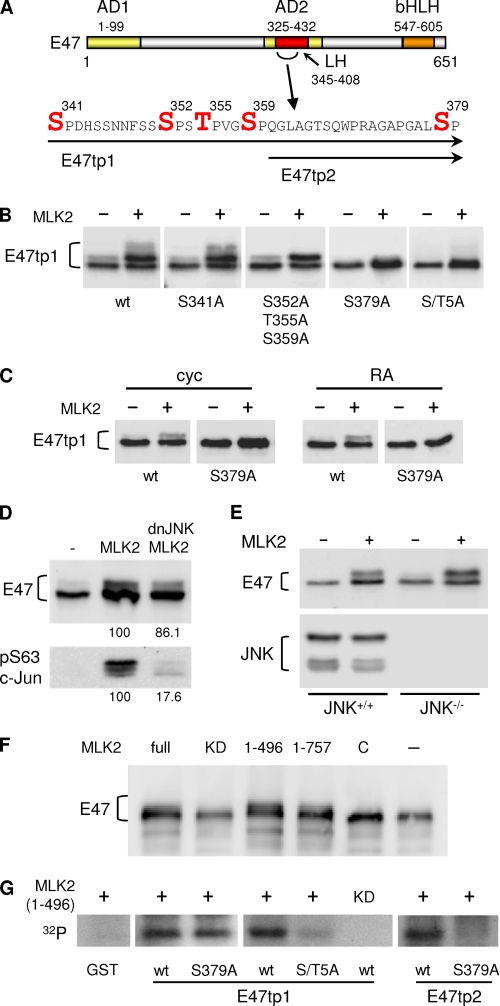
A close inspection of the shift caused by MLK2 co-expression in TAP-tagged full and partial-degradation products (data not shown), suggested that major phosphorylation events should take place within the N-terminal 460 aa of E47 (Fig. 3A). By means of an ordered deletion analysis (Fig. 3B), we delimited a region spanning amino acids 331–460 in E47 that corresponded to the AD2 activation domain and included the LH motif previously identified (7). Because MLKs have been shown to recognize sequence-specific 24-aa docking sites in downstream kinases (40), we scrutinized the identified region that was highly phosphorylated by MLK2 and found a significant similarity to the MLK docking site between residues 387 and 408 of E47 (Fig. 3A). On the other hand, although MLKs show similarities to serine-threonine and tyrosine kinases, so far they have been shown to phosphorylate only serine and threonine residues in downstream kinases (17, 19). Thus, we searched for serine and threonine residues in E47 near the putative MLK docking site that were conserved from fish to human. Although there are no strictly conserved serines or threonines C-terminal from the docking site, there are many conserved Ser/Thr residues between positions 331 and 386, with Ser379 being the closest to the MLK docking sequence (Fig. 4A). First, the S379A mutation abolished most of the shift caused by MLK2 on E47 in both HEK293T (Fig. 4B) and SH-SY5Y (Fig. 4C) cells. Although MLKs do not require a proline next to the Ser/Thr residue (36, 41, 42), because Ser379 is followed by proline, we decided to screen for additional (S/T)P sites in a close serine-rich patch between amino acids 350 and 359 (Fig. 4A). We also took into account the fact that MLKs likely target (S/T)XXXS as consensus sequence for autophosphorylation. Whereas the most prominent shift was still observed in a triple S352A/T355A/S359A mutant, the smear that can be observed in the wild-type peptide was lost in the triple mutant (Fig. 4B), suggesting that MLK2 phosphorylates additional sites to Ser379. Finally, mutating Ser341 only affected very slightly the complex mobility shift caused by MLK2.
FIGURE 3.
The AD2 activation domain of E47 is phosphorylated in the presence of MLK2. A, scheme showing the different domains in E47. AD1 and AD2, activating domains. Ordered deletions used to delimit the main phosphorylation region of E47 are depicted. The sequence of a putative MLK docking site is aligned with those defined in several MLK substrates. Chemically identical (*), highly conserved (:), or less conserved (.) amino acids are indicated. Chemical characteristics of conserved amino acids are indicated in red (acid), blue (basic), magenta (polar), and green (hydrophobic). B, analysis of E47 regions phosphorylated by MLK2 in vivo. TAP-tagged E47 constructs were transfected in HEK293T in the absence or presence of MLK2 and E47 phosphorylation was assessed by Western blot.
