Abstract
We previously reported that catechins of green tea have different antiproliferative effects on cell lines derived from gender-dependent cancers; epicatechin 3-gallate (ECG) had the strongest inhibitory effect. In the present study, we examined the effects of epigallocatechin (EGC), epicatechin-gallate (ECG) and EGC 3-gallate (EGCG) on the viability, density, doubling time and cycle number of cell lines derived from melanoma metastasized to lymph nodes (MB-1133 and SE-0154) or distant organs (CH-0356, JK-0346, SA-1171, GE-0208, NS-1176 and LF-0023). These catechins have been documented to have no growth suppressive or apoptotic effects on normal melanocytes (Nihal et al., Int J Cancer 2005;114:513–21). EGCG (50 μM) showed greater inhibitory potency than EGC (50 μM) in SE-0154, NS-1176, GE-0208 and LF-0023 cell lines but the two catechins produced similar inhibitory effects in CH-0356, JK-0346 and SA-1171 cell lines. The IC50 (50% inhibitory concentration) was lower for EGC than EGCG in MB-1133 and CH-0356 cells, higher for EGC than EGCG in GE-0208 cells and comparable (11–12 μM) for both the catechins in LF-0023 cells. When compared with EGC, the cytotoxic effect (% dead cell counts) and the suppression of the growth (change in cell number) of all melanoma cell lines tested were pronounced with EGCG. This investigation validates the hypothesis that anticancer action of the various catechins may vary with the type of malignancy and provides a model for tumor cell heterogeneity based on susceptibility and resistance of tumor cells to different green tea catechins. Therefore, this information is critical for undertaking chemopreventive or chemotherapeutic trials against melanoma and gender-based cancers.
Keywords: EC 3-gallate (ECG), epigallocatechin (EGC), EGC 3-gallate (EGCG), green tea, metastatic melanoma
Introduction
The four major catechins in green tea (Camellia sinensis) are epicatechin (EC), epicatechin 3-gallate (ECG), epigallocatechin (EGC) and EGC 3-gallate (EGCG). The dry weights of EC, ECG, EGC and EGCG are 792 ± 3, 1702 ± 16, 1695 ± 1 and 8295 ± 92 mg/100 g, in green tea, and 240 ± 1, 761 ± 4, 1116 ± 24 and 1199 ± 0.12 mg/100 g, in black tea (1). Green tea catechins have chemopreventive potential against cancer (2–7), and they protect against the damaging effects of ultraviolet radiation (8).
Although ECG, EGC and EGCG can potentially suppress the proliferation of cancer cells (7,9–16), EGCG is reportedly the most promising and is under clinical investigation in chemoprevention trials (3,7). We previously found that the anticancer effects of the various catechins in ovarian and prostate cancer cell lines varied with the type and stage of malignancy (17). ECG was more effective than EGCG in suppressing the growth of these gender-based carcinomas. In contrast, recent studies of human melanoma cell lines indicate that EGCG is more potent than other catechins, and that neither EGCG nor other catechins affect growth of normal melanocytes (7).
We hypothesize that different cancers may differ in their susceptibility to the antitumor activity of different green tea catechins. While prostate and ovarian carcinomas are susceptible to antitumor activity of ECG, human melanoma cells may be more susceptible to the antitumor activity of EGCG. Testing of the earlier-mentioned hypothesis requires comparing the tumor-killing activity of different green tea catechins on melanoma cell lines developed from locoregional lymph nodes, the same areas (in transit) and different distant organs.
In this investigation, we examined the ability of different catechins to suppress the growth of eight different human cell lines derived from locoregional lymph nodes (two cell lines), the same areas (in transit; two cell lines) and distant organs (four cell lines), after establishing the cell viability, doubling time and number of cell cycles for each cell line, as these parameters are known to vary under different growth conditions. The goal was to obtain data that would be useful for developing chemopreventive and possibly therapeutic measures for melanoma patients.
Materials and Methods
Human Cutaneous Melanoma Cell Lines
Eight cell lines developed from operative specimens of regional node or distant metastatic melanoma were used in this investigation (Table 1). All cell lines were developed by and obtained from Ms Estella Famatiga at the Tissue Culture Facility of John Wayne Cancer Institute (JWCI).
