Abstract
Aims: Chronic alcohol and drug dependence leads to neuroadaptations in hypothalamic–pituitary–adrenal (HPA) and sympathetic adrenal medullary (SAM) stress systems, which impact response sensitivity to stress and alcohol cue and facilitates risk of relapse. To date, gender variations in these systems have not been fully assessed in abstinent alcohol-dependent individuals who also met criteria for cocaine abuse. Methods: Forty-two (21 M/21 F) early abstinent treatment-seeking substance-abusing (SA) men and women and 42 (21 M/21 F) healthy control (HC) volunteers were exposed to three 5-min guided imagery conditions (stress, alcohol/drug cue, neutral relaxing), presented randomly, one per day across three consecutive days. Alcohol craving and anxiety ratings were obtained as well as measures of heart rate (HR), blood pressure, plasma ACTH, cortisol, norepinephrine (NE) and epinephrine (EPI). Results: SA males showed increased ACTH and EPI basal tone compared with HC males and SA females. However, they demonstrated no increase in ACTH and cortisol levels following stress and alcohol cue imagery exposure compared to the neutral condition. SA females demonstrated a typically increased stress response in both measures. In addition, SA males showed no increase in cardiovascular response to either stress or cue, and no increase in catecholamine response to cue compared with their response to neutral imagery. Again, this dampening was not observed in HC males who produced significantly higher levels of cue-related HR and EPI, and significantly higher stress-related DBP. In contrast, SA females showed an enhanced ACTH and cortisol response to stress and cue compared with neutral imagery and this was not observed in the HC females. They also demonstrated a reduced increase in NE and EPI compared with both SA males and HC females as well as reduced HR compared with HC females. Conclusions: While SA males showed a generalized suppression of HPA, SAM system and cardiovascular markers following both stress and cue, SA women demonstrated a selective sympatho-adrenal suppression to stress only and an enhanced HPA response to both stress and cue. These gender variations are discussed in terms of their potential impact on relapse vulnerability and treatment outcome.
INTRODUCTION
Acute, chronic and withdrawal-related effects of heavy drinking and cocaine abuse are associated with structural and functional alterations to hypothalamic–pituitary–adrenal (HPA), sympathoadrenomedullary (SAM) and mesolimbic dopaminergic stress and reward systems (Koob et al., 2004; Koob and Le Moal, 2008). Notably, these neuroadaptations are characterized by altered emotional and autonomic sensitivity to stress and alcohol cue (Fox et al., 2007; Sinha et al., 2008) and are associated with compulsive alcohol and drug seeking in animals (Valdez and Koob, 2004; Funk et al., 2006) and relapse factors in humans (Adinoff et al., 2005; Sinha et al., 2006; Sinha, 2007). In addition, the impact of gender and sex hormones on the psychobiology of stress and addiction etiology suggests that these stress and reward system changes may be sex-specific (Back et al., 2005; Fox et al., 2006, Fox and Sinha, 2009).
To date, sex differences in the stress and alcohol cue-related craving state have not been thoroughly assessed in alcohol-dependent males and females who also met criteria for cocaine abuse. Assessing gender variation in this co-dependent population of alcohol abusers is particularly important as the simultaneous abuse of cocaine is common in alcohol dependence (Hearn et al., 1991; McCance-Katz et al., 1999). Moreover, the use of cocaine in combination with alcohol has a unique biochemical profile (Jatlow et al., 1996) and chronic cocaine and alcohol abuse are known to differentially alter neuroendocrine response to stress (Fox et al., 2008; Sinha et al., 2008). As such, gender variations in a co-morbid population may be markedly different from those previously observed in predominantly cocaine only (Back et al., 2005; Fox et al., 2006) and alcohol only (Brady et al., 2006) dependent individuals.
Assessing sex differences in response to primary relapse factors, such as stress and alcohol cue exposure, was also an aim of the current study. In addition to HPA system changes, chronic alcohol and cocaine use is associated with a unique sympathetic profile, which is marked by increased noradrenergic activity (Linnoila et al., 1987; Hawley et al., 1994) and which, along with the mesolimbic dopaminergic system, is implicated in the rewarding and sensitizing effects of both drugs (Platt et al., 2007; Sofuoglu and Sewell, 2008; Smith and Aston-Jones, 2008). Findings from our own laboratory have also shown that higher stress and cue-related cocaine craving is accompanied with increases in plasma epinephrine (EPI) and norepinephrine (NE) (Sinha et al., 2003).
Prior research indicates sex differences in mood, vascular drive and HPA axis sensitivity to stress and cue in cocaine abusers (Back et al., 2005; Fox et al., 2006), alcohol abusers (Brady et al., 2006) and social drinkers (Chaplin et al., 2008) as well as gender variation in catecholamine and HPA axis activity following stress in healthy subjects (Frankenhaeuser et al., 1976, 1978; Lundberg and Frankenhaeuser, 1999; Seeman et al., 2001; Zimmer et al., 2003; Kudielka and Kirschbaum, 2005). However, thus far, sex-specific sympatho-adrenal response to stress and alcohol cue in substance-abusing men and women are not well documented. Furthermore, as women have tended to report significantly enhanced ratings of anxiety and sadness compared with men in many of these previous studies (Back et al., 2005; Brady et al., 2006; Chaplin et al., 2008), and sex hormones such as estrogen and progesterone are known to modulate autonomic responsiveness (Saleh and Connell, 2003; Kajantie and Phillips, 2006), gender variation in both HPA and catecholamine response to stress and alcohol cue might be expected.
Clarifying sexual dimorphism in stress system engagement following exposure to stress and alcohol cue is clinically relevant as enhanced stress and cue-related craving and associated changes in HPA axis markers are associated with the development of alcohol dependence (Dai et al., 2002) and propensity to relapse in early abstinent alcohol- (Adinoff et al., 2005; Breese et al., 2005; Junghanns et al., 2005) and cocaine-dependent (Sinha et al., 2006) patients. This, in turn, may aid the development of uniquely tailored pharmacological and clinical treatment therapies for both male and female substance abusers. The current study therefore aims to compare a group of alcohol-dependent males and females who also meet criteria for cocaine abuse with a group of well-matched socially drinking healthy control (HC) males and females by exposing them to personalized stressful, alcohol/drug cue-related and relaxing guided imagery.