FIGURE 4.
MLK2 phosphorylates Ser/Thr residues within the LH motif of the AD2 activation domain. A, serine or threonine residues mutated in the AD2 activation domain of E47 are indicated in red. E47tp1 and E47tp2 indicate the extent of target peptides analyzed. B and C, effects of serine or threonine mutations to alanine on E47 phosphorylation by MLK2. TAP-tagged E47 constructs were transfected in the presence or absence of MLK2 in HEK293T (B) or SH-SY5Y (C) cells either cycling (cyc) or RA-treated (RA), and E47 mobility was analyzed by Western blot. The quintuple S/T5A mutant contains the following changes: S341A, S352A, T355A, S359A, and S379A. D, E47 phosphorylation by MLK2 and JNK in HEK293T cells. Cell extracts from HEK293T transfected with E47, MLK2, and a dominant-negative form of JNK (dnJNK) were used to analyze phosphorylation of E47 as a shift in mobility by Western blot. Ser63-phosphorylated c-Jun is shown as control for phosphorylation efficiencies, which are shown as relative percentage values below the corresponding lanes. E, E47 phosphorylation by MLK2 in JNK-deficient MEFs. Western blot analysis of E47 in the presence of transfected MLK2 in JNK+/+ and JNK−/− (double null JNK1 JNK2 mutant) MEFs. JNK proteins were also detected as control. F, analysis of E47 full-length phosphorylation by different MLK2 constructs. E47 was transfected in HEK293T together with constructs carrying different fragments of MLK2. full, N-terminal 1–496 aa or 1–757 aa fragments, a C-terminal 327–954 aa fragment (C), and a kinase-dead (KD) mutant. E47 phosphorylation was assessed by Western blot. G, MLK2 in vitro kinase assay. Bacterially expressed E47tp1 and E47tp2 target peptides, as well as their S379A or S/T5A mutants, were purified as GST fusions and subjected to in vitro kinase assays with recombinant N-terminal MLK2 (1–496 aa). Corresponding autoradiographies are shown. wt, wild type; KD, kinase-dead.
Two lines of evidence indicated that MLK2 enhances E47 phosphorylation independently of the downstream kinase JNK. First, co-transfection of a dominant-negative JNK inhibited MLK2-driven phosphorylation of c-Jun to 18%, whereas that of E47 only decreased to 86% (Fig. 4D). Second, MLK2 enhanced phosphorylation of E47 with a similar efficiency in either wild-type or double null JNK1,2 mutant MEFs (Fig. 4E). In summary, our results indicate that MLK2 induces E47 phosphorylation in vivo in a JNK-independent manner.
To test whether MLK2 is able to phosphorylate E47 directly, we carried out in vitro kinase assays with an N-terminal recombinant fragment (1–496 aa) of MLK2 that retains its kinase competence (Ref. 43 and see Fig. 4F) and exhibits a sufficient solubility in E. coli for efficient purification under native conditions (data not shown). We found that this fragment of MLK2, but not a kinase-dead mutant, strongly phosphorylated the abovementioned E47 peptide spanning residues 331–460 (Fig. 4G). Although the S379A mutant was still efficiently phosphorylated by MLK2, phosphorylation of the S/T5A quintuple mutant was severely reduced (Fig. 4G), indicating that the identified Ser/Thr residues are all important phosphorylation targets of MLK2. Although MLK2 phosphorylated very efficiently a shorter peptide that only contained Ser379, phosphorylation of a S379A mutant in this shorter context was undetectable. In summary, MLK2 phosphorylates in vitro at least five Ser/Thr residues within the LH motif that plays a key role as a transcriptional activation domain of E47 (7).
