Abstract
We investigated sex differences in the resting-state neural correlates of Openness to Experience, a universal personality trait defined by cognitive flexibility, attention to feelings, creativity, and preference for novelty. Using resting-state positron-emission tomography from 100 older individuals (>55 years of age), we identified associations between Openness and resting-state regional cerebral blood flow that replicated across 2 assessments of the same sample, approximately 2 years apart. Openness correlated positively with prefrontal activity in women, anterior cingulate activity in men, and orbitofrontal activity in both sexes, which suggests that areas linked to cognitive flexibility (women), monitoring processes (men), and reward and emotional processing (both) underlie individual differences in Openness. The results challenge the implicit assumption that the same trait will rely on the same neural mechanisms across all who express it.
Keywords: Five-Factor Model, neuroimaging, Openness to Experience, personality, sex differences
Introduction
In working to identify the neural underpinnings of behavioral traits, researchers have traditionally relied on combined-sex samples. This practice is understandable; the sheer cost and time-demanding nature of neuroimaging research necessitates such an approach. Yet, combining men and women into a single sample implicitly assumes that the neural substrate of psychological traits does not differ by sex. Evidence that challenges such assumptions has critical implications for pharmacological interventions, hypothesized neural circuits, and, more generally, is crucial for understanding differences between people.
Differences between men and women have been documented across a variety of contexts, from the physiological to the psychological. Sex differences are apparent in brain structure (Cosgrove et al. 2007), volume (Carne et al. 2006), and metabolic activity at rest (Gur et al. 1995). In the early stages of Alzheimer's disease, men have greater reductions in glucose metabolism than women, even in the presence of similar symptomatology (Perneczky et al. 2007). Elevated glucocorticoid hormones enhance memory for men but not women (Andreano and Cahill 2006). In the psychological realm, although similarities may outnumber differences (Hyde 2005), some differences are robust. It is well established, for example, that women are more susceptible to anxiety and depression than men. However, even when there are no sex differences at the behavioral level, the neurological underpinnings of psychological traits may still differ by sex (Cahill 2006).
Combining men and women to increase sample size and statistical power may obscure differences between the sexes and contribute to conflicting findings often observed in the literature. Neuroimaging studies of individual differences typically use small sample sizes and, with a median sample size of 13 (Sergerie et al. 2008), it is difficult to identify and replicate meaningful differences between people. Only large-scale projects with sufficiently large sample sizes will have the statistical power to detect potential sex differences in the neural underpinning of psychological constructs, such as personality traits.
Most previous research on the neurological basis of personality has focused on traits related to Neuroticism (Zald et al. 2002) and Extraversion (Johnson et al. 1999), to the neglect of the other traits within the Five-Factor Model (FFM) of personality. Equally important within the FFM, the trait Openness to Experience captures individual differences in cognitive flexibility, need for variety, and depth of emotional experience (Costa and McCrae 1992). It is a universal personality trait that “is seen in the breadth, depth, and permeability of consciousness, and in the recurrent need to enlarge and examine experience” (p. 826) (McCrae and Costa 1997). Open individuals are distinguished by their creativity, intellectual independence, and esthetic sensitivity. Openness is a broad trait that shapes not only an individual's experience of the world but also social interactions from friendship and marriage to cultural innovation (McCrae 1996).
Open individuals’ tolerance of ambiguity, ability to make remote associations, and curiosity touch nearly every aspect of functioning. They are verbally fluent, humorous, and expressive in interpersonal interactions (Sneed et al. 1998). Openness has been associated positively with crystallized intelligence (Bates and Shieles 2003) and entrepreneurialism (Zhao and Seibert 2006) and even has a place in politics: Across the first 42 US presidents, Openness correlates moderately with leader performance (r = 0.34) (Simonton 2006). Although women tend to score slightly higher on Openness than men, the correlates and consequences of Openness typically do not differ by sex.
Similar to the other traits within the FFM, approximately 50% of the variance in Openness is heritable (Bouchard 1994). This genetic influence suggests a strong biological basis; yet, the proximal biological underpinnings of this trait, including its neural substrate, have yet to be identified. One neuropsychological model of Openness (DeYoung et al. 2005) implicates the dopaminergic system, specifically projections to the prefrontal cortex (PFC) and the anterior cingulate cortex (ACC). Although neuropsychological tests that assess function of the dorsolateral prefrontal cortex (DL-PFC) (DeYoung et al. 2005; Higgins et al. 2007) lend support to this model, there is no direct evidence for the association between Openness and activity in specific brain regions.
