Abstract
Concerns have been raised regarding the availability of National Institute for Occupational Safety and Health (NIOSH)-certified N95 filtering facepiece respirators (FFRs) during an influenza pandemic. One possible strategy to mitigate a respirator shortage is to reuse FFRs following a biological decontamination process to render infectious material on the FFR inactive. However, little data exist on the effects of decontamination methods on respirator integrity and performance. This study evaluated five decontamination methods [ultraviolet germicidal irradiation (UVGI), ethylene oxide, vaporized hydrogen peroxide (VHP), microwave oven irradiation, and bleach] using nine models of NIOSH-certified respirators (three models each of N95 FFRs, surgical N95 respirators, and P100 FFRs) to determine which methods should be considered for future research studies. Following treatment by each decontamination method, the FFRs were evaluated for changes in physical appearance, odor, and laboratory performance (filter aerosol penetration and filter airflow resistance). Additional experiments (dry heat laboratory oven exposures, off-gassing, and FFR hydrophobicity) were subsequently conducted to better understand material properties and possible health risks to the respirator user following decontamination. However, this study did not assess the efficiency of the decontamination methods to inactivate viable microorganisms. Microwave oven irradiation melted samples from two FFR models. The remainder of the FFR samples that had been decontaminated had expected levels of filter aerosol penetration and filter airflow resistance. The scent of bleach remained noticeable following overnight drying and low levels of chlorine gas were found to off-gas from bleach-decontaminated FFRs when rehydrated with deionized water. UVGI, ethylene oxide (EtO), and VHP were found to be the most promising decontamination methods; however, concerns remain about the throughput capabilities for EtO and VHP. Further research is needed before any specific decontamination methods can be recommended.
Keywords: decontamination, filtering facepiece respirator, healthcare workers, N95 respirator, pandemic influenza, respirator reuse
INTRODUCTION
During an influenza pandemic, a shortage of filtering facepiece respirators (FFRs) may occur if manufacturing production is unable to meet the demand or if FFR stockpiles become depleted. According to a 2006 report from the National Academies’ Institute of Medicine, over 90 million N95 FFRs will be needed to protect workers in the healthcare sector during a 42-day influenza pandemic outbreak (Bailar et al., 2006). Guidance provided by the Centers for Disease Control and Prevention (CDC) states that once an FFR is worn in the presence of an infected patient, it should be considered potentially contaminated and not be reused by the same person or a coworker (CDC, 2007). A contaminated FFR could potentially serve as a fomite and lead to self-inoculation or spread of the organism to patients and other healthcare workers. Guidance from the Occupational Safety and Health Administration (OSHA) considers FFRs to be one-time-use devices when used in the presence of infected patients and advises employers and employees to only reuse FFRs during a pandemic if FFRs are in short supply and the device has not been obviously soiled or damaged (e.g. creased or torn), and it retains its ability to function properly (OSHA, 2007).
One possible strategy to reduce the impact of a respirator shortage would be to apply a biological decontamination process (e.g. such as those used in hospital settings for infection control) to inactivate the influenza virus that may be on the FFR. If the treatment did not deteriorate the FFR or leave potentially toxic residues on the FFR, then it could be available for subsequent reuse by the original user. Until recently, no data were published on the effects of decontamination on FFR performance. Viscusi et al. (2007) measured the laboratory filtration performance of one N95 model and one P100 model FFR that were exposed to 20 different biological decontamination treatments. They found that filtration performance after one-time decontamination treatments using bleach, ethylene oxide (EtO), microwave oven irradiation, ultraviolet germicidal irradiation (UVGI), and hydrogen peroxide (vaporized and liquid forms) was observed to have filter aerosol penetration values that remained less than the National Institute for Occupational Safety and Health (NIOSH) certification criteria. It was also found that decontamination using an autoclave, 160°C dry heat, 70% isopropyl alcohol, and soap and water (20-min soak) caused significant degradation to filtration efficiency.
Expanding on that research, the goal of this study was to further evaluate five of the decontamination methods examined in the previous study using a more diverse set of nine models of NIOSH-certified FFRs to determine which decontamination methods should be considered for future research studies. The biological decontamination methods used in this study include: (i) UVGI, (ii) EtO, (iii) vaporized hydrogen peroxide (VHP), (iv) microwave oven irradiation, and (v) 0.6% aqueous solution of sodium hypochlorite (hereafter referred to as ‘bleach’). Following treatment by each decontamination method, FFRs were evaluated for changes in physical appearance/odor (observational analysis) and laboratory performance (filter aerosol penetration and filter airflow resistance). Additional experiments were then conducted to examine the material properties of the FFRs in an attempt to rationalize some of the findings in the laboratory performance evaluation and observational analysis. The advantages and disadvantages of the various decontamination methods (including throughput capacity and possible health risk to the user) were also assessed.
METHODS
Respirator selection
Nine respirator models were used in this study, of which six models [three N95 FFR models (N95-A, N95-B, and N95-C) and three surgical N95 respirator models (SN95-D, SN95-E, and SN95-F)] constitute a random sampling from those N95 FFR models present in the US Strategic National Stockpile (SNS). Healthcare workers often use surgical N95 respirators, which are NIOSH-approved N95 FFRs that also have been cleared by the US Food and Drug Administration (FDA) for marketing as medical devices. Surgical N95 respirators are designed to be fluid resistant to splash and spatter of blood and other infectious materials and thus may respond differently to the decontamination processes than N95 FFRs. Three models of P100 FFRs (P100-G, P100-H, and P100-I) were randomly selected from models commercially available at the time of the study and included because they were considered likely to be more resistant to filtration efficiency degradation and thus offer a more rigorous basis of comparison. All respirators were purchased and verified to be from the same respective manufacturing lot at the beginning of the study to minimize any lot-to-lot variation as well as to ensure consistency during FFR filtration performance testing. FFRs used in this study consisted of electrostatically charged polypropylene filters (electret filter media).
Decontamination methods
The experimental conditions and parameters for the five decontamination methods and the ‘as-received’ (control) method are summarized in Table 1. All laboratory experiments were conducted under standard laboratory conditions (21 ± 2°C and relative humidity of 50 ± 10%) on triplicate sets of FFRs.
Table 1.
