Abstract
The long-term benefits of antenatal iron supplementation in child survival are not known. In 1999–2001, 4,926 pregnant women in rural Nepal participated in a cluster-randomized, double-masked, controlled trial involving 4 alternative combinations of micronutrient supplements, each containing vitamin A. The authors examined the impact on birth weight and early infant mortality in comparison with controls, who received vitamin A only. They followed the surviving offspring of these women at approximately age 7 years to study effects of in utero supplementation on survival. Of 4,130 livebirths, 209 infants died in the first 3 months and 8 were lost to follow-up. Of those remaining, 3,761 were followed, 150 died between ages 3 months and 7 years, and 152 were lost to follow-up. Mortality rates per 1,000 child-years from birth to age 7 years differed by maternal supplementation group, as follows: folic acid, 13.4; folic acid-iron, 10.3; folic acid-iron-zinc, 12.0; multiple micronutrients; 14.0; and controls, 15.2. Hazard ratios were 0.90 (95% confidence interval (CI): 0.65, 1.22), 0.69 (95% CI: 0.49, 0.99), 0.80 (95% CI: 0.58, 1.11), and 0.93 (95% CI: 0.66, 1.31), respectively, in the 4 supplementation groups. Maternal iron-folic acid supplementation reduced mortality among these children by 31% between birth and age 7 years. These results provide additional motivation for strengthening antenatal iron-folic acid programs.
Keywords: child, dietary supplements, folic acid, iron, micronutrients, pregnancy, prenatal care, survival
Globally, pregnant women and young children are at the highest risk of anemia, with iron deficiency contributing to 50% of this risk (1, 2). The global prevalence of anemia among pregnant women is estimated at 41.8% (1). There exists an international policy for antenatal iron-folic acid supplementation in many developing countries with high rates of anemia and iron deficiency (3). Recent studies have shown antenatal iron (with or without folic acid) to reduce rates of low birth weight (4–6) and preterm birth (6), and anemia during pregnancy is associated with increased risk of perinatal and maternal mortality (7, 8). Recently there has been a global move towards expanding international policy recommendations to promote antenatal multiple micronutrient supplementation. Evidence that micronutrient deficiencies beyond iron-folic acid are common during pregnancy exists but is sparse (9–11). A number of randomized controlled trials of multiple micronutrient supplements versus iron-folic acid have been conducted. Although some results remain unpublished, these studies were recently included in a meta-analysis by Haider and Bhutta (12), who found little benefit of supplementation on pregnancy outcomes, including low birth weight (relative risk (RR) = 0.94, 95% confidence interval (CI): 0.83, 1.06), small-for-gestational-age birth (RR = 1.04, 95% CI: 0.93, 1.17), preterm birth (RR = 0.88, 95% CI: 0.76, 1.03), and perinatal mortality (RR = 1.16, 95% CI: 0.95, 1.42).
In Sarlahi District, Nepal, we conducted a randomized, double-masked trial of administration of 4 alternative combinations of micronutrients during pregnancy (folic acid, folic acid-iron, folic acid-iron-zinc, and a multiple micronutrient supplement that contained the foregoing plus 11 other micronutrients), all including vitamin A, versus vitamin A alone as the control. We found that iron-folic acid supplementation relative to vitamin A alone significantly reduced the prevalence of low birth weight (<2,500 g) by 16% (RR = 0.84, 95% CI: 0.72, 0.99) (4) and the prevalence of maternal anemia during pregnancy and the postpartum period by approximately 50% (95% CI: 34, 66) (13). The multiple micronutrient supplement also lowered these outcomes but not more so than iron-folic acid. There was no impact on fetal loss, and a small apparent reduction in 3-month infant mortality was not significant (RR = 0.80, 95% CI: 0.55, 1.17) for iron-folic acid (14). The relative risk for the multiple micronutrient supplement was 1.14 (95% CI: 0.82, 1.56) for the 3-month infant mortality outcome, indicating little benefit despite the increase in birth weight (14).
We recently completed a follow-up study of the offspring born to women who participated in the original trial in rural Nepal (4, 14), to examine the long-term impact of antenatal/postnatal micronutrient supplementation on childhood survival, growth, and early clinical and biochemical markers of chronic disease. We examined the effects of this intervention on survival among children through early school age (approximately 7 years).
MATERIALS AND METHODS
In 1999–2001, we conducted a randomized, double-masked controlled community trial of antenatal and postnatal micronutrient supplementation in the rural southern plains district of Sarlahi, Nepal, where we have been conducting research over the past 20 years. Details on the trial were published in previous papers (4, 14). The study area was divided into 426 communities called sectors, which served as the unit of randomization in this cluster-randomized trial. Pregnant women received one of the following 4 daily micronutrient supplements in the form of identically shaped, sized, and colored tablets: folic acid (400 μg), folic acid-iron (60 mg of iron in the form of ferrous fumarate), folic acid-iron-zinc (30 mg of zinc sulfate), or a multiple micronutrient supplement containing folic acid-iron-zinc plus vitamin D (10 μg), vitamin E (10 mg), vitamin B1 (1.6 mg), vitamin B2 (1.8 mg), niacin (20 mg), vitamin B6 (2.2 mg), vitamin B12 (2.6 μg), vitamin C (100 mg), vitamin K (65 μg), copper (2.0 mg), and magnesium (100 mg); all 4 supplements included vitamin A. Women who received vitamin A alone (1,000 μg) served as the control group. The supplements were tested midway through the study, and micronutrient concentrations were found to be within 4% of the concentrations expected.
