Abstract
Posterior fossa syndrome is characterized by cerebellar dysfunction, oromotor/oculomotor apraxia, emotional lability and mutism in patients after infratentorial injury. The underlying neuroanatomical substrates of posterior fossa syndrome are unknown, but dentatothalamocortical tracts have been implicated. We used pre- and postoperative neuroimaging to investigate proximal dentatothalamocortical tract involvement in childhood embryonal brain tumour patients who developed posterior fossa syndrome following tumour resection. Diagnostic imaging from a cohort of 26 paediatric patients previously operated on for an embryonal brain tumour (13 patients prospectively diagnosed with posterior fossa syndrome, and 13 non-affected patients) were evaluated. Preoperative magnetic resonance imaging was used to define relevant tumour features, including two potentially predictive measures. Postoperative magnetic resonance and diffusion tensor imaging were used to characterize operative injury and tract-based differences in anisotropy of water diffusion. In patients who developed posterior fossa syndrome, initial tumour resided higher in the 4th ventricle (P = 0.035). Postoperative magnetic resonance signal abnormalities within the superior cerebellar peduncles and midbrain were observed more often in patients with posterior fossa syndrome (P = 0.030 and 0.003, respectively). The fractional anisotropy of water was lower in the bilateral superior cerebellar peduncles, in the bilateral fornices, white matter region proximate to the right angular gyrus (Tailerach coordinates 35, –71, 19) and white matter region proximate to the left superior frontal gyrus (Tailerach coordinates –24, 57, 20). Our findings suggest that multiple bilateral injuries to the proximal dentatothalamocortical pathways may predispose the development of posterior fossa syndrome, that functional disruption of the white matter bundles containing efferent axons within the superior cerebellar peduncles is a critical underlying pathophysiological component of posterior fossa syndrome, and that decreased fractional anisotropy in the fornices and cerebral cortex may be related to the abnormal neurobehavioural symptoms of posterior fossa syndrome.
Keywords: posterior fossa, cerebellum, mutism, medulloblastoma
Introduction
Under normal circumstances, the cerebral cortex and cerebellum are in circuit via afferent and efferent signalling. Impulses originating within the cerebral cortex travel along axons via the cortico-ponto-cerebellar pathway, ultimately terminating within the Purkinje cell layer of cerebellum. Axons from these neurons form inhibitory synapses onto the deep cerebellar nuclei. Subsequent excitatory projections from these nuclei to various targets within the spinal column, brainstem and cerebral cortex are well-recognized for their importance in affecting coordination of movement. The efferent pathway which ultimately reciprocates with the cerebral cortex is the dentatothalamocortical pathway. This tract is comprised of axons originating within the dentate nuclei that project through the ipsilateral superior cerebellar peduncle and, after decussation in the midbrain tegmentum, synapse within the contralateral ventrolateral nucleus of the thalamus. From the thalamus, second order axons terminate in the primary motor cortex as well as secondary and tertiary association areas within the frontal and parietal lobes. Thus, in addition to its influence on planned motor activity, the cerebellum, via the dentatothalamocortical tract, is believed to modulate cognition and behaviour (Schmahmann and Pandya, 1997; Schmahmann, 2004; Baillieux et al., 2008).
This non-traditional view of cerebellar function is supported by the recognition of cognitive, linguistic and behavioural–affective impairments in children and adults with an injury or functional disturbance of the cerebellum and/or the proximal dentatothalamocortical pathway (Martin and Albers, 1995; Pollack et al., 1995; Chugani et al., 1997; Muller et al., 1998; Schmahmann and Sherman, 1998; Baillieux et al., 2007) Posterior fossa syndrome (PFS) is an example of infratentorial injury leading to neurobehavioural dysfunction and is best described in children who have undergone surgical resection for an infratentorial tumour (Aguiar et al., 1995; Pollack et al., 1995; Gelabert-Gonzalez and Fernandez-Villa, 2001; Wells et al., 2008). In medulloblastoma, the most common malignant brain tumour of childhood, PFS is estimated to occur in 25% of patients treated with surgery (Gelabert-Gonzalez and Fernandez-Villa, 2001; Robertson et al., 2006; Wells et al., 2008). Mutism, oromotor/oculomotor apraxia, emotional lability, axial hypotonia and cerebellar/brainstem dysfunction are the principal symptoms that typify this syndrome. Interestingly, symptom onset is usually 24–48 h after an otherwise unremarkable postoperative period. Treatment is primarily supportive, including prolonged inpatient rehabilitation as well as gastrostomy and/or tracheostomy placement for severe cases. Recovery is variable. The most distressing symptoms—mutism and emotional lability—are generally transient, abating weeks to months after onset; but significant long-term cognitive, behavioural and/or motor deficits are frequently recognized in these children (Doxey et al., 1999; Steinbok et al., 2003; Steinlin et al., 2003; Huber et al., 2006).
Clinical experience suggests the risk for developing PFS is increased in patients with midline embryonal tumours. Otherwise, there is no identified patient/tumour characteristic, surgical approach or postoperative injury pattern that is universally accepted as a predictor of PFS (Aguiar et al., 1995; Catsman-Berrevoets et al., 1999; Kusano et al., 2006; Ozgur et al., 2006; Baillieux et al., 2007). The disrupted physiology underlying the striking neurobehavioural deficits is also largely unknown. Unfortunately, deficient understanding of PFS has limited the implementation of aggressive preventative and/or ameliorative measures.
