Abstract
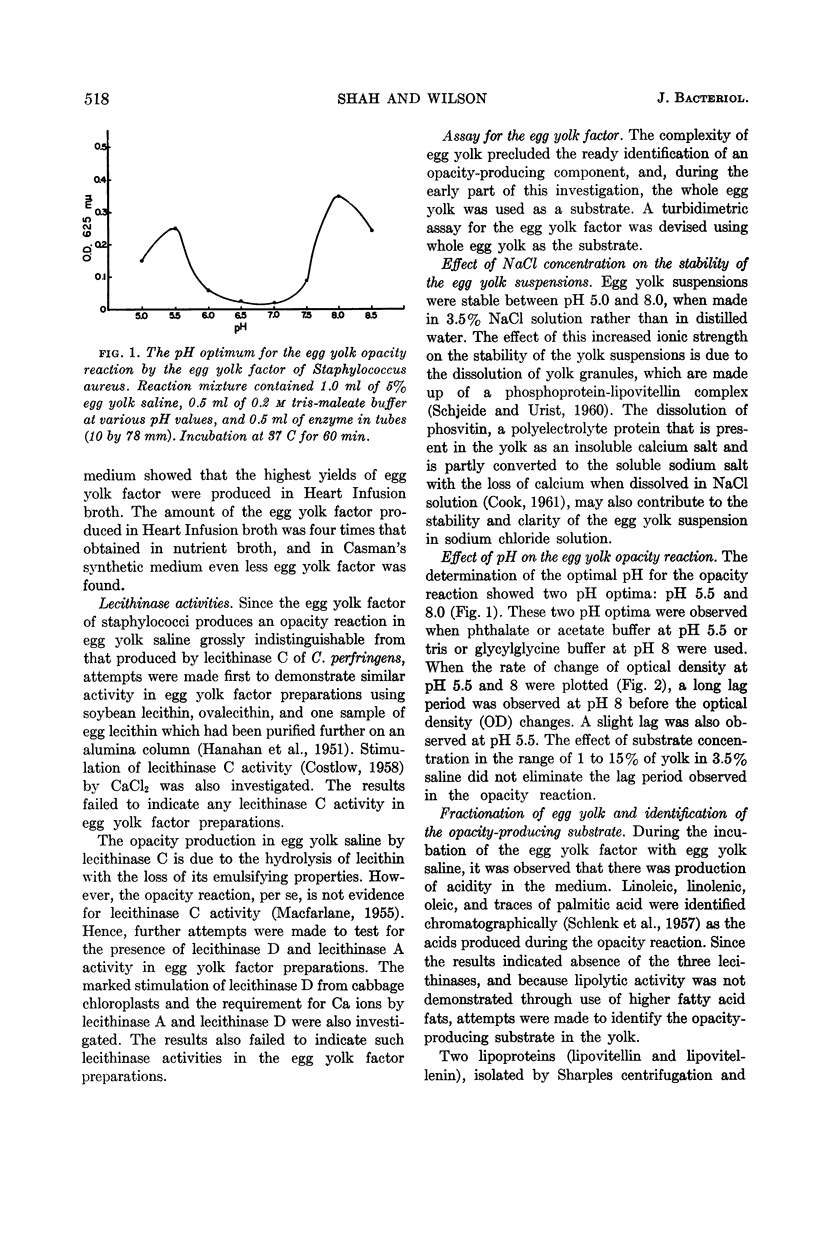
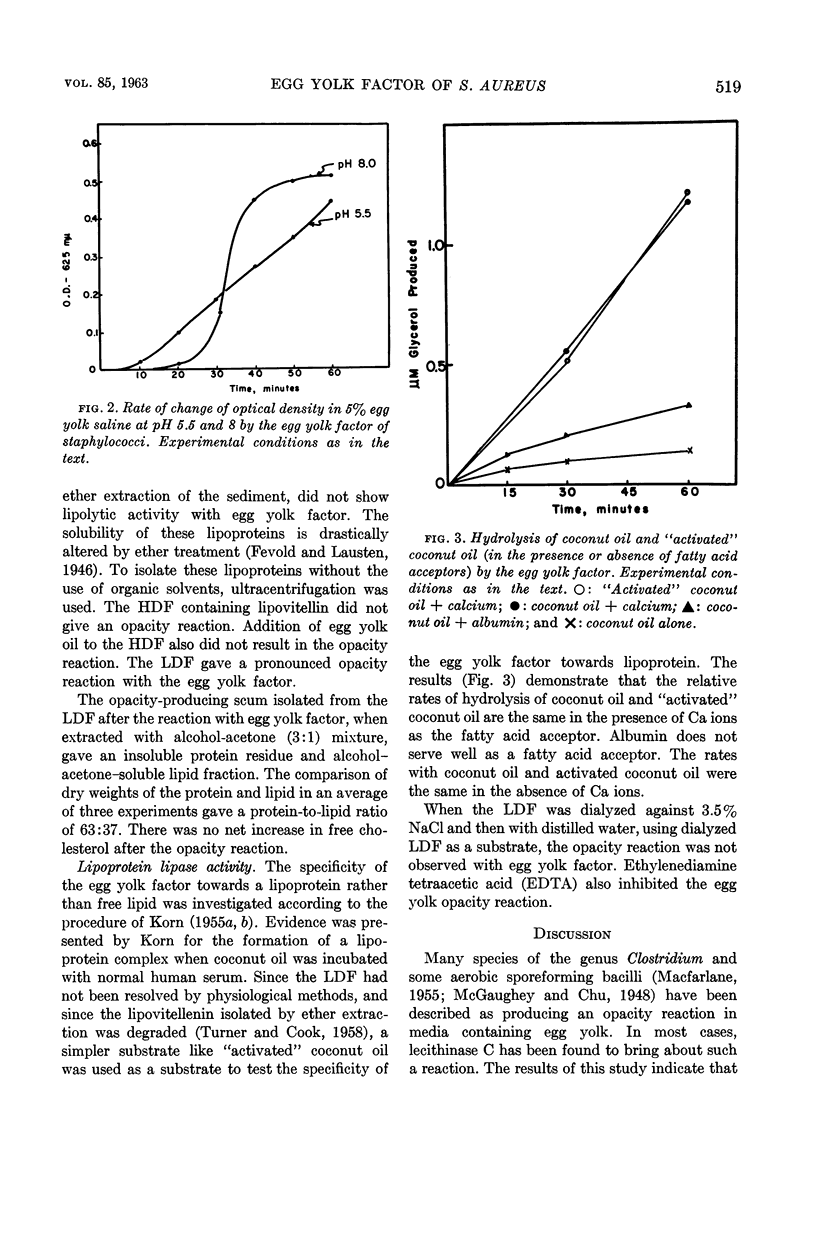
Shah, D. B. (University of Wisconsin, Madison) and J. B. Wilson. Egg yolk factor of Staphylococcus aureus. I. Nature of the substrate and enzyme involved in the egg yolk opacity reaction. J. Bacteriol. 85:516–521. 1963.—Some pathogenic staphylococci produce an opacity reaction when grown in media containing egg yolk. The egg yolk factor is a lipase with a requirement for a fatty acid acceptor, rather than any of the three lecithinases. Highest amounts of egg yolk factor were obtained in Heart Infusion broth rather than nutrient broth or Casman's synthetic medium. The lowest density lipo-protein (lipovitellenin) isolated by ultracentrifugation of egg yolk has been identified as the opacity-producing substrate. The lipase acts on the lipid moiety, resulting in alterations in the solubility of lipovitellenin. Lipolysis is optimal at pH 8. The optimal pH for the opacity reaction is at pH 5.5 and 8. The protein (vitellenin) in lipovitellenin has an isoelectric point at pH 5.5. It is proposed that slight alterations in the stabilizing lipid due to lipolysis and the very low solubility of vitellenin may drastically alter the solubility of lipovitellenin at pH 5.5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDER V. G., GILLESPIE W. A., HERDAN G. Production of opacity in egg-yolk broth by Staphylococci from various sources. J Pathol Bacteriol. 1953 Jul;66(1):205–210. doi: 10.1002/path.1700660123. [DOI] [PubMed] [Google Scholar]
- CASMAN E. P. Serologic studies of staphylococcal enterotoxin. Public Health Rep. 1958 Jul;73(7):599–609. [PMC free article] [PubMed] [Google Scholar]