Women in the study were identified early in pregnancy using a urine test-based pregnancy identification surveillance system. After providing consent, women received their daily allocated supplements throughout pregnancy and until 3 months postpartum from female project workers at twice-weekly home visits. The project workers monitored the women's compliance using tablet counts at each visit and encouraged regular intake of the supplements. Over 1.5 years, 4,926 pregnancies were included in the trial. The pregnancies resulted in 4,130 livebirths, 34 of which were of liveborn twins. A total of 209 children had died by age 3 months (14). The mean number of pregnant women per sector was 11.6 (standard deviation, 5.6). The mean gestational age at enrollment was 11 weeks (standard deviation, 5.1), and the median percentage of compliance with supplement intake (number of supplements consumed out of the total number of days eligible from pregnancy enrollment to 3 months postpartum) was 82 (interquartile range, 63–101) and comparable across treatment groups.
From September 2006 to March 2008, we conducted a follow-up study of all surviving children of women who had participated in the original trial. Because of our continued research in the same study area in the interim period, we had routinely updated our information on household addresses, tracked moves, and conducted vital surveillance. In addition, 3,857 of the surviving children also participated in a placebo-controlled trial of iron-folic acid and/or zinc supplementation during 2001–2005 (15, 16) and were routinely visited for supplementation and vital status assessment, among other things. Thus, in 2006, at the outset, we generated a list of all surviving children for a systematic cross-sectional follow-up.
Three separate teams visited the homes of the children over a period of approximately 18 months. After obtaining consent, the first team collected basic data on household members and their vital status and socioeconomic status. They also conducted blood pressure measurements, interviewed the parents regarding the child's schooling, and measured the child's middle upper arm circumference. A second team of trained anthropometrists measured waist circumference, weight, height, and the triceps and subscapular skinfold thicknesses of the children at a second home visit. Dietary intake was assessed using 1-year and 7-day food frequency questionnaires with a list of selected foods generated from our years of collecting similar data in this study area. A 7-day morbidity history of 10 morbidity symptoms was also elicited. A third team conducted fasting venous blood draws and collected other biologic specimens.
At each visit, migrations, refusals, and deaths were recorded. In the event of death, a short history of the death, including the date of death and a parental report of whether the death was due to injury, severe acute illness, or chronic illness or was a sudden death was ascertained. For deaths that occurred during the follow-up period of the parent trial, a detailed verbal autopsy interview was conducted with the parents, followed by review by 2 physicians and assignment of a consensus cause of death. Cause-of-death information was available for 57 deaths, out of which 11 deaths were assigned an “uncertain” cause. The follow-up study received ethical approval from the institutional review boards at Johns Hopkins School of Public Health (Baltimore, Maryland) in the United States and the Institute of Medicine (Kathmandu) in Nepal.
We compared characteristics of children and their households at the time of follow-up by treatment group. Children's anthropometric data were converted to weight-for-age, height-for-age, and weight-for-height z scores using the international World Health Organization growth standards (17). Twins were included in the analysis, as was done previously, since their exclusion did not change the results. Analyses were conducted on an intent-to-treat basis. Data on mortality from ages 3 months to 7 years and from birth to age 7 years were examined for each of the treatment groups in comparison with controls. Hazard ratios and 95% confidence intervals for both mortality outcomes were calculated using a Cox proportional hazards model with a robust variance estimator, which uses the independence working model for its correlation structure (18) to account for the cluster-randomization design. The mortality rate in the control group was used as the reference category. We conducted the same analyses to examine treatment effects for iron-folic acid and the multiple micronutrient group by category of birth weight (<2,500 g vs. ≥2,500 g), gestational age (<37 weeks vs. ≥37 weeks), maternal age (≤19 years vs. >19 years), and body mass index (weight (kg)/height (m)2; <18.5 vs. ≥18.5) at enrollment in the trial. Interaction terms for these variables and the intervention groups were included in the models and tested at the 10% significance level. In addition, maternal intervention effects were adjusted for iron-folic acid and/or zinc supplementation at preschool age, which occurred as part of the subsequent study that enrolled children included in the current analysis, but the child interventions did not change the hazard ratios and were excluded. We also tested the interaction between maternal and child supplementation but found it to be nonsignificant. Across the 2 interventions, there were many cells, and most of the deaths included in the analysis occurred prior to the start of the child supplementation study.
We plotted Kaplan-Meier survival curves (19) by treatment group to visualize the survival differences by treatment group. For each child, we calculated the duration of follow-up in days, using the date of birth and the date of censorship (date of death for those who died). For those lost to follow-up, the date on which their vital status was last known was used as the date of censorship. Thirteen children had missing dates of death. For these children, age at death in years as reported by the parents was used to calculate age of death, assuming that the death had occurred in the middle of the year.
Analyses were performed using SAS, version 9.0 (SAS Institute Inc., Cary, North Carolina), and Stata, version 10.0 (Stata Corporation, College Station, Texas).