Transcriptional Repression by MLK2 Requires the Ser/Thr Target Residues within the LH Motif of E47
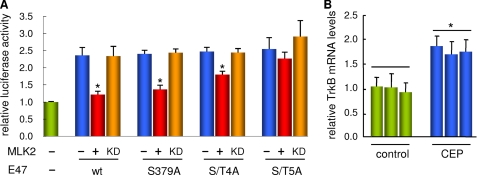
To test whether MLK2 would inhibit the transcriptional activation domain of E47 by phosphorylation of the identified target residues within the LH motif, we co-expressed different E47 mutants with wild-type and kinase-dead MLK2 proteins and analyzed TrkB promoter-driven expression by the aforementioned luciferase assay (Fig. 5A). Although the single S379A mutant was not significantly resistant to MLK2-mediated inhibition, expression produced by a quadruple mutant (S352A/T355A/S359A/S379A, referred to as S/T4A) was significantly higher in the presence of MLK2. Moreover, expression levels attained by the S/T5A quintuple mutant were almost unaffected by co-expression of MLK2, indicating that MLK2-mediated repression effects require the same Ser/Thr residues in E47 that are phosphorylated both in vitro and in vivo by MLK2. The different E47 mutants stimulated TrkB promoter-driven expression very similarly in the absence of MLK2, which suggests that the serine/threonine to alanine substitutions introduced do not cause important functional or structural alterations in E47. In summary, these results give support to the notion that MLK2 inhibits E47 transcriptional activity by direct phosphorylation of Ser/Thr residues within the LH motif.
FIGURE 5.
Transcriptional repression by MLK2 requires the Ser/Thr target residues identified within the LH motif of E47. A, effects of Ser/Thr mutations on the ability of E47 to activate TrkB promoter-driven expression in the presence of MLK2. SH-SY5Y cells treated for 3–4 days with RA were transfected with a TrkB-promoter luciferase reporter and the indicated E47 constructs in the presence or absence of either wild-type (wt) or a kinase-dead (KD) mutant of MLK2 and harvested 1 day after transfection to determine luciferase activities. The quadruple S/T4A mutant contains the following changes: S352A, T355A, S359A, and S379A. The quintuple S/T5A mutant contains S341A as an additional change. The mean values and standard errors of five independent transfection experiments are plotted. Significant differences (α = 0.05) within sets of same E47 variant are indicated with an asterisk. B, effect of MLK inhibitor CEP11004 on TrkB mRNA expression in SH-SY5Y cells. Proliferating SH-SY5Y cells were deprived from trophic factors in the presence of RA and treated or not with 400 nm CEP11004 for 24 h. Reverse transcription real time PCR analysis of TrkB was made relative to TFRC as control. The mean values and standard errors of three independent determinations from three independent experiments are shown. Significant differences (α = 0.05) with control are indicated with an asterisk.
MLK Inhibits TrkB Expression in Neuronal Cells
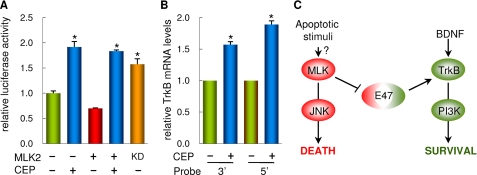
Inhibition of MLKs by CEP11004 has been shown to increase TrkB protein levels in cerebellar granule neurons (32), and our results suggest that, because MLK2 inhibits E47 activity, MLKs could regulate TrkB expression at a transcriptional level. To test the functional relevance of MLKs on endogenous TrkB transcription in a neuronal differentiation paradigm, we used SH-SY5Y cells in the presence or absence of RA to stimulate or not E47-driven transcription, respectively (34), and samples were collected from three independent experiments to determine TrkB and TFRC (as control) mRNA levels by reverse transcription real time PCR. Relative TrkB expression levels in the absence of RA were very low and insensitive to MLK inhibition (data not shown), but, as expected from our previous work (34), they were easily detected under RA-elicited differentiation conditions. More importantly, TrkB mRNA expression levels showed a clear increase in cells treated with CEP11004 (Fig. 5B). Similar results were obtained in cerebellar granule neurons. Thus, TrkB promoter-driven expression was clearly stimulated by the addition of CEP11004, even under MLK2 overexpression conditions (Fig. 6A). Because MLKs require dimerization for autophosphorylation and full activation (17), we used the MLK2 kinase-dead protein as a dominant mutant and found that it also stimulated TrkB promoter-driven expression (Fig. 6A). Finally, MLK inhibition by CEP11004 caused a clear increase of TrkB mRNA levels in cerebellar granule neurons levels as determined with two different probes targeted to 5′ and 3′ sequences of the TrkB mRNA (Fig. 6B). These results demonstrate that the MLK inhibitor CEP11004 increases expression of TrkB at the mRNA level and support our findings that point to MLK as a key repressor of E47-driven expression in neurons.
FIGURE 6.
