Abstract
Aims: The study used an animal model of fetal alcohol spectrum disorders (FASD) to investigate the impact of alcohol exposure during a period equivalent to all three trimesters in humans on social recognition memory. It was hypothesized that the effects on specific aspects of social recognition memory would be sexually dimorphic. Methods: This study exposed rats to ethanol during both the prenatal and early postnatal periods. Two control groups included a group exposed to the administration procedures but not ethanol and a non-treated group. At ∼90 days, all rats were tested repeatedly in a test of social recognition memory with a juvenile animal of the same sex. Experimental rats of both sexes were allowed to investigate an unknown juvenile for either 2, 3 or 5 min and then, after a delay of 30, 60, 120 and 180 min, were allowed to investigate the same juvenile for 5 min. Results: Male rats investigated the juvenile for much longer than female rats. Ethanol-exposed male rats showed a deficit in recognition memory that was evident with longer delays when the initial investigation time was either 2- or 3-min long. In contrast, ethanol-exposed female rats showed a deficit in recognition memory only when the initial investigation period was of 2 min. Measurement of oxytocin receptor binding in the amygdala region indicated that ethanol exposure lowered oxytocin receptor binding in females but not males. Conclusions: The results suggest that ethanol exposure during development caused a deficit in memory duration but not encoding in males and a deficit in encoding but not memory duration in females. The deficit in ethanol-exposed females may be related to changes in oxytocin receptors in the amygdala.
INTRODUCTION
Fetal alcohol spectrum disorders (FASD) can occur in the offspring of women who drink heavily during pregnancy and can include a wide array of behavioral and cognitive deficits (Streissguth et al., 1980; 1994). Social behavior in individuals with FASD has been shown to be disrupted to a greater degree than can be explained by a reduction in the IQ (Thomas et al., 1998) and may contribute to the high prevalence of secondary disabilities such as trouble with the law, juvenile delinquency and depression (Streissguth et al., 1996; Streissguth and O’Malley, 2000). Animal models of FASD have also shown that social behavior is disrupted by alcohol exposure during development and that this effect is due to the teratogenic effects of alcohol (Kelly et al., 2000). While social behavior is very complex in humans, it is possible to use animal models to investigate the nature and neural basis of deficits in the social behavior induced by alcohol exposure during development.
Social recognition memory has been shown to be impaired in both sexes by alcohol exposure during development (Kelly and Tran, 1997). While the impact of alcohol is to the same degree in both sexes, social recognition memory is clearly sexually dimorphic with respect to behavior (Thor and Holloway, 1982; Hliñák, 1993) and also the neural bases (Bluthé et al., 1990; van Wimersma Greidanus and Maigret, 1996). Adult male rats investigate a juvenile for longer than female adult rats yet females tend to have a longer lasting memory of the juvenile. The social recognition memory in males has been shown to be dependent upon the vasopressin fibers in the bed nucleus of the stria terminalis (BNST) and these fibers are testosterone dependent. Social recognition memory in females has not been as well studied but has been suggested to be dependent upon oxytocin and estrogen levels (Hliñák, 1993; van Wimersma Greidanus and Maigret, 1996). Given the behavioral and neural differences in the social recognition memory between the sexes and given that alcohol exposure during development has been shown to reduce testosterone in males and estrogen in females, it is quite likely that the specific manner in which alcohol exposure during development impacts social recognition memory in the two sexes may differ.
In order to determine the behavioral mechanism underlying alcohol-induced impairment in social recognition memory in the two sexes, aspects of the behavioral test are manipulated systematically. Tests of social recognition memory involve an initial investigation period and a delay period between the two presentations of the same juvenile. The task involves encoding the sensory aspects of the juvenile, mostly using the olfactory sense (Thor and Holloway, 1982; Burman and Mendl, 2002), and then forming and retrieving a memory. Clearly, sensory processing is occurring during the first investigation period, typically 5 min (Thor and Holloway, 1982; Bluthé et al., 1990; Kelly and Tran, 1997), and if this time is shortened, the challenge to the sensory system to process the information is increased. The longer the delay, the greater the challenge to the associative memory system. Manipulation of the initial investigation time affects the amount of sensory processing of the juvenile odor whereas manipulation of the delay should result in a detection of the strength of the associative memory of the juvenile. Both associative strength (Gallo and Weinberg, 1982; Gentry and Middaugh, 1988; Stanton and Goodlett, 1998) and the processing of sensory cues can be impaired by ethanol exposure during development and thus, it is possible that impairments in both processes are disrupted during social recognition memory.