RESULTS
Out of 4,130 livebirths, 209 deaths occurred by age 3 months and 8 children were lost to follow-up—data that were previously reported and analyzed by treatment group (14). Of the remaining 3,913 surviving children, we had information from our ongoing studies that 137 children had died and 128 had migrated out of the study area prior to the follow-up study in 2006–2008, when a total of 3,648 children's homes were visited (Figure 1). Of these children, 24 more had migrated and 13 had died. The numbers were comparable by treatment group for each follow-up. Thus, vital status was known for 3,761 (96.1%) of the 3,913 children whose survival was known at 91 days, whereas 152 (3.9%) were lost to follow-up. Of those 3,761 children, 150 (4.0%) had died between ages 3 months and 7 years, and in all, 359 deaths had occurred since birth.
Figure 1.
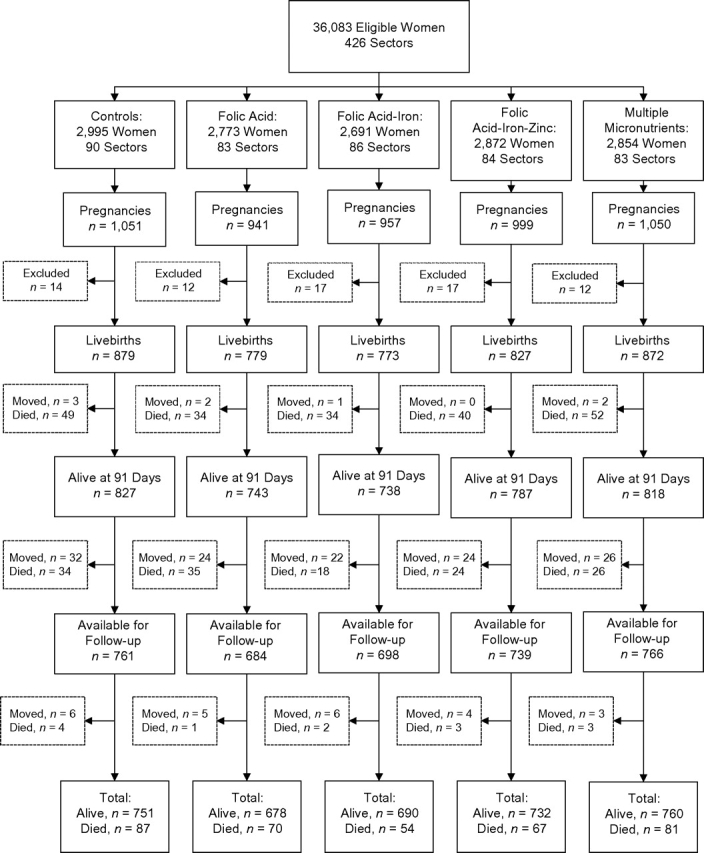
Population follow-up and participation in a controlled trial involving alternative combinations of antenatal micronutrient supplements, Nepal, 1999–2008. Excluded women were those who had a false-positive pregnancy test result, an unknown pregnancy outcome, or an induced abortion.
Previously, we showed that the intervention groups did not differ with regard to a range of baseline maternal characteristics (4, 14). At follow-up, the mean age of surviving children was 7.5 years (standard deviation, 0.5) and did not vary by treatment group (Table 1). Out of 3,761 children, information on the child's household and socioeconomic status was available for 3,753. Mean weight-for-age, height-for-age, and body mass index-for-age z scores and middle upper arm circumference at follow-up revealed significant undernutrition in the population. Approximately 60%–70% of the children had received some schooling, but only about 16%–17% were considered to be literate as reported by the parents. A majority of the population belonged to the artisan and low Hindu caste and to the Madheshi ethnic group. Ownership of land, cattle, and other assets did not differ across treatment groups. Children also did not differ by their dietary intakes or morbidity history in the past 7 days.
Table 1.
Characteristics of Children and Their Households at Follow-up, by Maternal Antenatal Micronutrient Supplementation Group, Nepal, 1999–2008a
| Controls |
Supplementation Group |
||||||||||||||
| Folic Acid |
Folic Acid-Iron |
Folic Acid-Iron-Zinc |
Multiple Micronutrients |
||||||||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Child characteristics | |||||||||||||||
| Age at follow-up, years | 749 | 7.5 (0.4) | 681 | 7.5 (0.4) | 689 | 7.4 (0.4) | 731 | 7.5 (0.5) | 761 | 7.5 (0.4) | |||||
| Height-for-age z score | 698 | −1.93 (0.89) | 629 | −1.89 (0.83) | 639 | −1.90 (0.88) | 661 | −1.83 (0.91) | 720 | −1.93 (0.89) | |||||
| Weight-for-age z score | 698 | −2.11 (0.88) | 629 | −2.06 (0.82) | 638 | −2.07 (0.92) | 661 | −2.08 (0.90) | 720 | −2.12 (0.90) | |||||
| Body mass indexb-for-age z score | 698 | −1.23 (0.87) | 629 | −1.19 (0.86) | 639 | −1.19 (0.88) | 659 | −1.27 (0.84) | 720 | −1.23 (0.83) | |||||
| Middle upper arm circumference, cm | 700 | 15.4 (1.1) | 630 | 15.4 (1.1) | 639 | 15.4 (1.2) | 662 | 15.4 (1.1) | 720 | 15.4 (1.1) | |||||
| Receipt of any schooling | 452 | 62.6 | 438 | 66.6 | 465 | 68.8 | 489 | 69.2 | 473 | 63.8 | |||||