Despite this paucity of direct evidence, associations with core features of Openness offer provocative hints into the possible role of these structures. The cognitive flexibility so characteristic of open individuals stems from their ability to store and manipulate information with ease (DeYoung et al. 2005). Working memory, attention, and the ability to shift sets, mechanisms necessary for such manipulation, are highly associated with activity in the DL-PFC and the ACC (MacDonald et al. 2000). Thus, a disposition toward cognitive flexibility may be dependent on engagement of brain regions typically activated during tasks designed to measure cognitive control. In addition, esthetic chills—transient emotional responses to the experience of beauty—a nearly universal indicator of Openness (McCrae 2007), correlate positively with activity in the ACC (Blood and Zatorre 2001).
Although Neuroticism and Extraversion may be the most commonly studied traits due to their relevance to mood, anxiety, and substance abuse disorders, Openness is strongly related to emotional processing, such as affect differentiation (Terracciano, McCrae, et al. 2003) and emotion recognition (Terracciano, Merritt, et al. 2003). Openness also has a strong negative association with alexithymia (Luminet et al. 1999), and this inability to differentiate emotions has been implicated in somatization (Mattila et al. 2008) and difficulties in social functioning (Vanheule et al. 2007). As such, identifying the neural correlates of Openness will help facilitate research on the neural mechanisms involved in emotional processing.
In the current study, we used a robust design to test whether individual differences in Openness were reliably associated with regions of stable brain activity over time and whether these associations differed by sex in a sample of older individuals. Participants (N = 100; 46 female; mean age at Year 1 baseline = 71.4) underwent resting-state positron-emission tomography (PET) scans twice, approximately 2 years apart. As members of the neuroimaging substudy of the Baltimore Longitudinal Study of Aging (Shock et al. 1984), participants were assessed annually for 9 years. In the current analyses, we use resting-state PET scans of regional cerebral blood flow (rCBF) from Years 1 and 3 to maximize the number of participants who also had multiple assessments of Openness. Each PET scan was paired with a separate administration of the NEO Personality Inventory (NEO-PI-R), a standard, comprehensive, and well-validated measure of Openness (Costa and McCrae 1992). Thus, each participant had 2 independent assessments of both rCBF and Openness to Experience. These measures were correlated to identify stable neural correlates of Openness that replicated across the 2 assessments.
Materials and Methods
Subjects and Procedures
PET data were analyzed from Years 1 and 3 of the neuroimaging substudy of the Baltimore Longitudinal Study of Aging (BLSA; Shock et al. 1984). In the current study, we included participants in the sample if they had valid resting-state PET scans from both Years 1 and 3 and had 2 valid personality assessments within 2 years of each scan. A total of 100 subjects (46 female; 91% white) met these criteria. Subjects in the BLSA neuroimaging study were limited to those 55 years of age or older; in the present sample, the average age was 71.4 (standard deviation [SD] = 7.7) overall, 72.3 (SD = 7.4) for men, and 70.4 (SD = 7.9) for women at the Year 1 scan; this difference in age was not statistically significant, F1,98 = 1.5, not significant (NS). On average, both men and women in this sample were similarly educated (men, M = 16.65 [SD = 3.05]; women, M = 15.75 [SD = 2.46]); no significant group difference, F1,98 = 2.51, NS. Men and women also did not differ on depressive symptomatology, as measured by the Center for Epidemiologic Studies Depression Scale, F1,98 = 3.21, NS. Approximately half of the women were on hormone therapy at either Year 1 (n = 23) or Year 3 (n = 25); hormone status was missing for 3 participants.
Participants completed a battery of 12 neuropsychological tests evaluating 6 cognitive domains. Memory was assessed using the California Verbal Learning Test and Benton Visual Retention Test. Word knowledge and verbal ability were measured using Primary Mental Abilities Vocabulary. Verbal fluency was assessed by Letter and Category fluency tests. Attention and working memory were measured by the Digit Span Test of the Wechsler Adult Intelligence Scale-Revised and the Trail Making Test. Digits Backward, Trails B, and Verbal Fluency (categories and letters) assessed executive function. The Card Rotations Test assessed visuospatial function. At each assessment, subjects were deemed to be cognitively normal by consensus diagnosis. The local Institutional Review Board approved the study, and all subjects provided written informed consent before each assessment.
Measure of Openness to Experience
Participants completed the Openness scale of the Revised NEO-PI-R, a widely used, objective measure of personality (Costa and McCrae 1992). The NEO-PI-R measures personality traits at both the broad factor level and at the more circumscribed facet level. Subjects responded on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Raw scores were converted to T-scores (M = 50, SD = 10) using the combined-sex norms for adults reported in the Manual. In the current sample, the Openness scale had an alpha reliability of 0.90 at Time 1 and 0.91 at Time 2; the retest correlation across the 2 assessments was 0.90. Mean Openness scores were significantly higher for women than men at Year 1 (M = 53.5 [SD = 9.5] vs. M = 48.9 [SD = 11.0]; F1,98 = 4.96, P < 0.05) but not at Year 3 (M = 52.8 [SD = 10.5] vs. M = 48.9 [SD = 11.2]; F1,98 = 3.14, NS). Across the 2 assessments, Openness scores did not change significantly for either women or men (t values = 1.11 and −0.04, respectively, both NS).
