Abstract
Aim: The aim of this study was to assess whether chronic alcohol misuse affects N-methyl-d-aspartate (NMDA) receptor subunit concentrations in human cases, and whether male and female subjects respond differently. Methods: Real-time RT-PCR normalized to GAPDH was used to assay NR1, NR2A and NR2B subunit mRNA in superior frontal (SFC) and primary motor (PMC) cortex tissue obtained at autopsy from chronic alcoholics with and without comorbid cirrhosis of the liver, and from matched controls. Results: The expression of all three subunits was significantly lower in both areas of cirrhotic alcoholics than in either controls or alcoholics without comorbid disease, who did not differ significantly. Values were also influenced by the subject's sex and genotype. The μ-opiate receptor C1031G polymorphism selectively modulated NMDA transcript expression in cirrhotic-alcoholic SFC, an effect that was more marked for NR1 and NR2A than for NR2B subunit transcripts. Contrasting 5HT1B genotypes affected NMDA mRNA expression differently in male and female SFC, but not PMC, in cirrhotic alcoholics. Conclusion: NMDA receptor subunit expression may differentially influence male and female cirrhotic alcoholics’ susceptibility to brain damage.
INTRODUCTION
Alcoholism is a complex disease with genetic and environmental influences. No genetic factor is predictive of its development; rather, predisposition for alcohol misuse and addiction is determined by polymorphisms in a large number of genes. Several loci associated with alcoholism have been identified; many of these are genes encoding neurotransmitter receptors and transporters (reviewed in Enoch (2003)).
The endogenous opioid system is implicated in alcohol misuse (reviewed in Oswald and Wand (2004)), in part because naltrexone blocks alcohol-induced increases in dopamine levels in rat nucleus accumbens (Benjamin et al., 1993). The latter process is central to many addictions (Pierce and Kumaresan, 2006). Opioids tonically inhibit the hypothalamic–pituitary–adrenal (HPA) axis in the stress response; opioid dysfunction may underlie atypical stress responses that could reinforce alcohol dependence, as has been proposed for heroin addiction (reviewed in Kreek et al. (2005)). The intronic C1031G SNP of the μ-opioid receptor gene OPRM1 is significantly associated with opioid dependence in a Chinese population (Szeto et al., 2001), but not with drug or alcohol dependence in European Americans (Zhang et al., 2006).
Serotonin (5HT) plays roles in mood, sleep and appetite; serotonergic dysfunction is associated with mood disorders and addiction. Its actions are mostly mediated by G-protein-coupled receptors (GPCRs) linked to Ca2+ and cAMP (Hoyer et al., 2002). Serotonergic antagonists, neurotoxins and uptake enhancers that reduce brain 5HT increase ethanol intake by animals; whereas the administration of 5HT, 5HT-releasing compounds or selective serotonin re-uptake inhibitors (SSRIs) can increase brain 5HT and reduce ethanol intake (LeMarquand et al., 1994). 5HT metabolite concentrations rise in human CSF and urine during acute alcohol exposure, and SSRIs have had some success in the treatment of alcohol misuse (Lovinger, 1997). The G861C polymorphism G allele of the 5HT1B receptor gene HTR1B is associated with alcoholism (Fehr et al., 2000) and alcoholism combined with antisocial personality disorder (Soyka et al., 2004), whereas the C allele is over-represented in alcoholics with inactive aldehyde dehydrogenase-2 (ALDH2*2; Hasegawa et al., 2002).
Substance misuse differs between the sexes. Whilst prevalence is lower in women, consumption of alcohol, marijuana, opioids and cocaine escalates more rapidly (reviewed in Becker and Hu (2008)). A given ethanol dose, adjusted for body weight, leads to a higher blood alcohol concentration in women, despite more rapid elimination. This has been attributed to sex differences in body fat, although there is evidence that differences in gastric ethanol metabolism also play a role (Baraona et al., 2001). Atrophy of the brain through alcohol misuse reaches similar levels in male and female subjects even though females have lower average alcohol consumption over a shorter period of time (Mann et al., 2005). The HTR1B G861C G allele is over-represented in male alcoholics, but not in females (Fehr et al., 2000).
The underlying mechanism of alcohol-induced brain damage, in particular the loss of neurones (Harper and Kril, 1990), may involve an imbalance in excitatory–inhibitory neurotransmission. Glutamate is the major excitatory neurotransmitter of the CNS; its action is mediated by post-synaptic membrane-bound receptors that include the ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate (KA) and NMDA classes.
The NMDA glutamate receptor (NMDAR) is a major target for ethanol. Binding of the native agonist, glutamate and the co-agonist, glycine, produces a conformational change to open an intrinsic ion channel that allows the influx of cations, notably calcium, into the cell. The NMDAR is probably a tetramer comprising two NR1 and two NR2 subunits (Laube et al., 1998; Furukawa et al., 2005). The NR1 subunit is encoded by a single gene (GRIN1) that has eight splice variants; NR2 subunits are coded by four separate genes (GRIN2A–D). Varying subunit combinations give NMDARs diverse pharmacokinetic properties (Hynd et al., 2004).
Acute exposure of NMDARs to ethanol reduces Ca2+ influx, to an extent not only dependent on the identities of the NR2 subunits but also influenced by the NR1 variants present (Jin and Woodward, 2006). Chronic exposure of animals to alcohol can lead to a compensatory increase in NMDAR functionality through increased expression of NMDAR subunits; however, the experimental paradigm used can greatly influence which subunit is affected and the magnitude of the change. The largest alteration in expression occurs during withdrawal following a constant exposure or repeated deprivation protocol (Gulya et al., 1991; Darstein et al., 2000; Nixon et al., 2004; Haugbol et al., 2005; Nelson et al., 2005; Raeder et al., 2008).
The influence of sex on molecular changes has not been extensively studied in human brain, despite its vulnerability (Harper et al., 1990). Rodent models of chronic ethanol dependence and withdrawal show sex differences in the expression of γ-aminobutyric acid (GABA) and NMDA receptor subunits (Devaud and Morrow, 1999; Devaud and Alele, 2004). NR1 subunit expression is greatly increased in the cortex of female rats, both whilst they are actively drinking and after 3 days of withdrawal, but is unchanged in males (Devaud and Morrow, 1999; Devaud and Alele, 2004). After 3 days of withdrawal, NR2A subunit expression is increased in male, but not female, rats, while NR2B expression is increased in both sexes (Devaud and Alele, 2004).