Table 1.
Human melanoma cell lines used in this study
| Sex | Age at diagnosis (years) | Primary site | Histologic type | Source of tumor cells | JWCI cell line ID | Passage number |
|---|---|---|---|---|---|---|
| M | 79 | Leg/hip | LMM | Inguinal lymph node (regional metastasis) | MB-1133 | 8 |
| M | 39 | Skin | NMM | Pulmonary lymph node | SE-0154 | 11 |
| F | 42 | Skin | SSM | Pancreas | CH-0356 | 12 |
| M | 30 | Buttocks skin | SSM | Liver | JK-0346 | 22 |
| M | 40 | Lower leg skin | NMM | Lower leg (in-transit metastasis) | SA-1171 | 6 |
| M | 61 | Nasal sinuses | Unknown | Nasal wall (in-transit metastasis) | GE-0208 | 8 |
| M | 69 | Neck anterior | Unknown | Spleen | NS-1176 | 5 |
| F | 22 | Lower leg skin | SSM | Proximal jejunum | LF-0023 | 13 |
LMM, lentigo malignant melanoma; NMM, nodular malignant melanoma; SSM, superficially spreading melanoma.
All cell lines were cryopreserved and cell viability was monitored as described earlier (17). Cells recovered from cryovials were grown in RPMI 1640 with glutamine (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, HEPES buffer, gentamycin (5 mg%) and fungizone (0.5 mg%), at 37°C in a humidified atmosphere of 5% CO2. Upon confluency, cells were detached with sterile ethylene diamine tetra acetate (EDTA)-dextrose (137 mM sodium chloride, 5.4 mM potassium chloride, 5.6 mM dextrose, 0.54 mM EDTA), 7.1 mM sodium bicarbonate] at 37°C for 5–15 min, recovered with cold RPMI 1640–10% FBS, and resuspended in the same medium. Cell viability and cell count were reassessed before the cells were seeded into culture flasks.
Tea Epicatechins
All three ECs used in this study were obtained from Sigma (ECG, Sigma E3892, FW 442.4; EGC, Sigma E3768, FW. 306.3; EGCG, Sigma E4143, FW. 458.4) and were >98% pure as assessed by high-performance liquid chromatography (by the commercial source) (17). Stock solutions were prepared under sterile conditions with 20, 40, 50 and/or 60 μM of each EC or with no EC (control) in culture medium.
Growth Conditions
In all experiments, 25 ml sterile polystyrene tissue culture flasks with a vented, blue plug seal cap (Beckton Dickinson, Franklin Lakes, NJ, Cat. No. 353107) were used (17). Each flask contained stock solution with or without EC in concentrations of 50 μM (four or five flasks for each EC and four or five flasks for control) and 20, 40 and 60 μM (four flasks for each EC and four flasks for control). Cells (x106) suspended in 10 ml of RPMI-1640-FBS solution described earlier were transferred to each flask and allowed to grow until the control cells reached confluency. The culture medium was not changed during the course of the experiment, either in control or in experimental flasks. The cells were detached with sterile EDTA-dextrose at 37°C for 5 min, recovered with cold RPMI 1640-FBS medium, and resuspended in the same medium. Cells were counted as described in our previous report (17). The time interval between seeding and confluent growth of control cells was divided by the number of folds increased from seeding to confluence to calculate the doubling time. The number of cell cycles was calculated by dividing the confluence time by the mean doubling time of the cell population. The 50% inhibitory concentration (IC50) of each catechin for each cell line was calculated using a software program (Microcal Origin Corp, OriginLab Corporation, Northampton, MA).
Statistics
The Kruskal–Wallis and Fisher's least significant difference (LSD) methods were used for pairwise comparisons of values significant at the 0.05 level. All the statistical results are provided in the Figs 1 and 2.