METHOD
Participants
All participants were recruited from the community via advertisements posted on the internet and in local area newspapers. Forty-two (21 males and 21 females) alcohol-dependent individuals who met DSM-IV criteria for current alcohol dependence as well as criteria for current and lifetime cocaine abuse were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) for 4–6 weeks of inpatient treatment and study participation. Forty-two (21 male and 21 female) socially drinking healthy controls (HCs), who consumed a range of 0.5–30 drinks in the prior month, matched with the SA group on age, gender and race were also recruited. Substance-abusing (SA) individuals who met current DSM-IV criteria for dependence on another psychoactive substance other than nicotine were excluded. Controls (HCs) with current or past diagnoses of any substance dependence were also excluded. All participants were excluded if they were on medications for medical or psychiatric problems. All subjects underwent a thorough medical evaluation to ensure good physical health. Study procedures were approved by the Human Investigation Committee of the Yale University School of Medicine.
Design
A mixed repeated measures design was used with drug group 2, (SA versus HC) and gender 2 (males versus females) as the between-subject factor and condition 3 (stress, cue and neutral) and timepoint (varying levels for each assessment) as the repeated measure factors. The stress, cue and neutral imagery conditions were presented on consecutive days with only one stimulus presentation per day. Imagery condition was assigned randomly and counterbalanced across subjects. All staff were blind to the imagery condition.
Procedures
SA patients were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) for study participation. The CNRU is a locked inpatient treatment research facility with no access to alcohol or drugs and limited and monitored access to visitors. Urine and breathalyzer testing was conducted every 3 days to ensure drug abstinence. Laboratory sessions were conducted ∼28 days after admission in the inpatient treatment unit in order to allow for normalization of neurobiological changes associated with acute withdrawal. HCs completed baseline assessments and imagery script development procedures during outpatient visits and were then admitted to the Yale Center for Clinical Investigations Hospital Research Unit (YCCI-HRU) for a three-night, four-day hospital stay for participation in the laboratory sessions.
Prior to laboratory sessions, all subjects were interviewed using the Structured Clinical Interview for the DSM-IV (SCID-IV, First et al., 1997) to assess psychiatric and substance abuse diagnoses and history. Detailed information on baseline levels of alcohol and drug use history was obtained using the Quantity Frequency Variability Index (QFVI; Cahalan et al., 1969) for HCs and using the Form-90 for the SA group (Miller and Del Boca, 1994).
Imagery scripts
In the second week, scripts for the guided imagery induction were written based on methods developed by Lang and colleagues (Lang et al., 1980; Miller et al., 1987) and further adapted in our previous studies (see Sinha et al. (2003) for full details). Briefly, the stress imagery script was based on subjects’ description of a recent personal stressful event that was experienced as ‘most stressful’ (determined by ratings on a 10-point Likert scale where 1 = ‘not at all stressful’ and 10 = ‘the most stress they felt recently in their life’). Only situations rated as 8 or above were accepted as appropriate for script development. The stress imagery scripts did not include scenarios either relating to or culminating in drug use. The alcohol/drug cue imagery script was developed by having subjects identify a recent situation that included alcohol and drug-related stimuli and resulted in subsequent drug use (i.e. being at a bar or watching others smoke crack and drink alcohol). Drug-related scenarios did not include scenarios that involved stressful events such as being arrested. All social drinkers were required to provide alcohol-related scripts. A neutral imagery script was developed from the subjects’ description of a personal non-drug-related relaxing situation. All scripts were then recorded onto an audiotape to be played in the laboratory sessions.
Habituation and imagery training session
On a day prior to the laboratory sessions, subjects were brought into the testing room to acclimatize themselves to specific aspects of the study procedures, including the stress of intravenous (IV) catheter insertion as well as the subjective rating forms and training in relaxation and imagery procedures. Details on the imagery script development procedures and the imagery and relaxation training procedures have been described previously (Sinha et al., 2003; Sinha, 2008).
Laboratory sessions
Each subject was tested in the same room for the training and three laboratory sessions. On each day of the laboratory session, subjects abstained from breakfast and were allowed a smoke break at 7:30 AM in order to reduce the effects of nicotine withdrawal. Subjects were then taken into the testing room at 8:00 AM. After settling in a sitting position on a hospital bed, a heparin-treated catheter was inserted by the research nurse in the antecubital region of the subject's non-preferred arm, in order to periodically obtain blood samples. A blood pressure cuff was placed on the subject's preferred arm to monitor blood pressure and a pulse sensor was placed on the subject's forefinger to obtain a measure of pulse. This was followed by a 45-min adaptation period during which subjects were asked to practice relaxation. Following the adaptation period, baseline bloods were drawn, HR and BP were taken, and alcohol craving and anxiety rating scales were administered. At 9:00 AM, subjects were provided headphones and given the following instructions for the imagery procedure: ‘Close your eyes and imagine the situation being described, “as if” it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation. Stop imagining when you hear the voice on the tape tell you to stop imaging’. The length of each script was exactly 5 min. HR and BP was continuously monitored during the imagery period. All measures were collected immediately following imagery exposure and again at regular 15-min recovery intervals until 1 h after imagery. If participants’ anxiety remained above baseline levels following the final timepoint, they were taken through another series of relaxation procedures until their ratings returned to baseline levels. After the last assessment at 10:35 AM, the subjects were disconnected from the apparatus and served breakfast.
All subjective, cardiac and blood measures were taken at baseline (−5), immediately following imagery (0 timepoint) and six recovery timepoints (+5, +10, +15, +30, +45, +60 min after imagery).
Laboratory assessments
Subjective measures
The desire for using alcohol was assessed using a 10-point visual analog scale (VAS) in which 1 = ‘not at all’ and 10 = ‘extremely high’. Anxiety was also measured using a similar 10-point VAS anchored as above.
Physiological measures
A Critikon Dinamap 120 Patient Monitor was used to assess blood pressure. A pulse sensor was attached to the subject's finger and connected to the Dinamap Monitor to provide a continuous measure of pulse.
Blood samples (HPA measures and catecholamines)
Blood collected for the analysis of ACTH and cortisol was obtained in heparinized tubes; blood samples for NE and EPI determination were collected in tubes containing EGTA and reduced glutathione. All tubes were placed on ice immediately after drawing and were centrifuged at 4°C within 30 min of collection, and then aliquoted. All HPA blood samples were stored at −70°C and shipped on dry ice to Rockefeller University where assays were conducted using standard radioimmuno-assay procedures. All catecholamine samples were analyzed in Dr Anderson's laboratory at Yale University. Catecholamines were alumina-extracted from 1.0 mL plasma samples, separated by reverse phase ion-pair high performance liquid chromatography (HPLC) and detected using coulometry (Coulochem II, ESA, Inc.).