The purpose of this study was to examine the behavioral mechanisms underlying deficits in social recognition memory in rats of both sexes exposed to alcohol during development. In addition, this study will also begin to identify the neural correlates to social recognition memory deficit in alcohol-exposed rats. Oxytocin has been suggested to be involved in social recognition memory at least in females (Hliñák, 1993; van Wimersma Greidanus and Maigret, 1996) and it is also involved in other social behaviors, such as maternal behavior, female sexual behavior and social attachment (Insel, 1992; Nelson and Panksepp, 1998; Cushing and Carter, 1999; Pfaff et al., 1999), that have been shown to be disrupted by ethanol exposure during development (Barron and Riley, 1985; Wilson et al., 1996; Marino et al., 2002; Lugo et al., 2003; Gass et al., 2007). Given the importance of oxytocin in social behaviors in general and the strong impact that alcohol exposure during development has on social behaviors both in animal models (for a review, see Kelly et al. (2000)) and in the clinical syndrome (Thomas et al., 1998), we chose to examine oxytocin receptor binding in neural tissue from the amygdala region in order to begin to identify correlates to the social recognition memory deficits, and possibly other social deficits, in alcohol-exposed rats.
METHODS
Subjects
All procedures and treatments were approved by the Institutional Animal Care and Use Committee of the University of South Carolina and in accord with guidelines from the National Institutes of Health. Experimental rats were produced by breeding Long-Evans rats (Harlan) in the animal quarters in the Department of Psychology. The room was maintained at 22°C and the light–dark cycle is a 12-h cycle with the lights on at 07:00 h. Rats were fed standard rat chow pellets (Tekland). Sexually naive female Long-Evans rats were housed with an experienced male overnight in cages with wire mesh floors. When a vaginal smear indicated the presence of sperm on the following morning, the female was housed singly in a polypropylene cage with wood shavings. The day of positive smear was designated as gestational day (GD) 1.
On GD 1, the fetuses in the dams were randomly assigned to one of three groups. The ET group was exposed to ethanol from GD 1 to 22 and PD 2 to 10; the IC group was exposed to the administration procedures and pair-fed to the ET group; the NC group was weighed daily and not treated in any other manner. The dams of the fetuses exposed to ethanol were intubated intragastrically (i.g.) with 4.5 g/kg of ethanol in a 20.0 mL/kg volume of water from gestational day 1 through 22. This procedure produces peak blood alcohol concentrations between 300 and 330 mg/dL in the dams (Marino et al., 2002). Each dam of the IC group was matched to a similar weight (±3 g) dam of the ET group and only allowed as much rat chow as its matched dam consumed on a particular gestational day. The IC dams were intubated daily with a maltose–dextrin solution (20 mL/kg) made isocaloric with the 4.5 g/kg ethanol solution. Treatments of the dams of the ET and IC groups were continued up to but did not include the day of birth (typically GD 23). The dams of the final group of fetuses (NC group) were allowed access to lab chow and water ad libitum throughout pregnancy and were not intubated.
The day of birth was designated as postnatal day (PD) 1. On PD 2, the litter was culled to four of each sex, when possible. All rat pups were marked with nontoxic marker until PD 10 and paw-marked with India ink (Geller and Geller, 1966) on PD 10. Postnatal treatments began on PD 2. Every day at approximately 14:00 h, experimental rat pups were removed from the dam one at a time, weighed and intubated i.g. if appropriate for that pup. This process took less than 2 min and care was taken to disrupt the litter and dam as little as possible. The intubation process involved dipping a PE 10 tube into corn oil for lubrication and then inserting it down the esophagus until the end of the tube was in the stomach. Rat pups from the ET group was given 3.0 g/kg of body weight of ethanol, respectively, administered in milk formula in a volume of 27.8 mL/kg once daily (Kelly and Tran, 1997) via the PE 10 tube. Two hours later, ET rat pups were intubated with a volume (27.8 mL/kg) of milk solution without the ethanol. This second intubation of milk ensures adequate nutrition during the postnatal period (Kelly and Tran, 1997; Tran et al., 2000). This ethanol exposure paradigm produces peak blood alcohol concentrations between 300 and 330 mg/dL during the postnatal period (Marino et al., 2002). Rat pups from the IC group were subjected to the intubation procedures at both times but not given any milk formula or ethanol. (If milk is given to the IC group, they overeat and become much heavier than the NC group (pilot data from this lab and Goodlett and Johnson (1997)). The intubations were done on postnatal day 2 through postnatal day 10. Rats from the NC dams were weighed at 14:00 h on postnatal day 2 through 10 (which takes less than 30 s) but they were not treated in any way. All rats were weaned at 21 days and housed in same-sex littermates in polycarbonate cages.