Perturbation of the cerebello-cerebral network is presumed to underlie many of the manifest symptoms of PFS (Schmahmann and Sherman, 1998; Levisohn et al., 2000; Riva and Giorgi, 2000). Given its role as the primary outflow tract for the cerebellum and the vulnerability of its more proximal aspects to surgical injury, with respect to PFS, the dentatothalamocortical pathway has garnered increased attention (Aguiar et al., 1995; Ozgur et al., 2006; Robertson et al., 2006; Wells et al., 2008). To investigate the involvement of the dentatothalamocortical tracts in PFS, we reviewed conventional magnetic resonance imaging (MRI) and diffusion tensor imaging in a cohort of paediatric embryonal tumour patients. We hypothesized that preoperative tumour position within the 4th ventricle and distance between the superior cerebellar peduncles may predict the development of PFS, and that anisotropy of water diffusion in proximal dentatothalamocortical tracts would be lower in those patients diagnosed with PFS.
Subjects and methods
Subjects
Between August 2003 and May 2006, 64 patients were enrolled on an IRB approved institutional protocol designed for the treatment of children and young adults ≤21 years of age with newly diagnosed embryonal tumours. The majority of patients (n = 62) enrolled on this protocol had tumours located within the posterior fossa. After surgical resection, 21% of these patients (medulloblastoma n = 10 and atypical teratoid/rhabdoid tumour n = 3) were prospectively diagnosed with PFS based upon clinical symptoms and signs. Retrospective review of postoperative clinical notes was often indeterminate with regard to PFS symptom onset, thus diagnosis of PFS was arbitrarily reported as the primary surgical date. Of the 49 patients who did not develop PFS after posterior fossa tumour resection, 13 were randomly selected for comparative analyses.
Diagnostic imaging
Image acquisition
MRI was performed with a Siemens symphony 1.5T or 3T scanner (Siemens Medical Solutions, Erlangen, Germany) using the standard quadrature headcoil. The imaging examination included standard T1-, T2-weighted and fluid attenuated inversion recovery diagnostic scans and diffusion-weighted images for diffusion tensor imaging. Diffusion weighted images were acquired with a single-shot, spin-echo, echo-planar acquisition sequence with eddy current balanced diffusion weighting gradient pulses to reduce distortion [six encoding directions with b = 1000 s/mm2; echo time (TE)/repetition time (TR) = 127 ms/10 s; matrix = 128 × 128 on 230 mm × 230 mm field of view; slices 3 mm without gap resulting in voxels of 1.8 × 1.8 × 3 mm] (Reese et al., 2003). Four measurements of the diffusion data were realigned and resliced (SPM2, http:www.fil.ion.ucl.ac.uk/spm/) before diffusion tensor processing and analysis.
Image processing and analysis
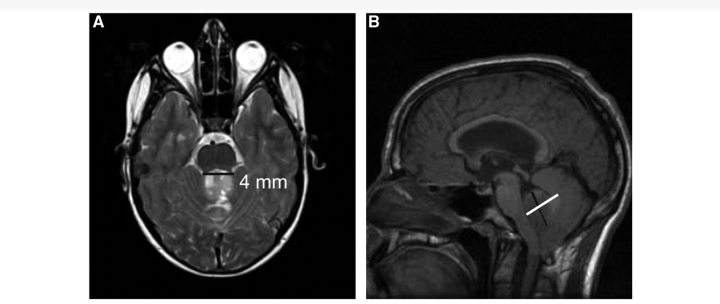
Both pre- and postoperative conventional MRI were completed on all patients. Unfortunately, metal artefact and patient movement sufficiently obscured imaging in three patients (all with PFS). Thus, 23 patients (10 with PFS, 13 without PFS) comprised the final cohort. A single neuroradiologist (FHL) who was blinded to the diagnosis of PFS evaluated all conventional imaging. On the preoperative imaging, tumour location was characterized as either midline or lateral (left versus right) and the location/laterality of tumour invasion represented by abnormal T2 signal within the cerebral hemispheres, thalami, midbrain, pons, medulla, inferior/middle/superior cerebellar peduncles and cerebellum was assessed. To characterize the relative cranial/caudal location of tumour, we measured on the sagittal T1-weighted image the extension of tumour above and below the line bisecting the 4th ventricle and calculated the above/below ratio for each patient (Fig. 1). To characterize preoperative stress on the superior cerebellar peduncles, we measured on the T2-weighted image, the distance (splay) between the superior cerebellar peduncles at the level of the rostral pons (Fig. 1). A radiological diagnosis of hydrocephalus was noted and the degree of hydrocephalus was calculated using the Evan's Index. We hypothesized that the above/below ratio and superior cerebellar peduncular splay would be greater in patients with PFS because tumours residing higher in the posterior fossa would cause more preoperative stress and require greater surgical manipulation of the cerebellar dentatothalamocortical outflow via the superior cerebellar peduncles. Two postoperative examinations, immediate and delayed, were reviewed. The degree of surgical resection, as well as the location/laterality of abnormal T2 signal within similar head regions as the preoperative scan, was assessed. One patient (non-PFS) did not have an immediate postoperative film available for review (N = 22). Fisher's exact test was used to compare proportions between groups (SAS v9.1®; SAS® Institute, Cary, NC).
Figure 1.
Depicts splay between superior cerebellar peduncles (A) and above/below ratio (B) in a patient with medulloblastoma.