| Literate | 129 | 17.8 | 100 | 15.2 | 100 | 14.8 | 140 | 19.8 | 110 | 14.8 | |||||
| Morbidity in the past 7 days | |||||||||||||||
| Fever | 56 | 8.0 | 57 | 9.0 | 61 | 9.4 | 66 | 9.9 | 79 | 11.0 | |||||
| Diarrhea (≥4 watery stools/day) | 19 | 2.7 | 14 | 2.2 | 10 | 1.6 | 15 | 2.3 | 17 | 2.4 | |||||
| Productive cough | 23 | 3.3 | 24 | 3.8 | 35 | 5.4 | 28 | 4.2 | 26 | 3.6 | |||||
| Rapid breathing or grunting | 18 | 2.6 | 22 | 3.5 | 16 | 2.5 | 28 | 4.2 | 20 | 2.9 | |||||
| Food intake (any) in the past 7 days | |||||||||||||||
| Dairy products | 587 | 83.2 | 505 | 79.6 | 501 | 77.5 | 527 | 79.5 | 570 | 79.1 | |||||
| Meat | 292 | 41.5 | 274 | 43.2 | 276 | 42.6 | 285 | 42.9 | 301 | 41.8 | |||||
| Fish | 237 | 33.7 | 234 | 36.9 | 207 | 31.9 | 225 | 33.8 | 212 | 29.4 | |||||
| Eggs | 111 | 15.8 | 89 | 14.0 | 92 | 14.2 | 114 | 17.1 | 94 | 13.1 | |||||
| Dark green leaves | 506 | 72.1 | 442 | 69.9 | 507 | 78.2 | 487 | 73.4 | 531 | 73.6 | |||||
| Tea | 236 | 33.6 | 179 | 28.3 | 208 | 32.1 | 228 | 34.4 | 217 | 30.1 | |||||
| Family characteristics | |||||||||||||||
| Religion/castec | |||||||||||||||
| Hindu | |||||||||||||||
| Brahmin | 68 | 8.7 | 53 | 7.4 | 37 | 5.2 | 53 | 7.0 | 45 | 5.7 | |||||
| Chettri | 65 | 8.3 | 34 | 4.8 | 67 | 9.4 | 57 | 7.6 | 49 | 6.2 | |||||
| Vaishya | 439 | 56.1 | 476 | 66.6 | 487 | 68.5 | 493 | 65.5 | 521 | 65.8 | |||||
| Shudra | 106 | 13.6 | 97 | 13.6 | 67 | 9.4 | 108 | 14.3 | 114 | 14.4 | |||||
| Muslim | 102 | 13.0 | 48 | 6.7 | 49 | 6.9 | 35 | 4.6 | 58 | 7.3 | |||||
| Buddhist/Christian | 2 | 0.3 | 7 | 1.0 | 4 | 0.6 | 7 | 0.9 | 4 | 0.5 | |||||
| Ethnic groupd | |||||||||||||||
| Pahadi | 221 | 28.3 | 230 | 32.2 | 211 | 29.7 | 239 | 31.7 | 199 | 25.1 | |||||
| Madheshi | 559 | 71.5 | 483 | 67.6 | 497 | 69.9 | 512 | 68.0 | 591 | 74.6 | |||||
| House construction | |||||||||||||||
| No walls, grass, thatch, mud walls | 596 | 76.2 | 560 | 78.3 | 539 | 75.9 | 612 | 81.4 | 613 | 77.4 | |||||
| Wood or cement walls | 186 | 23.8 | 155 | 21.7 | 171 | 24.1 | 140 | 18.6 | 179 | 22.6 | |||||
| Land ownership | |||||||||||||||
| No land or <2 kathase | 328 | 42.4 | 321 | 45.2 | 288 | 40.6 | 316 | 42.1 | 334 | 42.6 | |||||
| ≥2 kathas | 445 | 57.6 | 389 | 54.8 | 421 | 59.4 | 434 | 57.9 | 451 | 57.4 | |||||
| Ownership of assets | |||||||||||||||
| Cattle | 534 | 68.5 | 493 | 69.0 | 506 | 71.2 | 517 | 68.8 | 534 | 67.4 | |||||
| Goats | 485 | 62.2 | 460 | 64.4 | 445 | 62.6 | 510 | 67.8 | 506 | 63.9 | |||||
| Bicycle | 463 | 59.2 | 414 | 57.9 | 417 | 58.6 | 457 | 60.7 | 510 | 64.4 | |||||
| Radio | 252 | 32.2 | 238 | 33.3 | 231 | 32.5 | 252 | 33.5 | 265 | 33.5 | |||||
| Television | 246 | 31.5 | 221 | 30.9 | 221 | 31.1 | 229 | 30.4 | 229 | 28.9 | |||||
| Availability of electricity | 422 | 54.0 | 327 | 45.7 | 363 | 51.0 | 376 | 49.9 | 380 | 48.0 | |||||
Abbreviation: SD, standard deviation.
The maximum n for household socioeconomic variables was 3,753; that for anthropometry was 3,351; and that for diet and morbidity questions was 3,370.
Weight (kg)/height (m)2.
Religion/caste was undeclared for 1 subject.
Pahadi are people from the hills, and Madheshi are people from India who have settled in the plains of Nepal. The ethnicity of 11 subjects was unknown.
1 katha = 0.084 acres.