Statistical analysis
The drug groups were compared on demographics and substance use measures using either analyses of variance (ANOVA) or chi-square and measures on which groups differed were included as covariates in all analyses.
Linear mixed-effect models (LME; Laird and Ware, 1982) were implemented to analyze the baseline and response data, using SAS, version 8 software (SAS Institute, Cary, NC). Between-subject factor of group (SA versus HC) and gender (males versus females) and within-subject factors of condition (stress, cue and neutral) and timepoint (varying levels) were the fixed effects, and subjects were the random effect. In order to control for individual baseline variability, changes from baseline data were used.
Secondary analyses using Pearson's product moment correlational coefficients were also conducted in order to assess the association between basal tone and drinking/drug patterns prior to treatment as well as basal tone and response to stress and cue. These correlations were performed separately in substance-abusing men and women.
RESULTS
Participants (Table 1)
Both SA and HC males and females were statistically matched for age and race. As HCs had spent a greater number of years in education (P = 0.0004) and were lighter smokers (P < 0.0001) compared with SA patients, both variables were included as random effects (covariates) in the LME model.
Table 1.
Drug use and demographic data for substance abusing and healthy control males and females
| SA Female | SA Male | HC Female | HC Male | P | ||
|---|---|---|---|---|---|---|
| N = 21 | N = 21 | N = 21 | N = 21 | |||
| Age | 35.76 ± 6.93 | 36.76 ± 7.87 | 34.19 ± 9.30 | 36.10 ± 8.35 | ns | |
| Race: | ||||||
| Caucasian | 10 (48%) | 15 (71%) | 8 (38%) | 14 (67%) | ns | |
| Other | 11 (52%) | 6 (29%) | 13 (62%) | 7 (33%) | ||
| Years in education | 12.48 ± 1.54 | 12.81 ± 1.75 | 14.09 ± 1.64 | 14.62 ± 2.0 | 0.0004 | SA vs HC |
| Alcohol/drug use | ||||||
| No. of days alcohol (last month) | 16.48 ± 11.66 | 23.1 ± 9.00 | 2.97 ± 4.56 | 7.8 ± 8.04 | <.0001 | SA vs HC |
| No. of drinks consumed per occasion (last month) | 9.62 ± 6.42 | 15.34 ± 8.0 | 1.5 ± 0.71 | 1.58 ± 0.86 | <.0001 | SA vs HC |
| No. of years alcohol used | 12.62 ± 8.16 | 18.19 ± 7.51 | 5.22 ± 6.02 | 12.11 ± 8.73 | <.0001 | (SAF, SAM, HCM) vs (HCF) |
| No. of years cocaine used | 8.63 ± 6.35 | 7.00 ± 6.40 | 1.00 ± 0.00 | 1.00 ± 1.41 | ns | SA vs HC |
| No. of regular smokers | 18 (86%) | 19 (90%) | 2 (10%) | 6 (29%) | <.0001 | SA vs HC |
| Psychiatric measures | ||||||
| Lifetime anxiety disorders | 3 (14%) | 3 (14%) | 0 | 0 | ns | |
| Lifetime depression disorders | 5 (24%) | 2 (10%) | 5 (24%) | 0 | ns | |
| Basal measures | ||||||
| Plasma ACTH | 16.44 ± 6.07 | 25.08 ± 7.06 | 16.49 ± 6.22 | 18.45 ± 6.08 | <.02 | SAM > HCM; SAM > SAF |
| Plasma norepinphrine | 206.97 ± 79.07 | 172.0 ± 54.87 | 198.0 ± 78.11 | 241.75 ± 91.48 | .02 | SAM < HCM; SAM < SAF |
| Plasma epinephrine | 15.89 ± 8.01 | 17.04 ± 8.41 | 13.53 ± 7.01 | 20.37 ± 8.20 | .03 | M > F |
| SBP | 108.03 ± 6.41 | 117.42 ± 6.86 | 112.91 ± 11.99 | 113.38 ± 6.85 | <.02 | SAF < SAM; SAF < HCF |
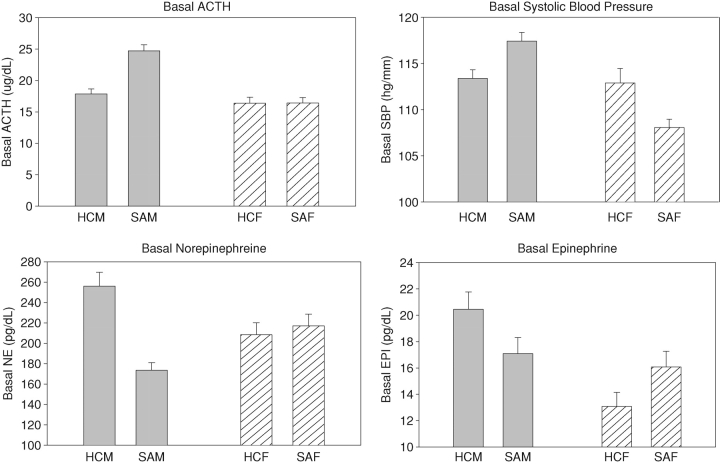
Baseline findings (Fig. 1)
Fig. 1.
Gender and group differences in basal measures. (a) Basal ACTH. SAM > HCM, P = 0.0009; SAM > SAF, P < 0.0001. (b) Basal SBP. M > F, P < 0.006; SAF < SAM, P = 0.0004; SAF < HCF, P = 0.03. (c) Basal norepinephrine. HCM > HCF, P = 0.07; HCM > SAM, P = 0.02. (d) Basal epinephrine. M > F, P = 0.03.
Note. HCM: Healthy Control Males; SAM: Substance Abusing Males; HCF: Healthy Control Females; SAF: Substance Abusing Females.
A main effect of gender was shown for EPI [1, 77 = 4.6; P = 0.03] and SBP [1, 79 = 8.1; P < 0.006] indicating that males had higher basal levels of both compared with females. Significant group × gender interactions were also observed for ACTH [1, 79 = 5.8; P = 0.01], SBP [1, 79 = 5.8; P < 0.02] and NE [1, 79 = 5.3; P = 0.02]. This indicated that SA males demonstrated significantly higher levels of ACTH (P < 0.0001) compared with both SA females (P < 0.0001) and HC males (P = 0.0009) while SA females demonstrated significantly lower SBP compared with both SA males (P = 0.0004) and HC females (P = 0.03). In addition, SA males demonstrated lower basal NE compared with HC males (P = 0.02) and SA women (P = 0.1), although the latter was only a trend.