Social recognition memory
Testing began on PD 90 and female rats were tested only when in diestrus as verified by vaginal smear. There were 3 days of testing (not necessarily consecutive) and on each day of testing, there were four tests of social recognition memory. Each test consisted of a first session of either 2, 3 or 5 min followed by a delay, and then a second session of 5 min. For the first session, the experimental adult rat was placed in a cage containing fresh bedding and then exposed to a novel juvenile rat of the same sex (21- to 30-day old) for either 2, 3 or 5 min in the first session and then returned to its home cage. After a delay, the same juvenile was used in the second session. Each test was conducted with differing lengths of the first session (2, 3 and 5 min) and delays (30, 60, 120 and 180 min) and the second session was always of 5 min. The various delays and first investigation periods were counter-balanced across and within groups and across days.
On the third day of testing, a control test was conducted after the four tests of social recognition memory. The control test was the same as the tests for social recognition memory except that the juvenile stimulus animal in the second session was different than that in the first period; this control condition would detect any differences in habituation or sensitization to the test situation among groups. The control condition used 5-min first and second sessions and a delay of 60 min.
For all sessions in both the tests of social recognition memory and the control test, investigation time in seconds, which includes both sniffing and grooming the juvenile rat, was recorded by a stop watch. The raw investigation times for each session were analyzed for the control test and for the first session in the tests of social recognition memory. The investigation ratio for social recognition memory was calculated as the investigation time in the second session divided by the average across the two sessions in the control condition for that specific animal. This approach was necessary since the durations of the first and second sessions were not necessarily the same in the tests of social recognition memory and the ratio allowed control over individual variability in investigation regardless of session.
Oxytocin receptor binding
Brains were removed from the animals between 90 and 120 days of age. The amygdala from both sides were dissected free and combined, and immersed into cold (4°C) 0.32 M sucrose. Tissue was only removed from females when they were in diestrus as verified through vaginal smear. The tissue was homogenized and centrifuged at 1000 g for 20 min. The supernatant was removed and centrifuged at 30,000 g for 20 min. The resulting pellet was sonicated in 1 mL of buffer, which was 50 mM Tris–HCl (pH 7.4) containing 0.5% BSA, 0.1% bacitracin and 5 mM MgSo4, and then spun at 30,000 g for 20 min. The pellet was suspended in 140 μL of the buffer. Then, 20 μL of this solution was added into 60 μL of the buffer containing 0.25 nM of oxytocin receptor antagonist d(CH2)5[Tyr(Me)2,Thr4,Tyr-NH29]OVT (OTA) labeled with 125I and 60 μL of buffer. Nonspecific binding was assayed by adding 10 μM of oxytocin (Sigma) to the tubes. The tubes were incubated for 30 min at 30°C and then bound radioactivity was separated from free radioactivity by filtration through Gelman GA-3 (Metricel, 1.2 μm) filters. The filter papers were counted and specific binding was calculated. Protein was assayed via the method of Lowry (Lowry et al., 1951). Receptor binding was expressed as fmoles/mg of protein.
RESULTS
Subjects
Only one male and one female per litter were used in the test of social recognition memory and in the oxytocin receptor-binding assay; remaining animals in the litters were assigned to different studies. Independent groups of animals were used for the behavioral testing and receptor assay. Subject numbers are indicated in the figure legends (Figs. 1 and 4). Analysis of body weight of dams indicated that while all dams gained weight over gestation, there was no difference in weight gain due to treatment (data not shown). A repeated measure ANOVA on body weights of the offspring on PD 2 through 10 and PD 90 indicated that body weights increased over development (main effect of days: F (9, 648) = 6868, P < 0.001) and that sex differences in body weights increased over days (interaction between sex and days: F (9, 648) = 373, P < 0.001). While there were no significant interactions of treatment group with days or sex, there was a main effect of group (F (2, 79) = 3.2, P < 0.05). However, post hoc tests (Tukey's HSD) failed to detect any significant specific effects among groups (see Table 1).