The hazard ratio for the period from birth to approximately 7 years of age was 0.69 (95% CI: 0.49, 0.99; P = 0.043) in the folic acid-iron group (Table 2). The hazard ratios for the combinations of supplements containing folic acid alone or iron-folic acid with zinc or other micronutrients were attenuated at 0.90, 0.80, and 0.93, respectively, with 95% confidence intervals that included 1. The mortality rate for the period from 91 days to approximately 7 years of age was 6.9 per 1,000 child-years in the control group, with the hazard ratio in the iron-folic acid group being 0.58 (95% CI: 0.34, 1.00; P = 0.049). Adjusting for childhood iron-folic acid and/or zinc supplementation as part of a subsequent study (15, 16) did not change the treatment effects of maternal supplementation, although this analysis was restricted to those who participated in the child supplementation trial and included only 100 deaths which occurred during this trial (data not shown). In a pure intent-to-treat analysis that combined fetal losses (n = 838)—both miscarriages (not a study outcome) and stillbirths—with child deaths, the hazard ratios in the treatment groups ranged from 0.94 to 0.98, with 95% confidence intervals including 1.0. We also combined only stillbirths (n = 186) with child deaths and found the hazard ratio for the iron-folic acid group to be 0.73 (95% CI: 0.54, 0.98).
Table 2.
Rates of Mortality From Birth to Age 7 Years Among Children of Women Who Received Antenatal Micronutrient Supplements, by Supplementation Group, Nepal, 1999–2008
| Controls | Supplementation Group |
||||
| Folic Acid | Folic Acid-Iron | Folic Acid-Iron-Zinc | Multiple Micronutrients | ||
| Deaths occurring from birth to age 7 years | |||||
| Child-years of follow-up | 5,731 | 5,203 | 5,219 | 5,562 | 5,793 |
| No. of deaths | 87 | 70 | 54 | 67 | 81 |
| Rate per 1,000 child-years | 15.2 | 13.4 | 10.3 | 12.0 | 14.0 |
| HR (95% CI)a | 1.00 | 0.90 (0.65, 1.22) | 0.69 (0.49, 0.99) | 0.80 (0.58, 1.11) | 0.93 (0.66, 1.31) |
| Deaths occurring between ages 91 days and 7 years | |||||
| Child-years of follow-up | 5,532 | 5,201 | 5,217 | 5,560 | 5,791 |
| No. of deaths | 38 | 36 | 20 | 27 | 29 |
| Rate per 1,000 child-years | 6.9 | 6.9 | 3.8 | 4.8 | 5.0 |
| HR (95% CI)a | 1.00 | 1.05 (0.66, 1.67) | 0.58 (0.34, 1.00) | 0.74 (0.46, 1.19) | 0.76 (0.47, 1.23) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Hazard ratios and 95% confidence intervals were calculated using Cox proportional hazards with a robust estimate of variance.
Stratified analyses showed that few factors modified the effect of folic acid-iron and multiple micronutrient supplementation (Figure 2). The P value for interaction was significant (P < 0.1) only for preterm birth and the multiple micronutrient supplement group. Neither supplement showed a hazard ratio indicative of adverse risk with a 95% confidence interval that excluded 1 in the substrata that were examined.
Figure 2.
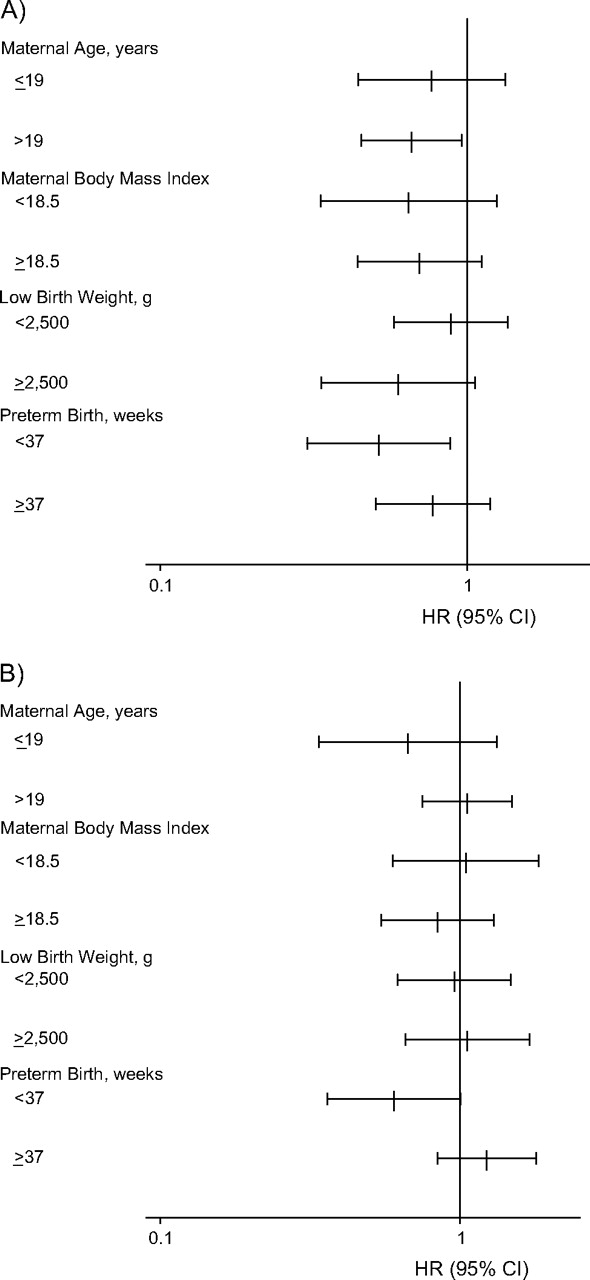
Effects of antenatal micronutrient supplementation on offspring mortality from birth to age 7 years, expressed as hazard ratios (HR) and 95% confidence intervals (CI), by supplementation group, Nepal, 1999–2008. A) Folic acid-iron supplementation versus vitamin A alone (control group); B) multiple micronutrient supplementation versus vitamin A alone (control group). For interaction between preterm birth and multiple micronutrient supplementation, P < 0.1 (Cox proportional hazards model with a robust variance estimate). Body mass index is calculated as weight (kg)/height (m)2.