The diffusion tensor was solved using a weighted linear least-square fit implementation using the diffusion tensor imaging fit algorithm as part of the Functional Magnetic Resonance Imaging of the Brain (FMRIB) diffusion toolbox (FDT v.2.0) (http:www.fmrib.ox.ac.uk/fsl/fdt/index.html) (FMRIB Centre, University of Oxford, Oxford, UK). Many patients undergo initial imaging and tumour resection at their local institution prior to referral to our institution for further cancer therapy. Because fractional anisotropy (FA) images were typically not obtained in the preoperative scans, preoperative diffusion tensor analysis was not attempted. FA images from the immediate postoperative scan were calculated from the diffusion tensor in each voxel. The tract-based spatial statistics (TBSS) tool in the FMRIB Software Library (FSL) was used to identify tract-based differences in FA values between the PFS and control patients. TBSS computed a group mean FA skeleton, which represented the centres of all fibre bundles that were common to the subjects involved in the study (Smith et al., 2007). The aligned fractional anisotropic image for each subject was projected onto the tract skeleton. The skeletonized FA maps for the groups were compared with the non-parametric permutation procedure called ‘randomize’ in FSL. Differences in FA between the groups were considered statistically significant for P ≤ 0.05, after correction for multiple comparisons.
Results
Cohort characteristics
All patients diagnosed with PFS were male in contrast to the control group which had seven females (54%). Otherwise, the two groups were similar with respect to age at diagnosis, treatment risk, extent of surgical resection and timing of all scans. Consistent with the diagnosis of PFS, all patients exhibited abnormal speech and 8/10 patients had documented irritability and/or agitation in the postoperative period (Table 1).
Table 1.
Patient characteristics
| PFS |
||
|---|---|---|
| No | Yes | |
| Gender, n (%) | ||
| Male | 6 (46) | 10 (100) |
| Surgery extent, n (%) | ||
| Gross total resection | 10 (77) | 9 (90) |
| Near total (90%) | 1 (8) | 0 (0) |
| Sub total (50–90%) | 2 (15) | 1 (10) |
| Age at tumour diagnosis median (range) | 7.8 (3.7–20.2) | 8.7 (5.4–16.9) |
| Days from surgery to baseline scan median (range) | –2 (–5, 13) | –2 (–11, 1) |
| Days from surgery to post-op scan median (range) | 1.5 (1, 14) | 1 (1, 10) |
| Days from surgery to follow-up scan median (range) | 16 (13, 26) | 17 (5 27) |
| Evan's index of hydrocephalus median (range) | 0.37 (0.31, 0.50) | 0.43 (0.31, 0.56) |
Preoperative imaging review
An increased above/below ratio that reflects a more rostral tumour position within the 4th ventricle was statistically associated with the future development of PFS (P = 0.035). Otherwise, no statistical differences were detected at the baseline study between the two groups with regard to tumour invasion or in the splay between superior cerebellar peduncles (P = 0.11). The denate nuclei, superior cerebellar peduncle and midbrain were rarely involved preoperatively. The majority of the patients (78%) within the cohort had radiological evidence for hydrocephalus and the degree of preoperative hydrocephalus was similar for both groups (Table 2).
Table 2.
Frequency of T2 involvement in baseline imaging
| Variables | PFS |
|||
|---|---|---|---|---|
| Total (n = 23) | No (n = 13) | Yes (n = 10) | P-value | |
| Cerebellum, n (%) | 0.604† | |||
| Bilateral | 1 (100) | 1 (100.0) | 0 | |
| None | 18 (100) | 9 (50.0) | 9 (50.0) | |
| Unilateral | 4 (100) | 3 (75.0) | 1 (25.0) | |
| Dentate, n (%) | 0.104† | |||
| None | 19 (100) | 9 (47.4) | 10 (52.6) | |
| Unilateral | 4 (100) | 4 (100.0) | 0 | |
| Inferior CP, n (%) | 0.656† | |||
| Bilateral | 6 (100) | 3 (50.0) | 3 (50.0) | |
| None | 15 (100) | 8 (53.3) | 7 (46.7) | |
| Unilateral | 2 (100) | 2 (100.0) | 0 | |
| Middle CP, n (%) | 0.360† | |||
| None | 17 (100) | 9 (52.9) | 8 (47.1) | |
| Unilateral | 5 (100) | 4 (80.0) | 1 (20.0) | |
| Superior CP, n (%) | 0.127† | |||
| None | 18 (100) | 12 (66.7) | 6 (33.3) | |
| Unilateral | 5 (100) | 1 (20.0) | 4 (80.0) | |
| Medulla, n (%) | 0.476† | |||
| Bilateral | 3 (100) | 1 (33.3) | 2 (66.7) | |
| None | 18 (100) | 10 (55.6) | 8 (44.4) | |
| Unilateral | 2 (100) | 2 (100.0) | 0 | |
| Pons, n (%) | 1.000† | |||
| None | 22 (100) | 12 (54.5) | 10 (45.5) | |
| Unilateral | 1 (100) | 1 (100.0) | 0 | |
| Midbrain, n (%) | 1.000† | |||
| None | 21 (100) | 12 (57.1) | 9 (42.9) | |
| Unilateral | 2 (100) | 1 (50.0) | 1 (50.0) | |
| Thalamus, n (%) | ||||
| None | 22 (100) | 13 (59.1) | 9 (40.9) | |
| Cerebral hemisphere, n (%) | ||||
| None | 22 (100) | 13 (59.1) | 9 (40.9) | |
†Exact test; CP = cerebellar peduncles.