FFR treatments
| Treatment | Experimental conditions and parameters |
| As-received | No decontamination treatment was performed (control group). |
| UVGI | FFRs placed on the working surface of a Sterilgard III laminar flow cabinet (The Baker Company, Sanford, ME, USA) fitted with a 40-W UV-C light (average UV intensity experimentally measured to range from 0.18 to 0.20 mW cm−2). Fifteen-minute exposure to each side (outer and inner), 176–181 mJ cm−2 exposure to each side of FFR. |
| EtO | Steri-Vac 5XL sterilizer (3M, St Paul, MN, USA). Single warm cycle (55°C and 725 mg l−1 100% EtO gas). FFRs and a chemical indicator placed in an individual standard poly/paper pouch. EtO exposure for 1 h followed by 4 h of aeration. FFRs were shipped to and from a commercial facility specializing in low-temperature sterilization methods and were tested within 72 h of receipt. |
| VHP | STERRAD® 100S H2O2 Gas Plasma Sterilizer (Advanced Sterilization Products, Irvine, CA, USA), single 55-min standard cycle. FFRs and a chemical indicator placed in an individual Mylar/Tyvek™ self-seal pouch. FFRs were shipped to and from a commercial facility specializing in low-temperature sterilization methods and were tested within 72 h of receipt. |
| Microwave oven irradiation | Commercially available 2450 MHz, Sharp Model R-305KS (Sharp Electronics, Mahwah, NJ, USA) microwave oven with revolving glass carousel, 1100 W (manufacturer rated); 750 W ft−3 experimentally measured; 2-min total exposure (1 min each side of FFR). A paper towel was placed on the revolving glass plate for insulation to protect the FFRs from melting onto the glass plate. Using a power setting of 10 (maximum power), FFRs were placed faceseal-side down, initially, to reduce the risk of faceseal component materials melting onto the paper towel due to elevated temperatures reached by the glass plate when microwaved for 2 min. Ambient cooling of the glass plate was maintained between trials. |
| Bleach | Thirty minutes submersion in 0.6% (one part bleach to nine parts of deionized water) aqueous solution of sodium hypochlorite (original concentration = 6% available as Cl2). Manufacturing specification: 6.00 ± 0.06% (w/w) available chlorine; Cat no. 7495.7-1, CAS no. 7732-18-5 (Ricca Chemical Company, Pequannock, NJ, USA). After treatment, FFRs were hung on a laboratory pegboard and allowed to air-dry overnight with assistance from a freestanding fan. |
Respirator test methods
Observational analysis.
All post-decontamination and control FFR samples were inspected and scrutinized carefully for any visible sign of degradation or changes that could be noted in texture or ‘feel’ of the respirator (softness, pliability, coarseness, roughness, etc.). All samples were sniffed for any discernible odor or smell.
Filter aerosol penetration.
A Model 8130 Automated Filter Tester (AFT) (TSI, Inc., St Paul, MN, USA) was used to measure initial filter aerosol penetration for all post-decontamination and control FFR samples. All tests were conducted at room temperature with a continuous airflow of 85 ± 4 l min−1 in accordance with NIOSH certification test procedures (NIOSH, 2007) for challenging N-series filters, with two exceptions: all filters were tested for filter aerosol penetration without any relative humidity pretreatment or NaCl aerosol loading. Collecting the data in this manner allows consistency with previous work (Viscusi et al., 2007). Filter aerosol penetration levels were determined using a Plexiglas test box as previously used and described by Viscusi et al. (2007) or an appropriately sized test fixture supplied by the respective FFR manufacturer, as was the case for models N95-C, SN95-D, and P100-H.
Filter airflow resistance.
For all control and post-decontamination FFR samples, a TSI Model 8130 AFT was also used to measure initial filter airflow resistance in millimeters of water column height pressure (mmH2O). It must be clarified that the NIOSH certification test for inhalation airflow resistance for FFRs is not performed using the TSI 8130 AFT but is executed in accordance with NIOSH Standard Test Procedure RCT-APR-STP-0007, which specifies the use of a different calibrated apparatus incorporating a vacuum source and manometer (NIOSH, 2005). For this evaluation, it was convenient to report the filter airflow resistance obtained from the TSI Model 8130 AFT because filter aerosol penetration and filter airflow resistance results are generated simultaneously and the intent is to determine changes in filter airflow resistance. This methodology was used previously by the National Personal Protective Technology Laboratory (NPPTL) (Viscusi et al., 2009).
Experimental design
The primary experimental design called for 162 FFRs (nine different FFR models × six test conditions × three samples per test condition) to be tested by observational analysis, for filter airflow resistance and for filter aerosol penetration. The 162 FFRs in the design included 135 post-decontamination FFRs and 27 control FFRs (no decontamination).
Statistical analysis
For statistical analysis, the six test conditions (see Table 1) comprised one control group and five decontamination treatments. A one-way analysis of variance (ANOVA) test was performed for each of the nine FFR models for filter aerosol penetration and filter airflow resistance (for 18 total tests). Thus, each model was treated independently due to its inherent uniqueness (difference in number of filter layers, hydrophobicity, materials of construction, etc.). Results were considered statistically significant if the P-value was <0.05. Statistical analyses were performed using Microsoft Excel (Microsoft Corporation, part of Microsoft Office Professional Edition 2003). No statistical analysis of the subjective observational analysis data was done.
Additional testing
Additional secondary experiments were subsequently conducted on the FFRs to understand better their material properties. This information can be used to further optimize the decontamination methods and/or explain some of the findings from the observational analyses or laboratory performance evaluation experiments.
Dry oven experiments.
To investigate the effects on filter aerosol penetration at various dry heat temperatures and to determine if these effects were similar to those of FFRs that underwent microwave oven irradiation, new FFRs were placed in a Fisher Scientific Isotemp 500 Series laboratory oven (Fisher Scientific, Pittsburgh, PA, USA) for 1 h at temperatures ranging from 80 to 120°C. Filter aerosol penetration was measured after samples cooled to ambient temperature.
Hydrophobicity testing.
A qualitative assessment of water affinity for each FFR filter media layer was performed to determine the hydrophobic/hydrophilic nature of the various layers for the nine different FFR models. For this experiment, it was hypothesized that the number of layers and the nature of the outer layer (surface of the FFR most distant from the wearer) and the inner layer (surface of the FFR closest to the breathing zone of the wearer) would provide insight into any model-specific effects associated with liquid chemical-based decontamination methods. A circular swatch (∼5 cm in diameter) was cut from additional, new as-received samples of each FFR model. Following layer separations, a 100 μl aliquot of deionized water was pipetted onto the surface of each side of each layer (front and back). Two FFR models incorporated layers of plastic webbing, presumably to support shape; these layers were not tested because they are not filtering layers. A layer was noted as hydrophilic when it absorbed the water droplet. A layer was noted as hydrophobic when the water droplet beaded on the layer's surface.