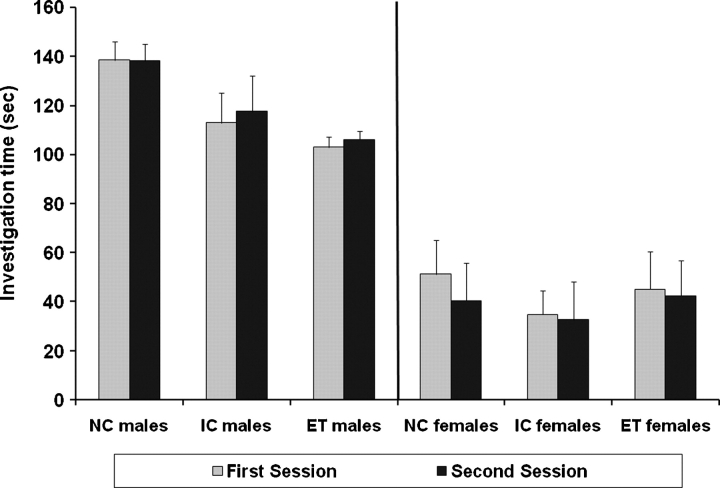
Fig. 1.
Mean investigation time in first and second sessions in the control condition. The juveniles used in the first and second sessions are different and thus, this is not a test of memory. Error bars represent the standard errors of the mean (SEMs). Subject numbers were 11 males and 11 females in the ET group, 12 males and 14 females in the IC group, and 15 males and 15 females in the NC group.
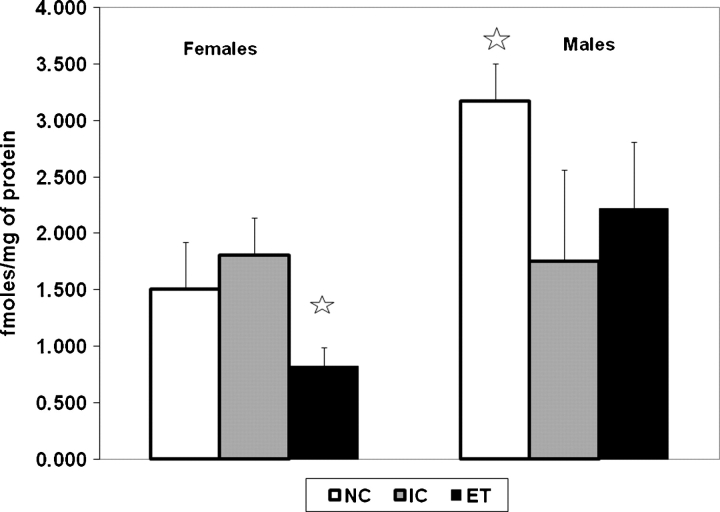
Fig. 4.
Mean oxytocin receptor binding in the amygdala region. Error bars represent SEMs. The stars indicate significant differences from the other two groups within that sex. Subject numbers were 9 males and 9 females in the ET group, 8 males and 10 females in the IC group, and 8 males and 7 females in the NC group.
Table 1.