Postoperative imaging review
On the immediate postoperative scan following surgery, patients with PFS were more likely to have abnormal T2 signal involving the pons (P = 0.029), midbrain (P = 0.003) and superior cerebellar peduncles (P = 0.030) than those without PFS. With respect to T2 signal abnormalities noted within the pons, dentate, superior cerebellar peduncles and midbrain: most patients without PFS (83%) had ≤1 of these structures involved. However, the majority of patients with PFS (90%) had abnormalities either in three or more of these structures. Furthermore, bilateral injury within these structures was more common in patients with PFS than those patients without PFS (80% versus 15%). On the delayed postoperative scan, no statistical differences between the two groups were observed. However, patients with PFS tended to have more abnormalities detected within the superior cerebellar peduncles (P = 0.10). Of note, because the dentate nuclei were involved frequently in both groups, no statistical differences were noted (Tables 3 and 4).
Table 3.
Frequency of T2 involvement in immediate postoperative imaging
| PFS |
||||
|---|---|---|---|---|
| Variables | Total (n = 22) | No (n = 12) | Yes (n = 10) | P-value |
| Cerebellum, n (%) | 0.099† | |||
| Bilateral | 13 (100) | 5 (38.5) | 8 (61.5) | |
| Unilateral | 9 (100) | 7 (77.8) | 2 (22.2) | |
| Dentate, n (%) | 0.646† | |||
| Bilateral | 2 (100) | 1 (50.0) | 1 (50.0) | |
| None | 5 (100) | 4 (80.0) | 1 (20.0) | |
| Unilateral | 15 (100) | 7 (46.7) | 8 (53.3) | |
| Inferior CP, n (%) | 0.721† | |||
| Bilateral | 13 (100) | 6 (46.2) | 7 (53.8) | |
| None | 5 (100) | 3 (60.0) | 2 (40.0) | |
| Unilateral | 4 (100) | 3 (75.0) | 1 (25.0) | |
| Middle CP, n (%) | 0.381† | |||
| Bilateral | 4 (100) | 1 (25.0) | 3 (75.0) | |
| None | 7 (100) | 5 (71.4) | 2 (28.6) | |
| Unilateral | 11 (100) | 6 (54.5) | 5 (45.5) | |
| Superior CP, n (%) | 0.008† | |||
| Bilateral | 1 (100) | 1 (100.0) | 0 | |
| None | 11 (100) | 9 (81.8) | 2 (18.2) | |
| Unilateral | 10 (100) | 2 (20.0) | 8 (80.0) | |
| Medulla, n (%) | 0.308† | |||
| Bilateral | 2 (100) | 1 (50.0) | 1 (50.0) | |
| None | 13 (100) | 9 (69.2) | 4 (30.8) | |
| Unilateral | 7 (100) | 2 (28.6) | 5 (71.4) | |
| Pons, n (%) | 0.029† | |||
| Bilateral | 3 (100) | 0 (0.0) | 3 (100.0) | |
| None | 18 (100) | 12 (66.7) | 6 (33.3) | |
| Unilateral | 1 (100) | 0 | 1 (100.0) | |
| Midbrain, n (%) | 0.003† | |||
| Bilateral | 3 (100) | 0 | 3 (100.0) | |
| None | 16 (100) | 12 (75.0) | 4 (25.0) | |
| Unilateral | 3 (100) | 0 | 3 (100.0) | |
| Thalamus, n (%) | ||||
| None | 21 (100) | 12 (57.1) | 9 (42.9) | |
| Cerebral hemisphere, n (%) | 0.686† | |||
| Bilateral | 1 (100) | 1 (100.0) | 0 | |
| None | 19 (100) | 11 (57.9) | 8 (42.1) | |
| Unilateral | 1 (100) | 0 | 1 (100.0) | |
†Exact test; CP = cerebellar peduncles.
Table 4.
Frequency of T2 involvement in delayed postoperative imaging
| PFS |
||||
|---|---|---|---|---|
| Variables | Total (n = 23) | No (n = 13) | Yes (n = 10) | P-value |
| Cerebellum, n (%) | 0.138† | |||
| Bilateral | 8 (100) | 3 (37.5) | 5 (62.5) | |
| None | 7 (100) | 3 (42.9) | 4 (57.1) | |
| Unilateral | 8 (100) | 7 (87.5) | 1 (12.5) | |
| Dentate, n (%) | 0.719† | |||
| Bilateral | 3 (100) | 1 (33.3) | 2 (66.7) | |
| None | 12 (100) | 7 (58.3) | 5 (41.7) | |
| Unilateral | 8 (100%) | 5 (62.5) | 3 (37.5) | |
| Inferior CP, n (%) | 0.371† | |||
| Bilateral | 11 (100) | 5 (45.5) | 6 (54.5) | |
| None | 9 (100) | 5 (55.6) | 4 (44.4) | |
| Unilateral | 3 (100) | 3 (100.0) | 0 | |
| Middle CP, n (%) | 0.589† | |||
| Bilateral | 3 (100) | 1 (33.3) | 2 (66.7) | |
| None | 14 (100) | 9 (64.3) | 5 (35.7) | |
| Unilateral | 6 (100) | 3 (50.0) | 3 (50.0) | |
| Superior CP, n (%) | 0.074† | |||
| Bilateral | 1 (100) | 1 (100.0) | 0 | |
| None | 14 (100) | 10 (71.4) | 4 (28.6) | |
| Unilateral | 8 (100) | 2 (25.0) | 6 (75.0) | |
| Medulla, n (%) | 0.265† | |||
| Bilateral | 2 (100) | 1 (50.0) | 1 (50.0) | |
| None | 16 (100) | 11 (68.8) | 5 (31.3) | |
| Unilateral | 5 (100) | 1 (20.0) | 4 (80.0) | |
| Pons, n (%) | 0.144† | |||
| Bilateral | 3 (100) | 0 | 3 (100.0) | |
| None | 18 (100) | 12 (66.7) | 6 (33.3) | |
| Unilateral | 2 (100) | 1 (50.0) | 1 (50.0) | |
| Midbrain, n (%) | 0.055† | |||
| Bilateral | 3 (100) | 0 | 3 (100.0) | |
| None | 18 (100) | 12 (66.7) | 6 (33.3) | |
| Unilateral | 1 (100) | 1 (100.0) | 0 | |
| Thalamus, n (%) | 0.429† | |||
| Bilateral | 1 (100) | 0 | 1 (100.0) | |
| None | 20 (100) | 12 (60.0) | 8 (40.0) | |
| Cerebral hemisphere, n (%) | 0.171† | |||
| Bilateral | 1 (100) | 0 | 1 (100.0) | |
| None | 19 (100) | 12 (63.2) | 7 (36.8) | |
| Unilateral | 1 (100) | 0 | 1 (100.0) | |
†Exact test; CP = cerebellar peduncles.