Different genetic markers are associated with increased risk of alcohol misuse, dependence, craving, tolerance and withdrawal severity. The effects of these polymorphisms can pre-dispose an individual to alcohol misuse, or make an individual more susceptible to its effects. Polymorphisms may not only alter the product of the parent gene (e.g. by changing the amino-acid codon) but may also have pleiotropic effects, i.e. the changes in one gene may affect the expression of, or activity of the product of, another gene. Since it is very difficult to acquire sufficient numbers of subjects at autopsy to perform detailed gene-frequency analyses, we rely on the literature to identify protective or risk genotypes (Enoch, 2003), and partition our transcript expression data according to these to determine their effects.
We used real-time RT-PCR to quantify the relative expression of NMDAR NR1, NR2A and NR2B subunit mRNA in human autopsy brain tissue. Superior frontal (SFC) and primary motor (PMC) cortex tissue was obtained from alcoholics without comorbid disease, alcoholics with cirrhosis of the liver and matched controls. We report significant differences in the patterns of expression of these transcripts between the sexes that were further modulated when cases were partitioned by genotype.
MATERIALS AND METHODS
Tissue
Unfixed frozen tissue samples were obtained from the Queensland Brain Bank and the NSW Tissue Resource Centre in collaboration with the Australian Brain Bank Network. Ethical clearance was obtained from the University of Queensland Medical Research Ethics Committee, HEC #2006000901. The subjects groups were expanded from, and overlapped to a large extent with, those used in two recent studies (Ridge et al., 2008; Ridge and Dodd, 2009), augmented by the availability of further genotype data. Full macroscopic and microscopic examinations were carried out at autopsy and the cause of death determined. For each case, information on general health, diet, alcohol intake, medication and the presence of alcohol-related diseases such as cirrhosis of the liver, was available from medical records and autopsy reports. No subject had clinical or pathological evidence of neurological or psychiatric disease (apart from alcoholism) prior to death. According to the reports, no patient was a polysubstance or intravenous drug user, and none was on prescribed neuroactive medication. Cases were divided into groups depending on alcohol intake and the presence of complicating disease. Full liver pathology, which differentiated cirrhotic and non-cirrhotic participants, was available in every case. Controls were teetotal or had low alcohol intake (<20 g of ethanol per day), whereas alcoholics consumed >80 g of ethanol per day. All the alcoholics had been misusing ethanol for most of their adult lives, and in almost all instances where information was available had been drinking up until the time of death: mean alcohol-misuse durations were 38 years for non-cirrhotic and 40 years for cirrhotic alcoholics. Full consumption details were not available in every case, but where they were, the average daily consumption by alcoholics without comorbid disease was 13 standard drinks (130 g ethanol); it was 25 standard drinks (250 g ethanol) for alcoholics with cirrhosis. At autopsy, one hemisphere was fixed in formalin for pathological examination, the other dissected in the mortuary to minimize the delay to cryoprotection. Pieces of unfixed cerebral cortex (1–5 cm3) were immediately immersed in ice-cold 0.32 M sucrose, brought to the laboratory, frozen slowly and stored at −80°C until analysis. These procedures are optimal for the preservation of tissue components (Dodd et al., 1986). Details of cases are shown in Table 1.
Table 1.
Details of subjects
| Group | N | Age (years) | PMI (hours) |
|---|---|---|---|
| Control | 21 | 59.27 ± 2.98 | 33.20 ± 5.05 |
| Female | 10 | 52.62 ± 4.31 | 34.54 ± 7.30 |
| Male | 11 | 65.92 ± 4.11 | 31.85 ± 6.96 |
| Alcoholic without comorbid disease | 23 | 51.07 ± 2.87 | 29.75 ± 4.86 |
| Female | 10 | 46.31 ± 4.31 | 27.53 ± 7.30 |
| Male | 13 | 55.82 ± 3.78 | 31.97 ± 6.41 |
| Cirrhotic alcoholic | 14 | 57.88 ± 3.68 | 30.15 ± 6.24 |
| Female | 6 | 58.35 ± 5.56 | 28.88 ± 9.43 |
| Male | 8 | 57.42 ± 4.82 | 31.43 ± 8.17 |
PMI, post-mortem interval. For both parameters, neither the group or sex main effect nor the group by sex interaction was statistically significant. Overall mean ages: females, 52.43 ± 2.75 years; males, 59.72 ± 2.46 years; overall mean PMI values: females: 30.31 ± 4.66 hours, males: 31.75 ± 4.17 hours. Values are means ± SEM.
RNA
Total RNA was extracted from brain tissue using Trizol reagent (Invitrogen, Mount Waverley, Victoria, Australia) following the manufacturer's protocol. RNA was quantified spectrophotometrically and stored at −80°C. cDNA was made from 2 μg of total RNA using oligo-dT primers and Superscript III Reverse Transcriptase® (Invitrogen) following the manufacturer's instructions. cDNA was diluted 50-fold for real-time PCR.
Real-time PCR
The level of mRNA transcripts of NR1, NR2A, NR2B and GAPDH were determined using the following primer pairs:
NR1-RTF, 5′-GTCCACCAGACTGAAGATTGTGAC-3′;
NR1-RTR, 5′-CTCCTCCTTGCATGTCCCA-3′;
NR2A-RTF, 5′-GCTCTTCTCCATCAGCAGGG-3′;
NR2A-RTR, 5′-GGATCCCGTCAGATTGAAGTCT-3′;
NR2B-RTF, 5′-GGTCTTCTCCATCAGCAGAGG-3′;
NR2B-RTR, 5′-TGTTGTTCATGGTTGCGGT-3′;
GAPDHF, 5′-GGCATGGACTGTGGTCATGAG-3′;
GAPDHR, 5′-TGCACCACCAACTGCTTAGC-3′.
Primers were used at 300 nM final concentration. Then, 2.5 μL diluted cDNA together with the appropriate primers was added to 12.5 μL SYBR® Green PCR Master Mix (Applied Biosystems, Scoresby, Victoria, Australia) in a total volume of 25 μL. Real-time PCR was carried out using an ABI Prism 7000 Sequence Detection System (Applied Biosystems) with cycling parameters of denaturation at 95°C for 10 min, then 45 cycles of 95°C for 15 s and 60°C for 1 min. All four transcripts were measured in each sample in duplicate. Replicates with cycle threshold (Ct) values that differed by >0.3 standard deviations were re-run until consistency was achieved. Expression values were normalized to GAPDH and are reported in units of 2−ΔCt, where ΔCt is the difference in Ct values between the target and GAPDH transcripts in the same sample.