Chlorine off-gassing experiments.
To quantify observations of discernable odor from FFRs following bleach decontamination, a series of off-gassing experiments was conducted using a Model 4340 Chlorine Gas Analyzer (Interscan Corp., Chatsworth, CA, USA). Chlorine off-gassing was measured from FFRs after bleach treatment as described in Table 1. A subset of four FFR models was chosen for testing based on the various combinations of water repellency discerned from the hydrophobicity experiments described previously: N95-A (outer hydrophobic layer/inner hydrophilic layer), N95-B (outer and inner hydrophilic layers), SN95-E (outer and inner hydrophobic layers), and SN95-F (outer hydrophobic layer/inner hydrophilic layer). Bleach off-gassing tests were conducted after a bleach decontamination treatment by immediately placing the FFR face up inside a plastic bag which was open to room air on one side. This setup was designed to minimize air fluctuation within the bag. The detector's sample tube inlet was positioned under the inside of the FFR and all tests were conducted at a flow rate of 0.5 l min−1. FFRs were tested under four conditions: (i) immediately after a 30-min submersion in bleach, (ii) dried overnight after a 30-min submersion in bleach, (iii) a 30-min submersion in bleach, immediately rinsed (under a flowing stream of deionized water for ∼1 min) and then dried overnight, and (iv) a 30-min submersion in bleach, then dried overnight followed by rinsing with deionized water.
RESULTS
Observational analysis
Changes to the FFR materials of construction caused by each decontamination treatment are summarized in Table 2. Respirator component materials melted on all six FFRs from two models (SN95-E and P100-I) during microwave oven irradiation. EtO and UVGI were the only methods that did not cause any observable physical changes to the FFRs.
Table 2.
Discernible observations caused by FFR decontamination treatments
| Decontamination treatment | Discernible observations |
| Bleach | Metallic nosebands were slightly tarnished and visibly not as shiny when compared with their as-received counterparts. SN95-E inner nose comfort cushion was discolored. Following air-drying overnight (16 h), all FFRs were dry to the touch and all still had a characteristic smell of bleach. |
| UVGI | No visible changes were observed for all samples. |
| EtO | No visible changes were observed for all samples. |
| VHP | Metallic nosebands were slightly tarnished and visibly not as shiny when compared with their as-received counterparts. |
| Microwave oven irradiation | All three physical samples of two different models (SN95-E and P100-I) melted partially. SN95-E filtration material melted in areas adjacent to the metallic nosebands. P100-I melted in various locations of the inner foam faceseal comfort lining. Both models were considered unwearable following treatment and subsequently were not evaluated for filter aerosol penetration or filter airflow resistance. |
Filter aerosol penetration
For each ‘FFR model/decontamination treatment’ combination, the average initial filter aerosol penetrations are summarized in Table 3. Not all the 135 post-decontamination FFR samples in the experimental design were tested for filter aerosol penetration as planned; the six FFRs that exhibited melting after microwave irradiation could not undergo laboratory performance evaluation. The remaining 129 post-decontamination FFRs were tested and demonstrated expected levels of filtration efficiency performance. These results indicate that for all tested FFR samples that did not melt, FFR filtration performance was not adversely affected by the decontamination process. Most of the ANOVA tests for initial filter aerosol penetration were non-significant (P > 0.05), (Table 4). In terms of average initial filter aerosol penetration, only P100-I yielded a significant difference by treatment (P = 0.0438), which appeared to be primarily driven by the increased filter aerosol penetration levels for the UVGI treatment (0.012 versus 0.008% for the control). Although statistically significant, this difference in levels of filter aerosol penetration is practically insignificant because the penetration levels still are far less than expected levels for this class of FFRs (<0.03%).
Table 3.
Summary data of filter aerosol penetration and filter airflow resistance for FFRs following various decontamination treatmentsa
| FFR model | Treatment | Average initial sodium chloride penetration (%P) | Standard deviation of penetration | Average initial resistance (mmH2O) | Standard deviation of resistance |
| N95 FFRs | |||||
| N95-A | As-received | 0.121 | 0.08 | 7.6 | 0.83 |
| UVGI | 0.072 | 0.04 | 7.6 | 0.29 | |
| EtO | 0.101 | 0.06 | 7.3 | 0.10 | |
| VHP | 0.071 | 0.04 | 7.8 | 0.21 | |
| Microwave | 0.105 | 0.07 | 7.9 | 0.06 | |
| Bleach | 0.262 | 0.18 | 8.1 | 0.47 | |
| N95-B | As-received | 1.00 | 0.64 | 9.4 | 0.68 |
| UVGI | 0.76 | 0.43 | 10.3 | 0.12 | |
| EtO | 0.667 | 0.39 | 9.7 | 0.10 | |
| VHP | 0.659 | 0.34 | 9.6 | 0.50 | |
| Microwave | 1.06 | 0.74 | 9.0 | 0.40 | |
| Bleach | 0.629 | 0.34 | 9.8 | 0.30 | |
| N95-C | As-received | 1.48 | 0.94 | 6.9 | 1.61 |
| UVGI | 1.77 | 0.96 | 7.1 | 1.68 | |
| EtO | 1.82 | 1.12 | 6.9 | 1.47 | |
| VHP | 1.47 | 0.91 | 6.5 | 2.37 | |