Diffusion tensor imaging
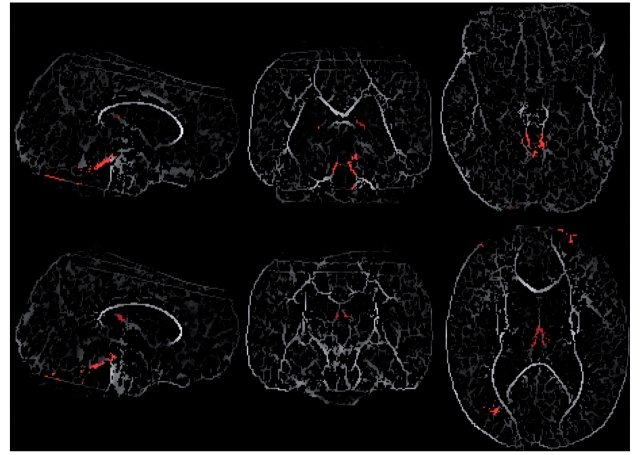
On the immediate postoperative scan, patients with PFS had significantly reduced FA in four areas: (i) bilateral superior cerebellar peduncles; (ii) bilateral fornices (column); (iii) white matter region proximate to the right angular gyrus (Tailerach coordinates 35, −71, 19) and (iv) white matter region proximate to the left superior frontal gyrus (Tailerach coordinates −24, 57, 20) (Fig. 2).
Figure 2.
On the immediate postoperative scan, patients with PFS had significantly reduced FA in four areas: (i) bilateral superior cerebellar peduncles; (ii) bilateral fornices (column); (iii) white matter region proximate to the right angular gyrus (Tailerach coordinates 35,−71, 19) and (iv) white matter region proximate to the left superior frontal gyrus (Tailerach coordinates −24, 57, 20). The tracts with reduced FA are shown as red overlay on the mean skeletonized FA map (some additional artefact can be seen at the edges of the images).
Discussion
Despite clinical heterogeneity, postoperative PFS in childhood brain tumour patients is one of the better described neurobehavioural syndromes that can occur after infratentorial injury (Aguiar et al., 1995; Pollack et al., 1995; Gelabert-Gonzalez and Fernandez-Villa, 2001; Wells et al., 2008). The diagnosis of PFS appears to be associated with worse neurological and cognitive outcomes (Doxey et al., 1999; Steinbok et al., 2003; Steinlin et al., 2003; Huber et al., 2006). One of the most intriguing aspects of this syndrome is that despite apparent presurgical similarities, only a subset of patients with posterior fossa tumours developed PFS after definitive resection. Surgical approach, tumour characteristics and various perioperative factors (concomitant hydrocephalus, infection, vasospasm and delayed ischaemia) have been posited as possible causative mechanisms (Pollack et al., 1995; Ersahin et al., 1996; Turgut, 1998; Catsman-Berrevoets et al., 1999; Kotil et al., 2008; Frassanito et al., 2009). However, none of these factors has been conclusively accepted. Preventative or ameliorative interventions are lacking, in part, because of this incomplete understanding. Given its projection to the cerebral cortex, proximal dentatothalamocortical tract involvement in PFS is presumed, but this hypothesis has not been systematically examined (Crutchfield et al., 1994; Koh et al., 1997; Riva and Giorgi, 2000; Robertson et al., 2006; Wells et al., 2008).