Mean body weights (±SEMs) of experimental subjects
| ET group | IC group | NC group | ||||
|---|---|---|---|---|---|---|
| Postnatal day | Males | Females | Males | Females | Males | Females |
| 2 | 6.25 ± 0.17 | 6.08 ± 0.22 | 6.90 ± 0.20 | 6.62 ± 0.18 | 7.30 ± 0.25 | 6.81 ± 0.20 |
| 3 | 7.17 ± 0.24 | 6.93 ± 0.26 | 7.93 ± 0.26 | 7.61 ± 0.20 | 7.76 ± 0.39 | 7.87 ± 0.25 |
| 4 | 8.22 ± 0.25 | 7.81 ± 0.29 | 9.42 ± 0.31 | 8.78 ± 0.26 | 9.36 ± 0.38 | 9.08 ± 0.26 |
| 5 | 9.33 ± 0.31 | 8.94 ± 0.34 | 10.9 ± 0.36 | 10.1 ± 0.28 | 11.0 ± 0.45 | 10.5 ± 0.30 |
| 6 | 10.4 ± 0.30 | 10.1 ± 0.38 | 12.5 ± 0.41 | 11.6 ± 0.31 | 12.4 ± 0.52 | 12.1 ± 0.39 |
| 7 | 11.8 ± 0.32 | 11.7 ± 0.37 | 14.1 ± 0.47 | 13.0 ± 0.37 | 14.0 ± 0.61 | 13.7 ± 0.44 |
| 8 | 13.6 ± 0.41 | 13.3 ± 0.46 | 15.9 ± 0.50 | 14.4 ± 0.43 | 15.2 ± 0.70 | 15.4 ± 0.41 |
| 9 | 15.5 ± 0.54 | 15.3 ± 0.55 | 17.6 ± 0.60 | 16.5 ± 0.50 | 17.3 ± 0.78 | 17.3 ± 0.51 |
| 10 | 17.6 ± 0.66 | 17.3 ± 0.66 | 19.5 ± 0.68 | 18.2 ± 0.57 | 19.0 ± 0.83 | 19.3 ± 0.57 |
| 90 | 398.3 ± 6.7 | 249.2 ± 4.9 | 422.3 ± 6.8 | 254.7 ± 6.1 | 401.9 ± 11 | 268.5 ± 13 |
Social recognition memory
Analysis of the investigation times of the control test revealed a large effect of sex (F (1, 79) = 89.1, P < 0.001) because males investigated more than females (see Fig. 1). However, there were no differences in investigation times across groups or sexes or between the first and second sessions in the control test (see Fig. 1).
In order to be sure that there were no effects of order or day of testing, the data were collapsed across group and sex and analyzed with either order of tests or day of testing as a between factor. Neither of these analyses revealed any effects of day or order of testing.
In order to be sure that there were no differences among groups in the amount of time spent on investigation in the first session of the tests of social recognition memory, an analysis of the investigation times (seconds) in the first session was conducted with group and sex as between factors and duration of the first session (2, 3 and 5 min) as the repeated measure. Males spent more time investigating the juvenile during the first session than females (F (1, 79) = 207.0, P < 0.001) and the investigation times increased with increasing session length (F (2, 158) = 124.1, P < 0.001). An interaction between sex and the duration of the first session (F (4, 158) = 36.7, P < 0.001) was due to a greater increase in the investigation time with increasing session duration in males than females. Importantly, there were no differences among groups with respect to time spent investigating in the first session regardless of the duration of the first session (data not shown).
Examination of the data revealed a high amount of variability among the male groups, particularly the ET males when the first session was of 3 min. Because increased variability in ethanol-treated subjects is expected, a conservative approach to the outlier analysis was taken. The analysis for extreme outliers (Explore function, SPSS 17.0) (defined as a data point whose distance from the nearest quartile is three times the interquartile range) found one particular ET male whose data points were extreme outliers for the 60, 120 and 180 min delays when the duration of the first session was of 3 min. These data points (a total of three) were discarded from the analyses and replaced with the group mean.
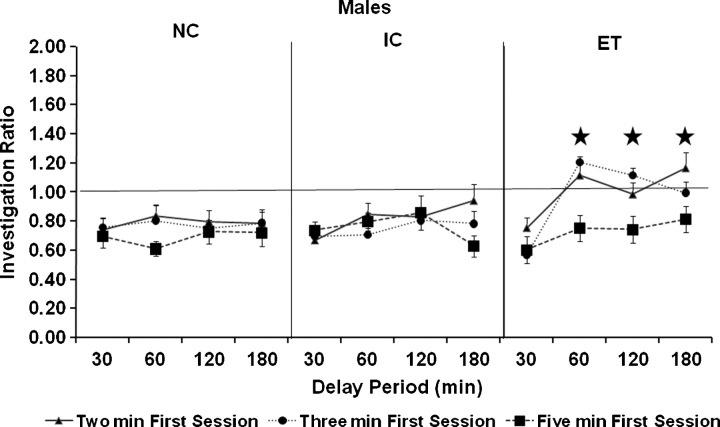
Because there were a priori reasons to expect substantial sex differences, the sexes were analyzed separately. A repeated measures ANOVA with group as a between factor and duration of first session and duration of delay as repeated measures on the ratios of the males in the social recognition test revealed main effects of duration of the first session (F (2, 79) = 5.96, P < 0.01) and duration of delay (F (3, 120) = 5.0, P < 0.01) and an interaction of duration of delay and group (F (6, 120) = 2.19, P < 0.05). The interaction of the duration of delay and group was because, when the data were collapsed across the duration of the first session, the ET males showed a higher ratio (indicating impaired memory) at delays of 60, 120 and 180 min than either the IC or NC males (Ps < 0.05, Tukey's HSD) (see Fig. 2). It should be noted that while the three-way interaction between duration of first session, duration of delay and group was not significant, the effects of duration of delay are much more prominent when the duration of the first session was of either 2 or 3 min.