| Microwave | 1.46 | 0.82 | 6.2 | 0.61 | |
| Bleach | 1.13 | 0.79 | 8.0 | 3.06 | |
| Surgical N95 respirators | |||||
| SN95-D | As-received | 1.57 | 0.83 | 8.4 | 0.50 |
| UVGI | 1.86 | 0.97 | 9.2 | 0.44 | |
| EtO | 0.90 | 0.49 | 8.1 | 0.32 | |
| VHP | 0.71 | 0.50 | 8.6 | 1.04 | |
| Microwave | 0.711 | 0.44 | 8.7 | 0.64 | |
| Bleach | 0.561 | 0.38 | 9.6 | 0.29 | |
| SN95-E | As-received | 0.335 | 0.19 | 6.1 | 0.15 |
| UVGI | 0.371 | 0.21 | 7.1 | 0.61 | |
| EtO | 0.498 | 0.32 | 6.7 | 0.40 | |
| VHP | 0.542 | 0.32 | 7.1 | 1.28 | |
| Microwave | Melted | Melted | Melted | Melted | |
| Bleach | 0.233 | 0.12 | 6.6 | 0.56 | |
| SN95-F | As-received | 0.716 | 0.37 | 6.7 | 0.17 |
| UVGI | 0.720 | 0.37 | 6.6 | 0.26 | |
| EtO | 0.687 | 0.35 | 6.3 | 0.25 | |
| VHP | 0.727 | 0.37 | 6.5 | 0.29 | |
| Microwave | 0.652 | 0.33 | 5.4 | 0.72 | |
| Bleach | 0.692 | 0.35 | 5.9 | 0.46 | |
| P100 FFRs | |||||
| P100-G | As-received | 0.009 | 0.01 | 13.1 | 0.79 |
| UVGI | 0.005 | 0.00 | 13.1 | 1.21 | |
| EtO | 0.003 | 0.00 | 12.8 | 0.57 | |
| VHP | 0.006 | 0.01 | 13.4 | 1.23 | |
| Microwave | 0.002 | 0.00 | 13.1 | 0.62 | |
| Bleach | 0.006 | 0.00 | 13.6 | 0.92 | |
| P100-H | As-received | 0.007 | 0.01 | 15.8 | 0.87 |
| UVGI | 0.007 | 0.01 | 16.0 | 1.82 | |
| EtO | 0.003 | 0.00 | 15.2 | 0.64 | |
| VHP | 0.010 | 0.01 | 15.0 | 1.27 | |
| Microwave | 0.000 | 0.00 | 15.8 | 0.30 | |
| Bleach | 0.010 | 0.01 | 15.1 | 0.81 | |
| P100-I | As-received | 0.008 | 0.00 | 16.4 | 0.85 |
| UVGI | 0.012 | 0.01 | 16.5 | 0.10 | |
| EtO | 0.006 | 0.00 | 15.9 | 0.76 | |
| VHP | 0.007 | 0.00 | 16.2 | 0.93 | |
| Microwave | Melted | Melted | Melted | Melted | |
| Bleach | 0.004 | 0.00 | 17.0 | 0.98 | |
Filter aerosol penetration and filter airflow resistance testing performed using a TSI 8130 AFT (n = 3).
Table 4.
One-way ANOVA test results for each FFR model
| FFR model | Penetration (P-value) | Resistance (P-value) |
| N95 FFRs | ||
| N95-Aa | 0.0635 | 0.1233 |
| N95-Ba | 0.5761 | 0.0035b |
| N95-Ca | 0.8067 | 0.7572 |
| Surgical N95 FFRs | ||
| SN95-Da | 0.7688 | 0.0170b |
| SN95-Ec | 0.2189 | 0.2448 |
| SN95-Fa | 0.9409 | 0.0014b |
| P100 FFRs | ||
| P100-Ga | 0.2185 | 0.7446 |
| P100-Ha | 0.3046 | 0.4970 |
| P100-Ic | 0.0438a | 0.2580 |
For each FFR model with the exceptions of SN95-E and P100-I, one-way ANOVAs compare observed filter aerosol penetration or filter airflow resistance values for six test treatments [five different decontamination treatments and one as-received (control) group].
Values in bold font are P-value <0.05. Probability (P-value) of observing the given F-statistic or larger by chance.
The one-way ANOVAs compare observed filter aerosol penetration and filter airflow resistance values for five test treatments [four different decontamination treatments and one as-received (control) group]. Respirator component materials melted for these FFRs during microwave oven irradiation and subsequently samples were not evaluated for initial filter aerosol penetration and initial filter airflow resistance.
Filter airflow resistance
For each ‘FFR model/decontamination treatment’ combination, the average initial filter airflow resistances are summarized in Table 3. The six FFRs in which melting occurred could not be tested for filter airflow resistance. For the remaining 129 post-decontamination samples tested, average initial filter airflow resistance measurements were ≤17.0 mm H2O. Previous studies using the same test method on 21 models of NIOSH-approved N95 FFRs observed filter airflow resistance levels between 7 and 30 mmH2O (Viscusi et al., 2009). For filter airflow resistance, three of the nine ANOVA tests, including N95-B (P = 0.0035), SN95-D (P = 0.0170), and SN95-F (P = 0.0014), showed significantly different means (see Table 4). Although statistically significant, the levels of differences in filter airflow resistance between treatments are not practically meaningful as small changes in filter airflow resistance are unlikely to be noticed by the user (Vojtko et al., 2008).
Dry oven experiments
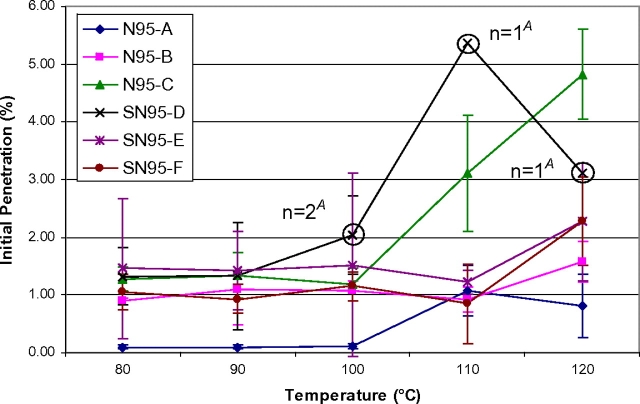
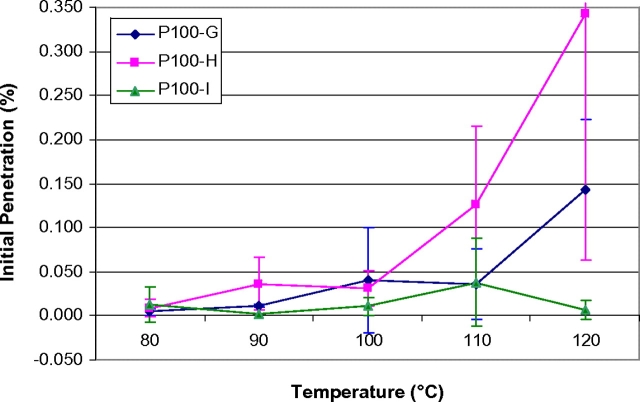
The degree to which temperature affects initial filter aerosol penetration and component melting was observed to be model specific (Figs 1 and 2). The average initial penetration (n = 3) for each N95 model is shown in Fig. 1. Only three tested N95 FFR samples had filter aerosol penetrations >5% (therefore failed to maintain their expected filtration efficiency level of ≥95%). These three failing samples were one SN95-D (5.37% at 110°C) and two N95-C (5.18 and 5.37%, both at 120°C). Five of the SN95-D samples could not be analyzed following treatments of 100°C (one sample), 110°C (two samples), and 120°C (two samples) because their inner moisture barrier melted into the filtration media rendering those samples unsuitable for testing. For the three P100 FFR models, average initial filter aerosol penetration values for P100-G and P100-H exceeded 0.03% beginning at 100°C for P100-G and beginning at 90°C for P100-H (Fig. 2). P100-I averaged an initial filter aerosol penetration value <0.03% for all evaluated temperature increments with the exception of one 110°C temperature experiment. This unexpectedly high average result was due to a single test (%P = 0.096).