MLK2 inhibits TrkB expression in cerebellar granule neurons. A, MLK2 inhibition up-regulates TrkB promoter-driven expression. Cerebellar granule neurons cultured for 6 days were transfected with a TrkB-promoter luciferase reporter and either wild-type or a kinase-dead (KD) mutant of MLK2 and treated or not with 400 nm CEP11004 for 18 h after transfection to determine luciferase activities. Mean values and standard errors of six independent transfection experiments are plotted. Significant differences (α = 0.05) with control are indicated with an asterisk. B, effect of MLK inhibition on TrkB expression in cerebellar granule neurons treated or not with 400 nm CEP11004 for 24 h. Reverse transcription real time PCR analysis of TrkB was done with two different probes targeting 5′ and 3′ sequences of the TrkB mRNA, and the results obtained were made relative to glyceraldehyde-3-phosphate dehydrogenase as control. Mean values and standard errors of six independent determinations are shown. Significant differences (α = 0.05) with control are indicated with an asterisk. C, scheme showing the molecular link established by E47 between the JNK and phosphatidylinositol 3-kinase pathways to determine the cell toward death or survival. When trophic signals are high, E47 would provide with a high level of Trk receptors to activate cell survival pathways. On the contrary, in the presence of apoptotic stimuli, increasingly active MLK proteins would lead to JNK-mediated cell death and, in addition, limit E47-dependent Trk expression to abate cell survival signals. Thus, E47 inhibition by MLK appears as an important link that would negatively coordinate cell death and survival pathways in neurons.
DISCUSSION
In a previous study we reported that bHLH protein E47 binds to E-box sequences within the promoter of the TrkB gene and activates its transcription (34). Here we show that MLK2 kinase, whose activity has been involved in the regulation of JNK pathway (17, 39), represses TrkB promoter activity by phosphorylating E47 in a JNK-independent manner. In particular, we show that phosphorylation of Ser/Thr residues within the LH motif of the AD2 activation domain inhibits E47 as a transcriptional activator of TrkB. Moreover, inhibition of MLK activity by CEP11004 (32) increases TrkB expression levels in cerebellar granule neurons and differentiating SH-SY5Y neuroblastoma cells. These findings point to a novel molecular mechanism by which MLK regulates TrkB expression by phosphorylating the E47 transcription factor.
The molecular mechanisms that control the transcriptional activity of E47 are not fully understood but appear to involve both positive and negative regulatory factors. Previous work has demonstrated that p38 MAPK activates muscle-specific gene transcription. Phosphorylation of E47 at Ser140 by p38 MAPK enhances MyoD/E47 heterodimerization and subsequent binding to E-box sequences (12). Other results may suggest that p38 MAPK could exert a negative effect by phosphorylating other residues in the N terminus of E47 (13). Although this post-translational modifications would involve the N-terminal sequences of E47 that contain the AD1 activation domain, studies conducted by Quong et al. (7) and Aronheim et al. (5) identified a LH region within the AD2 activation domain capable of forming a loop structure between amino acids 345 and 394, and an amphipathic α helix between amino acids 395 and 408. Interestingly, this region showed different transactivation properties depending on the cell line used (5), suggesting that it would likely be regulated by lineage-specific co-activators and/or co-repressors. As a relevant example, c-Jun has been reported to inhibit the transactivation potential of the AD2 activation domain of E47 (8). Although the mechanism by which c-Jun represses E47 transactivation activity remained to be elucidated, these authors demonstrated that serines at positions 63 and 73 in c-Jun were not involved in the repression mechanism. Phosphorylation of these serine residues by JNK is essential for c-Jun activation (44), but not for binding to activators such as ATF-2 (45), which would rule out the possibility that the MLK2 effect on E47 activity is mediated by downstream activation of JNK.
MLK2 is predominantly expressed in brain, and it is localized in both the cytoplasm and nucleus (26, 46). Previous studies have reported interactions between MLK2 and components of transcriptional complexes. Thus, MLK2 has been shown to phosphorylate and enhance the silencing activity of Alien, a co-repressor for nuclear receptors. The authors suggested that this mechanism would represent a link between MLK2 and transcriptional repression of target genes during neuronal differentiation (26). On the other hand, it has been reported that MLK2 interacts with and phosphorylates NeuroD via the Huntingtin protein in mouse N2A neuroblastoma cells (25). Unfortunately, because NeuroD activity was assayed very indirectly as the fraction of Xenopus embryos displaying ectopic neurons after mRNA injection, a direct consequence on the transactivation properties of NeuroD was not demonstrated. In any event, the domains of MLK2 required for binding NeuroD and E47 are different. Although NeuroD requires the LZ domain of MLK2 (25), it is totally dispensable for E47 interaction.