Fig. 2.
Mean investigation ratio in males. Error bars represent SEMs. Stars indicate the delay intervals where the ratio of the ET males was greater than both the NC and IC males, regardless of the duration of the first session. This effect was most prominent when the duration of the first session was 2 or 3 min.
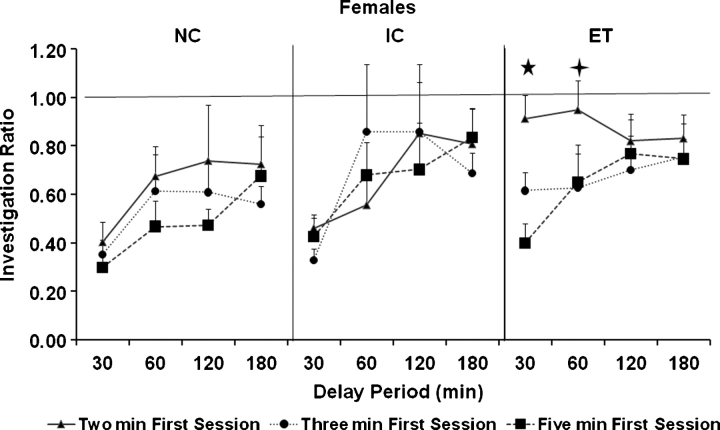
A repeated measures ANOVA with group as a between factor and duration of session and duration of delay as repeated measures on the ratios of the females in the social recognition tests revealed main effects of duration of first session (F (2, 74) = 6.4, P < 0.01) and duration of delay (F (3, 111) = 6.2, P < 0.001) and a three-way interaction among duration of first session, duration of delay and group (F (12, 222) = 7.3, P < 0.05). The three-way interaction was because, when the duration of the first session was of 2 min, ET females showed a higher ratio (indicating impaired memory) at the 30 and 60 min delay compared to the IC females and at the 30 min delay compared to the NC females (Ps < 0.05, Tukey's HSD) (see Fig. 3).
Fig. 3.
Mean investigation ratio in females. Error bars represent SEMs. The five-point star indicates that delay interval where the ratio of the ET females was greater than both the NC and IC females when tested with a first session duration of 2 min. The four-point star indicates that delay interval where the ratio of the ET females was greater than the IC females only when tested with the first session duration of 2 min.
Oxytocin receptor binding
An ANOVA with group and sex as between factors was conducted on oxytocin receptor binding in the amygdala region and we found a significant interaction between group and sex (F (2, 45) = 6.43, P < 0.01) (see Fig. 4). ET females had lower oxytocin receptor binding compared to both IC and NC controls that did not differ from each other (Ps < 0.05, Tukey's HSD). In contrast, the oxytocin receptor binding is reduced in both the IC and ET male rats compared to the NC males (Ps < 0.05, Tukey's HSD), indicating an effect of the administration procedure. There was an overall gender difference in oxytocin receptor binding that is greater in male than in female rats in the amygdala region (F (1, 45) = 5.23, P < 0.05).
DISCUSSION
Ethanol exposure during development impaired social recognition memory in a sexually dimorphic manner. Social recognition memory in males was impaired by ethanol exposure during long delays particularly when the duration of the first session was 2–3 min. In contrast, social recognition memory in females was impaired by ethanol exposure only when the first investigation period was short and only with short delays (2 min). Since all groups of animals within each sex investigated for equal amounts on time during the control condition, these findings cannot be attributed to differences in reactivity to the presentation of the juvenile during the second period and must be attributed to some deficit in the formation of the social recognition memory itself. Oxytocin receptor binding in the amygdala region was reduced by ethanol exposure during development in females only.