This study represents the first blinded review of magnetic resonance and diffusion weighted imaging obtained prospectively in a cohort of paediatric patients after recent resection of a posterior fossa embryonal tumour. Comparing patients who did and did not develop PFS, perturbation of the proximal dentatothalamocortical tract was identified to be a potentially important determinant of this debilitating disorder. On immediate postoperative evaluation, conventional imaging showed evidence of proximal dentatothalamocortical tract involvement in those patients diagnosed with PFS. Previous studies have suggested that injury to the dentate nuclei is critical to the development of PFS (Ersahin et al., 1996; Kusano et al., 2006; Puget et al., 2009). Our findings are generally supportive; however, neither unilateral nor bilateral damage to the dentate was required for development of PFS. Rather, patients with PFS were distinguished by bilateral, multi-focal postoperative injury (increased T2 signal suggestive of oedema) to structures within the posterior fossa. When examining the putative anatomical structures (pons, dentate nuclei, superior cerebellar peduncles and midbrain), the majority of patients with PFS (90%) were noted to have signal abnormalities within three or more of these structures. In contrast, none of the unaffected patients had ≥3 structures involved. Furthermore, bilaterality may be an important associative finding, as 80% of patients with PFS versus 15% of patients without PFS had evidence of bilateral injury within these structures. Thus, ‘multiple, bilateral hits’ within posterior fossa components of the cortico-cerebellar-cortical circuit may be required for PFS development. In a separate analysis utilizing the same cohort of patients, Miller et al. (2008) observed a positive association between PFS and bilateral damage to the dentatothalamocortical tracts as patients with bilateral damage were ∼12 times more likely to develop PFS than those with unilateral injury only. These findings are consistent with Crutchfield's early hypothesis that bilateral interruption of the dentatothalamocortical pathways may be responsible for the development of PFS (Crutchfield et al., 1994).
To the best of our knowledge, the use of diffusion tensor imaging in PFS has not been previously reported. Consistent with our initial hypothesis, decreased integrity (as measured by decreased FA) among the white matter bundles comprising both superior cerebellar peduncles was also evident in patients with PFS. Interestingly, whereas bilateral involvement of the superior cerebellar peduncles is apparent on diffusion tensor imaging, on postoperative conventional imaging, unilateral signal abnormalities in these structures were typically observed. This discrepancy between conventional and diffusion tensor imaging suggests that a functional disturbance of the proximal efferent tract without a direct neuroanatomical correlate on conventional magnetic resonance imaging may be present. As supported by conventional imaging, the observed decreased anisotropy within bilateral superior cerebellar peduncles may not necessarily reflect direct, but rather additive effects of remote, injury (pons and/or dentate nuclei). Furthermore, as Pollock et al. (1995) have previously suggested, the delayed onset of symptoms in some patients indicates that the involved structures in PFS are not likely to be directly damaged in surgery (Pollack et al., 1995). In the future, it will be important to correlate the timing of PFS onset with the pattern of injury to determine if patients with direct injury to bilateral superior cerebellar peduncles exhibit symptoms sooner than patients with more remote, albeit salient injury. Irrespective of timing and pattern of injury, our findings support the suggestion that disruption of white matter tracts within the superior cerebellar peduncles (either through direct injury or remote perturbation) may represent the underlying pathophysiological substrate for PFS in patients undergoing posterior fossa surgery.
Based on a hypothesized link between proximal dentatothalamocortical tract injury and PFS, we also evaluated two novel preoperative measures (tumour above/below ratio and superior cerebellar peduncular splay) that were designed to predict development of PFS. Indeed, patients with tumours that resided higher in the fourth ventricle were statistically more likely to develop PFS. Tumour in this location may predispose to PFS because there is more preoperative stress, and increased vulnerability within the proximal dentatothalamocortical tract to operative injury. In this study, there was also a trend towards larger superior cerebellar peduncular splay in patients with PFS. In light of our findings noted above, a change in surgical approach based on these measures is not warranted. However, increased postoperative surveillance of patients with tumours that reside high in the 4th ventricle and/or widen the splay of the superior cerebellar peduncles should be given. A larger prospective study is required to determine the accuracy and thresholds of these measures for predicting PFS development.
Perhaps unexpectedly, diffusion tensor imaging also identified three supratentorial tracts where FA was lower in patients with PFS than in the control group. Detection of these areas highlights the value of TBSS for efficient and unbiased group comparison of diffusion tensor imaging data. Although speculative, the observed disordered water diffusion within the fornices and white matter tracts proximate to the right angular gyrus and left superior frontal gyrus, areas known to process spatial and temporal information essential for normal cognitive, linguistic and motor behaviour, is provocative and may represent or suggest specific cortical regions relevant to the neurobehavioural manifestations of PFS. Indeed, with respect to language, comparable findings using single photon emission tomography (SPECT) in patients with cerebellar injury are reported (Marien et al., 1996, 2001). In these studies, expressive syntax disturbance, agrammatism and impaired prosody reminiscent of patients with acquired left frontal lesion were observed. Interestingly, a corresponding left frontal perfusion deficit was noted in these patients. To explain how a remote cerebellar injury may affect cortical functioning, a cerebello-cerebral diaschisis model has been proposed (Marien et al., 1996, 2001; Riva and Giorgi, 2000). Using diffusion tensor imaging, our findings further support this model and are not dissimilar from other studies that have utilized SPECT in patients with PFS (Sagiuchi et al., 2001; Clerico et al., 2002; Ersahin et al., 2002). These latter studies, as well as our current data, may suggest critical cortical regions involved in additional aspects of PFS such as apraxia and behavioural lability. Further elaboration of the neural substrates of PFS, including grey matter and white matter, may help to clarify the role of the cerebellum in cognitive function (Glickstein, 2006).