Response findings (difference from baseline)
With the exception of anxiety, significant three-way, drug group × gender × condition interactions were observed for all measures and these are reported in full in the text. Two-way interactions are also reported for anxiety. All main effects of drug group (SA versus HC), gender (males versus females) and condition (stress, cue, neutral) are displayed in Table 2.
Table 2.
Main effect of drug group, gender and condition for each dependent variable
| Dependent variable | Main effect of condition | Main effect of drug group | Main effect of gender |
|---|---|---|---|
| Alcohol craving | [2, 160 = 69.8; P < 0.0001] | [1, 79 = 13.6; P = 0.0004] | |
| S > N, P <.0001; C > N, P <.0001; C > S, P =.0008 | SA > HC | ||
| Anxiety | [2, 159 = 116.2; P < 0.0001] | [1, 79 = 10.9; P = 0.001] | |
| S > N, P <.0001; C > N, P <.0001; S > C, P <.0001 | SA > HC | ||
| Heart rate | [2, 159 = 116.2; P < 0.0001] | [1, 79 = 3.7; P < 0.06] | |
| S > N, P <.0001; C > N, P <.0001 | SA < HC | ||
| Systolic blood pressure | [1, 79 = 8.1; P < 0.006] | ||
| M > F | |||
| Diastolic blood pressure | [1, 79 = 10.3; P < 0.002] | ||
| M > F | |||
| ACTH | [2, 147 = 6.2; P < 0.003] | ||
| S > N, P =.0006; S > C, P <.03 | |||
| Cortisol | [2, 147 = 10.9; P < 0.0001] | [1, 79 = 5.9; P < 0.02] | [1, 79 = 10.7; P < 0.02] |
| S > N, P =.0002; C > N, P <.0001 | SA < HC | M > F | |
| NE | [2, 145 = 6.2; P < 0.003] | ||
| C > S, P =.0006 | |||
| EPI | [2, 140 = 12.3; P < 0.0001] | ||
| C > S, P =.0009 |
Note: S: Stress, C: Cue, N: Neutral. SA: Substance Abusers, HC: Healthy Controls. M: Males, F: Females.
Subjective data
Alcohol craving
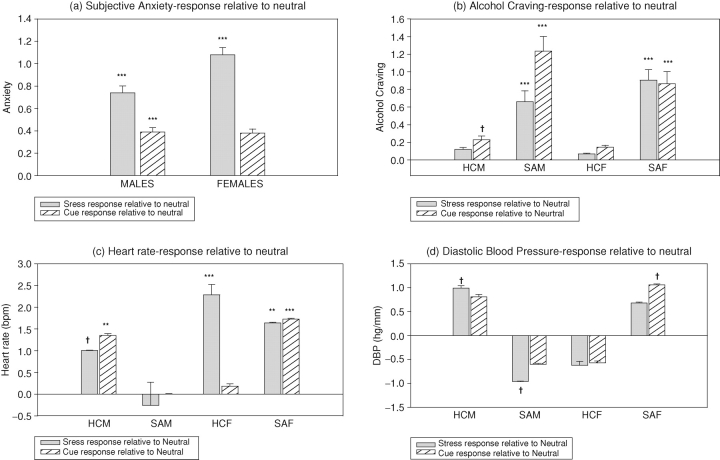
A drug group × gender × condition interaction [2, 160 = 4.1; P < 0.02] showed that SA females reported significantly higher alcohol craving compared with HC females following exposure to stressful (P < 0.0001) and cue-related (P = 0.0007) imagery. Similarly, SA males reported significantly higher alcohol craving in the stress (P = 0.01) and cue (P < 0.0001) conditions compared with HC males. No differences in craving were noted between the SA males and females. Greater alcohol craving was reported following exposure to cue imagery compared with exposure to neutral imagery in SA males (cue > neutral: C > N: P < 0.0001), SA females (C > N: P < 0.0001) and HC males (C > N: P < 0.04), but not HC females (Fig. 2b). No group or sex differences were observed in the neutral imagery condition.
Fig. 2.
Subjective and cardiovascular response to stress and cue-related imagery relative to neutral imagery in substance-abusing and healthy men and women. (a) Anxiety. All means show change from baseline scores and data are collapsed across timepoints. SA > HC in stress, P = 0.0007; SA > HC in cue, P < 0.0001; F > M in stress, P = 0.02. (b) Alcohol craving. All means show change from baseline scores and data are collapsed across timepoints. SAF > HCF in stress, P < 0.0001; SAF > HCF in cue, P = 0.0007; SAM > HCM in stress, P = 0.01, SAM > HCM in cue, P < 0.0001. (c) Heart rate. All means show change from baseline scores and data are collapsed across timepoints. HCF > SAF in stress, P = 0.002; HCF > SAF in neutral, P < 0.004; HCF > HCM in stress, P = 0.03. (d) Diastolic blood pressure. All means show change from baseline scores and data are collapsed across timepoints. SAM > SAF in stress, P = 0.04; SAM > SAF in cue, P < 0.03; SAM > SAF in neutral, P < 0.0001.
Note. HCM: Healthy Control Males; SAM: Substance Abusing Males; HCF: Healthy Control Females; SAF: Substance Abusing Females. †P <.05; *P <.01; **P <.001; ***P <.0001. P values represent difference between stress and neutral or cue and neutral imagery conditions.
Anxiety
A drug group × condition interaction was observed for anxiety [2, 159 = 18.7; P < 0.0001] showing that SA patients reported higher anxiety ratings following stress (P = 0.0007) and cue (P < 0.0001) exposure compared with HCs. No group differences were observed in the neutral imagery condition. Additionally, an increase in anxiety following cue exposure was only observed in the SA group (C > N, P < 0.0001) not the HCs. A significant gender × condition interaction [2, 159 = 10.2; P < 0.0001] also showed that females reported greater anxiety following exposure to stress compared with males (P = 0.02; Fig. 2a). No sex differences were observed following exposure to either cue or neutral imagery.
Physiological data
Heart rate
A drug group × gender × condition interaction [2, 159 = 6.65; P < 0.002] indicated that SA males showed no HR response to either stress or cue-related imagery relative to neutral imagery. In contrast, the SA females demonstrated an increased HR response to both stress (stress > neutral: S > N, P = 0.0006) and cue (C > N, P < 0.0001) compared with neutral imagery. HC males also showed an increased response to stress (S < N, P < 0.02) and cue (C > N, P = 0.001; Fig. 2c). Although SA females showed an increase in HR following stress relative to neutral (S > N, P = 0.0006), their response was significantly lower compared to that observed in the HC females (P = 0.002). HC females also demonstrated a significantly greater HR response to stress compared with HC males (P = 0.03).