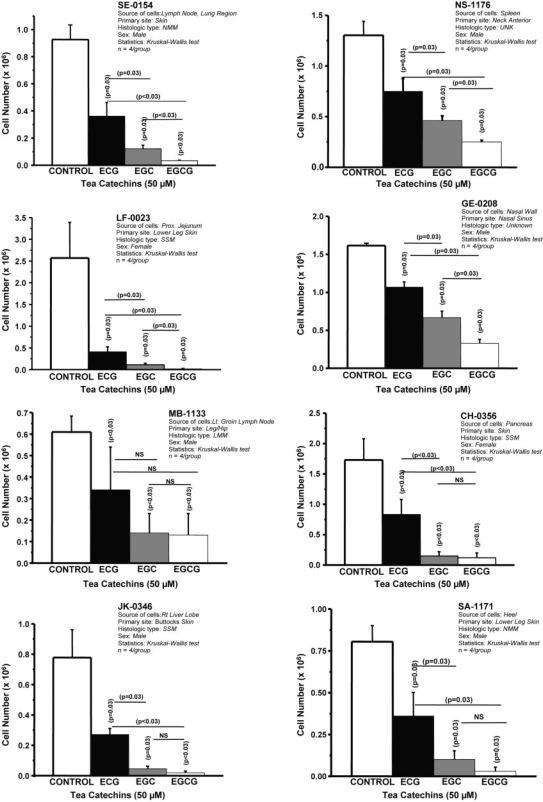
Figure 1.
Antiproliferative effect of purified tea epicatechins on eight different human melanoma cell lines. Density of melanoma cells seeded (0.216 × 106 cells/CH0356; 0.297 × 106 cells/MB1133; 0.250 × 106/GE0208; 0.300 × 106/all other cell lines) into four or five flasks containing culture medium (RPMI-1640 with 10% FBS-antibiotics) with or without catechins (50 μM). When growth of untreated cells reached confluency, cells from each flask were harvested and viable/dead cells were counted. Mean and standard deviation are represented. P-values were obtained with pair-wise comparison.
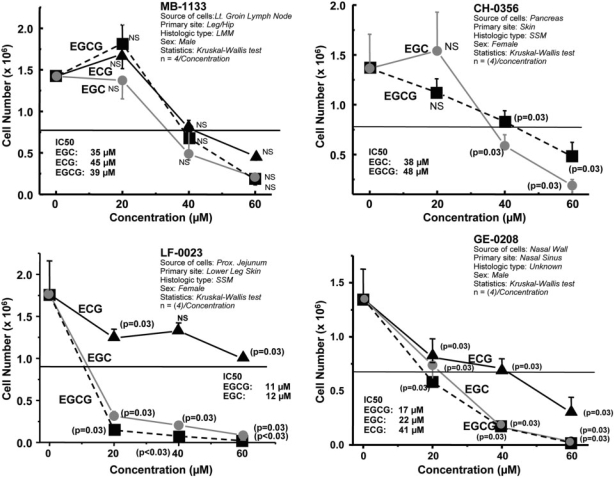
Figure 2.
Dosimetric growth suppression of human melanoma cell lines by ECG, EGC and EGCG. Cells (0.25 × 106/line) were seeded in flasks containing culture medium (RPMI-1640 with 10% FBS-antibiotics) with or without ECG or EGC or EGCG at concentrations of 0, 20, 40 and 60 μM (four flasks for each dose). The horizontal line in the middle of the graph indicates IC-50. When untreated cells reached confluency, cell monolayers in each flask were harvested and counted. P-values (Kruskal–Wallis test) indicate significant differences between mean values of treated and untreated cells.
Results
Two Catechins Strongly Suppress Growth of Human Melanoma Cells
Figure 1 summarizes the mean density of cells treated with 50 μM of ECG, EGC, EGCG or no catechin. Each mean represents four separate measurements (flasks). In each case, the density (cell number) of treated cells was measured when untreated (control) cells reached confluency. Although all three catechins significantly affected cell density, pair-wise comparison clearly identified EGC and EGCG as more potent in seven of the eight lines. In MB-1133 cells, the inhibitory effect of EGC or EGCG was not significantly greater than that of ECG. Statistically, the inhibitory effects of EGC and EGCG were comparable in CH-0356, JK-0346, SA-1171 and MB-1133 cells. However, EGCG was more potent than EGC in SE-0154, NS-1176, GE-0208 and LF-0023 cells. LF-0023 cells were remarkably susceptible to all three catechins.