The fornices are not a classical component of the dentatothalamocortical pathway. Indeed, decreased FA in these structures may be confounded by increased hydrocephalus in the PFS patients (Evan's index = 0.43 versus 0.37 in non-PFS patients). Furthermore, to our knowledge, anterograde amnesia has not been reported in patients with PFS (although this would be very difficult to assess), as in other patients with bilateral fornical damage. However, several brainstem nuclei, most notably the ventral tegmental area within the midbrain, have reciprocal connections with limbic pathways that include the fornical columns (Morgane et al., 2005). Therefore, surgical or functional disruption of these nuclei, with subsequent alteration of limbic homeostasis, may underlie aspects of the behavioural–affective disorder commonly encountered in PFS. This suggested mechanism is speculative and needs to be further evaluated.
Several other limitations should be kept in mind when interpreting our data. First, despite well-established cardinal signs and symptoms, PFS is a heterogeneous syndrome that has thus far defied a strict diagnostic criterion. Although our patients with PFS were identified prospectively, onset, duration and level of impairments were not prospectively recorded. Thus, like other reports which characterize a PFS population, clinical heterogeneity within this cohort is expected and is not strictly accounted with respect to outcomes. Also, three patients (two non-PFS, one PFS) had multiple surgeries prior to definitive resection; baseline scan was arbitrarily assigned as the one prior to the initial surgical resection. Furthermore, because our institution serves as a national referral centre, onset of PFS could not always be accurately determined as some patients were referred after primary surgery and onset of symptoms. Related to this issue, the preoperative magnetic resonance images were often completed at other centres and thus were not of uniform technique or quality. This heterogeneity could have affected the interpretation of our predictive measures. In addition, preoperative FA data were not available for analysis. Systematic collection of such data would potentially enrich our understanding of the impact of preoperative stress upon the dentatothalamocortical tract. All patients with PFS in this study were male. We believe this to be a clinical anomaly rather than an important pathological finding. Nevertheless, gender differences could influence our findings. Finally, the diffusion tensor imaging findings in the supratentorium should be interpreted cautiously. Decreased FA observed proximate to the right angular gyrus (Tailerach coordinates 35, −71, 19) and left superior frontal gyrus (Tailerach coordinates −24, 57, 20) could potentially be the result of susceptibility artefacts related to ventricular blood present after surgery, air/tissue interfaces within the frontal sinuses and/or external ear canal, as well as metal devices (shunt, ommaya) secured on the surface of the skull.
Despite these limitations, our study adds significantly to the growing body of research that implicates the proximal dentatothalamocortical tracts in PFS. Using conventional magnetic resonance and diffusion tensor imaging, our findings suggest multiple bilateral injuries to the proximal dentatothalamocortical pathways may predispose the development of PFS and that functional disruption of the white matter bundles containing efferent axons within the superior cerebellar peduncles is a critical underlying pathophysiological component of PFS. When postoperative injury is remote from the superior cerebellar peduncles, the cause or nature of this perturbation remains uncertain. With regard to clinical decision-making in patients with posterior fossa tumours, although measurements of splay and location were not robustly predictive, heightened concern should be given to those patients that harbour tumours high in the 4th ventricle. Prospective studies, including preoperative diffusion tensor imaging, intra-operative or postoperative conventional, functional and vascular imaging in conjunction with careful neurological and neurocognitive evaluations are needed to validate our findings and clarify further the neuroanatomical and functional substrates of this puzzling syndrome.
Funding
National Institutes of Health Cancer Center Support (CORE) Grant (P30 CA 21765); the National Institute of Child Health and Human Development grant (HD49888); the American Lebanese Syrian Associated Charities (ALSAC).
Glossary
Abbreviations
- FA
fractional anisotropy
- FMRIB
Functional Magnetic Resonance Imaging of the Brain
- PFS
posterior fossa syndrome
- SPECT
single photon emission tomography
- TBSS
tract-based spatial statistics
References
- Aguiar PH, Plese JP, Ciquini O, Marino R. Transient mutism following a posterior fossa approach to cerebellar tumors in children: a critical review of the literature. Childs Nerv Syst. 1995;11:306–10. doi: 10.1007/BF00301766. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Marien P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–73. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Baillieux H, Weyns F, Paquier P, De Deyn PP, Marien P. Posterior fossa syndrome after a vermian stroke: a new case and review of the literature. Pediatr Neurosurg. 2007;43:386–95. doi: 10.1159/000106388. [DOI] [PubMed] [Google Scholar]