Systolic blood pressure
A drug group × gender × condition interaction [2, 159 = 3.4; P < 0.04], indicated that SA males showed higher SBP across all three imagery conditions compared with SA females (stress, P = 0.06; cue, P < 0.002; neutral, P = 0.03) and higher SBP compared with HC males in the cue condition (P = 0.004). HC males also showed higher SBP compared with HC females following stress (P < 0.04).
Diastolic blood pressure
A drug group × gender × condition interaction [2, 159 = 7.2; P = 0.001], indicated that SA males demonstrated higher levels of DBP across all conditions compared with SA females (stress, P = 0.04; cue, P = 0.03; neutral, P < 0.0001). Despite this higher DBP drive, they showed a suppressed response to stress relative to their response to neutral imagery (N > S, P = 0.05), which was not seen in HC males who demonstrated a typically increased DBP response to stress (S > N, P < 0.04; Fig. 2d). In contrast to SA males, SA females demonstrated an enhanced DBP response to cue-related imagery compared with neutral (C > N, P = 0.03). Again, this was not observed in either the HC females or SA males, who both showed a trend toward a suppressed cue-related response.
HPA axis measures
ACTH
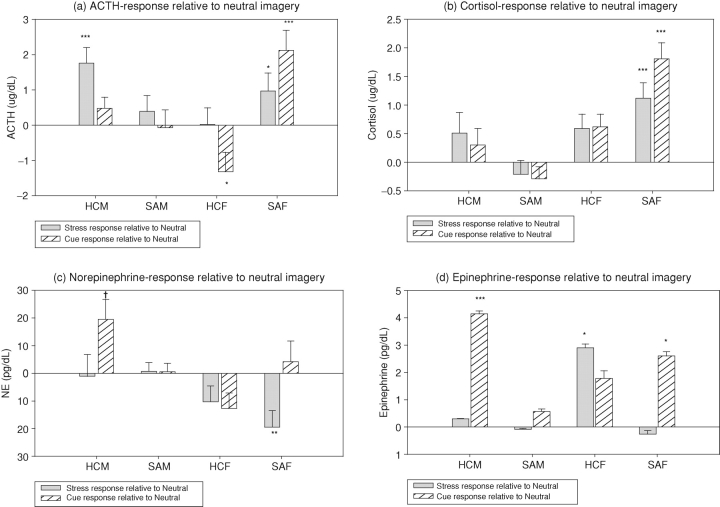
Drug group × gender × condition interactions [2, 147 = 11.7; P < 0.0001], indicated that SA females demonstrated an enhanced ACTH response to both stress (S > N, P < 0.005) and cue (C > N, P < 0.0001) relative to their response to the neutral imagery condition. In contrast, this stress and cue-related increase in ACTH was not observed in the HC females (Fig. 3a). HC males also showed an increased ACTH response to stress (S > N, P < 0.0001). While SA females and HC males demonstrated an enhanced ACTH response to stress, which was not observed in the SA males who showed no significant difference in ACTH response across all three imagery conditions. In addition, SA males demonstrated significantly lower levels of ACTH compared with HC males in the stress condition (P < 0.006) and lower levels of ACTH compared with both HC males (P < 0.05) and SA females (P = 0.005) in the cue condition. In contrast, HC males demonstrated higher ACTH levels compared with HC females following stress exposure (P = 0.04).
Fig. 3.
HPA and SAM response to stress and cue-related imagery relative to neutral imagery in substance-abusing and healthy men and women. (a) ACTH. All means show change from baseline scores and data are collapsed across timepoints. SAM < HCM in stress, P < 0.006; SAM < HCM in cue, P < 0.05; SAM < SAF in cue, P = 0.005; HCM > HCF in stress, P = 0.04. (b) Cortisol. All means show change from baseline scores and data are collapsed across timepoints. SAM < HCM in stress, P < 0.005; SAM < HCM in cue, P = 0.0009; HCM > HCF in stress, P = 0.006; HCM > HCF in cue, P < 0.004; HCM > HCF in neutral, P < 0.007. (c) Norepinephrine. All means show change from baseline scores and data are collapsed across timepoints. SAF < HCF in stress, P = 0.03; SAF < SAM in stress, P = 0.003. (d) Epinephrine. All means show change from baseline scores and data are collapsed across timepoints. SAF < HCF in stress, P < 0.05; SAF > SAM in cue, P < 0.05; HCM > SAM in cue, P = 0.001; HCM > HCF in cue, P = 0.02.
Note. HCM: Healthy Control Males; SAM: Substance Abusing Males; HCF: Healthy Control Females; SAF: Substance Abusing Females. †P <.05; *P <.01; **P <.001; ***P <.0001. P values represent difference between stress and neutral or cue and neutral imagery conditions.
Cortisol
A drug × gender × condition interaction [2, 147 = 7.8; P = 0.0006] showed that SA females showed an increased response to both stressful (S > N, P < 0.0001) and cue-related (C > N, P < 0.0001) imagery compared with their response to neutral imagery. In contrast, this significantly enhanced response to stress and cue was not observed in either HC females or HC males (Fig. 3a). Again, while SA females demonstrated stress and cue-related increases in cortisol levels, this was not observed in SA males. In addition, SA males showed significantly lower cortisol levels compared with HC males following both stress (P < 0.005) and cue (P = 0.0009) exposure. In contrast, HC males showed significantly higher cortisol levels following all three imagery conditions compared with HC females (stress, P = 0.006; cue, P < 0.004; neutral, P < 0.007).
Catecholamines
Norepinephrine
A drug group × gender × condition interaction [2, 145 = 4.5; P = 0.01] indicated that SA females showed a suppressed NE response to stress compared with the neutral imagery condition (N > S, P = 0.001; Fig. 3c). They also demonstrated significantly lower levels of NE following exposure to stress, compared with both HC females (P = 0.03) and SA males (P = 0.003). While a significant cue-related increase in NE was observed in the HC males (C > N, P < 0.02); SA males again failed to demonstrate an increase in NE levels following exposure to cue relative to their response in the neutral imagery condition.
Epinephrine
A drug group × gender × condition interaction [2, 140 = 7.3; P = 0.001] indicated that the SA females showed no EPI response to stress compared to the neutral imagery condition, while HC females demonstrated an appropriate increase in EPI following exposure to stress compared with neutral imagery (S > N, P = 0.01) (Fig. 3d). SA females also demonstrated significantly lower levels of EPI following exposure to stress compared with HC females (P < 0.05). In relation to cue exposure, SA males demonstrated no increased response to cue, while HC males showed an appropriate increased EPI response to the cue-related imagery relative to neutral (C > N, P < 0.0001) (Fig. 3d). SA males also showed lower levels of EPI compared with both SA females (P < 0.05) and HC males (P = 0.001). Conversely, HC males showed higher cue-related EPI levels compared with HC females (P = 0.02).