PET Scanning
Subjects underwent the same PET scanning procedure at both scanning occasions (i.e., Years 1 and 3). During the resting-state scan, subjects were instructed to keep their eyes open and focused on a computer screen covered by a black cloth. In addition to this scan, subjects underwent 2 additional CBF scans, during verbal and figural memory recognition tasks. Scan order was counterbalanced across subjects.
PET measures of rCBF were obtained using [15O]water. For each scan, 75 mCi of [15O] water were injected as a bolus. Images were acquired for 60 s on a GE 4096 + PET scanner, starting from when radioactivity in the brain was detected to surpass threshold level. The scans provided 15 slices of 6.5 mm thickness. Each PET scan was realigned and spatially normalized into standard stereotactic space and smoothed to a full width at half maximum of 12, 12, and 12 mm in the x, y, and z planes. To control for variability in global flow, rCBF values at each voxel were ratio adjusted to the mean global flow and scaled to 50 mL/100 g per min for each image.
The image data were analyzed using Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK), where voxel by voxel comparisons determined significant associations between rCBF and Openness. We correlated Openness scores with patterns of CBF for men and women separately at Years 1 and 3. Second-level conjunction analyses were then used to detect significant associations between Openness and rCBF that replicated across the 2 scans. Significant effects were based on the magnitude (P ≤ 0.005) and spatial extent (≥40 voxels) of brain activity. The rCBF values were also extracted from regions of significant correlation in each group to directly compare differences in activity levels between men and women. All analyses were adjusted for baseline age.
Results
In the current sample, sex differences were apparent at the behavioral level: A repeated measures analysis indicated that women scored slightly higher on Openness than men (F1,98 = 4.18, P < 0.05), but neither men nor women changed across the 2 assessments (F1,98 = 0.53, NS), and there was no sex × time interaction (F1,98 = 0.62, NS).
Using the PET data, we correlated Openness scores with rCBF separately in men and women at Years 1 and 3. Second-level conjunction analyses across the 2 scans revealed that Openness correlated with blood flow in specific regions of the brain at both assessments (Table 1). Controlling for hormone replacement status among the women did not alter the pattern of correlations in this group.
Table 1.
Replicated rCBF correlates of Openness to Experience by sex
| Side | Coordinate |
t | kE | P | rOpenness | |||
| x | y | z | ||||||
| Women | ||||||||
| Positive correlations | ||||||||
| Mid frontal gyrus (9) | R | 24 | 40 | 36 | 3.95 | 200 | <0.001 | 0.53 |
| Mid frontal gyrus (10) | R | 36 | 48 | 10 | 3.48 | 62 | <0.005 | 0.51 |
| Orbitofrontal gyrus (11) | L | −18 | 48 | −18 | 3.32 | 60 | <0.001 | 0.35 |
| Negative correlations | ||||||||
| Inferior temporal gyrus (20) | L | −30 | −18 | −30 | 4.47 | 543 | <0.001 | −0.53 |
| Inferior temporal gyrus (20) | R | 26 | −18 | −26 | 3.83 | 172 | <0.001 | −0.44 |
| Inferior temporal gyrus (20) | R | 52 | −22 | −38 | 3.62 | 159 | <0.001 | −0.45 |
| Inferior temporal gyrus (20) | R | 52 | −22 | −16 | 3.45 | 68 | <0.001 | −0.39 |
| Men | ||||||||
| Positive correlations | ||||||||
| Anterior cingulate gyrus (32) | L | −2 | 40 | 16 | 4.31 | 198 | <0.001 | 0.39 |
| Orbitofrontal gyrus (11) | L | −12 | 12 | −20 | 3.56 | 126 | <0.001 | 0.42 |
| Insula | R | 38 | 12 | 8 | 3.56 | 44 | <0.001 | 0.31 |
| Negative correlations | ||||||||
| Cuneus | L | −8 | −98 | 16 | 3.01 | 46 | <0.005 | −0.32 |
| Combined sample | ||||||||
| Positive correlations | ||||||||
| Mid frontal gyrus (10) | R | 34 | 50 | 8 | 3.53 | 108 | <0.001 | 0.21 |
| Mid frontal gyrus (8) | R | 36 | 3 | 40 | 3.53 | 418 | <0.001 | 0.33 |
| Insula | R | 38 | 14 | 6 | 4.09 | 101 | <0.001 | 0.15 |
| Negative correlations | ||||||||
| Inferior temporal gyrus (20) | L | −44 | −24 | −34 | 4.18 | 339 | <0.001 | −0.44 |
| Cuneus | L | −8 | −100 | 18 | 3.01 | 47 | <0.005 | −0.23 |
Note: Regions correlated with Openness to Experience across the 2 assessments. Stereotaxic coordinates are listed; BAs are indicated in parentheses. kE represents the number of voxels.