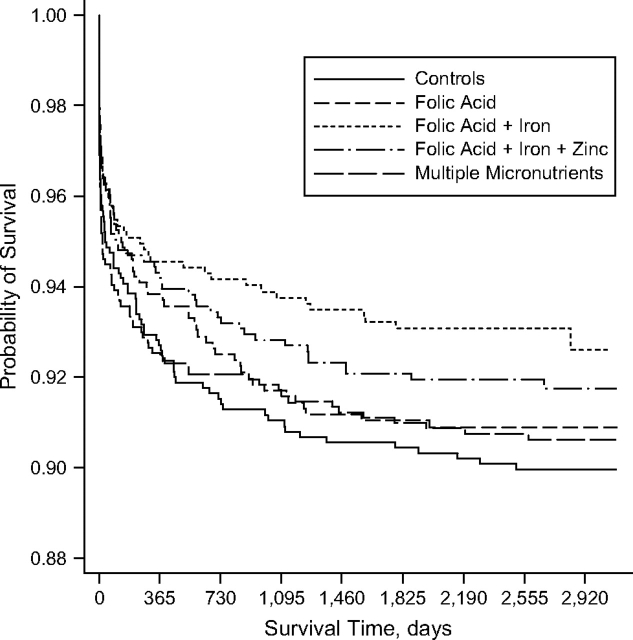
Kaplan-Meier survival curves showed that the offspring of women who had received folic acid-iron supplementation during pregnancy had the highest probability of survival (Figure 3). The survival curves appeared to diverge through about 1,800 days (approximately 5 years), after which the lines seemed to become more parallel.
Figure 3.
Kaplan-Meier curve for probability of survival among children of women who received antenatal micronutrient supplements, by supplementation group, Nepal, 1999–2008.
We examined cause-of-death assignment by physician review for 46 deaths on which data were recorded previously and type of death (categorized as injury-related, due to severe acute illness, due to long-term illness, or sudden) reported by parents at the time of follow-up for another 90 deaths (Table 3). When we combined acute lower respiratory illness, diarrhea/dysentery, sepsis, hepatitis, and severe acute illness as causes of death, suggestive of an “infection-related/acute” cause, the proportionate mortality ratios in the control and folic acid-iron groups were 74 and 55, respectively.
Table 3.
Distribution of Child Deaths by Cause of Deatha and Antenatal Micronutrient Supplementation Group, Nepal, 1999–2008
| Cause of Deathbc | Controls (n = 38) | Supplementation Group |
|||
| Folic Acid (n = 36) | Folic Acid-Iron (n = 20) | Folic Acid-Iron-Zinc (n = 27) | Multiple Micronutrients (n = 29) | ||
| Diarrhea/dysentery | 2 | 3 | 1 | 1 | 2 |
| Acute lower respiratory infection | 5 | 4 | 3 | 3 | 5 |
| Sepsis/hepatitis | 2 | 1 | 1 | 1 | 1 |
| Acute severe illness | 19 | 10 | 6 | 11 | 11 |
| Long-term chronic illness | 2 | 2 | 2 | 4 | 3 |
| Injury | 2 | 5 | 2 | 2 | 4 |
| Sudden death | 0 | 6 | 2 | 2 | 1 |
| Otherd | 1 | 3 | 1 | 0 | 0 |
Determined using physician reviews of verbal autopsies and types of deaths ascertained at the time of follow-up by parental interviews.
Forty-six causes were ascertained using physician reviews of verbal autopsy data for deaths that occurred during the antenatal (6) and preschool (25) supplementation trials. Ninety “causes” were ascertained using parental reports of category of cause, including injury, acute and chronic illness, and sudden death, at the time of the follow-up study.
Data on cause of death were missing for 14 deaths (5 in the control group, 2 in the folic acid group, 2 in the folic acid-iron group, 3 in the folic acid-iron-zinc group, and 2 in the multiple micronutrient group).
Included severe malnutrition, retinoblastoma, and “uncertain cause.”
DISCUSSION
In this study, we found a 31% reduction in childhood mortality due to maternal antenatal/postnatal supplementation with iron-folic acid plus vitamin A as compared with vitamin A alone, in a setting where maternal iron deficiency and anemia are common. To our knowledge, this is the first time that the long-term effect of maternal iron-folic acid supplementation, normally a global policy for pregnant women, on childhood survival has been observed. The randomized, controlled design of the study provided statistical strength for making causal inferences regarding this effect. The study also achieved a high rate of follow-up of children.
Intermittent administration of large doses of vitamin A to preschool children has been shown to reduce mortality among children under age 5 years by 30% (20). However, beyond this intervention, few trials have been undertaken to examine the impact of direct supplementation with micronutrients on childhood mortality. Iron-folic acid or zinc supplementation to children from ages 1 month to 36 months in the same population in Nepal had no impact on survival (15, 16). A reduction in mortality resulting from an intervention such as antenatal/postnatal iron-folic acid supplementation, as currently exists in many malnutrition settings, provides a new and previously unreported benefit to offspring during childhood.