- Catsman-Berrevoets CE, Van Dongen HR, Mulder PG, Paz y Geuze D, Paquier PF, Lequin MH. Tumour type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatr. 1999;67:755–7. doi: 10.1136/jnnp.67.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Rothermel R, Behen M, Chakraborty P, Mangner T, et al. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann Neurol. 1997;42:666–9. doi: 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- Clerico A, Sordi A, Ragni G, Festa A, Cappelli C, Maini CL. Brief report: transient mutism following posterior fossa surgery studied by single photon emission computed tomography (SPECT) Med Pediatr Oncol. 2002;38:445–8. doi: 10.1002/mpo.1361. [DOI] [PubMed] [Google Scholar]
- Crutchfield JS, Sawaya R, Meyers CA, Moore BD., 3rd Postoperative mutism in neurosurgery. Report of two cases. J Neurosurg. 1994;81:115–21. doi: 10.3171/jns.1994.81.1.0115. [DOI] [PubMed] [Google Scholar]
- Doxey D, Bruce D, Sklar F, Swift D, Shapiro K. Posterior fossa syndrome: identifiable risk factors and irreversible complications. Pediatr Neurosurg. 1999;31:131–6. doi: 10.1159/000028848. [DOI] [PubMed] [Google Scholar]
- Ersahin Y, Mutluer S, Cagli S, Duman Y. Cerebellar mutism: report of seven cases and review of the literature. Neurosurgery. 1996;38:60–5. doi: 10.1097/00006123-199601000-00015. discussion 66. [DOI] [PubMed] [Google Scholar]
- Ersahin Y, Yararbas U, Duman Y, Mutluer S. Single photon emission tomography following posterior fossa surgery in patients with and without mutism. Childs Nerv Syst. 2002;18:318–25. doi: 10.1007/s00381-002-0614-z. [DOI] [PubMed] [Google Scholar]
- Frassanito P, Massimi L, Caldarelli M, Di Rocco C. Cerebellar mutism after spontaneous intratumoral bleeding involving the upper cerebellar vermis: a contribution to the physiopathogenic interpretation. Childs Nerv Syst. 2009;25:7–11. doi: 10.1007/s00381-008-0711-8. [DOI] [PubMed] [Google Scholar]
- Gelabert-Gonzalez M, Fernandez-Villa J. Mutism after posterior fossa surgery. Review of the literature. Clin Neurol Neurosurg. 2001;103:111–14. doi: 10.1016/s0303-8467(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Glickstein M. Thinking about the cerebellum. Brain. 2006;129:288–90. doi: 10.1093/brain/awh728. [DOI] [PubMed] [Google Scholar]
- Huber JF, Bradley K, Spiegler BJ, Dennis M. Long-term effects of transient cerebellar mutism after cerebellar astrocytoma or medulloblastoma tumor resection in childhood. Childs Nerv Syst. 2006;22:132–8. doi: 10.1007/s00381-005-1223-4. [DOI] [PubMed] [Google Scholar]
- Koh S, Turkel SB, Baram TZ. Cerebellar mutism in children: report of six cases and potential mechanisms. Pediatr Neurol. 1997;16:218–9. doi: 10.1016/s0887-8994(97)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotil K, Eras M, Akcetin M, Bilge T. Cerebellar mutism following posterior fossa tumor resection in children. Turk Neurosurg. 2008;18:89–94. [PubMed] [Google Scholar]
- Kusano Y, Tanaka Y, Takasuna H, Wada N, Tada T, Kakizawa Y, et al. Transient cerebellar mutism caused by bilateral damage to the dentate nuclei after the second posterior fossa surgery. Case report. J Neurosurg. 2006;104:329–31. doi: 10.3171/jns.2006.104.2.329. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–50. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang. 2001;79:580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- Marien P, Saerens J, Nanhoe R, Moens E, Nagels G, Pickut BA, et al. Cerebellar induced aphasia: case report of cerebellar induced prefrontal aphasic language phenomena supported by SPECT findings. J Neurol Sci. 1996;144:34–43. doi: 10.1016/s0022-510x(96)00059-7. [DOI] [PubMed] [Google Scholar]
- Martin P, Albers M. Cerebellum and schizophrenia: a selective review. Schizophr Bull. 1995;21:241–50. doi: 10.1093/schbul/21.2.241. [DOI] [PubMed] [Google Scholar]
- Miller N, Kocak M, Glass J, Ji Q, Lobel U, Morris EB, et al. MRI evaluation of the proximal efferent cerebellar pathway in pediatric patients with posterior fossa syndrome. 46th Annual Meeting, American Society of Neuroradiology. New Orleans, LA. 2008 [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–60. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Muller RA, Chugani DC, Behen ME, Rothermel RD, Muzik O, Chakraborty PK, et al. Impairment of dentato-thalamo-cortical pathway in autistic men: language activation data from positron emission tomography. Neurosci Lett. 1998;245:1–4. doi: 10.1016/s0304-3940(98)00151-7. [DOI] [PubMed] [Google Scholar]
- Ozgur BM, Berberian J, Aryan HE, Meltzer HS, Levy ML. The pathophysiologic mechanism of cerebellar mutism. Surg Neurol. 2006;66:18–25. doi: 10.1016/j.surneu.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37:885–93. doi: 10.1227/00006123-199511000-00006. [DOI] [PubMed] [Google Scholar]
- Puget S, Boddaert N, Viguier D, Kieffer V, Bulteau C, Garnett M, et al. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer. 2009;115:1338–47. doi: 10.1002/cncr.24150. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–61. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg. 2006;105:444–51. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- Sagiuchi T, Ishii K, Aoki Y, Kan S, Utsuki S, Tanaka R, et al. Bilateral crossed cerebello-cerebral diaschisis and mutism after surgery for cerebellar medulloblastoma. Ann Nucl Med. 2001;15:157–60. doi: 10.1007/BF02988609. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Steinbok P, Cochrane DD, Perrin R, Price A. Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg. 2003;39:179–83. doi: 10.1159/000072468. [DOI] [PubMed] [Google Scholar]
- Steinlin M, Imfeld S, Zulauf P, Boltshauser E, Lovblad KO, Ridolfi Luthy A, et al. Neuropsychological long-term sequelae after posterior fossa tumour resection during childhood. Brain. 2003;126:1998–2008. doi: 10.1093/brain/awg195. [DOI] [PubMed] [Google Scholar]
- Turgut M. Transient “cerebellar” mutism. Childs Nerv Syst. 1998;14:161–6. doi: 10.1007/s003810050204. [DOI] [PubMed] [Google Scholar]
- Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev. 2008;14:221–8. doi: 10.1002/ddrr.25. [DOI] [PubMed] [Google Scholar]