Genotyping
The MOR C1031G and 5HT1B G861C polymorphisms were genotyped using PCR followed by restriction enzyme digestion as described by Lappalainen et al. (1998) and Szeto et al. (2001), respectively. In brief, for MOR C1031G, a 145 bp fragment was amplified using the following primers: (F) 5′-GCT CTG GTC AAG GCT AAG AAT-3′ and (R) 5′-CCA GGT TGG ATG AGA GAG AAT G-3′. The PCR product was digested by Hinf I at 37°C overnight and the fragments displayed in 4% agarose gels by electrophoresis. The G allele was indicated by an uncut 145 bp band, and the C allele gave a 124 bp and a 21 bp band. For 5HT1B G861C, PCR amplified a 452 bp fragment with the following primers: (F) 5′-GAA ACA GAC GCC CAA CAG GAC-3′ and (R) 5′-CCA GAA ACC GCG AAA GAA GAT-3′. The PCR product was digested by HincII at 37°C overnight and the fragments resolved on a 2% agarose gel. The G allele was represented by an uncut 452 bp band; the C allele gave a 310 bp and a 142 bp band.
Statistical analysis
Normal probability plots showed that the ΔCt values, but not the 2−ΔCt values, were normally distributed (Ridge et al., 2008). Hence, ΔCt values were used in all statistical analyses, and then the computed means were converted to 2−ΔCt values for presentation. Analyses of variance (ANOVA) and covariance (ANCOVA) were performed using SPSS software (SPSS Inc, Chicago, IL, USA). Pairwise comparisons used Newman–Keuls post hoc tests. Exact probabilities are quoted where possible; statistical significance was accepted at P < 0.05.
RESULTS
Overall effects
Preliminary analysis showed that mRNA expression values were quite stable over the entire range of post-mortem intervals encountered here (Ridge et al., 2008). Neither the subject's age at death nor the length of time the tissue had been in storage at −80°C were significant confounds (Ridge et al., 2008), though we took care to ensure that frozen tissue samples had never been thawed prior to extraction. In previous work, we found no significant difference in GAPDH Ct values between different groups of alcoholics and controls, nor between brain areas within subjects, nor in the interactions between these factors, i.e. GAPDH was a suitable housekeeper for this work (Ridge et al., 2008). When we further sub-divided cases by sex we found that this partitioning of the variances revealed differences between case groups and sexes (Table 2). It may be seen that both sets of alcoholic subjects had slightly higher Ct values (lower levels of GAPDH mRNA) than controls, while females had slightly higher Ct values than males. These two effects were additive (sex × group interactions were not significant), and there were no significant within-subject differences between brain regions.
Table 2.
GAPDH Ct values
| Female | Male | Overall group | Combined group | |||
|---|---|---|---|---|---|---|
| SFCf | PMCf | SFCf | PMCf | Meana,c | Meand,e | |
| Control | 20.23 ± 0.32 | 20.86 ± 0.39 | 19.70 ± 0.31 | 19.99 ± 0.37 | 20.20 ± 0.21 | |
| Non-cirrhotic alcoholic | 21.31 ± 0.32 | 21.01 ± 0.39 | 20.36 ± 0.28 | 20.77 ± 0.34 | 20.86 ± 0.20 | 20.54 ± 0.15 |
| Cirrhotic alcoholic | 21.12 ± 0.38 | 21.05 ± 0.46 | 20.69 ± 0.38 | 20.92 ± 0.46 | 20.94 ± 0.26 | 20.89 ± 0.15 |
| Overall sex meanb,c | 20.93 ± 0.19 | 20.40 ± 0.18 | ||||
aMain effect for group, F2,52 = 3.601, p = 0.034; bmain effect for sex, F1,52 = 4.193, p = 0.046; cgroup × sex interaction, F2,52 = 0.21, p = 0.81; dmain effect for combined controls plus alcoholics without comorbid disease versus cirrhotic alcoholics, F1,54 = 1.742, p = 0.14; emain effect for combined alcoholics versus control, F1,54 = 7.277, p = 0.009; fmain effect for area, F1,52 = 4.193, p = 0.22; all group or sex interactions with area were non-significant, p > 0.3. Values are means ± SEM.
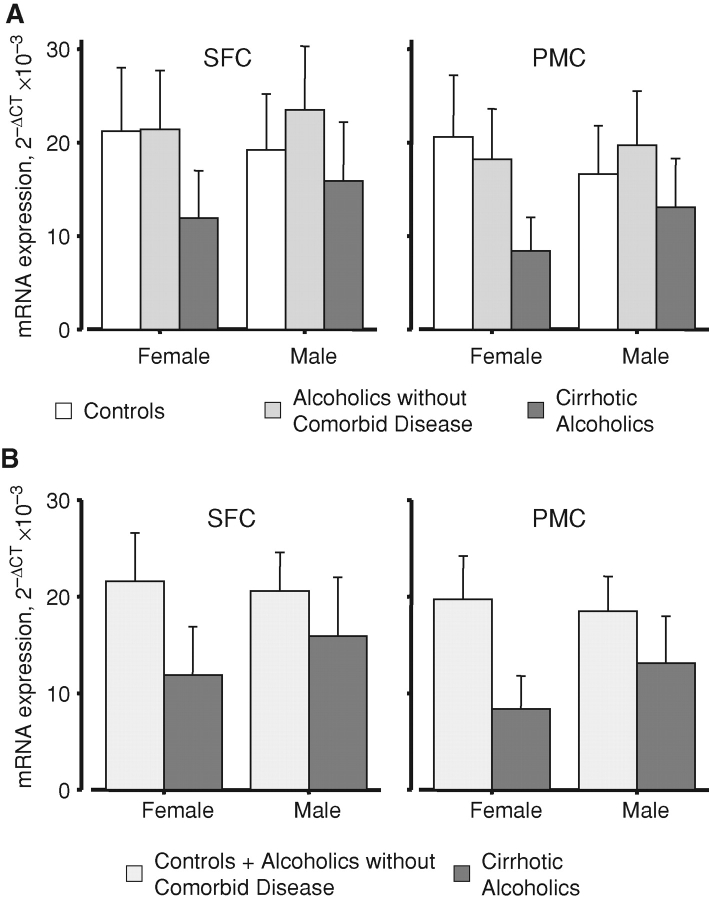
We have previously shown that the expression of NMDA receptor transcripts is significantly lower in both SFC and PMC in cirrhotic alcoholics than in the homotropic regions in either controls or alcoholics without comorbid disease, and that the latter two groups do not differ significantly from each other (Ridge et al., 2008). When the sexes were separated, the lack of difference between controls and alcoholics without comorbid disease remained (Fig. 1A). When these two groups were combined, the decrease in expression in male cirrhotic alcoholics was less marked than that in female cirrhotic alcoholics, in both cortical areas (Fig. 1B). Indeed, the pairwise differences only attained statistical significance in females in post hoc tests (Fig. 1B, legend). It should be emphasized that the combined group means in Fig. 1B are least-squares geometric averages of the controls plus non-comorbid alcoholic means shown in Fig. 1A.