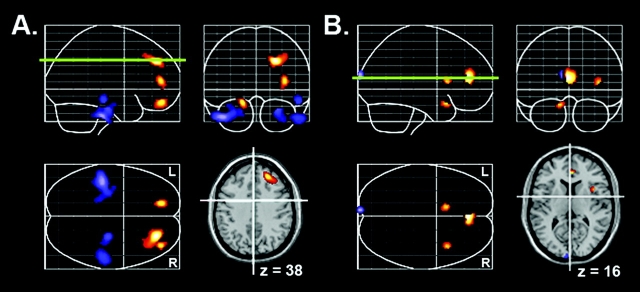
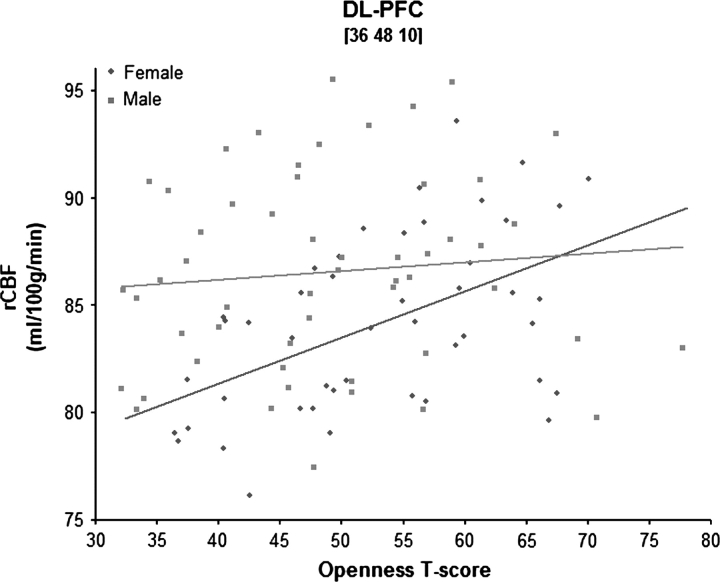
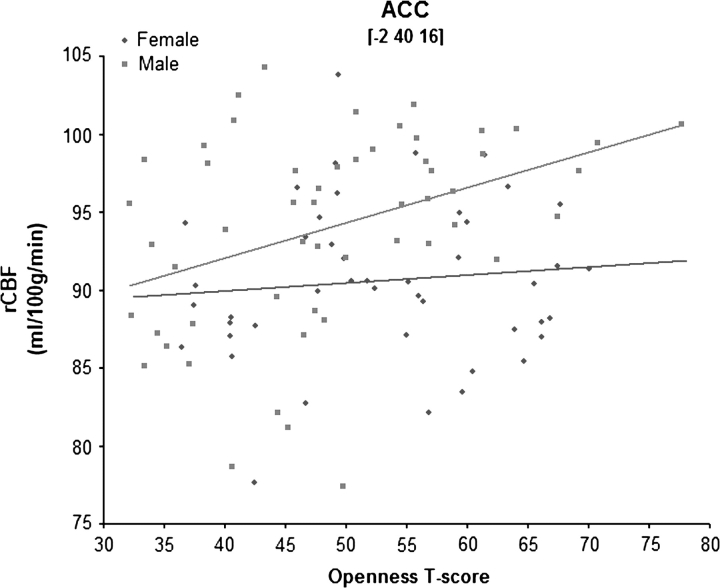
As expected, open individuals had greater blood flow in both the DL-PFC and the ACC; these relations, however, were sex specific. For women, higher Openness scores were associated with greater rCBF in the PFC, specifically the right DL-PFC (Brodmann Area [BA] 9/10; Fig. 1A), whereas for men, higher Openness scores were associated with greater activity in the left ACC (BA 32; Fig. 1B). Using the Fisher’s r-to-z transformation, direct comparisons of the correlations between Openness scores and blood flow levels confirmed that brain activity differed significantly between men and women within these regions (all z values > 1.98, P values < 0.05). Sample scatter plots of these correlations are shown in Figures 2 and 3.
Figure 1.
(A, B) Sex-specific positive (red) and negative (blue) correlations between rCBF and higher scores on Openness to Experience for women (A) and men (B). The green line represents the location or slice of the brain shown in the lower right-hand corner.