Secondary analyses
As robust gender variation was observed in relation to basal autonomic and HPA axis measures, secondary analyses were conducted to ascertain (i) whether basal tone was related to alcohol and drug use prior to treatment, and (ii) whether basal tone was associated with response to stress and cue in the substance-abusing men and women.
Substance-abusing men
In SA males increased basal levels of EPI (r = 0.54, P = 0.01) and SBP (r = 0.48, P < 0.03) were both significantly associated with a higher number of days spent drinking in the month prior to inpatient admission. Moreover, high basal EPI tone was significantly associated with reduced EPI levels following exposure to cue-related imagery (r = −0.54, P = P < 0.02) and increased levels of EPI following exposure to stressful imagery (r = 0.58, P = 0.005). Although significantly higher basal SBP in the SA men was not associated with SBP response, it was associated with significantly reduced HR response following exposure to stress (r = −0.34, P < 0.03) and neutral (r = −0.31, P < 0.05) imagery. High levels of basal ACTH and low levels of basal NE in the SA men were not associated with either drinking or drug use patterns prior to treatment or with HPA and SAM response to stress and cue. Prior use of cocaine was also not associated with any baseline alterations.
Substance-abusing women
In SA females, lower basal levels of SBP were not associated with any patterns of drinking or cocaine use and did not correlate significantly with response SBP or HR. A trend toward increased basal NE tone in the SA women was significantly associated with reduced NE response following exposure to both stressful (r = −0.45, P < 0.05) and neutral (r = −0.54, P < 0.02) imagery.
DISCUSSION
Consistent with previous research both SA men and women in the present study reported significantly higher levels of stress and cue-induced anxiety and alcohol craving compared with HCs. However, accompanying emotional and bio-physiological stress system engagement in these SA men and women was shown to be highly sex-specific.
Substance-abusing males
In SA males, increases in the stress and cue-related craving state were characterized by significantly heightened anxiety and a failure to demonstrate a typical autonomic and cardiovascular arousal response. For example, following exposure to stress and cue-related imagery, increases in alcohol craving and anxiety were accompanied by significantly lower ACTH and cortisol levels compared with both HC males as well as relative to a relaxing imagery. SA men also demonstrated blunted sympathadrenal (NE and EPI) and cardiovascular (HR, DBP) responses to cue-related imagery along with increases in anxiety and alcohol craving. In contrast, cue-related increase in alcohol craving was not accompanied by autonomic stress system dampening in HC males.
This failure to mount appropriate stress system engagement in response to stress and cue is also clearly illustrated by the contrast in SAM and HPA axis activity in HC males, and between HC males and females. First, HC males showed a significant increase in alcohol craving, NE, EPI, HR and DBP following exposure to cue compared with neutral imagery, and second HC males demonstrated higher stress-related alcohol craving and ACTH levels as well as a higher cortisol drive across all three imagery conditions compared with HC females. Moreover, these findings are consistent with those typically seen in healthy males and females across a number of stress and cue-related paradigms (Kirschbaum et al., 1992, 1999; Kudielka et al., 1998; Chaplin et al., 2008; Fox and Sinha, 2009).
SA men also demonstrate increased ACTH, EPI and SBP basal tone compared with both SA women and HC men. Although high baseline cortisol levels are typically reported following recent alcohol withdrawal (Costa et al., 1996; Keedwell et al., 2001), the baseline levels observed in this study may reflect a unique profile indicative of alcohol-dependent men who also met abuse criteria for cocaine. This would corroborate prior findings showing higher basal levels of ACTH in cocaine-dependent men compared with cocaine-dependent women (Fox et al., 2006). Enhanced HPA basal tone has been associated with a poor ability to respond physiologically to threat and stress system challenge in alcohol-dependent individuals (Wand and Dobs, 1991; Adinoff et al., 1998; Sinha et al., 2008) that, in turn, has been associated with relapse factors in alcoholics (Adinoff et al., 2005; Junghanns et al., 2005; Sinha, 2007; Fox et al., 2008) and dependence in individuals with a positive family history for alcoholism (Zimmerman et al., 2004; Sorocco et al., 2006). Similarly, in support of these findings, prior research from our own laboratory has also shown that weakened HPA and autonomic response to stress in alcoholic men is accompanied by increases in craving (Sinha et al., 2008).
In the current sample of SA men, findings are consistent with previous research in reflecting an HPA profile that is impaired at multiple levels of HPA axis function (Adinoff et al., 2005). High levels of basal ACTH and a blunted HPA response to stress and cue may be consistent with alcohol and cocaine abuse potentially serving to increase ACTH levels by modulating CRH release and facilitating glucocorticoid negative feedback (see Rivier et al. (1984) and Sarnyai et al. (2001) for a review). Increased ACTH, EPI and SBP basal tone in SA men may also potentially reflect a ceiling level of adrenal and physiological response undermining the ability to respond effectively to stress or cue. The potential effects of the enhanced basal profile on the stress and cue-related craving state in SA men is also supported in this study by the fact that increased basal EPI tone was associated with blunted EPI response to cue and increased basal SBP was associated with a lower HR drive also known to accompany increased alcohol craving in dependent populations (Sinha et al., 2008). Although increased basal EPI tone was not different to that observed in HC males, increased baseline levels in men compared to women may still reflect a risk marker for poor stress response in SA men. Notably, current findings also indicated that these increased basal alterations in EPI and SBP tone were significantly associated with increased alcohol consumption prior to inpatient treatment, highlighting the direct effects of drinking-related modulations on basal stress systems in men.
Substance-abusing females
In contrast to both SA males and HC females, increases in stress and cue-related alcohol craving in SA females were accompanied by a significantly enhanced HPA response. In addition, SA females also showed significantly higher levels of cue-related ACTH compared with SA males and increases in cue-related NE, HR and DBP compared with neutral imagery. This effect of cue was not observed in either the HC females or SA males.