Fig. 1.
Overall mean NMDAR mRNA expression. The expression of each transcript was calculated in units of ΔCt relative to GAPDH; these values were then averaged across the three transcripts and converted to 2−ΔCt for presentation. SFC, superior frontal cortex; PMC, primary motor cortex. (A) Expression in the three case groups (control, white bars; alcoholic without comorbid disease, lightly shaded bars; cirrhotic alcoholic, darkly shaded bars), F2, 41 = 3.478; P = 0.04. It may be seen that values in controls and non-comorbid alcoholics were largely similar. (B) Cirrhotic alcoholics (darkly shaded bars) versus all other subjects (control plus alcoholic without comorbid disease; lightly shaded bars), F1, 46 = 6.125; P = 0.017. In post hoc testing, expression was significantly lower overall in female cirrhotic alcoholic subjects than in female combined control plus non-cirrhotic alcoholic subjects, P = 0.046, but the equivalent comparison failed to reach statistical significance for male subjects. Values are means ± SEM.
Alcoholics without comorbid disease
Cortical NR1 levels are reportedly higher in female, but not male, alcohol-dependent rats than in the respective controls (Devaud and Morrow, 1999; Devaud and Alele, 2004). We did not find a comparable effect in human subjects: if anything, the opposite tendency was seen in the pathologically relevant SFC. NR1 expression was non-significantly lower in female non-cirrhotic alcoholic SFC than in control female SFC, whereas NR1 expression trended higher in male non-cirrhotic alcoholic SFC than in control male SFC (Fig. 2A). In the PMC, NR1 expression differed little or trended lower than controls in alcoholics without comorbid disease of either sex (Fig. 2A). NR1 expression levels in the PMC of both uncomplicated alcoholics and controls were lower than those in the respective SFC of these two groups (Fig. 2A).
Fig. 2.
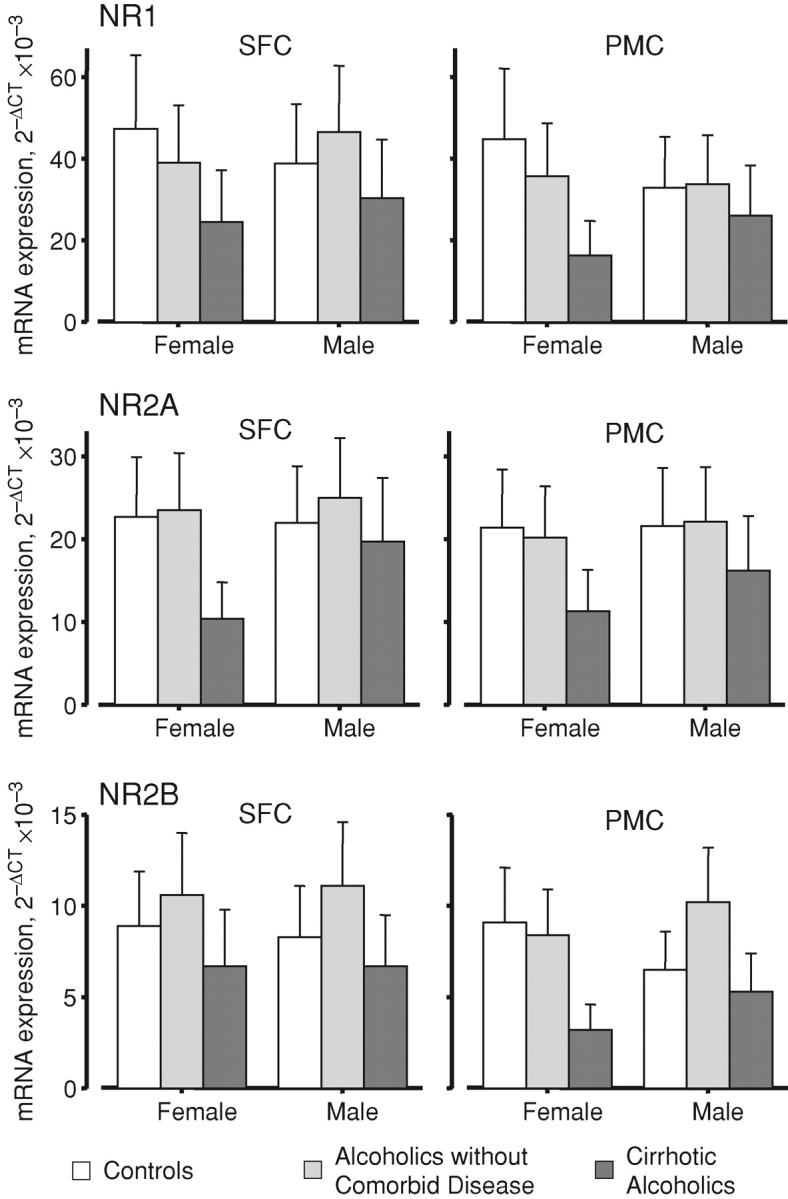
Case group and sex differentially modulate the regional expression of NMDAR subunit transcripts. Key as for Fig. 1. The interaction was significant, F4, 82 = 3.371; P = 0.013. Values are means ± SEM.
The NR2A transcript showed no significant variations when alcoholics without comorbid disease were compared with controls. In line with the NR1 data, there was slightly higher NR2A expression in male non-cirrhotic alcoholic SFC than in control male SFC (Fig. 2B). Values in females were almost identical in both brain regions; this was also true of male PMC (Fig. 2B).