Fig. 1.
N95 FFR average initial sodium chloride filter aerosol penetration versus temperature. Each data point represents the average initial penetration of three samples (n = 3), unless otherwise noted. ‘A’ indicates five SN95-D FFRs melted, one at 100°C, two at 110°C, and two at 120°C and could not be penetration or airflow resistance tested.
Fig. 2.
P100 FFR average initial sodium chloride filter aerosol penetration versus temperature. Each data point represents the average initial penetration of three samples (n = 3).
Hydrophobicity testing
All nine FFR models demonstrated differences in their number of media layers and the hydrophobicity of their filter media (Table 5). Common to all three models of surgical N95 respirator was the fact that their outer layer was hydrophobic. This is not surprising since surgical N95 respirators cleared by the US FDA undergo fluid resistance testing and are used as barriers against disease transmission by airborne respiratory fluids, including blood, and other small infectious droplets (Bailar et al., 2006). The N95 FFRs and P100 FFRs varied by having either hydrophobic or hydrophilic outer and inner layers. All middle layers, with the exception of those that were plastic webbing, were hydrophobic on both sides.
Table 5.
FFR media layer hydrophobicity
| FFR model | Total layers | Outer layer | Middle layers | Inner layer |
| N95 FFRs | ||||
| N95-A | 4 | — | Second, — | + |
| Third, — | ||||
| N95-B | 2 | +/— | No middle layer | —/+ |
| N95-C | 5 | Plastic webbing | Second, — | — |
| Third, — | ||||
| Fourth, plastic webbing | ||||
| Surgical N95 respirators | ||||
| SN95-D | 5 | — | Second, — | — |
| Third, — | ||||
| Fourth, — | ||||
| SN95-E | 5 | — | Second, — | — |
| Third, — | ||||
| Fourth, — | ||||
| SN95-F | 4 | — | Second, — | + |
| Third, — | ||||
| P100 FFRs | ||||
| P100-G | 5 | — | Second, — | + |
| Third, — | ||||
| Fourth, — | ||||
| P100-H | 12 | Plastic webbing | Second, — | — |
| Third, — | ||||
| Fourth, — | ||||
| Fifth, — | ||||
| Sixth, plastic webbing | ||||
| Seventh, — | ||||
| Eighth, — | ||||
| Ninth, — | ||||
| 10th, — | ||||
| 11th, plastic webbing | ||||
| P100-I | 6 | + | Second, — | + |
| Third, — | ||||
| Fourth, — | ||||
| Fifth, — | ||||
—, both sides of layer are hydrophobic; +, both sides of layer are hydrophilic; +/—, outer side of layer is hydrophilic and inner side of layer is hydrophobic; —/+, outer side of layer is hydrophobic and inner side of layer is hydrophilic; plastic webbing, not tested.
Chlorine off-gassing experiments
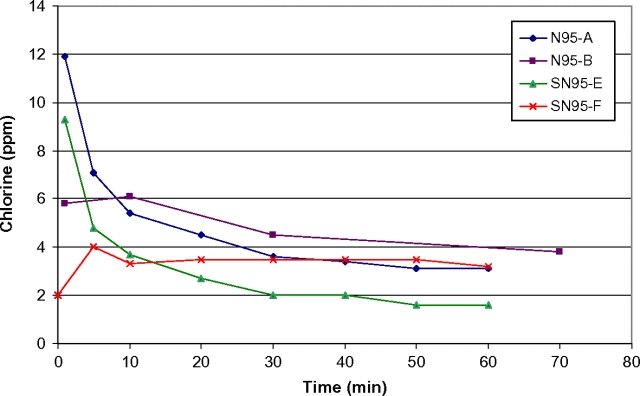
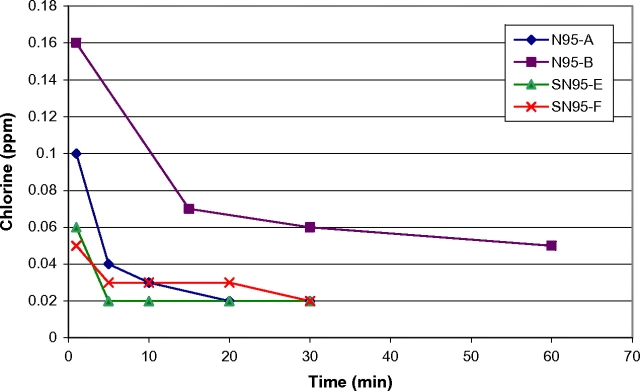
Initial concentrations of chlorine gas (2–12 p.p.m.) were measured on FFRs wet with bleach immediately following submersion for 30 min (Fig. 3). FFRs that were treated using bleach and allowed to air-dry overnight (as described in Table 1) had initial concentrations of ∼0.05 p.p.m. followed by no detectable off-gassing (0 p.p.m.) after the initial data point. FFRs which were submerged in bleach, immediately rinsed (entirely under a stream of deionized water for ∼1 min) and then allowed to air-dry overnight had concentrations similar to FFRs which were not rinsed, indicating that the water rinse had no effect. When FFRs were rehydrated by rinsing with deionized water following overnight air-drying, low-level chlorine off-gassing concentrations were measured at ∼0.1 p.p.m. (Fig. 4).
Fig. 3.
Chlorine off-gassing of FFRs after 30 min submersion in bleach (tested wet).
Fig. 4.
Chlorine off-gassing of FFRs submerged in bleach (dried overnight then rinsed with deionized water and tested wet).