Despite demonstrating an enhanced HPA axis response to both stress and cue, SA females exhibited reduced SAM system engagement and cardiovascular response to stress. They produced significantly lower levels of NE and EPI following stress compared with both SA men and HC females as well as a significantly dampened NE response following exposure to stressful compared with relaxing imagery. Although they produced a normal HR response to stress, they showed significantly lower HR levels compared with HC females. In relation to reduced HR response in the current SA women, the contrast with controls is important, as HC women displayed a significantly higher stress-induced HR response compared with HC men. Again, this is consistent with previous studies using healthy volunteers that have shown a tendency for females to exhibit higher basal heart rate and increased heart rate in response to acute stress compared with men (Stoney et al., 1988; Allen et al., 1993; Wright et al., 1997).
A decrease in sympathetic drive and increase in HPA axis response to stress as well as a sensitized HPA and autonomic response to cue may reflect selective neuroadaptations specific to the stress and cue-related alcohol craving state in SA women. As such, they may also have important implications regarding relapse risk. First, research from both preclinical and healthy human studies have shown that increases in sympathetic activity are a robust marker of stress and anxiety states (see Koob, (1999) for a review; Rasmussen et al., 2006). Furthermore, as the functional integrity of the autonomic system is compromised in both alcohol- and cocaine-dependent individuals (Adinoff et al., 2005; Fox et al., 2008; Sinha et al., 2008), the stress-related suppression in sympathetic regulation observed in the current SA females may represent a dysfunctional response to distress, potentially associated with increased relapse factors. For example, reduced stress-related HR response has been associated with increased alcohol craving in alcohol-dependent individuals (Sinha et al., 2008). Second, an enhanced craving and HPA axis response to stress has also been associated with relapse factors in cocaine-dependent individuals (Sinha et al., 2006). In addition, heightened cardiovascular response to cue has also been shown to accompany increases in drug craving in co-morbid cocaine- and alcohol-dependent individuals (Fox et al., 2005).
In relation to autonomic basal tone, SA women showed significantly lower SBP compared with HC females and SA males. However, these blood pressure differences were not associated with either stress or cue-related response, or drinking patterns. Although basal levels of NE in SA females were similar to those in HC females, they were higher than the levels in SA males and significantly associated with the suppressed NE levels following exposure to stress. As such, although basal NE may not be dysregulated in SA females per se, the adrenergic system may represent a vulnerability maker for dysregulated response to stress in SA women. This is also consistent with the increased levels of stress-related anxiety in both the SA and HC women. It is important to note, however, that while secondary analyses were conducted to assess the nature of gender variation in basal tone, one of the study limitations regards that fact that data were not prospective. Without prospective pre-morbid data, it is difficult to ascertain for certain whether the changes in basal stress arousal markers and stress responses are solely due to neuroadaptations associated with chronic substance abuse. Although basal EPI and SBP upregulation in SA men was shown to be significantly associated with drinking levels it may also be indicative of stress system adaptations to more severe alcohol withdrawal symptomology, especially as changes in autonomic and cardiovascular systems are characteristic of alcohol withdrawal (Kähkönen et al., 2008; Monte Secades et al., 2008).
Implications
In view of the fact that many pharmacological treatments for both alcohol and cocaine abuse target the stress and reward brain pathways, clarifying sex-specific dissociations in pituitary adrenal and sympathetic adrenal endocrine response to stress is paramount. This is particularly in view of the fact that the majority of prior research documenting high basal HPA drive and blunted ACTH and cortisol response to laboratory stressors and pharmacological challenge have been conducted in all-male alcohol-dependent samples (Errico et al., 1993; Vescovi et al., 1997; Lovallo et al., 2000; Anthenelli et al., 2001; Adinoff et al., 2005).
While our findings support this research in SA men, it is clear that SA women show a very different stress-related craving state. This may have implications for the efficacy of various pharmacological treatments in SA men and women. For example, opioid receptor antagonists such as naltrexone reduce drinking by decreasing alcohol craving, which is thought to be associated with the drug's disinhibitory effect on the HPA axis function and subsequent heightening of hypothalamic tone (Adinoff et al., 1998; King et al., 2002; O’Malley et al., 2002). A dampened HPA system in the current SA males is therefore consistent with the fact that naltrexone has demonstrated good efficacy in large multi-site treatment studies comprising predominantly male subjects (Berglund et al., 1997; Anton et al., 2006; Pettinati et al., 2006). Notably, however, an improvement in overall drinking outcomes has not been observed in a recent naltrexone trial comprising females only (O’Malley et al., 2007). In view of current findings, this may potentially be due to the fact that SA women have a sensitized rather than dampened HPA system.
Summary
This study shows that the nature of stress and cue-related relapse vulnerability in SA men and women may involve uniquely sex-specific basal and response processes. While the stress-related craving state is accompanied by a general non-specific reduction in HPA and autonomic function in SA males, stress system suppression is selectively sympathetic in SA females and accompanied by sensitized HPA activity. Similarly, while cue-related craving is associated with a general dampened autonomic response in SA males, it is associated with a non-specific enhanced autonomic function in SA females. Notably, the relapse-related hypo-response to stress and cue in SA males may also be driven by a drinking-related upregulation in basal markers. This basal profile may therefore reflect a unique sex-specific marker of relapse vulnerability particular to SA men. As studies outlining the stress and cue-related craving state in SA populations have predominantly been conducted in all-male samples, current data also emphasize the need for the over-sampling of women in treatment studies.
Acknowledgments
This study was supported in part by grants R0I-AA13892 (R.S.), K02-DA17232 (R.S.), P50-DA16556 (R.S.) and UL1 RR024139 (Yale NIH/NCRR/CTSA Program Grant). The ACTH and cortisol measures were processed at the Kreek Laboratory on the Biology of Addictive Diseases at the Rockefeller University (P60-DA 05130) and the peripheral catecholamine assays were conducted in the Anderson Laboratories at the Yale Child Study Center. We also wish to thank the staff at the Clinical Neuroscience Research Unit and the Hospital Research Unit of the Yale Center for Clinical Investigation (YCCI) at the Yale University School of Medicine for their assistance in completing this study.