- COSTLOW R. D. Lecithinase from Bacillus anthracis. J Bacteriol. 1958 Sep;76(3):317–325. doi: 10.1128/jb.76.3.317-325.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE W. A., ALDER V. G. Production of opacity in egg-yolk media by coagulase-positive staphylococci. J Pathol Bacteriol. 1952 Jan;64(1):187–200. doi: 10.1002/path.1700640119. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., TURNER M. B., JAYKO M. E. The isolation of egg phosphatidyl choline by an adsorption column technique. J Biol Chem. 1951 Oct;192(2):623–628. [PubMed] [Google Scholar]
- KATES M. Lecithinase activity of chloroplasts. Nature. 1953 Oct 31;172(4383):814–815. doi: 10.1038/172814a0. [DOI] [PubMed] [Google Scholar]
- KATES M. Lecithinase systems in sugar beet, spinach, cabbage, and carrot. Can J Biochem Physiol. 1954 Sep;32(5):571–583. [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. II. Substrate specificity and activation of coconut oil. J Biol Chem. 1955 Jul;215(1):15–26. [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane M. G., Knight B. C. The biochemistry of bacterial toxins: The lecithinase activity of Cl. welchii toxins. Biochem J. 1941 Sep;35(8-9):884–902. doi: 10.1042/bj0350884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID W. B., WILSON J. B. A study of the staphylococci associated with the bovine udder. Am J Vet Res. 1959 Sep;20:825–831. [PubMed] [Google Scholar]
- SCHJEIDE O. A., URIST M. R. Proteins induced in plasma by oestrogens. Nature. 1960 Oct 22;188:291–294. doi: 10.1038/188291a0. [DOI] [PubMed] [Google Scholar]
- SHAPIRO B. Purification and properties of a lysolecithinase from pancreas. Biochem J. 1953 Mar;53(4):663–666. doi: 10.1042/bj0530663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- TURNER K. J., COOK W. H. Molecular weight and physical properties of a lipoprotein from the floating fraction of egg yolk. Can J Biochem Physiol. 1958 Sep;36(9):937–949. [PubMed] [Google Scholar]