Studies with neuronal models have involved MLKs as mediators of apoptosis induced by trophic factor deprivation (31, 38). On the one hand, perhaps with the participation of Cdc42, MLK would sense trophic factor limiting conditions to activate JNK and induce c-Jun-dependent and -independent processes leading to apoptosis (31). On the other hand, Wang et al. (32) have shown that the MLK inhibitor CEP11004 increases TrkA and TrkB protein levels in both central and peripheral nervous systems, this activation process important being for phosphatidylinositol 3-kinase-mediated long term survival. Thus, the authors of this work proposed that MLK inhibitors would not only inhibit the JNK apoptotic pathway but, as they raised Trk receptor protein levels, they would also increase cell responsiveness to trophic factors. This dual effect could explain the protective action of MLK inhibitors in models of neuronal injury and neurodegeneration such as Alzheimer's disease, where basal forebrain cholinergic neurons down-regulate TrkA expression in aged rats (47). We show here that MLK2 phosphorylates E47 and represses its activity as a transcription factor, thus reducing TrkB expression levels as a direct consequence. Our results provide with a direct molecular link between the JNK and phosphatidylinositol 3-kinase pathways and would explain the effects caused by MLK inhibitors on Trk receptor levels and long term survival (see scheme in Fig. 6C).
It has been shown that the interaction between the SH3 domain of MLK2 and the proline-rich N terminus of Huntingtin inhibits MLK2 activity in HEK293T and HN33 cells (48), and it has been proposed that, in normal cells, MLK2 would be sequestered in an inactive form by binding of its SH3 domain to the N terminus of Huntingtin (17, 48). In Huntington disease, the polyglutamine-expanded mutant versions of Huntingtin would fail to bind MLK2 and, as a consequence, cause JNK activation and apoptosis (48). Interestingly, Ginés et al. (49) have shown that levels of TrkB receptor protein are diminished in a knock-in mouse model of Huntington disease. This observation could also be explained taking into account that, as we show here, unwanted MLK activation should inhibit E47-dependent expression of TrkB, raising the question as to whether enforced expression of TrkB could delay Huntington disease progression.
Neuron survival during development of the nervous system is at least in part determined by the limited availability of target-derived growth factors, which act to inhibit programmed cell death (50). Then, to ensure that apoptosis is only triggered once trophic factor levels fall below a precise threshold, mechanisms should exist that add robustness and convert linear response systems into switch-like devices. In our scheme (Fig. 6C), when trophic signals are high, E47 would ensure plenty expression levels of Trk receptors and positively contribute to activate cell survival pathways. On the contrary, when trophic factor availability becomes limiting, increasing levels of active MLK by apoptotic signals would raise JNK-mediated effects to boost cell death and, in parallel, reduce E47-dependent Trk expression levels to decrease cell survival signals even further. Thus, E47 would serve as a link between phosphatidylinositol 3-kinase and JNK pathways to promptly incline neurons toward survival or death.
Acknowledgments
We gratefully acknowledge Sònia Rius and Roser Pané for technical assistance, Enrique Calvo (Centro Nacional de Investigaciones Cardiovasculares, Instituto de Salud Carlos III) for mass spectrometry analysis, Carme Caelles (Institut de Recerca Biomèdica Barcelona) for the dominant-negative form of JNK cDNA, and E. Wagner and A. Muñoz for providing mouse JNK−/− fibroblasts. We sincerely thank Gary L. Johnson, Pura Muñoz-Cánovas, and Eugene Johnson, Jr., for helpful comments. We are also thankful to Masataka Nakamura, Syu-ichi Hirai, and Elisa Izaurralde for generously providing us with plasmids or reagents. We appreciate the help received from the members of our group.
This work was supported by the Ministry of Science and Innovation of Spain, Consolider-Ingenio 2010, Fundació La Caixa, and the European Union (Fondo Europeo de Desarrollo Regional).