References
- Adinoff B, Iranmanesh A, Veldhuis J, et al. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, et al. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–5. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MT, Stoney CM, Owens JF, et al. Hemodynamic adjustments to laboratory stress: the influence of gender and personality. Psychosom Med. 1993;55:505–17. doi: 10.1097/00006842-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Maxwell RA, Geracioti TD, Jr, et al. Stress hormone dysregulation at rest and after serotonergic stimulation among alcohol-dependent men with extended abstinence and controls. Alcohol Clin Exp Res. 2001;25:692–703. [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, et al. COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, et al. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–76. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Berglund M, Balldin J, Bendtsen P, et al. Pharmacological treatment of alcohol dependence. Acamprosate and naltrexone offer new approach. Lakartidningen. 1997;94:2645–8. [PubMed] [Google Scholar]
- Brady KT, Waldrop AE, McRae AL, et al. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J Stud Alcohol. 2006;67:700–6. doi: 10.15288/jsa.2006.67.700. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29:185–95. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices.A national study of drinking behaviors and attitudes. Monographs of the Rutgers Center of Alcohol Studies. 1969;6:260. [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, et al. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–50. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, et al. An assessment of hypothalamo–pituitary–adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–75. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology. 2002;27(3):442–52. doi: 10.1016/S0893-133X(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, et al. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol. 1993;54:393–8. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for the DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0, 4/97 revision) New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- Fox HC, Berquist KL, Hong KI, et al. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M, Kemp K, et al. Gender differences in cardiovascular and corticoadrenal response to stress and drug-cue in cocaine dependent individuals. Psychopharmacology. 2006;185:348–57. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Siedlarz KM, et al. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17:103–19. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, et al. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and to drug-related cues. Psychoneuroendocrinology. 2005;30:880–91. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M, Dunne E, Lundberg U. Sex differences in sympathetic adrenal medullary reactions induced by different stressors. Psychopharmacology. 1976;47:1–5. doi: 10.1007/BF00428693. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M, von Wright MR, Collins A, et al. Sex differences in psychoneuroendocrine reactions to examination stress. Psychosom Med. 1978;40:334–43. doi: 10.1097/00006842-197806000-00006. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Lê ACD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–43. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Hawley RJ, Nemeroff CB, Bissette G, et al. Neurochemical correlates of sympathetic activation during severe alcohol withdrawal. Alcohol Clin Exp Res. 1994;18:1312–6. doi: 10.1111/j.1530-0277.1994.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Hearn WL, Rose S, Wagner J, et al. Cocaethylene is more potent than cocaine in mediating lethality. Pharmacol Biochem Behav. 1991;39:531–3. doi: 10.1016/0091-3057(91)90222-n. [DOI] [PubMed] [Google Scholar]
- Jatlow P, McCance EF, Bradberry CW, et al. Alcohol plus cocaine: the whole is more than the sum of its parts. Ther Drug Monit. 1996;18:460–4. doi: 10.1097/00007691-199608000-00026. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, et al. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–5. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Bondarenko BB, Lipsanen J, et al. Effects of verapamil, an antagonist of L-type calcium channels, on cardiovascular symptoms in alcohol withdrawal. Neuropsychobiology. 2008;58:123–7. doi: 10.1159/000170393. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Poon L, Papadopoulos AS, et al. Salivary cortisol measurements during a medically assisted alcohol withdrawal. Addict Biol. 2001;6:247–56. doi: 10.1080/13556210120056580. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, et al. Hypothalamic–pituitary–adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002;26:778–88. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, et al. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 1999;61:154–62. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–57. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–80. doi: 10.1016/s0006-3223(99)00164-x. Review. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27(8):739–49. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer J, Hellhammer DH, et al. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. J Clin Endocrinol Metab. 1998;83:1756–61. doi: 10.1210/jcem.83.5.4758. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- Lang AR, Searles J, Lauerman R, et al. Expectancy, alcohol, and sex guilt as determinants of interest in and reaction to sexual stimuli. J Abnorm Psychol. 1980;89:644–53. doi: 10.1037//0021-843x.89.5.644. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Mefford I, Nutt D, et al. NIH conference. Alcohol withdrawal and noradrenergic function. Ann Intern Med. 1987;107:875–89. doi: 10.7326/0003-4819-107-6-875. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, et al. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–8. [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M. Stress and workload of men and women in high-ranking positions. J Occup Health Psychol. 1999;4:142–51. doi: 10.1037//1076-8998.4.2.142. [DOI] [PubMed] [Google Scholar]
- McCance-Katz M, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. Am J Addict. 1999;8:300–11. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–8. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Miller G, Levin DN, Kozak MJ, et al. Individual differences in imagery and the psychophysiology of emotion. Cogn Emotion. 1987;1:367–90. [Google Scholar]
- Monte Secades R, Casariego Vales E, Pértega Díaz S, et al. Clinical course and features of the alcohol withdrawal syndrome in a general hospital. Rev Clin Esp. 2008;208:506–12. doi: 10.1157/13128675. Spanish) [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, et al. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Sinha R, Grilo CM, et al. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: a randomized controlled trial. Alcohol Clin Exp Res. 2007;31:625–34. doi: 10.1111/j.1530-0277.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26:610–25. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2007;322:894–902. doi: 10.1124/jpet.107.121806. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during “abstinence”. Alcohol. 2006;38:173–7. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic–pituitary–adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–31. [PubMed] [Google Scholar]
- Saleh TM, Connell BJ. Central nuclei mediating estrogen-induced changes in autonomic tone and baroreceptor reflex in male rats. Brain Res. 2003;961:190–200. doi: 10.1016/s0006-8993(02)03928-8. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–43. Review. [PubMed] [Google Scholar]
- Seeman TE, Singer B, Wilkinson CW, et al. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–40. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–95. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2008;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, et al. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34(5):1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic–pituitary–adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, et al. Hypothalamic–pituitary–adrenal axis and sympatho–adreno–medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14(2):119–29. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, et al. Blunted hypothalamic–pituitary–adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59:210–7. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoney CM, Matthews KA, McDonald RH, et al. Sex differences in lipid, lipoprotein, cardiovascular, and neuroendocrine responses to acute stress. Psychophysiology. 1988;25:645–56. doi: 10.1111/j.1469-8986.1988.tb01902.x. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–89. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, DiGennaro C, Coiro V. Hormonal (ACTH, cortisol, beta-endorphin, and met-enkephalin) and cardiovascular responses to hyperthermic stress in chronic alcoholics. Alcohol Clin Exp Res. 1997;21:1195–8. [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic–pituitary–adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–5. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wright RA, Murray JB, Storey PL, et al. Ability analysis of gender relevance and sex differences in cardiovascular response to behavioral challenge. J Pers Soc Psychol. 1997;73:405–17. doi: 10.1037//0022-3514.73.2.405. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Basler HD, Vedder H, et al. Sex differences in cortisol response to noxious stress. Clin J Pain. 2003;19:233–9. doi: 10.1097/00002508-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Zimmerman U, Spring K, Koller G, et al. Hypothalamic–pituitary–adrenal system regulation in recently detoxified alcoholics is not altered by one week of treatment with acamprosate. Pharmacopsychiatry. 2004;37:98–102. doi: 10.1055/s-2004-818986. [DOI] [PubMed] [Google Scholar]