The formation of social recognition memory is clearly a sexually dimorphic process with dramatic differences in the total investigation time between the sexes and a process that is clearly testosterone dependent in males (Bluthé et al., 1990). It has been shown that testosterone levels are reduced in males exposed to ethanol during development (McGivern et al., 1988; Lugo et al., 2006) and this may provide the key alteration that underlies the deficits in the formation of social recognition memory. The current behavioral results in males suggest that alcohol exposure does not impact the ability to encode the sensory information of the juvenile but instead most strongly impacts the length of time that the memory lasts. It would be of interest to examine the impact of ethanol exposure during development on the vasopressin fibers in the bed nucleus of the stria terminalis (Bluthé et al., 1990), which have been shown to be testosterone dependent (de Vries and Panzica, 2006), and also the impact of testosterone supplementation on the behavior and brain of ethanol-exposed males.
In females, the behavioral deficit was only found when the first investigation time was limited to 2 min and the delay was short. This finding suggests that, while ethanol exposure did not impact the length of time the recognition memory lasts, there was an impact on the ability to encode the sensory information of the juvenile when the interval was short and perhaps there was an impact on short-term rather than long-term memory. Other studies have found that ethanol exposure during development impairs the ability to encode sensory information specifically related to social behavior (Gass et al., 2007; Lawrence et al., 2007) and this may be another example of such an impairment. The finding of ethanol-induced reduction in oxytocin receptor binding in the amgydala is only found in females. Oxytocin receptors have also been shown to be dependent upon estrogen levels (Champagne et al., 2001) and estrogen levels have been shown to be reduced in ethanol-exposed females (Wilson et al., 1996). Given that both estrogen and oxytocin have been suggested to underlie social recognition memory in females (Hliñák, 1993; van Wimersma Greidanus and Maigret, 1996), there may be a link between these receptor and hormone levels and the behavioral deficit observed in the current study.
Ethanol exposure during development lowered the weight of the offspring both on postnatal day 2 and 10, a finding that has been reported before and can be attributed to effects of ethanol during gestational days 11–22 (Tran et al., 2000). Importantly, this reduction in weight is no longer detectable by adulthood suggesting catch-up growth and the inability to attribute behavioral differences among adult groups to reduced body weight. The finding of greater oxytocin receptor binding in the amygdala region in males than that in females was not expected. However, given that there may be cross-reactivity between oxytocin and vasopressin receptors in this type of assay (Chini et al., 2008) and that the dissection of the amygdala region may have included the adjacent tissue (which could vary across groups if alcohol impacts the overall volume of the amygdala), it is important that these results be interpreted cautiously. It will be necessary to follow-up these findings with a study using autoradiography and ligands for both oxytocin and vasopressin receptors. Nevertheless, given the importance of both neuropeptides in complex social behavior (Donaldson and Young, 2008) and the impact of ethanol exposure during development on complex social behavior (Kelly et al., 2000), the link between these systems and behavioral deficits in FASD should be elucidated. Importantly, these findings suggest that the neural and behavioral deficits induced by ethanol exposure during development may be different between the sexes.
Acknowledgments
This research was funded by NIAAA grant AA1566 to S.J.K. We would like to thank Dr Marlene A. Wilson for consulting on the receptor binding assay and Taylor Alexander for help with the behavioral testing.