NR2B expression showed a different pattern from the other two transcripts, with higher levels in the SFC of non-cirrhotic alcoholics of both sexes than in control SFC. NR2B expression was also higher in the PMC of male alcoholics than in male control PMC, but this trend was not seen in female PMC, where NR2B expression did differ from that in female control PMC (Fig. 2C).
Cirrhotic alcoholics
To varying degrees, cirrhotic alcoholics showed lower expression of all transcripts than controls. In females, the differences were most marked for NR1 and NR2A in both areas, and for NR2B in PMC, but not SFC (Fig. 2). None of the differences between male cirrhotic alcoholics and male controls, in either area, were large. NR2B expression was notably lower in cirrhotic alcoholics than in non-cirrhotic alcoholics (Fig. 2C).
Genotype effects
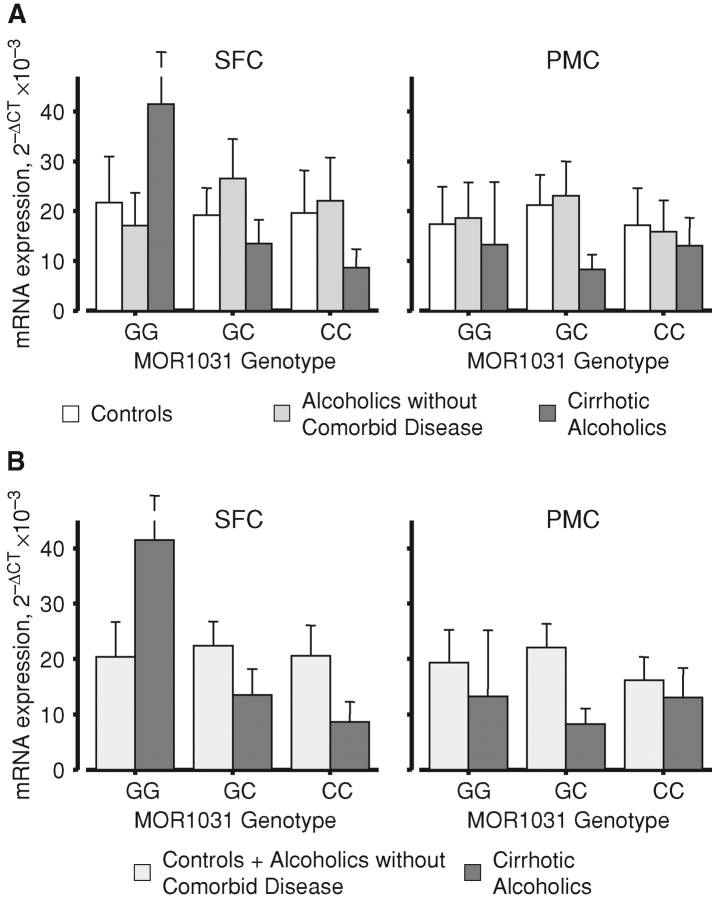
Subjects were assayed for a number of single-nucleotide polymorphisms (SNPs) and the expression data partitioned according to these genotypes. The μ-opioid receptor polymorphism MOR C1031G (rs2075572) is reportedly associated with heroin and alcohol addiction (reviewed in Kreek et al. (2005)). Partitioning of our data according to this genotype delineated no basal sex differences in expression (lower-order interactions were not significant, see Fig. 3, legend). However, when the subjects were also divided by the case group, there were marked differences in the pattern of expression in the SFC of cirrhotic alcoholics compared with the other subjects (Fig. 3). For the controls and uncomplicated alcoholics, the C1031G genotype did not modulate the pattern of expression of combined transcripts in any significant way. However, whilst cirrhotic alcoholics that were heterozygous at this locus conformed to the pattern reported above (Fig. 1), GG homozygotes showed markedly higher expression than controls in SFC, whilst expression was notably lower in CC homozygotes (Fig. 3A). No such effect was seen in PMC. It is apparent from the strength of statistical interaction that the effect is confined to cirrhotic alcoholics (Fig. 3B). When the transcripts were analysed separately, the higher-order interaction was also significant (Fig. 3, legend). NR1 and NR2A expression patterns were very similar to the averages shown in Fig. 3A, but MOR C1031G genotype had much less of a differential effect on NR2B expression in cirrhotic alcoholic SFC (not shown). The patterns shown in Fig. 3 did not differ between the sexes (not shown).
Fig. 3.
μ-Opioid receptor genotype modulates the regional expression of NMDAR mRNA. The mean ΔCt values averaged across the NR1, NR2A and NR2B transcripts were calculated as for Fig. 1; key as for Fig. 1. There were no basal or sex differences in expression when combined subjects were divided by MOR1031 genotype (main effect for genotype, F2, 41 = 0.31; P = 0.73; genotype × sex, F2, 41 = 0.08; P = 0.03), and no overall differences in patterns between the three transcripts (transcript × genotype, F4, 82 = 1.49; P = 0.21; transcript × sex × genotype, F4, 82 = 0.50; P = 0.73). However, when the subjects were separated into their respective case groups, clear differences emerged. (A) Expression in the three case groups averaged across transcripts, F4, 41 = 7.312; P < 0.001. It may be seen that values in controls and non-comorbid alcoholics were comparable. The patterns differed somewhat when the transcripts were considered separately, F4, 82 = 2.909, P = 0.007 (see the text). (B) Expression in cirrhotic alcoholics versus combined controls and alcoholics without comorbid disease, averaged across transcripts, F2, 46 = 13.323; P < 0.001. Values are means ± SEM.