Figure 2.
Scatter plot of Openness and activity in the DL-PFC.
Figure 3.
Scatter plot of Openness and activity in the ACC.
In addition to the positive correlation with the DL-PFC, women higher in Openness also had less blood flow in both their left and especially their right inferior temporal gyri (BA 20) relative to women lower in Openness (Fig. 1A). These correlations were not significant for men, and direct comparison of rCBF in these regions indicated that these correlations differed significantly between men and women (all z values > 3.28, P values < 0.05). In contrast, men higher in Openness had more blood flow in their right insula and less blood flow in their cuneus relative to men lower in Openness (Fig. 1B). Although these correlations were not significant for women, the correlations between rCBF in these regions and Openness did not differ significantly between men and women (z values = 1.46 and −0.82, respectively, both NS).
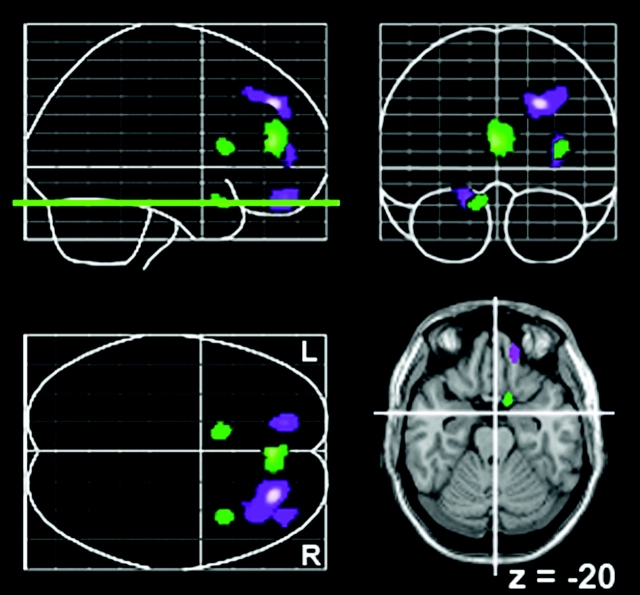
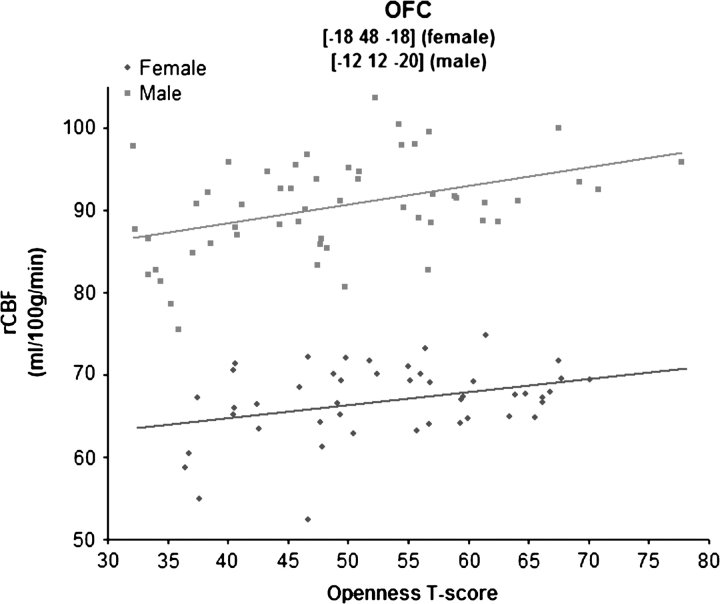
Not all associations, however, were sex specific: Both men and women higher on Openness had greater rCBF in the left orbitofrontal cortex (OFC; BA 11; Fig. 4). A scatter plot of the correlation is shown in Figure 5. The peak activation was in slightly different places in the OFC for men and women, but the correlation between Openness and these 2 peaks did not differ significantly between the sexes (z values = −0.05 and −1.63, both NS).
Figure 4.
Common correlates between rCBF and higher scores on Openness to Experience for women (purple) and men (green). All correlations are positive. Correlations did not differ significantly between men and women.
Figure 5.
Scatter plot of Openness and activity in the OFC.
As a comparison, we combined men and women together and correlated Openness with rCBF in the whole sample. Some of the strongest correlations observed in the sex-specific analyses were apparent in the full-sample analyses (see Table 1, bottom panel). In the full sample, higher Openness scores were correlated positively with activity in the right middle frontal gyrus and insula and negatively with the left inferior temporal gyrus and cuneus. Lowering the threshold slightly (P < 0.01) also revealed the association between Openness and the OFC. Although the local maxima of these full sample regional correlations were in slightly different areas than previously observed in the sex-specific correlations, the middle frontal and inferior temporal correlations were primarily driven by women, and the correlations between Openness and rCBF in the insula and cuneus were driven by men. For example, the correlation between Openness and activity in the middle frontal gyrus (10) was 0.46 for women, but 0.15 for men. Likewise, the correlation between Openness and activity in the cuneus was −0.37 for men but −0.15 for women. These findings suggest that sex-specific contributions can play a role in mixed group results, but that collapsing men and women together also potentially obscures sex-specific correlations.