Dosimetric Analysis of Growth Suppression
Proliferation of MB-1133, CH-0356, LF-0023 and GE-0208 cells was assessed at EC concentrations of 0, 20, 40 and 60 μM (four flasks/dose). The dosimetric results plotted in Fig. 2 indicate that suppression of cell growth was greatest in cells treated with high concentrations (≥40 μM) of EGC or EGCG. Catechin-mediated growth suppression can be ranked as follows: EGCG>EGC≥ECG. The corresponding IC50 values, summarized in Table 2, show a pattern distinctly different from that found in prostate and ovarian cancer cells (17).
Table 2.
IC50 values of tea catechins in melanoma, prostate and ovarian cancer cell lines
| Tumor cell line | IC50 (μM) |
||
|---|---|---|---|
| ECG | EGC | EGCG | |
| Melanoma | |||
| Nodal metastasis | |||
| • MB-1133 | 45 | 35 | 39 |
| Organ metastasis | |||
| • CH-0356 | ND | 38 | 48 |
| • GE-0208 | 41 | 22 | 17 |
| • LF-0023 | ND | 12 | 11 |
| Prostate cancer [17] | |||
| Organ-confined (HH870) | 27 | 45 | |
| Metatastic (DU145) | 24 | 89 | |
| Epithelial ovarian cancer [17] | |||
| Metastatic (HH450) | 29 | 62 | |
| Metastatic (HH639) | 30 | 42 | |
ND, not done.
Tumor Cell Doubling Time: EGC Versus EGCG
Table 3 summarizes the influence of the ECs on cell viability, percentage of dead cell count, doubling time and cell cycle. For most of the cell lines, doubling was not observed for cells treated with EGC or EGCG; instead the cell number decreased, indicating cell death. The number of cell cycles and the time taken for confluency differed among the cell lines. EGCG was the potent inhibitor of growth than EGC and other catechins for CH-0356, GE-0208, JK-0346, LF-0023 and NS-1176 (mean viable cell counts after culture).
Table 3.
Effects of four epicatechins (50 μM) on mean viable cell count, cell cycle number, % dead cell count and doubling time of different melanoma cell lines
| Parameters | Control | ECG | EGC | EGCG |
|---|---|---|---|---|
| CH-0356 (216 × 103 seeding/flask; 168 h for confluency; five flasks/control or catechins) | ||||
| Mean viable cell count (×103) | 1720 ± 347 | 820 ± 250 | 150 ± 69 | 110 ± 81 |
| Dead cell count (%) | 6.0 ± 1.6 | 7.0 ± 1.0 | 12.5 ± 4.9 | 20.3 ± 5.9 |
| Number of cell cycles | 2.95 ± 0.30 | 1.79 ± 0.45 | 0 | 0 |
| Mean doubling time (h) | 56.93 ± 5.94 | 94 ± 24 | 0 | 0 |
| MB-1133 (297 × 103 seeding/flask; 168 h for confluency; five flasks/control or catechins) | ||||
| Mean viable cell count (×103) | 613 ± 70 | 137 ± 90 | N/A | 131 ± 100 |
| Dead cell count (%) | 19.78 ± 2.0 | 39.77 ± 14.8 | N/A | 36.65 ± 22.9 |
| Number of cell cycles | 1.02 ± 0.16 | 0 | N/A | 0 |
| Mean doubling time (h) | 164.81 ± 21.6 | 0 | N/A | 0 |
| GE-0208 (250 × 103 seeding/flask; 145 h for confluency; four flasks/control or catechins) | ||||
| Mean viable cell count (×103) | 1616 ± 310 | 1069 ± 69.1 | 666 ± 86.1 | 329 ± 52.3 |
| Dead cell count (%) | 8.47 ± 0.96 | 6.25 ± 0.31 | 5.31 ± 1.95 | 21.84 ± 5.84 |
| Number of cell cycles | 2.65 ± 0.26 | 2.01 ± 0.09 | 1.38 ± | 0 |
| Mean doubling time (h) | 54.69 ± 5.0 | 72 ± 6.8 | 105 ± 13.7 | 0 |
| JK-0346 (300 × 103 seeding/flask; 120 h for confluency; four flasks/control or catechins) | ||||
| Mean viable cell count (×103) | 779 ± 183 | 271 ± 40.5 | 46.0 ± 17.1 | 18.0 ± 11.8 |
| Dead cell count (%) | N/A | N/A | N/A | N/A |
| Number of cell cycles | 1.28 ± 0.34 | 0 | 0 | 0 |
| Mean doubling time (h) | 93.56 ± 23.4 | 0 | 0 | 0 |
| LF-0023 (300 × 103 seeding/flask; 185 h for confluency; four flasks/control or catechins) | ||||
| Mean viable cell count (×103) | 2570 ± 821 | 409 ± 117 | 111 ± 32.9 | 21.0 ± 7.22 |
