Abstract
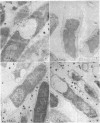
Cota-Robles, Eugene H. (University of California, Riverside). Electron microscopy of plasmolysis in Escherichia coli. J. Bacteriol. 85:499–503. 1963.—Escherichia coli cells plasmolyzed in 0.35 m sucrose reveal plasmolysis at one tip of a cell or in the center of dividing cells in which protoplast partition has been complete. Central plasmolysis reveals that protoplast separation can be completed before the invagination of the cell wall is complete. These studies support the concept that these cells divide by constriction. The strength of the union between cell wall and cytoplasm is not uniform around the entire cell. It is strongest along the sides of these rod-shaped cells and weakest at one tip of the single cell. Thus, a single cell generally forms one cup-shaped vacuole in which the cytoplasm has collapsed away from one tip of the cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COTA-ROBLES E. H., DUNCAN P. H. The effect of D-glutamic acid upon spheroplast formation in Escherichia coli B. Exp Cell Res. 1962 Nov;28:342–349. doi: 10.1016/0014-4827(62)90288-4. [DOI] [PubMed] [Google Scholar]
- Conti S. F., Gettner M. E. ELECTRON MICROSCOPY OF CELLULAR DIVISION IN ESCHERICHIA COLI. J Bacteriol. 1962 Mar;83(3):544–550. doi: 10.1128/jb.83.3.544-550.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. J Biophys Biochem Cytol. 1958 Nov 25;4(6):727–730. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]