- bHLH
- basic helix-loop-helix
- MLK
- mixed lineage kinase
- JNK
- c-Jun N-terminal kinase
- LH
- loop-helix
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated kinase
- CRIB
- Cdc42/Rac interactive binding
- aa
- amino acid(s)
- TAP
- tandem affinity purification
- GST
- glutathione S-transferase
- MEF
- mouse embryonic fibroblast
- RA
- retinoic acid
- DTT
- dithiothreitol.
REFERENCES
- 1.Massari M. E., Murre C. (2000) Mol. Cell Biol. 20, 429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwell T. K., Weintraub H. (1990) Science 250, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 3.Davis R. L., Weintraub H., Lassar A. B. (1987) Cell 51, 987–1000 [DOI] [PubMed] [Google Scholar]
- 4.Norton J. D. (2000) J. Cell Sci. 113, 3897–3905 [DOI] [PubMed] [Google Scholar]
- 5.Aronheim A., Shiran R., Rosen A., Walker M. D. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 8063–8067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massari M. E., Jennings P. A., Murre C. (1996) Mol. Cell Biol. 16, 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quong M. W., Massari M. E., Zwart R., Murre C. (1993) Mol. Cell Biol. 13, 792–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson G. L., Henderson E., Massari M. E., Murre C., Stein R. (1995) Mol. Cell Biol. 15, 1398–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sloan S. R., Shen C. P., McCarrick-Walmsley R., Kadesch T. (1996) Mol. Cell Biol. 16, 6900–6908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson S. E., Wang X., Hardy S., Taparowsky E. J., Konieczny S. F. (1996) Mol. Cell Biol. 16, 1604–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole F., Zhang W., Geyra A., Kang J. S., Krauss R. S. (2004) Dev. Cell 7, 843–854 [DOI] [PubMed] [Google Scholar]
- 12.Lluís F., Ballestar E., Suelves M., Esteller M., Muñoz-Cánoves P. (2005) EMBO J. 24, 974–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page J. L., Wang X., Sordillo L. M., Johnson S. E. (2004) J. Biol. Chem. 279, 30966–30972 [DOI] [PubMed] [Google Scholar]
- 14.Neufeld B., Grosse-Wilde A., Hoffmeyer A., Jordan B. W., Chen P., Dinev D., Ludwig S., Rapp U. R. (2000) J. Biol. Chem. 275, 20239–20242 [DOI] [PubMed] [Google Scholar]
- 15.Nie L., Xu M., Vladimirova A., Sun X. H. (2003) EMBO J. 22, 5780–5792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King A. M., Van der Put E., Blomberg B. B., Riley R. L. (2007) J. Immunol. 178, 3521–3529 [DOI] [PubMed] [Google Scholar]
- 17.Gallo K. A., Johnson G. L. (2002) Nat. Rev. Mol. Cell Biol. 3, 663–672 [DOI] [PubMed] [Google Scholar]
- 18.Zhao Z. S., Manser E. (2005) Biochem. J. 386, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handley M. E., Rasaiyaah J., Chain B. M., Katz D. R. (2007) Int. J. Exp. Pathol. 88, 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorow D. S., Devereux L., Dietzsch E., De Kretser T. (1993) Eur. J. Biochem. 213, 701–710 [DOI] [PubMed] [Google Scholar]
- 21.Ing Y. L., Leung I. W., Heng H. H., Tsui L. C., Lassam N. J. (1994) Oncogene 9, 1745–1750 [PubMed] [Google Scholar]
- 22.Sakuma H., Ikeda A., Oka S., Kozutsumi Y., Zanetta J. P., Kawasaki T. (1997) J. Biol. Chem. 272, 28622–28629 [DOI] [PubMed] [Google Scholar]
- 23.Swenson K. I., Winkler K. E., Means A. R. (2003) Mol. Biol. Cell 14, 156–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chadee D. N., Kyriakis J. M. (2004) Nat. Cell Biol. 6, 770–776 [DOI] [PubMed] [Google Scholar]