Finally, to ensure that all the associations found in the current study were specific to Openness, we correlated the other 4 FFM traits with rCBF in the Openness associated regions; none of the other 4 traits correlated significantly with rCBF in these areas. In addition, we tested whether the rCBF correlates of Openness replicated during both the verbal and figural memory task performance. During the verbal task, Openness correlated positively with activity in the ACC in men and positively with OFC activity in women. Women also showed a negative correlation between Openness and rCBF in the inferior temporal gyrus during both the verbal and figural tasks. Although these correlations were observed during both rest and cognitive performance, associations between Openness and rCBF in other resting-state regions were not observed. This may be due, in part, to patterns of task-specific activity masking the underlying associations observed during the resting condition.
Discussion
The aim of the present research was to test whether stable individual differences in the universal personality trait Openness to Experience were associated with stable rCBF in a sample of older adults. Higher Openness scores correlated positively with resting-state rCBF in the DL-PFC, the ACC, and in the OFC. These relations were both specific and common across the sexes: Openness correlated with regions of the brain related to working memory for women, attention and reward for men, and emotional and reward processing for both the sexes. It is striking that Openness correlated positively with brain regions linked to working memory and attention as these are the 2 executive functions crucial for the core characteristic of Openness to Experience—cognitive flexibility.
The DL-PFC and ACC are associated with the executive functions that allow for flexible interactions with the environment. Open individuals are known for their cognitive flexibility and are skilled at finding novelty in the midst of familiarity, such as discovering new uses for everyday objects. Open women may derive this ability through chronic activation of the DL-PFC, an area associated with both response inhibition (i.e., the ability to override a preprogrammed response; Garavan et al. 2002) and the active manipulation of information held in storage (i.e., working memory; Smith and Jonides 1999). Open men, in contrast, may derive this ability through chronic activation of the ACC, an area associated with attentional control, conflict detection, and the monitoring of internal states that require adjustments in control (Kerns et al. 2004). This latter finding is consistent with the negative correlation observed between the ACC and alexithymia (Larsen et al. 2003), where lower ACC activity is associated with an inability to differentiate emotions, which is a strong negative correlate of Openness.
Our findings converge with neuropsychological models of Openness that implicate the PFC and ACC in the neural underpinnings of this trait (DeYoung et al. 2005). Specifically, DeYoung et al. (2005) suggest that dopaminergic projections to both of these areas may be partially responsible for the cognitive exploratory tendencies that characterize open individuals. This reasoning is consistent with others who have argued that such projections to the PFC are what allow new information to enter working memory (Braver and Barch 2002) and such projections to the ACC enhance both working memory and creative thinking (Ashby et al. 1999). Notably, we found dispositional cognitive flexibility to be associated with greater blood flow in these 2 areas—but these relations were sex specific. This divergence suggests that the 2 sexes rely on different neural pathways for the operations that define the trait Openness to Experience.
In some cases, sex differences in brain structure and function may generate overt sex differences in behavior. However, it has also been argued that sexual dimorphisms in the brain may compensate for differences in the effects of hormones on the brain (de Vries 2004). In the current study, because adjusting for hormone therapy status in our postmenopausal female sample did not affect our findings, circulating estrogen levels alone cannot account for the observed sex differences in associations. Rather, organizational effects of sex hormones during development or chromosomal differences may have contributed to differences in function/structure regardless of the postmenopausal level of circulating estrogen.
In contrast to the sex-specific correlates, correlations with the OFC point to commonalities between men and women in the neural underpinnings of Openness. The OFC is implicated in a variety of processes integral to this personality trait, including sensory integration, the processing of emotional information, and emotion recognition and differentiation. The OFC underlies reward and the anticipation of reward and some have argued that this area is responsible for binding situations with consequences. As such, the OFC may be involved in how individuals learn the emotional consequences of stimuli (Viskontas et al. 2007). This region of the OFC, in particular, is also thought to act as a mediator between working memory and attentional processes in the lateral prefrontal areas and the emotional and motivational processes in the more caudal regions of the OFC (Petrides 2007). These areas may be responsible for attention to novel stimuli, deeper processing, and ultimately better encoding of the environment (Petrides 2007). Working together, activation of the DL-PFC and ACC may orient open individuals to exploration and novelty, whereas activity in the OFC serves to reward such tendencies. Individuals high in Openness may be particularly sensitive to reward due to chronic OFC (men and women) and ACC (men) activity. That is, these individuals may seek out new and novel experiences because they are consistently rewarded by them.