| Dead cell count (%) | 9.16 ± 3.21 | 9.26 ± 1.0 | 22.36 ± 5.66 | 60.80 ± 9.15 |
| Number of cell cycles | 2.97 ± 0.51 | 0 | 0 | 0 |
| Mean doubling time (h) | 62.38 ± 11.6 | 0 | 0 | 0 |
| NS-1176 (300 × 103 seeding/flask; 142 h for confluency; four flasks/control or catechins) | ||||
| Mean viable cell count (×103) | 1303 ± 139 | 750 ± 125 | 461 ± 46.6 | 249 ± 17.8 |
| Dead cell count (%) | 2.92 ± 0.44 | 4.75 ± 1.00 | 7.04 ± 0.98 | 18.0 ± 5.6 |
| Number of cell cycles | 2.11 ± 0.15 | 1.27 ± 0.24 | 0 | 0 |
| Mean doubling time (h) | 67.47 ± 4.86 | 112 ± 21.2 | 0 | 0 |
| SA-1171 (250 × 103 seeding/flask; 192.5 h for confluency; four flasks/control or catechins) | ||||
| Mean viable cell count (×103) | 805 ± 95.7 | 361 ± 141 | 101 ± 52.3 | 30 ± 24.8 |
| Dead cell count (%) | 19.90 ± 3.58 | 28.11 ± 2.31 | 25.58 ± 4.30 | 42.15 ± 31.3 |
| Number of cell cycles | 1.67 ± 0.17 | 0 | 0 | 0 |
| Mean doubling time (h) | 115.59 ± 12.3 | 0 | 0 | 0 |
| SE-0154 (300 × 103 seeding/flask; 144 h for confluecy; four flasks/control or catechins) | ||||
| Mean viable cell count (×103) | 926 ± 108 | 361 ± 101 | 121 ± 25.9 | 33 ± 5.0 |
| Dead cell count (%) | 10.28 ± 3.75 | 10.64 ± 2.63 | 20.27 ± 2.81 | 50.01 ± 8.59 |
| Number of cell cycles | 1.61 ± 0.17 | 0 | 0 | 0 |
| Mean doubling time (h) | 89.66 ± 9.29 | 0 | 0 | 0 |
N/A, not applicable.
Discussion
More effective, minimally toxic systemic agents are needed to decrease the morbidity and mortality of melanoma. Tea catechins appear to inhibit human melanoma cells; both EGC and EGCG suppressed growth of human melanoma cell line UACC-375 (10). In a recent study, treatment with EGCG caused a dose-dependent decrease in cell proliferation, induced apoptosis and cell cycle arrest, and significantly inhibited colony formation of a malignant amelanotic cell line (A-375) and a metastatic melanoma cell line (Hs-294T), but did not affect normal melanocytes (7). Based on these findings, it was concluded that EGCG alone could be useful for the management of melanoma.
Results of the present investigation based on eight metastatic melanoma cell lines favor the view that not a single catechin can totally arrest the growth of tumor cells in view of the heterogeneity of cell types in a tumor population. The data suggest that EGC together with EGCG could be more beneficial for the management of melanoma, because not all melanoma cells respond identically to EGCG or any particular tea catechin. For example, melanoma cell line LF-0023 was uniformly susceptible to ECG, EGC and EGCG, whereas the other cell lines differed in their susceptibility to these catechins. The percentage dead cells observed for all the melanoma cell lines were greater for EGCG than for any other catechins. EGCG was, indeed, the most potent inhibitor of cell line GE-0208; both EGCG and EGC inhibited the growth of cell lines MB-1133, CH-0566 and LF-0023. Overall, it appears that EGCG may be a potent inhibitor of cell proliferation and promoter of cell death of all the melanoma cell lines. It should be noted that the cell population in a tumor lesion or in cell culture could be heterogeneous in their antigen and cell surface receptor expression and the differences observed with different catechins may reflect such heterogeneity of the cell population. Although it is well known that cell population in tumor lesions or biopsies are heterogeneous, we do not have any direct or indirect evidence to suggest heterogeneity of the cell population in culture. Since these cell lines used in this investigation belong to early passages, one may anticipate prevalence of tumor cell heterogeneity in culture.