DISCUSSION
The goal of this study was to evaluate five decontamination methods using nine FFR models from three FFR types (three N95 models, three surgical N95 respirator models, and three P100 models) to determine which methods should be considered for future research studies. The five decontamination methods were selected based on previous research from the NPPTL laboratory (Viscusi et al., 2007). Criteria for assessing methods of decontaminating disposable N95 FFRs have been suggested by the National Academies (Bailar et al., 2006); the decontamination method must remove the viral threat, be harmless to the user, and not compromise the integrity of the various elements of the respirator. This manuscript utilizes and expands upon the second and third criteria. For purposes of discussion, a successful FFR decontamination method is considered to be a physical or chemical treatment which does not degrade laboratory performance (filter aerosol penetration and filter airflow resistance) beyond expected performance levels, is able to be performed on enough FFRs in a short period of time to be practical in the event of a pandemic-induced shortage, and should not pose any additional health risk to the user. In this study, assessment of potential health risks (e.g. possible dermal contact with residuals and/or inhalation of off-gassing residuals) was done using the observational analysis data, off-gassing test results, and general knowledge of the physical/chemical characteristics of the decontamination method. Chemical off-gassing is of particular concern because of the close proximity of the FFR to the wearer's face and breathing zone. A limited assessment of the throughput capability was also done using general knowledge of the various decontamination methods. Additional studies on dry heat laboratory oven exposure and FFR media layer hydrophobicity were conducted to collect data on various aspects of FFR resilience and construction in order to further optimize decontamination strategies and assess the practicality for FFR decontamination during a shortage. In the following sections, the results of laboratory performance testing and observational analysis, additional testing, and assessment of throughput and health concerns will be discussed for each of the five decontamination methods evaluated in order to provide recommendations on which decontamination methods should be considered in future research studies.
Bleach
Bleach is available as an aqueous solutions containing 5–15% sodium hypochlorite (active ingredient) which is a highly active oxidizing agent known to be effective against a broad spectrum of bacteria and viruses (,Rutala and Weber, 1997; McDonnell and Russell, 1999). Bleach decontamination did not affect the FFRs’ filter aerosol penetration and filter airflow resistance. The metallic nosebands of all models that had them were slightly tarnished following decontamination and the inner nose cushion on the SN95-E FFRs was discolored. Throughput capability of a bleach method similar to the one used in this study is likely to be high; the main limiting factors are the size of the vessel containing the bleach and FFRs, adequate space to dry the FFRs, and sufficient time for air-drying.
All FFR models had a scent of bleach following overnight air-drying. Residual bleach remaining on FFRs is of concern given its known health effects. Hypochlorite powder, solutions, and vapor can be irritating and corrosive to the eyes, skin, and respiratory tract. For example, Nixon et al. (1975) reported that a 5.25% sodium hypochlorite solution caused severe irritation to human skin over a 4-h exposure. Other studies also reported skin irritation for long-term exposure down to a 1% solution (Eun et al., 1984; Habetes et al., 1986; Hostynek et al., 1990). Low concentrations of bleach have been shown to trigger respiratory events in asthmatics and sensitized individuals (,Medina-Ramon, 2005; Mirabelli et al., 2007). The chlorine off-gassing measurements showed that overnight air-drying significantly reduced off-gassing; however, when the FFR was rehydrated with deionized water, an increase in off-gassing was measured. This observation may be significant when viewed in light of the moisture in the exhaled breath of an individual; it gives rise to the possibility of an individual being exposed to low levels of chlorine (<0.2 p.p.m.) from a bleach-decontaminated FFR. Comparing Table 5 and data shown in Fig. 4, a relationship between hydrophobicity of outer and inner respirator surface layers to off-gassing concentration could not be established.
Considering the potential health risks, the bleach method evaluated in this study is not recommended for further study without modification. Possible modifications worth further investigation would include reduced initial bleach concentration, chemical methods for neutralizing residuals, additional rinse steps, and more aggressive air-drying procedures.
Ethylene oxide
EtO is used in a wide range of work settings as a sterilant or fumigant, including healthcare, diagnosis, and treatment facilities; medical products manufacturing; and libraries and museums (NIOSH, 1981). EtO decontamination did not affect the filter aerosol penetration, filter airflow resistance, or physical appearance of the FFRs in this study. The EtO process used in this study has a 5-h total processing cycle (1-h EtO exposure followed by 4 h of aeration) and has a 4.8 ft3 (0.14 m3) chamber volume (3M, 2007). The 5-h total processing time may be a limiting factor in the timely processing of a large volume of FFRs. Residual EtO remaining on FFRs following EtO vapor-phase decontamination is not believed to be a concern because the sterilization process includes a final aeration cycle of 4 h to remove residual EtO gas.
Vaporized hydrogen peroxide
VHP has been shown to be sporicidal at temperatures ranging from 4 to 80°C, with sterilant concentrations ranging from 0.5 to <10 mg l−1 (Joslyn, 1991). VHP decontamination for a single warm cycle did not significantly affect FFR filter aerosol penetration or filter airflow resistance. The only visible physical effect on the FFRs was a slight tarnishing of the metallic nosebands. The VHP process used in this study has a short cycle time (55 min) and a usable processing volume of 3.5 ft3 (0.1 m3) (Advanced Sterilization Products, 2007). Although the 55-min cycle time is short compared to the lengthy EtO total process time, the throughput capability of VHP processing is limited by the fact that cellulose-based products (e.g. cotton, which may be present in some head straps or some FFR layers) absorb hydrogen peroxide and can cause the STERRAD® cycle to abort due to low hydrogen peroxide vapor concentration. Significant levels of residual hydrogen peroxide vapors off-gassing from FFR materials following the STERRAD® process are unlikely and not of concern because the vapors decompose readily into water vapor and oxygen, both of which are environmentally benign (Advanced Sterilization Products, 2007).
Microwave oven irradiation
Biological decontamination of FFRs using a domestic microwave oven is an attractive idea since it has the advantages of convenience and short treatment times. The decontamination method used here treats the microwave oven as a source of dry heat, similar to other studies. Elhafi et al. (2004) demonstrated that four avian viruses (infectious bronchitis virus, avian pneumovirus, Newcastle disease virus, and avian influenza virus) were inactivated on dried cotton swab samples using a domestic microwave oven for as little as 20 s. Rosaspina et al. (1994) demonstrated destruction of Mycobacterium bovis dried onto scalpel blades after 4 min of microwave exposure.