The brain undergoes both structural and functional changes as it ages. For example, additional regions may be recruited during cognitive tasks to maintain performance, presumably to compensate for deficits in regions responsible for cognitive functioning (Cabeza et al. 2002; Beason-Held et al. 2008). Few studies have examined whether the neural correlates of personality change as the brain ages, but some differences in the structural correlates of Neuroticism and Extraversion between younger and older adults have been reported (Wright et al. 2006, 2007). It is of note, however, that the neural regions which we found to be associated with Openness, particularly the DL-PFC, ACC, and OFC, are implicated in executive functions and emotional processing in both younger and older adults; these processes are the core characteristics of trait Openness. Thus, these regions would likely also be associated with Openness in other age groups. It will be of interest in future research as to whether these correlates hold true across the life span.
The present research has several strengths and improves upon previous neuroimaging studies of personality traits in several ways. Perhaps, the most notable aspect of our design is the multiple assessments of both resting-state rCBF and Openness. Multiple assessments of a trait through repeated administration of the same measure, multiple informants, or different measures of the same trait tend to increase reliability of the measurement of the trait. Thus, multiple assessments of both resting-state rCBF and Openness should produce more robust, replicable associations that are less dependent on state-specific variation and provide greater sensitivity to more enduring, trait-like resting-state correlates of Openness.
In addition to our multiple assessments, we use a comprehensive, well-validated measure of Openness to Experience and have an unusually large sample size for a neuroimaging study of personality traits. Our large sample size gave us the statistical power to test men and women separately and therefore identify potential sex differences in the neural underpinnings of personality. Despite these strengths, some limitations need to be addressed in future research. For example, our sample is not representative. Members of the Baltimore Longitudinal Study of Aging, particularly the neuroimaging substudy, tend to be relatively homogeneous with regard to race, education, and health status. In addition, as discussed above, our sample was drawn from an older population. Although there is not necessarily any reason to suspect that correlations between Openness and blood flow should change as individuals age, the results may be specific to this age group. Future research needs to confirm these relations in younger populations.
How individuals structure their experiential worlds has profound consequences for social behavior (McCrae 1996); the present research suggests that the brain regions responsible for such structuring are different for men and women. That is, when the brain is not actively involved in a task, the proclivity to understand and appreciate a wide range of experiences is associated with areas related to working memory for women and areas related to attention for men. Given that the correlates and consequences of Openness typically do not differ by sex, men and women may recruit different brain regions to achieve the same observable ends.
More generally, our findings challenge the practice of using combined-sex samples, which implicitly assume that the expression of a trait should rely on the same neural structures across both men and women. Sex differences in brain structure and function are well known and include differences in brain volume (Carne et al. 2006), regional cerebral glucose metabolism (Gur et al. 1995), and neurotransmitter receptor systems (Andreano and Cahill 2006; Cosgrove et al. 2007; Jovanovic et al. 2008). Here, we demonstrate sex differences and commonalities in the neural correlates of Openness to Experience, a crucial first step in deciphering the different neural regions associated with personality for men and women. Ultimately, the similarities between men and women may far outnumber the differences, but assuming that men and women are interchangeable obscures meaningful differences between the sexes.
Funding
This research was supported in part by the Intramural Research Program of NIH, National Institute on Aging. Revised NEO Personality Inventory (to P.T.C.).
Acknowledgments
We thank Robert R. McCrae, Antonio Terracciano, and Jason Sutin for helpful suggestions on earlier versions of this manuscript. Conflict of Interest: Paul T. Costa receives royalties from the Revised NEO Personality Inventory.