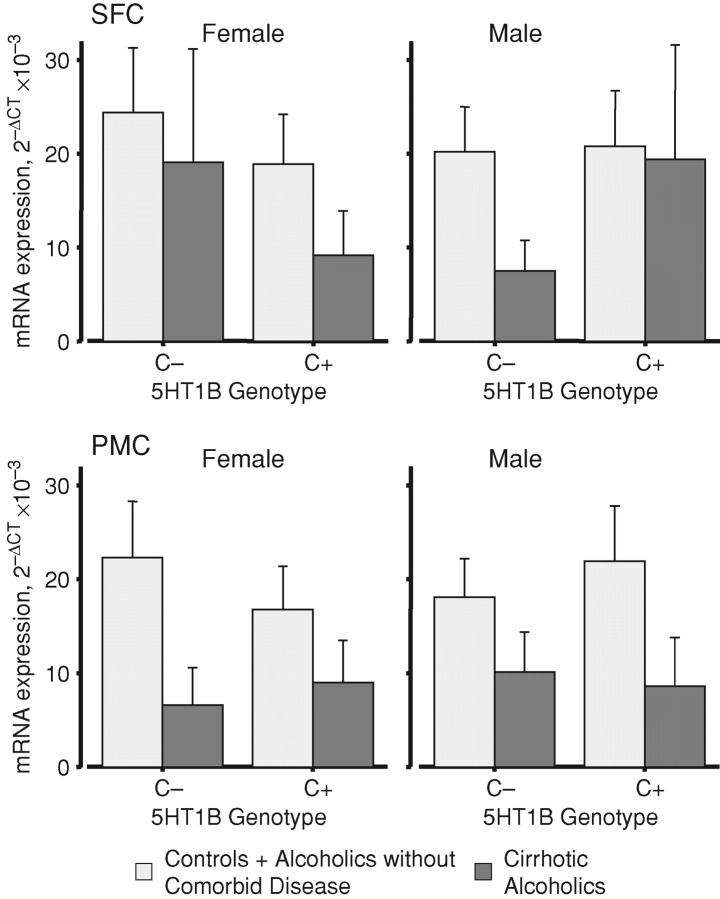
The data were divided according to the G861C polymorphism of the 5HT1B receptor (rs6296); as the minor (C) zygosity is relatively rare, subjects were classified according to possession of, or lack of, this allele. When average NMDAR transcript expression values were compared, males and females differed according to this genotype. In the SFC of cirrhotic alcoholic females lacking a C allele the averaged expression of NMDAR transcripts was comparable to that in controls (Fig. 4A). In contrast, SFC expression in cirrhotic alcoholic females positive for the C allele was lower than that in control female SFC (Fig. 4A). The SFC of male subjects showed a mirror-image pattern: cirrhotic alcoholics lacking a C allele had lower averaged transcript expression than non-cirrhotic subjects, whereas averaged transcript levels were similar across all subjects that possessed a C allele (Fig. 4A). The levels of all transcripts in the PMC of cirrhotic alcoholics were lower than those in the other two groups, with no notable effect of the genotype (Fig. 4B).
Fig. 4.
5HT1B receptor genotype modulates the regional expression of NMDAR mRNA differentially according to sex. Details as for Fig. 1; expression averaged across the NR1, NR2A and NR2B transcripts varied across the three case groups divided by sex and simplified genotype, F2, 43 = 8.003, P = 0.001 (not shown). Inspection showed that controls and alcoholics without comorbid disease gave similar values, so they were combined to clarify presentation (values for the combined group, lighter bars) and compared with cirrhotic alcoholics (darker bars). The revised interaction was significant, F1, 47 = 15.262; P < 0.001. Values are means ± SEM.
DISCUSSION
There is a wealth of studies to show that mediators of amino acid transmission, particularly GABAA receptors, are aberrant in pathologically relevant regions of the brain in alcoholics. GABAA receptor binding, pharmacology and subunit expression in SFC differ most markedly from controls in alcoholics without comorbid disease; cirrhotic alcoholics show more muted differences, despite more-severe pathology (Harper and Kril, 1990; Dodd et al., 1992; Thomas and Dodd, 1993; Lewohl et al., 1996, 1997a, 1997b, 2001). It is therefore of interest that this scenario is reversed for NMDA receptors, i.e. cirrhotic alcoholics show marked differences whereas alcoholics without comorbid disease show no, or muted, effects (Ridge et al., 2008). Given the growing problem of female drinking in our societies, and the increased vulnerability of the female brain, the present study explored the latter result further. From a panel of over 20 SNPs in a range of genes reportedly associated with alcohol misuse, we found two that gave the strongest interactions with the case group, sex and regional transcript expression. It is noteworthy that subjects who start abusing alcohol early in life, and who are thus more likely to develop severe, chronic alcohol misuse, exhibit problems with mood and aggression control and may have a serotonin deficit (Buydens-Branchey et al., 1989).
The effect of sustained ethanol exposure on the expression of NMDAR subunits has been studied in animal models. The majority of these studies have only used male animals (Gulya et al., 1991; Trevisan et al., 1994; Darstein et al., 2000; Raeder et al., 2008). Changes in NMDAR expression are highly dependent on the experimental paradigm used; there are differing reports of which subunits are altered and in which areas this occurs. Several groups have found changes in sub-cortical regions, but not in cortex (Trevisan et al., 1994; Nixon et al., 2004; Raeder et al., 2008). When studies were extended to both sexes, NR1 subunit expression was unaffected by alcohol administration in the cortex of males but significantly increased in the cortex of females (Devaud and Morrow, 1999; Devaud and Alele, 2004).
To perform comparative studies in human brain tissue requires several conditions to be met (Hynd et al., 2003). In particular, the relevant transcripts must be sufficiently stable post-mortem to allow consistent PCR amplification and analysis. We have generally found GAPDH to be a suitable housekeeper for studies of autopsy cortex from subjects undifferentiated by sex. The significant differences in GAPDH ΔCt shown here reflect both an unexpected difference between males and females, and the high level of precision achievable with real-time PCR. The slightly higher GAPDH ΔCt values (lower mRNA expression) in both groups of alcoholics are consistent with a mild degree of neurodegeneration, especially in SFC, and a widespread loss of dendritic arbour (Harper and Kril, 1990). This could reduce mRNA integrity, and thus affirm that GAPDH is a good housekeeper in this context. Alternatively, cellular intermediary metabolism may be somewhat compromised in alcoholics. Whether the latter is the explanation for the overall difference between males and females is not clear. Note that the average difference between alcoholics and controls (∼0.7 Ct units) or between males and females (∼0.5 Ct units) is not trivial, since one PCR cycle doubles the amount of amplimer when cycling parameters are optimal—as was the case here. In future studies, it would be of value to assess alternative housekeepers; from a statistical viewpoint, it would probably also be preferable to analyse target expression values in Ct units using GAPDH Ct as a covariate, to conform more closely to transcript copy numbers.