References
- Barron S, Riley EP. Pup-induced maternal behavior in adult and juvenile rats exposed to alcohol prenatally. Alcohol Clin Exp Res. 1985;9:360–5. doi: 10.1111/j.1530-0277.1985.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Schoenen J, Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990;519:150–7. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Burman OHP, Mendl M. Recognition of conspecific odors by laboratory rats (Rattus norvegicus) does not show context specificity. J Comp Psychol. 2002;116:247–52. doi: 10.1037/0735-7036.116.3.247. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Diorio J, Sharma S, et al. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci. 2001;98:12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Manning M, Guillon G. Affinity and efficacy of selective agonists and antagonists for vsopressin and oxytocin receptors: an “easy guide” to receptor pharmacology. Prog Brain Res. 2008;170:513–7. doi: 10.1016/S0079-6123(08)00438-X. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Prior exposure to oxytocin mimics the effects of social contact and facilitates sexual behaviour in females. J Neuroendocrinol. 1999;11:765–9. doi: 10.1046/j.1365-2826.1999.00382.x. [DOI] [PubMed] [Google Scholar]
- de Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–55. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Neuromotor development and response inhibition following prenatal ethanol exposure. Neurobehav Toxicol Teratol. 1982;4:505–13. [PubMed] [Google Scholar]
- Gass JT, Jenkins WJ, Marino MD, et al. Alcohol exposure during development: analysis of effects on female sexual behavior. Alcohol Clin Exp Res. 2007;31:2065–72. doi: 10.1111/j.1530-0277.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- Geller LM, Geller EH. A simple technique for the permanent marking of newborn rats. Psychol Rep. 1966;18:221–2. doi: 10.2466/pr0.1966.18.1.221. [DOI] [PubMed] [Google Scholar]
- Gentry GD, Middaugh LD. Prenatal ethanol weakens the efficacy of reinforcers for adult mice. Neurotoxicol Teratol. 1988;37:135–44. doi: 10.1002/tera.1420370206. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson RB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–46. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Hliñák Z. Social recognition in ovariectomized and estradiol-treated female rats. Horm Behav. 1993;27:159–66. doi: 10.1006/hbeh.1993.1012. [DOI] [PubMed] [Google Scholar]
- Insel TR. Oxytocin—a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–9. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Tran TD. Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol Teratol. 1997;19:383–9. doi: 10.1016/s0892-0362(97)00064-0. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Bonner HC, Newsom RJ, et al. Effects of alcohol exposure during development on play behavior and c-Fos expression in response to play. Behav Brain Res. 2007;188:209–18. doi: 10.1016/j.bbr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OJ, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Lugo JN, Jr, Marino MD, Cronise K, et al. Effects of alcohol exposure during development on social behavior in rats. Physiol Behav. 2003;78:185–94. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- Lugo JN, Jr, Marino MD, Gass JT, et al. Ethanol exposure during development reduces resident aggression and testosterone in rats. Physiol Behav. 2006;87:330–7. doi: 10.1016/j.physbeh.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MD, Cronise K, Lugo JN, Jr, et al. Ultrasonic vocalizations and maternal–infant interactions in a rat model of fetal alcohol syndrome. Dev Psychobiol. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Raum WJ, Salido E, et al. Lack of prenatal testosterone surge in fetal rats exposed to alcohol: alterations in testicular morphology and physiology. Alcohol Clin Exp Res. 1988;12:243–7. doi: 10.1111/j.1530-0277.1988.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant–mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–52. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Ogawa S, Kow LM. Neural oxytocinergic systems as genomic targets for hormones and as modulators of hormone-dependent behaviors. Results Probl Cell Differ. 1999;26:91–105. doi: 10.1007/978-3-540-49421-8_5. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res. 1998;22:270–5. [PubMed] [Google Scholar]
- Streissguth AP, Barr AM, Kogan FL, et al. Understanding the occurrence of secondary disabilities in clients with Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Effects (FAE) 1996. Final Report to the Centers for Disease Control and Prevention (CDC). Seattle, University of Washington, Fetal Alcohol and Drug Unit.
- Streissguth AP, Barr HM, Sampson PD, et al. Prenatal alcohol and offspring development: the first fourteen years. Drug Alcohol Depend. 1994;36:89–99. doi: 10.1016/0376-8716(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Landesman-Dwyer S, Martin JC, et al. Teratogenic effects of alcohol in humans and laboratory animals. Science. 1980;209:353–61. doi: 10.1126/science.6992275. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, O’Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 2000;5:177–90. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, et al. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–33. [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Social memory in the male laboratory rat. J Comp Physiol Psychol. 1982;96:1000–6. [Google Scholar]
- Tran TD, Cronise K, Marino MD, et al. Critical periods for the effects of alcohol exposure on brain weight, body weight, activity, and investigation. Behav Brain Res. 2000;116:99–110. doi: 10.1016/s0166-4328(00)00263-1. [DOI] [PubMed] [Google Scholar]
- van Wimersma Greidanus TB, Maigret C. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 1996;713:153–9. doi: 10.1016/0006-8993(95)01505-1. [DOI] [PubMed] [Google Scholar]
- Wilson JH, Kelly SJ, Wilson MA. Early postnatal alcohol exposure in rats: maternal behavior and estradiol levels. Physiol Behav. 1996;59:287–93. doi: 10.1016/0031-9384(95)02094-2. [DOI] [PubMed] [Google Scholar]