- 25.Marcora E., Gowan K., Lee J. E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9578–9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckey M., Tenbaum S. P., Muñoz A., Baniahmad A. (2003) Mol. Cell Endocrinol. 213, 71–78 [DOI] [PubMed] [Google Scholar]
- 27.Leung I. W., Lassam N. (2001) J. Biol. Chem. 276, 1961–1967 [DOI] [PubMed] [Google Scholar]
- 28.Teramoto H., Coso O. A., Miyata H., Igishi T., Miki T., Gutkind J. S. (1996) J. Biol. Chem. 271, 27225–27228 [DOI] [PubMed] [Google Scholar]
- 29.Wang L. H., Besirli C. G., Johnson E. M., Jr. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 451–474 [DOI] [PubMed] [Google Scholar]
- 30.Maroney A. C., Glicksman M. A., Basma A. N., Walton K. M., Knight E., Jr., Murphy C. A., Bartlett B. A., Finn J. P., Angeles T., Matsuda Y., Neff N. T., Dionne C. A. (1998) J. Neurosci. 18, 104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris C. A., Deshmukh M., Tsui-Pierchala B., Maroney A. C., Johnson E. M., Jr. (2002) J. Neurosci. 22, 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L. H., Paden A. J., Johnson E. M., Jr. (2005) J. Pharmacol. Exp. Ther. 312, 1007–1019 [DOI] [PubMed] [Google Scholar]
- 33.Lei L., Parada L. F. (2007) Cell Mol. Life Sci. 64, 522–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Encinas M., Comella J. X., Aldea M., Gallego C. (2004) Mol. Cell Biol. 24, 2662–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funato N., Ohtani K., Ohyama K., Kuroda T., Nakamura M. (2001) Mol. Cell Biol. 21, 7416–7428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirai S., Katoh M., Terada M., Kyriakis J. M., Zon L. I., Rana A., Avruch J., Ohno S. (1997) J. Biol. Chem. 272, 15167–15173 [DOI] [PubMed] [Google Scholar]
- 37.Encinas M., Iglesias M., Liu Y., Wang H., Muhaisen A., Ceña V., Gallego C., Comella J. X. (2000) J. Neurochem. 75, 991–1003 [DOI] [PubMed] [Google Scholar]
- 38.Silva R. M., Kuan C. Y., Rakic P., Burke R. E. (2005) Mov. Disord. 20, 653–664 [DOI] [PubMed] [Google Scholar]
- 39.Handley M. E., Rasaiyaah J., Barnett J., Thakker M., Pollara G., Katz D. R., Chain B. M. (2007) Int. Immunol 13, 923–933 [DOI] [PubMed] [Google Scholar]
- 40.Takekawa M., Tatebayashi K., Saito H. (2005) Mol. Cell 18, 295–306 [DOI] [PubMed] [Google Scholar]
- 41.Yan M., Dai T., Deak J. C., Kyriakis J. M., Zon L. I., Woodgett J. R., Templeton D. J. (1994) Nature 372, 798–800 [DOI] [PubMed] [Google Scholar]
- 42.Tibbles L. A., Ing Y. L, Kiefer F., Chan J., Iscove N., Woodgett J. R., Lassam N. J. (1996) EMBO J. 15, 7026–7035 [PMC free article] [PubMed] [Google Scholar]
- 43.Phelan D. R., Price G., Liu Y. F., Dorow D. S. (2001) J. Biol. Chem. 276, 10801–10810 [DOI] [PubMed] [Google Scholar]
- 44.Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. (1991) Nature 354, 494–496 [DOI] [PubMed] [Google Scholar]
- 45.van Dam H., Wilhelm D., Herr I., Steffen A., Herrlich P., Angel P. (1995) EMBO J. 14, 1798–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phelan D. R., Loveland K. L., Devereux L., Dorow D. S. (1999) Mol. Reprod. Dev. 52, 135–140 [DOI] [PubMed] [Google Scholar]
- 47.Boissiere F., Faucheux B., Ruberg M., Agid Y., Hirsch E. C. (1997) Exp. Neurol. 145, 245–252 [DOI] [PubMed] [Google Scholar]
- 48.Liu Y. F., Dorow D., Marshall J. (2000) J. Biol. Chem. 275, 19035–19040 [DOI] [PubMed] [Google Scholar]
- 49.Ginés S., Bosch M., Marco S., Gavaldà N., Díaz-Hernández M., Lucas J. J., Canals J. M., Alberch J. (2006) Eur. J. Neurosci. 23, 649–658 [DOI] [PubMed] [Google Scholar]
- 50.Deshmukh M., Johnson E. M., Jr. (1997) Mol. Pharmacol. 51, 897–906 [DOI] [PubMed] [Google Scholar]