Of the nine FFR models that underwent microwave oven irradiation, filter aerosol penetration and filter airflow resistance were not affected for seven models. Material components melted on the two remaining models. Correlation could not be established for filter aerosol penetration results between dry oven-treated and microwave oven-irradiated samples. In microwave oven irradiation tests, all three SN95-D samples had penetration values <5% and did not melt; however, some SN95-D samples partially melted at 100, 110, and 120°C during dry oven treatment (Fig. 1). All SN95-E samples and all P100-I samples partially melted in the microwave oven, but no melting was observed for these two models, even at 120°C following dry oven treatment (Table 3, Figs 1 and 2).
The throughput capability of a method similar to the one in this study was limited by microwaving one FFR at a time; however, the 2-min treatment time per FFR was relatively short. Although it is likely that processing more than one FFR at a time is feasible (limited only by the internal volume of the oven), maximizing throughput was beyond the scope of this investigation. No known health risks to the user were identified. The data presented here suggest that the dry microwave oven irradiation method requires improvement before it could be recommended for decontamination and subsequent reuse. Possible modifications worth further investigation would include microwave irradiation of wet FFRs, shorter exposure times, and lower power settings.
Ultraviolet germicidal irradiation
UVGI has been demonstrated to be effective for the disinfection of drinking water and wastewater (Sykes, 1965; Angehrn, 1984; Lazarova et al., 1999; Craik et al., 2001; Lazarova and Savoye, 2004; Wu et al., 2005) and for hospital air disinfection as a method for controlling airborne infectious disease (Macher et al., 1992; Nardell, 1993; CDC, 1994; Gorsuch et al., 1998; Miller and Macher, 2000). This study found that UVGI treatment did not affect the filter aerosol penetration, filter airflow resistance, or physical appearance of the FFRs. Throughput capability of a method similar to the one in this study is benefited by a relatively short irradiation time (30 min); however, it is limited by the available working surface area of a biosafety cabinet equipped with a UV-C source or other area being irradiated by a UVGI source. No known health risks to the user were identified.
Study limitations
These findings are exploratory and the data presented in this study are applicable only to the FFRs and decontamination methods tested; other FFRs may be more easily degraded while others may be less affected and slight modifications to the decontamination methods could result in different findings. Future studies are still needed to evaluate whether the decontamination processes evaluated in this study will inactivate infectious microorganisms (or appropriate surrogates), if FFR decontamination influences respirator fit, and the effect of multiple decontamination treatments on FFR performance. Future studies should also investigate the depths that infectious organisms (or appropriate surrogates) penetrate into each FFR layer, assess the relative cost of various decontamination strategies, and determine how effective various decontamination methods are at reducing the number of viable virus in all layers of the FFRs. Recent work in the NPPTL laboratory toward developing a system for studying the virucidal capability of decontamination methods for FFRs appears promising (Fisher et al., 2009).
CONCLUSIONS
The effects of the various decontamination methods on the laboratory performance (filter aerosol penetration and filter airflow resistance) and physical appearance of FFRs were found to be model specific. The respirators tested have differences in their design, materials of construction, and hydrophobicity of their layers (including the filter media layers). Microwave oven irradiation melted all six samples from two FFR models. The remainder of the FFR samples that were evaluated exhibited average initial filter airflow resistances ≤17.0 mmH2O and average initial sodium chloride filter aerosol penetration values ≤1.86% for N95 FFRs and ≤0.012% for P100 FFRs. Although there were statistically significant differences found between control respirators and those that have undergone decontamination for both filter aerosol penetration and filter airflow resistance, the practical significance is minimal as the range of numerical differences is quite small. The scent of bleach remained noticeable on all FFR models following overnight drying and low levels of chlorine were found to off-gas from bleach-decontaminated FFRs when rehydrated with deionized water, thus giving rise to the possibility of low-level exposure to a subsequent wearer.
In light of these results, the microwave oven irradiation and bleach decontamination methods investigated in this study were determined to be the least desirable among the five methods tested for consideration in future studies. UVGI, EtO, and VHP were found to be the most promising decontamination methods; however, concerns remain about the throughput capabilities for EtO and VHP. Further research is needed before any specific decontamination methods can be recommended.
FUNDING
National Institute for Occupational Safety and Health (CAN #921Z6PT).
Acknowledgments
The authors would like to express their sincere gratitude to Charles Hughes and Donald Tumminelli (SPSmedical Supply Corp., Rush, NY, USA), Douglas P. Landsittel (Duquesne University, NIOSH/NPPTL), Samy Rengasamy (NIOSH/NPPTL), Evanly Vo (NIOSH/NPPTL), Debra Novak (NIOSH/NPPTL), Ziqing Zhuang (NIOSH/NPPTL), and Edward Fisher (EG&G Technical Services, Inc.) for their contributions and consultation. The authors would also like to extend thanks to Les Boord (NIOSH/NPPTL), Roland Berry Ann (NIOSH/NPPTL), Maryann D'Alessandro (NIOSH/NPPTL), Lisa Delaney (NIOSH/OD), Ray Roberge (NIOSH/NPPTL), Jay Parker (NIOSH/NPPTL), and Brian Heimbuch (Applied Research Associates) for their reviews, comments, and suggestions.
Disclaimer—The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the NIOSH. Mention of company names or products does not constitute endorsement by NIOSH.