While the combination of iron-folic acid also contained vitamin A, the impact on mortality is probably due to iron-folic acid alone, since the control group receiving vitamin A alone had a higher mortality rate. However, a positive interaction between the nutrients cannot be ruled out.
Previously, multiple micronutrients have shown no effect or modest effects on birth weight (12), and based on results from 2 South Asian trials that were not independently powered to find an impact on mortality, administration of multiple micronutrients may elevate risks of neonatal and perinatal mortality relative to iron-folic acid (14, 21, 22). One trial conducted in Indonesia, however, showed a significant 18% reduction in early infant mortality (<3 months) that was attributed to antenatal/postnatal micronutrient supplementation, as compared with iron-folic acid (23). In the present analysis, the hazard ratio for mortality from birth to age 7.5 years was 0.93 for multiple micronutrients versus controls, suggesting that no long-term adverse effect on mortality occurred. Similarly, the combination of folic acid-iron-zinc had a hazard ratio of 0.80, suggesting potential inhibition of iron with zinc, which was also seen with the birth-weight outcome in the original trial (4). Iron was present at the same dosage in all 3 preparations. We have previously suggested a potential role of negative nutrient-nutrient interactions in causing this (14). The hazard ratio for folic acid alone was 0.89.
Plausible mechanisms for iron supplementation's reducing mortality risk are not known at present, although they may include its effect on birth outcomes such as decreased low birth weight (4–6) and preterm birth (6), as well as increases in infant iron stores (24), all of which may have an impact on long-term survival. Whether maternal iron status plays a role in the development of fetal immunity or early programming is not well established, but the plausibility of such a mechanism cannot be overruled. Given that in this setting of high iron deficiency, where direct supplementation to these children from ages 1 month to 36 months had no impact on childhood mortality (15, 16), it is likely that there exists a critical window of time in the fetal period when iron nutriture can influence future health and survival—a window that may close during the postnatal period. As such, the intervention effect on long-term survival is probably due to supplementation mostly during pregnancy, not the postpartum period.
Our study suffered from our being unable to obtain medical diagnoses for causes of death. A medical determination of cause of death is rare in this environment, where most deaths occur at home and are unattended by physicians. Relying on parental recall for verbal autopsies has been considered valid, and we used these data for deaths when available. However, since many deaths had occurred several years prior to follow-up, we were able to use only crudely categorized causes of death as reported by the parents. However, the available data seemed to indicate that fewer infectious and severe acute deaths occurred in the folic acid-iron group than in the control group; the proportionate mortality ratios for these deaths were 55 and 74, respectively. We also did not collect data on iron status among children, although we did not expect iron supplementation during pregnancy to affect the status of children at school ages. It is also unlikely that the impact on survival was related to the 3 months of postnatal supplementation in women, since breast milk is a poor source of iron for infants. Overall, despite the significant findings, the sample size we had was still limited for observation of mortality outcomes, and we had 50% power to detect a difference of 30% or more with α = 0.05, assuming a mortality rate of 100 per 1,000 livebirths. In addition, comparison between treatment groups could not be done because of the overlapping and wide confidence intervals. For more efficiency, one could increase the size of the control arm; however, we did not do this in the original study, which limited our ability to examine between-group differences for a rarer outcome such as mortality.
We undertook sensitivity analyses to examine the impact of losses to follow-up on the study findings, applying 3 different assumptions regarding the survival of those lost to follow-up: that all survived, that all died, or that half died. We had 161 children who were lost to follow-up. The relative risks for iron + folic acid ranged from 0.71 (all lost survived) to 0.73 (all lost died), and the 95% confidence interval around 0.73 was (0.50, 0.99), after adjustment for clustering using generalized estimating equations Poisson regression analysis with exchangeable correlation. This analysis suggests that the approximately 30% reduction in mortality we observed in the iron-folic acid group was a robust estimate and not vulnerable to the uncertain vital experience among children lost to follow-up.
We know of 1 other study which involved follow-up of the offspring (at age 2 years) of women who participated in a multiple micronutrient supplementation trial (25). In that study, Vaidya et al. (25) found small but significant increases in body size and weight among children whose mothers had received multiple micronutrients during pregnancy as compared with iron-folic acid alone. More such follow-ups will be required to examine the long-term effects of maternal nutrient interventions on a range of outcomes. These studies would contribute to our understanding of the role of nutrition in the developmental origins of health and disease.
The findings of this study may be generalizable to a large swath of the South Asian population living on the Indian subcontinent, including northern India and Bangladesh, where similar burdens of maternal malnutrition, low birth weight, and childhood infectious morbidity and mortality exist.
In conclusion, these high-compliance follow-up data from a randomized, placebo-controlled trial cohort provide strong evidence for a beneficial effect of antenatal/postnatal iron-folic acid supplementation on childhood survival through early school age, extending previously observed beneficial effects on birth size, anemia, infant iron status, and early infant survival. Currently, use of antenatal iron and folic acid supplement is low, despite existing policies and persistent maternal iron deficiency and anemia, in many regions of the world. The findings reported here provide new impetus for programs to extend and improve coverage with iron and folic acid supplementation as part of routine antenatal care in undernourished and underserved populations in rural South Asia.