Male human alcoholics without comorbid disease conformed to the rodent models in that SFC NR1 and NR2A subunit expression was modestly higher than that in controls; controls and non-cirrhotic alcoholics showed comparable NR1 and NR2A expression in PMC. Levels of NR2B subunit transcripts were higher in both areas and accounted for almost all the increase in the mean NMDAR mRNA in PMC. The NR2B finding supports the hypothesis that it is the most responsive to alcohol exposure: non-human models show the greatest changes in this subunit (Nelson et al., 2005; Sheela Rani and Ticku, 2006). Increased NR2B levels in both sexes of a rodent model have been reported (Devaud and Alele, 2004).
Female alcoholics without comorbid disease showed an unexpected pattern. In contrast to the rodent models, all three subunits showed no significant differences from controls in either area. NR1 transcript expression was slightly lower in both areas, while expression of the other two either did not differ or was slightly higher, depending on cortical area. For both males and females, differences in subunit expression prior to drinking cannot be ruled out. Alternatively, the patterns may reflect selective differences in responsiveness of NMDAR subtypes to alcohol: as a proportion of SFC neurones are lost in severe chronic alcoholics (Kril et al., 1997), the observed patterns may reflect the surviving cellular architecture.
On considerations like this, it might be expected that cirrhotic alcoholics would show similar, but more marked, changes than alcoholics without comorbid disease, because cirrhotic alcoholics show, on average, greater levels of alcohol misuse and a more severe pathology; the incidence of cirrhosis rises with alcohol consumption (Kril and Harper, 1989; Saunders and Latt, 1993). Cirrhotic alcoholics may thus be more severely diseased, and it is notable that in our case groups their average daily consumption was indeed higher than that of alcoholics without comorbid disease (see the Materials and methods section). Effects that are more marked in cirrhotic alcoholics may reflect lifetime alcohol use, and alcoholics with varying degrees of neuropathological abnormalities may represent stages on a dosage continuum. However, our findings do not accord with this, as the cirrhotic alcoholics showed saltatory differences from both controls and alcoholics without comorbid disease. Liver damage may have additive effects to alcohol neurotoxicity, because the damaged liver is unable to process a range of toxins (especially ammonia) that can penetrate the brain (Butterworth et al., 1987). It is difficult to model these effects in laboratory rodents, since chronic administration of alcohol alone, even for periods of many months, does not induce cirrhosis (Leo and Lieber, 1983). There are also differences between males and females in vulnerability to cirrhosis, most likely because of differences in first-pass metabolism (Buydens-Branchey et al., 1989). Alcoholics with liver damage may be more susceptible to alcohol neurotoxicity as a result of a genetic predisposition (Dodd and Lewohl, 1998). Findings presented here suggested that the subject's genotype modulates NMDAR subunit mRNA expression—particularly in subjects with cirrhosis of the liver.
Opioids
Our data on the set of cases from which the current subjects were drawn show no association between the OPRM1 C1031G polymorphism and alcoholism (not shown), although analysis is still at a preliminary stage. However, cirrhotic alcoholic subjects homozygous for the C1031G G allele showed higher expression of all three NMDAR subunits. This may reflect differences in the pattern and frequency of drinking in GG subjects, which was further influenced by comorbid liver disease to lead to a large compensatory increase in NMDAR expression.
Serotonin
The 5HT1B receptor modulates the release of other neurotransmitters, including acetylcholine, glutamate, dopamine and GABA (Hoyer et al., 2002). Studies in mice suggest it mediates the intoxicating effects of alcohol (Crabbe et al., 1996). Division of our data using the G861C genotype showed distinct, complementary differences in NMDAR transcript expression between the male and female cirrhotic alcoholic cases in SFC. Female cirrhotic alcoholics showed similar NMDAR expression to controls in SFC if they lacked a C allele. In male cirrhotic alcoholic SFC, possession of a C allele led to expression levels similar to controls. Sex differences in the 5HT system, or its dysfunction, may affect drinking patterns. This may underlie differences in psychiatric disorders amongst alcoholics, where males are more likely to present with antisocial personality disorders, whereas female are more likely to present with depressive disorders (Enoch, 2003).
CONCLUSIONS
Male and female subjects present different treatment issues when they misuse alcohol. At least some of these differences may reflect differing susceptibility to brain damage, or differences in plasticity in dependence pathways. The ability to identify subjects at greatest risk, perhaps by assaying simple genetic markers, may prove of value in counseling and the development of targeted treatment paradigms.
Acknowledgments
We are grateful to neuropathologists from the Queensland Brain Bank, SCMB, University of Queensland, and from the NSW Tissue Resource Centre, for providing tissue samples; and to the next of kin, for informed written consent for the studies. The tissue banks are part of Australian Brain Bank Network supported by the National Health and Medical Research Council (NHMRC). The NSW Centre and Australian Brain Donor Program are supported by the University of Sydney, NHMRC, Schizophrenia Research Institute, National Institutes of Alcoholism and Alcohol Abuse USA (NIAAA) and NSW Department of Health. Financial support was provided by the NIAAA under grant NIH AA12404 and the NHMRC under grant no. 401551.