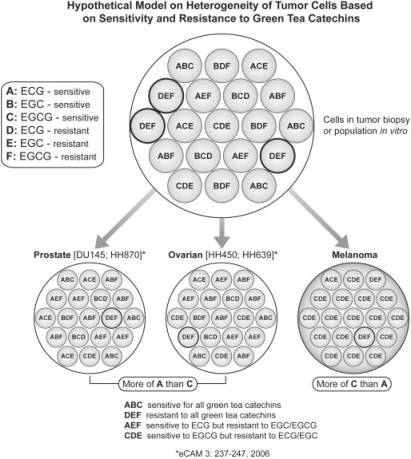
The heterogeneous nature of the cells in a patient's melanoma is a strong argument for broad-based, multi-agent systemic therapies that minimize the risk of chemoresistance. In our studies of prostate cancer cell lines, about 2–5% of metastatic cells failed to respond to individual catechins (17). Although our dosimetric analyses indicate that increasing the dose of catechins might promote better tumor killing, the resistance remains a potential problem. Heterogeneity of tumor cell population from the perspective of susceptibility and resistance to different green tea catechins is developed into a model (Fig. 3). This model provides a better understanding of the differential growth suppression of melanoma, prostate and ovarian cancer to three different catechins. Prostate and ovarian cancer cells are more susceptible for ECG (A) than for EGCG (C); on the other hand, melanoma cells appear to be more sensitive to EGCG (C). The model elaborates the earlier-mentioned contention. While some cells may be susceptible to all the catechins (ABC cells in the model), one may reasonably expect some cells to be resistant to all the catechins (DEF cells in the model). DEF cell population may require other phytotherapies involving soy genistein or turmeric curcumin or gingerol and shagalol from wet and dry ginger or chemotherapy. The results of this investigation suggest that a combination of catechins might improve response and even have a synergistic antitumor effect. Other naturally occurring, minimally toxic agents with antitumor properties, such as genistein, curcumin, gingerol-shagalol, crocin (saffron) and apigenins, might also be incorporated in this combination.
Figure 3.
Hypothetical model on heterogeneity of tumor cells based on sensitivity and resistance to green tea catechins. (1) ABC: A, ECG-sensitive, B, EGC-sensitive, C, EGCG-sensitive; (2) DEF: D, ECG-resistant, E, EGC- resistant, F, EGCG- resistant; (3) AEF: A, ECG-sensitive, E, EGC- resistant, F, EGCG- resistant; (4) BDF: B, EGC-sensitive, D, ECG-resistant, F, EGCG- resistant; (5) CDE: C, EGCG-sensitive; D, ECG-resistant, E, EGC- resistant; (6) ACE: A, ECG-sensitive, C, EGCG-sensitive, E, EGC- resistant; (7) ABF: A, ECG-sensitive, B, EGC-sensitive, F, EGCG- resistant; (8) BCD: B, EGC-sensitive, C, EGCG-sensitive, D, ECG-resistant.
Administration of catechins to melanoma patients would be an important issue. Work of Lee et al. (18) showed that plasma levels of EGCG and EGC in healthy volunteers increased to 78 and 223 ng/ml, respectively, 20 min after drinking brewed green tea (1.2 g of tea solids in 200 ml hot water). This suggests that drinking more than 10 cups of green tea may be necessary to maintain a plasma concentration of EGCG equivalent to that used to produce in vitro by a dose of 50 μM or 22.5 mg. Kaegi (19) suggested a daily intake of 13 cups of green tea as a chemopreventive measure. Since this level of tea consumption is impractically high, chemoprevention of melanoma with catechins may require administration of the appropriate catechin in a purified form. Green tea catechins are prepared by several QC/QA-controlled facilities in US (Nutrilite Corporation, Buena Park, CA and possibly Life Extension Foundation, Lauderdale, FL). However, in this in vitro study, we have only used commercially purified green tea catechins (Sigma, Saint Louis, MO). These purified components of green tea catechins can be more beneficial for chemoprevention of cancer.