Acknowledgments
Author affiliations: Center for Human Nutrition, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Parul Christian, Christine P. Stewart, Steven C. LeClerq, Lee Wu, Joanne Katz, Keith P. West); and Nepal Nutrition Intervention Project, Sarlahi, Kathmandu, Nepal (Subarna K. Khatry).
This work was carried out by the Center for Human Nutrition of the Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland) in collaboration with the National Society for the Prevention of Blindness (Kathmandu, Nepal), with funding from the Bill and Melinda Gates Foundation (Seattle, Washington). The antenatal micronutrient supplementation study was conducted under Micronutrients for Health cooperative agreement HRN-A-00-97-00015-00 and Global Research Activity cooperative agreement GHS-A-00-03-00019-00 between the Johns Hopkins University and the Office of Health, Infectious Diseases, and Nutrition, US Agency for International Development (Washington, DC). The Bill and Melinda Gates Foundation and the Sight and Life Research Institute (Baltimore, Maryland) provided additional support for the study. The preschool child iron and zinc supplementation study was funded by grant HD 38753 from the National Institutes of Health (NIH) (Bethesda, Maryland), by the Bill and Melinda Gates Foundation, and by cooperative agreement HRN-A-00-97-00015-00 between the Johns Hopkins University and the Office of Health, Infectious Diseases, and Nutrition, US Agency for International Development. Drs. Joanne Katz and Christine Stewart were supported by NIH grant R03 HD049406-01. Drs. Parul Christian, Joanne Katz, Subarna K. Khatry, and Steven C. LeClerq were supported by NIH grant R01 HD050254-01.
All members of the Nepal study team helped in the successful implementation of the study, including the field managers, supervisors, area coordinators, and team leader interviewers.
The funding agencies played no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- RR
relative risk
References
- 1.McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 2.Stoltzfus RJ. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. J Nutr. 2001;131(suppl):697S–700S. doi: 10.1093/jn/131.2.697S. [DOI] [PubMed] [Google Scholar]
- 3.Stoltzfus RJ, Dreyfuss ML. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. Washington, DC: International Life Sciences Institute; 1998. [Google Scholar]
- 4.Christian P, Khatry SK, Katz J, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003;326(7396):571–576. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cogswell ME, Parvanta I, Ickes L, et al. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr. 2003;78(4):773–781. doi: 10.1093/ajcn/78.4.773. [DOI] [PubMed] [Google Scholar]
- 6.Siega-Riz AM, Hartzema AG, Turnbull C, et al. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. Am J Obstet Gynecol. 2006;194(2):512–519. doi: 10.1016/j.ajog.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, et al., editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Vol 1. Geneva, Switzerland: World Health Organization; 2004. pp. 163–209. [Google Scholar]
- 8.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131(suppl):604S–615S. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 9.Jiang T, Christian P, Khatry SK, et al. Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr. 2005;135(5):1106–1112. doi: 10.1093/jn/135.5.1106. [DOI] [PubMed] [Google Scholar]
- 10.Christian P, Jiang T, Khatry SK, et al. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. Am J Clin Nutr. 2006;83(4):788–794. doi: 10.1093/ajcn/83.4.788. [DOI] [PubMed] [Google Scholar]
- 11.Pathak P, Kapil U, Kapoor SK, et al. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr. 2004;71(11):1007–1014. doi: 10.1007/BF02828117. [DOI] [PubMed] [Google Scholar]
- 12.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy [electronic article] Cochrane Database Syst Rev. 2006;(4):CD004905. doi: 10.1002/14651858.CD004905.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Christian P, Shrestha J, LeClerq SC, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. J Nutr. 2003;133(11):3492–3498. doi: 10.1093/jn/133.11.3492. [DOI] [PubMed] [Google Scholar]
- 14.Christian P, West KP, Khatry SK, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78(6):1194–1202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 15.Tielsch JM, Khatry SK, Stoltzfus RJ, et al. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community-based, cluster-randomised, placebo-controlled trial. Lancet. 2006;367(9505):144–152. doi: 10.1016/S0140-6736(06)67963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tielsch JM, Khatry SK, Stoltzfus RJ, et al. Effect of daily zinc supplementation on child mortality in southern Nepal: a community-based, cluster randomised, placebo-controlled trial. Lancet. 2007;370(9594):1230–1239. doi: 10.1016/S0140-6736(07)61539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 19.Kahn HA, Sempos CT. Statistical Methods in Epidemiology. New York, NY: Oxford University Press; 1989. [Google Scholar]
- 20.Sommer A, West KP., Jr . Vitamin A Deficiency: Health, Survival, and Vision. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 21.Osrin D, Vaidya A, Shrestha Y, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365(9463):955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 22.Christian P, Osrin D, Manandhar DS, et al. Antenatal micronutrient supplements in Nepal [letter] Lancet. 2005;366(9487):711–712. doi: 10.1016/S0140-6736(05)67166-8. [DOI] [PubMed] [Google Scholar]
- 23.Shankar AH, Jahari AB, Sebayang SK, et al. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group. Lancet. 2008;371(9608):215–227. doi: 10.1016/S0140-6736(08)60133-6. [DOI] [PubMed] [Google Scholar]
- 24.Preziosi P, Prual A, Galan P, et al. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. Am J Clin Nutr. 1997;66(5):1178–1182. doi: 10.1093/ajcn/66.5.1178. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya A, Saville N, Shrestha BP, et al. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet. 2008;371(9611):492–499. doi: 10.1016/S0140-6736(08)60172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]