References
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Bates TC, Shieles A. Crystallized intelligence as a product of speed and drive for experience: the relationship of inspection time and openness to g and Gc. Intelligence. 2003;31:275–287. [Google Scholar]
- Beason-Held LL, Kraut MA, Resnick SM. II. Temporal patterns of longitudinal change in aging brain function. Neurobiol Aging. 2008;29:497–513. doi: 10.1016/j.neurobiolaging.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TJ. Genes, environment, and personality. Science. 1994;264:1700–1701. doi: 10.1126/science.8209250. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carne RP, Vogrin S, Litewka L, Cook MJ. Cerebral cortex: an MRI-based study of volume and variance with age and sex. J Clin Neurosci. 2006;13:60–72. doi: 10.1016/j.jocn.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and the NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa (FL): Psychological Assessment Resources; 1992. [Google Scholar]
- de Vries GJ. Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Higgins DM. Sources of Openness/Intellect: Cognitive and neuropsychological correlates of the fifth factor of personality. J Pers. 2005;73:825–858. doi: 10.1111/j.1467-6494.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, Gur RE. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267:528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- Higgins DM, Peterson JB, Pihl RO, Lee AGM. Prefrontal cognitive ability, intelligence, big five personality, and the prediction of advanced academic and workplace performance. J Pers Soc Psychol. 2007;93:298–319. doi: 10.1037/0022-3514.93.2.298. [DOI] [PubMed] [Google Scholar]
- Hyde JS. The gender similarities hypothesis. Am Psychol. 2005;60:581–592. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- Johnson DL, Wiebe JS, Gold SM, Andreasen NC, Hichwa RD, Watkins GL, Boles Ponto LL. Cerebral blood flow and personality: a positron emission tomography study. Am J Psychiatry. 1999;156:252–257. doi: 10.1176/ajp.156.2.252. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Halldin C, Nordström AL. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald Iii AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Larsen JK, Brand N, Bermond B, Hijman R. Cognitive and emotional characteristics of alexithymia: a review of neurobiological studies. J Psychosom Res. 2003;54:533–541. doi: 10.1016/s0022-3999(02)00466-x. [DOI] [PubMed] [Google Scholar]
- Luminet O, Bagby RM, Wagner H, Taylor GJ, Parker JDA. Relation between alexithymia and the five-factor model of personality: a facet-level analysis. J Pers Assess. 1999;73:345–358. doi: 10.1207/S15327752JPA7303_4. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mattila AK, Kronholm E, Jula A, Salminen JK, Koivisto AM, Mielonen RL, Joukamaa M. Alexithymia and somatization in general population. Psychosom Med. 2008;70:716–722. doi: 10.1097/PSY.0b013e31816ffc39. [DOI] [PubMed] [Google Scholar]
- McCrae RR. Social consequences of experiential openness. Psychol Bull. 1996;122:323–337. doi: 10.1037/0033-2909.120.3.323. [DOI] [PubMed] [Google Scholar]
- McCrae RR. Aesthetic chills as a universal marker of openness to experience. Motiv Emot. 2007;31:5–11. [Google Scholar]
- McCrae RR, Costa PT. Conceptions and correlates of Openness to Experience. In: Hogan R, Johnson JA, Briggs SR, editors. Handbook of Personality Psychology. Orlando (FL): Academic Press; 1997. pp. 825–847. [Google Scholar]
- Perneczky R, Drzezga A, Diehl-Schmid J, Li Y, Kurz A. Gender differences in brain reserve: an 18F-FDG PET study in Alzheimer's disease. J Neurol. 2007;254:1395–1400. doi: 10.1007/s00415-007-0558-z. [DOI] [PubMed] [Google Scholar]
- Petrides M. The orbitofrontal cortex: novelty, deviation from expectation, and memory. Ann N Y Acad Sci. 2007;1121:33–53. doi: 10.1196/annals.1401.035. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta E, Tobin JD. Normal human aging: the Baltimore longitudinal study of aging. Washington: U.S. Government Printing Office; 1984. [Google Scholar]
- Simonton DK. Scientific status of disciplines, individuals, and ideas: empirical analyses of the potential impact of theory. Rev Gen Psychol. 2006;10:98–112. [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sneed CD, McCrae RR, Funder DC. Lay conceptions of the five-factor model and its indicators. Pers Soc Psychol Bull. 1998;24:115–126. [Google Scholar]
- Terracciano A, McCrae RR, Hagemann D, Costa PT. Individual difference variables, affective differentiation, and the structures of affect. J Pers. 2003;71:669–703. doi: 10.1111/1467-6494.7105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Merritt M, Zonderman AB, Evans MK. Personality traits and sex differences in emotion recognition among African Americans and Caucasians. Ann N Y Acad Sci. 2003;1000:309–312. doi: 10.1196/annals.1280.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheule S, Desmet M, Meganck R, Bogaerts S. Alexithymia and interpersonal problems. J Clin Psychol. 2007;63:109–117. doi: 10.1002/jclp.20324. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci. 2007;1121:528–545. doi: 10.1196/annals.1401.025. [DOI] [PubMed] [Google Scholar]
- Wright CI, Feczko E, Dickerson B, Williams D. Neuroanatomical correlates of personality in the elderly. Neuroimage. 2007;35:263–272. doi: 10.1016/j.neuroimage.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, Wedig MM. Neuroanatomical correlates of extraversion and neuroticism. Cereb Cortex. 2006;16:1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci USA. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Seibert SE. The big five personality dimensions and entrepreneurial status: a meta-analytical review. J Appl Psychol. 2006;91:259–271. doi: 10.1037/0021-9010.91.2.259. [DOI] [PubMed] [Google Scholar]