Acknowledgements
This study is supported by grants received from the Scientific Review Committee and carried out in the Department of Glycoimmunotherapy at John Wayne Cancer Institute. We thank Adam Blackstone for preparation of the figures, and Ms Cindy Sumobay for her assistance in tissue culture experiments. We also thank Ms Gwen Berry for editorial assistance.
References
- 1.Belsville, MD: U.S. Department of Agriculture; 2003. USDA Database for the Flavonoid Content of Selected Foods, Prepared by the Nutrient Data Laboratory, Food Composition Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service. [Google Scholar]
- 2.Okabe S, Ochiai Y, Aida M, Park K, Kim SJ, Nomura T, et al. Mechanistic aspects of green tea as a cancer preventive: effect of components on human stomach cancer cell lines. Jpn J Cancer Res. 1999;90:733–9. doi: 10.1111/j.1349-7006.1999.tb00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–55. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–80. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 6.Moyers SB, Kumar NB. Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr Rev. 2004;62:204–11. doi: 10.1111/j.1753-4887.2004.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 7.Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–21. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf N, Irby C, Katiyar SK, Elmets CA. Photoprotective effects of green tea polyphenols. Photodermatol Photoimmunol Photomed. 2007;23:48–56. doi: 10.1111/j.1600-0781.2007.00262.x. [DOI] [PubMed] [Google Scholar]
- 9.Kinjo J, Nagao T, Tanaka T, Nonaka G, Okawa M, Nohara T, et al. Activity-guided factionation of green tea extract with antiproliferative activity against human stomach cancer cells. Biol Pharm Bull. 2002;25:1238–40. doi: 10.1248/bpb.25.1238. [DOI] [PubMed] [Google Scholar]
- 10.Valcic S, Timmermann BN, Alberts DS, Wachter GA, Krutzsch M, Wymer J, et al. Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anticancer Drugs. 1996;7:461–8. doi: 10.1097/00001813-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Okabe S, Suganuma M, Hayashi M, Sueoka E, Komori A, Fujiki H. Mechanisms of growth inhibition of human lung cancer cell line, PC-9, by tea polyphenols. Jpn J Cancer Res. 1997;88:639–43. doi: 10.1111/j.1349-7006.1997.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elattar TM, Virji AS. Effect of tea polyphenols on growth of oral squamous carcinoma cells in vitro. Anticancer Res. 2000;20:3459–65. [PubMed] [Google Scholar]
- 13.Fujimoto N, Sueoka N, Sueoka E, Okabe S, Suganuma M, Harada M, et al. Lung cancer prevention with (-)-epigallocatechin gallate using monitoring by heterogeneous nuclear ribonucleoprotein B1. Int J Oncol. 2002;20:1233–9. [PubMed] [Google Scholar]
- 14.Chisholm K, Bray BJ, Rosengren RJ. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anticancer Drugs. 2004;15:889–97. doi: 10.1097/00001813-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Babich H, Krupka ME, Nissim HA, Zuckerbraun HL. Differential in vitro cytotoxicity of (-)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol In Vitro. 2005;19:231–42. doi: 10.1016/j.tiv.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J Nutr. 2003;133:2417S–24S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- 17.Ravindranath MH, Saravanan TS, Monteclaro CC, Presser N, Ye X, Selvan SR, et al. Epicatechins purified from green tea (Camellia sinensis) differentially suppress growth of gender-dependent human cancer cell lines. Evid Based Complement Alternat Med. 2006;3:237–47. doi: 10.1093/ecam/nel003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M-J, Lambert JD, Prabhu S, Meng X, Lu H, Maliakal P, et al. Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extract. Cancer Epidemiol Biomarkers Prev. 2004;13:132–7. doi: 10.1158/1055-9965.epi-03-0040. [DOI] [PubMed] [Google Scholar]
- 19.Kaegi E. Unconventional therapies for cancer: 2. Green Tea Can Med Assoc J. 1998;158:1033–5. [PMC free article] [PubMed] [Google Scholar]