References
- Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–7. [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D, Grant ER, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res. 1993;621:137–40. doi: 10.1016/0006-8993(93)90309-b. [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Giguère JF, Michaud J, et al. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987;6:1–12. doi: 10.1007/BF02833598. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey MH, Noumair D, et al. Age of alcoholism onset: II. Relationship to susceptibility to serotonin precursor availability. Arch Gen Psychiatry. 1989;46:231–6. doi: 10.1001/archpsyc.1989.01810030037005. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, et al. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- Darstein MB, Landwehrmeyer GB, Feuerstein TJ. Changes in NMDA receptor subunit gene expression in the rat brain following withdrawal from forced long-term ethanol intake. Naunyn-Schmiedeberg's Arch Pharmacol. 2000;361:206–13. doi: 10.1007/s002109900180. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Alele P. Differential effects of chronic ethanol administration and withdrawal on γ-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol Clin Exp Res. 2004;28:957–65. doi: 10.1097/01.alc.0000128225.83916.40. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Morrow AL. Gender-selective effects of ethanol dependence on NMDA receptor subunit expression in cerebral cortex, hippocampus and hypothalamus. Eur J Pharmacol. 1999;369:331–4. doi: 10.1016/s0014-2999(99)00103-x. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Hardy JA, Baig EB, et al. Optimization of freezing, storage, and thawing conditions for the preparation of metabolically active synaptosomes from frozen rat and human brain. Neurochem Pathol. 1986;4:177–98. doi: 10.1007/BF02834357. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Lewohl JM. Cell death mediated by amino acid transmitter receptors in human alcoholic brain damage: conflicts in the evidence. Ann NY Acad Sci. 1998;844:50–8. [PubMed] [Google Scholar]
- Dodd PR, Thomas GJ, Harper CG, et al. Amino acid neurotransmitter receptor changes in cerebral cortex in alcoholism: effect of cirrhosis of the liver. J Neurochem. 1992;59:1506–15. doi: 10.1111/j.1471-4159.1992.tb08467.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA. Pharmacogenomics of alcohol response and addiction. Am J Pharmacogenomics. 2003;3:217–32. doi: 10.2165/00129785-200303040-00001. [DOI] [PubMed] [Google Scholar]
- Fehr C, Grintschuk N, Szegedi A, et al. The HTR1B 861G>C receptor polymorphism among patients suffering from alcoholism, major depression, anxiety disorders and narcolepsy. Psychiatry Res. 2000;97:1–10. doi: 10.1016/s0165-1781(00)00215-8. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, et al. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–92. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Gulya K, Grant KA, Valverius P, et al. Brain regional specificity and time-course of changes in the NMDA receptor–ionophore complex during ethanol withdrawal. Brain Res. 1991;547:129–34. [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol Alcohol. 1990;25:207–16. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Harper CG, Smith NA, Kril JJ. The effects of alcohol on the female brain: a neuropathological study. Alcohol Alcohol. 1990;25:445–8. [PubMed] [Google Scholar]
- Hasegawa Y, Higuchi S, Matsushita S, et al. Association of a polymorphism of the serotonin 1B receptor gene and alcohol dependence with inactive aldehyde dehydrogenase-2. J Neural Transm. 2002;109:513–21. doi: 10.1007/s007020200042. [DOI] [PubMed] [Google Scholar]
- Haugbol SR, Ebert B, Ulrichsen J. Upregulation of glutamate receptor subtypes during alcohol withdrawal in rats. Alcohol Alcohol. 2005;40:89–95. doi: 10.1093/alcalc/agh117. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Lewohl JM, Scott HL, et al. Biochemical and molecular studies using human autopsy brain tissue. J Neurochem. 2003;85:543–62. doi: 10.1046/j.1471-4159.2003.01747.x. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int. 2004;45:583–95. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res. 2006;30:673–9. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, et al. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, et al. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathol (Berlin) 1989;79:200–4. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Long JC, Eggert M, et al. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55:989–94. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–61. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Leo MA, Lieber CS. Hepatic fibrosis after long-term administration of ethanol and moderate vitamin A supplementation in the rat. Hepatology. 1983;3:1–11. doi: 10.1002/hep.1840030101. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Crane DI, Dodd PR. Alcohol, alcoholic brain damage, and GABAA receptor isoform gene expression. Neurochem Int. 1996;29:677–84. doi: 10.1016/s0197-0186(96)00089-7. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Crane DI, Dodd PR. Expression of the α1, α2 and α3 isoforms of the GABAA receptor in human alcoholic brain. Brain Res. 1997a;751:102–12. doi: 10.1016/s0006-8993(96)01396-0. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Crane DI, Dodd PR. Zolpidem binding sites on the GABAA receptor in brain from human cirrhotic and non-cirrhotic alcoholics. Eur J Pharmacol. 1997b;326:265–72. doi: 10.1016/s0014-2999(97)85422-2. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Huygens F, Crane DI, et al. GABAA receptor α subunit proteins in human chronic alcoholics. J Neurochem. 2001;78:424–34. doi: 10.1046/j.1471-4159.2001.00414.x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Serotonin's role in alcohol's effects on the brain. Alcohol Health Res World. 1997;21:114–20. [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, et al. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL. Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Res. 2005;1048:69–79. doi: 10.1016/j.brainres.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Nixon K, Hughes PD, Amsel A, et al. NMDA receptor subunit expression after combined prenatal and postnatal exposure to ethanol. Alcohol Clin Exp Res. 2004;28:105–12. doi: 10.1097/01.ALC.0000106311.88523.7B. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–58. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Raeder H, Holter SM, Hartmann AM, et al. Expression of N-methyl-d-aspartate (NMDA) receptor subunits and splice variants in an animal model of long-term voluntary alcohol self-administration. Drug Alcohol Depend. 2008;96:16–21. doi: 10.1016/j.drugalcdep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Dodd PR. Cortical NMDA receptor expression in human chronic alcoholism: influence of the TaqIA allele of ANKK1. Neurochem Res. 2009;34:1775–82. doi: 10.1007/s11064-009-9941-8. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Ho AM-C, Innes DJ, et al. The expression of NMDA receptor subunit mRNA in human chronic alcoholics. Ann NY Acad Sci. 2008;1139:10–19. doi: 10.1196/annals.1432.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Latt N. Epidemiology of alcoholic liver disease. In: Hayes PC, editor. Clinical Gastroenterology. Sydney: Ballière Tindall; 1993. pp. 555–79. [DOI] [PubMed] [Google Scholar]
- Sheela Rani CS, Ticku MK. Comparison of chronic ethanol and chronic intermittent ethanol treatments on the expression of GABAA and NMDA receptor subunits. Alcohol. 2006;38:89–97. doi: 10.1016/j.alcohol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Koller G, et al. Association of 5-HT1B receptor gene and antisocial behavior in alcoholism. J Neural Transm. 2004;111:101–9. doi: 10.1007/s00702-003-0064-0. [DOI] [PubMed] [Google Scholar]
- Szeto CY, Tang NL, Lee DT, et al. Association between μ opioid receptor gene polymorphisms and Chinese heroin addicts. Neuroreport. 2001;12:1103–6. doi: 10.1097/00001756-200105080-00011. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Dodd PR. Transmitter amino acid neurochemistry in chronic alcoholism with and without cirrhosis of the liver. Drug Alcohol Rev. 1993;12:91–8. doi: 10.1080/09595239300185771. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Fitzgerald LW, Brose N, et al. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–8. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Luo X, Kranzler HR, et al. Association between two μ-opioid receptor gene (OPRM1) haplotype blocks and drug or alcohol dependence. Hum Mol Genet. 2006;15:807–19. doi: 10.1093/hmg/ddl024. [DOI] [PMC free article] [PubMed] [Google Scholar]