References
- 3M. 3M Steri-Vac ethylene oxide sterilization systems and accessories. St Paul, MN: 3M Inc; 2007. Available at http://www.3M.com/medicalspecialties. Accessed 24 March 2009. [Google Scholar]
- Advanced Sterilization Products. STERRAD® 100S. 2007. Available at http://www.sterrad.com/Products_&_Services/STERRAD/STERRAD_100S/Literature/100S_compare_eto.pdf. Accessed 24 March 2009. [Google Scholar]
- Angehrn M. Ultraviolet disinfection of water. Aqua. 1984;2:109–15. [Google Scholar]
- Bailar JC, Brosseau LM, Cohen HJ, et al. Reusability of facemasks during an influenza pandemic. Washington, DC: Institute of Medicine, National Academies Press; 2006. [Google Scholar]
- CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. MMWR Recomm Rep. 1994;43:1–132. [PubMed] [Google Scholar]
- CDC. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings 2007. Atlanta, GA: J.D. Siegel and the Healthcare Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention.; 2007. Available at http://www.cdc.gov/ncidod/dhqp/gl_isolation.html. Accessed 24 March 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik SA, Weldon D, Finch GR, et al. Inactivation of Cryptosporidium parvum oocysts using medium- and low-pressure ultraviolet radiation. Water Res. 2001;35:1387–98. doi: 10.1016/s0043-1354(00)00399-7. [DOI] [PubMed] [Google Scholar]
- Elhafi G, Naylor CJ, Savage CE, et al. Microwave or autoclave treatments destroy the infectivity of infectious bronchitis virus and avian pneumovirus but allow detection by reverse transcriptase–polymerase chain reaction. Avian Pathol. 2004;33:303–6. doi: 10.1080/0307945042000205874. [DOI] [PubMed] [Google Scholar]
- Eun HC, Lee AY, Lee YS. Sodium hypochlorite dermatitis. Contact Dermatitis. 1984;11:45. doi: 10.1111/j.1600-0536.1984.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Fisher E, Rengasamy S, Viscusi DJ, et al. Development of a test system to apply virus containing particles to air permeable materials for the evaluation of decontamination procedures for filtering facepiece respirators. J Appl Environ Microbiol. 2009;75:1500–7. doi: 10.1128/AEM.01653-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsuch EL, Grinshpun SA, Willeke K, et al. Method for evaluating germicidal ultraviolet inactivation of biocontaminated surfaces. Int J Occup Saf Ergon. 1998;4:287–97. doi: 10.1080/10803548.1998.11076395. [DOI] [PubMed] [Google Scholar]
- Habetes JMW, Geursen-Reitsma AM, Stolz E, et al. Sensitization to sodium hypochlorite causing contact dermatitis. Contact Dermatitis. 1986;15:140–2. doi: 10.1111/j.1600-0536.1986.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Hostynek JJ, Wilhelm KP, Cua AB, et al. Irritation factors of sodium hypochlorite solutions in human skin. Contact Dermatitis. 1990;23:316–24. doi: 10.1111/j.1600-0536.1990.tb05165.x. [DOI] [PubMed] [Google Scholar]
- Joslyn LJ. Gaseous chemical sterilization. In: Block SS, editor. Disinfection, sterilization and preservation. 4th. Philadelphia, PA: Lea and Febiger; 1991. pp. 344–5. [Google Scholar]
- Lazarova V, Savoye P. Technical and sanitary aspects of wastewater disinfection by UV irradiation for landscape irrigation. Water Sci Technol. 2004;50:203–9. [PubMed] [Google Scholar]
- Lazarova V, Savoye P, Janex ML, et al. Advanced wastewater disinfection technologies: state of the art and perspective. Water Sci Technol. 1991;40:203–13. [Google Scholar]
- Macher JM, Alevantis LE, Chang YL, et al. Effect of ultraviolet germicidal lamps on airborne microorganisms in an outpatient waiting room. Appl Occup Environ Hyg. 1992;7:505–13. [Google Scholar]
- McDonnell D, Russell D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Ramon M. Asthma, chronic bronchitis, and exposure to irritant agents in occupational domestic cleaning: a nested case-control study. Occup Environ Med. 2005;62:598–606. doi: 10.1136/oem.2004.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Macher JM. Evaluation of a methodology for quantifying the effect of room air ultraviolet germicidal irradiation on airborne bacteria. J Aerosol Sci Technol. 2000;33:274–5. [Google Scholar]
- Mirabelli MC, Zock J, Plana E, et al. Occupational risk factors for asthma among nurses and related healthcare professionals in an international study. Occup Environ Med. 2007;64:474–9. doi: 10.1136/oem.2006.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardell EA. Fans, filters, or rays? Pros and cons of the current environmental tuberculosis control technologies. Infect Cont Hosp Epidemiol. 1993;14:681–5. doi: 10.1086/646669. [DOI] [PubMed] [Google Scholar]
- NIOSH. National Institute for Occupational Safety and Health; 1981. Current intelligence bulletin 35-ethylene oxide. Available at http://www.cdc.gov/niosh/81130_35.html. Accessed 24 March 2009. [Google Scholar]
- NIOSH. Determination of inhalation resistance (Procedure RCT-APR-STP-0007) Pittsburgh, PA: National Institute for Occupational Safety and Health, National Personal Protective Technology Laboratory; 2005. Available at http://www.cdc.gov/niosh/npptl/stps/respirator_testing.htm#STP_APR. Accessed 24 March 2009. [Google Scholar]
- NIOSH. Determination of particulate filter efficiency level for N95 series filters against solid particulates for non-powered, air-purifying respirators (procedure TEB-APR-STP-0059) Pittsburgh, PA: National Institute for Occupational Safety and Health, National Personal Protective Technology Laboratory; 2007. Available at http://www.cdc.gov/niosh/npptl/stps/respirator_testing.htm#STP_APR. Accessed 24 March 2009. [Google Scholar]
- Nixon GA, Tyson CA, Wertz WC. Interspecies comparisons of skin irritancy. Toxicol Appl Pharmacol. 1975;31:481–90. doi: 10.1016/0041-008x(75)90272-0. [DOI] [PubMed] [Google Scholar]
- OSHA. Pandemic influenza preparedness and response guidance for healthcare workers and healthcare employers. Washington, DC: Occupational Safety and Health Administration; 2007. Pub no. OSHA 3328-05. [Google Scholar]
- Rosaspina S, Salvatorelli G, Anzanel D. The bactericidal effect of microwaves on Mycobacterium bovis dried on scalpel blades. J Hosp Infect. 1994;26:45–50. doi: 10.1016/0195-6701(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Rutala WA, Weber DJ. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Rev. 1997;10:597–610. doi: 10.1128/cmr.10.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes G. Disinfection and sterilization. 2nd. London: E & EN Spon; 1965. [Google Scholar]
- Viscusi DJ, Bergman MS, Sinkule EJ, et al. Evaluation of the filtration performance of 21 N95 filtering facepiece respirators after prolonged storage. Am J Infect Control. 2009;37:381–6. doi: 10.1016/j.ajic.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi DJ, King WP, Shaffer RE. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Respir Prot. 2007;24:93–107. [Google Scholar]
- Vojtko MR, Roberge MR, Vojtko RJ, et al. Effect of breathing resistance of a surgical mask worn over a N95 filtering facepiece respirator. J Int Soc Respir Prot. 2008;25:1–8. [Google Scholar]
- Wu Y, Clevenger T, Deng B. Impacts of goethite particles on UV disinfection of drinking water. Appl Environ Microbiol. 2005;71:4140–3. doi: 10.1128/AEM.71.7.4140-4143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]