Abstract
Propolis is a sticky dark-colored material showing a very complex chemical composition that honeybees collect from plants. It has been used in folk medicine since ancient times, due to several biological properties, such as antimicrobial, anti-inflammatory, antioxidant and immunomodulatory activities, among others. Its antitumor action in vivo and in vitro has also been reported, using propolis extracts or its isolated compounds. The goal of this work was to evaluate propolis's cytotoxic action in vitro on human laryngeal epidermoid carcinoma (Hep-2) cells. These cells were incubated with different concentrations of this bee product for different time periods, and morphology and the number of viable HEp-2 cells analyzed. Data showed that propolis exhibited a cytotoxic effect in vitro against HEp-2 cells, in a dose- and time-dependent way. Propolis solvent had no effects on morphology and number of viable cells, proving that the cytotoxic effects were exclusively due to propolis components. Since humans have been using propolis for a long time, further assays will provide a better comprehension of propolis's antitumor action.
Keywords: antitumor action, HEp-2 cells, propolis
Introduction
Propolis has attracted researchers’ interest in the last decades, because of its several biological and pharmacological properties, such as immunomodulatory, antimicrobial, antitumor, anti-inflammatory, antioxidant, among others (1). Besides, propolis-containing products have been intensely marketed by the pharmaceutical industry and health-food stores (2).
Propolis is a resinous material collected by bees from bud and exudates of plants, which is mixed with products of their salivary glands and wax. Its color varies from green, red to dark brown. Propolis has a characteristic smell and shows adhesive properties, because it strongly interacts with oils and proteins of the skin.
Several researchers have reported the antitumoral property of propolis both in vivo and in vitro. Propolis's antiproliferative activity on tumor cells has been demonstrated and some responsible compounds have been isolated (2,3).
Our group verified that propolis (10% treatment for 3 days) increased the cytotoxic activity of natural killer cells against murine lymphoma (4). This finding confirmed a previous observation that propolis administration over a short term to animals increases the immunological response (5). In our projects, we also evaluated the potential of propolis in carcinogenesis and mutagenesis assays (6,7). Propolis cytotoxic effects in vitro were also found on canine transmissible venereal tumor (8).
HEp-2 cell line is derived from laryngeal carcinoma cells of human nasopharyngeal mucosa. Being slow-growing tumors, these cells develop in animal hosts as well as in tissue culture (9). Propolis effects on these cells have not been investigated. Thus, the present work was carried out in order to verify a possible cytotoxic action of propolis on HEp-2 cells, evaluating its morphology and the number of viable cells after incubation with propolis in different concentrations and time periods.
Methods
Propolis Sample
Propolis was collected in the Beekeeping Section, UNESP, Campus of Botucatu, Brazil. Propolis was ground and 30% ethanolic extracts of propolis were prepared (30 g of propolis, making the volume 100 ml with 70% ethanol), in the absence of bright light, at room temperature, with moderate shaking. After a week, extracts were filtered and the dry weight of the extracts was calculated (120 mg/ml) (10). Propolis's chemical composition was investigated using thin-layer chromatography (TLC), gas-chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) analysis (11–13).
Propolis was diluted in minimum essential media (MEM) supplemented with 0.1 g/l of l-glutamine, 2.2 g/l sodium bicarbonate, 10 ml/l non-essential amino acids and 10% fetal calf serum (Sigma), and specific dilutions of this solution were prepared for each assay in order to achieve different propolis concentrations: 5, 10, 25, 50 and 100 μg/well. The same procedure was carried out with 70% ethanol (propolis solvent), in order to obtain 0.03, 0.06, 0.15, 0.29 and 0.59% ethanol, which are the respective concentrations of alcohol in propolis concentrations.
HEp-2 Cells Culture
HEp-2 cells were grown in MEM as described earlier and cultivated at 37°C and 5% CO2. First, cells in 25 cm2 flasks were washed with 5–10 ml of MEM, and afterwards 1–2 ml of trypsin (0, 2% trypsin in 5% EDTA) were added to each flask until cell detachment. Cells were counted using a hemocytometer and cultivated in a 96 well, U-bottom plate (Corning) at a final concentration of 2 × 105 cells/well (8).
Propolis Cytotoxicity Assay
HEp-2 cell cultures, after 80% confluent monolayers, were incubated with propolis or its solvent (ethanol) in MEM, using a 96 well plate, incubating at 37°C and 5% CO2, for 6, 24, 48 and 72 h. Control wells contained only the same cell number in the same media. Each propolis or ethanol concentration was assayed in triplicate. After each period, cell morphology was evaluated microscopically, as well as counting the number of viable cells in treated and untreated cultures, by trypan blue dye exclusion after trypsinization (8). Viable HEp-2 cells are epithelioid-shaped cells with few rounded ones.
Statistical Analysis
Friedman non-parametric test was employed in order to compare propolis effects along time. Kruskal–Wallis test was used to evaluate propolis concentrations effect in each time period (14).
Results
Propolis Exhibited a Cytotoxic Effect In Vitro in a Dose- and Time-dependent Manner
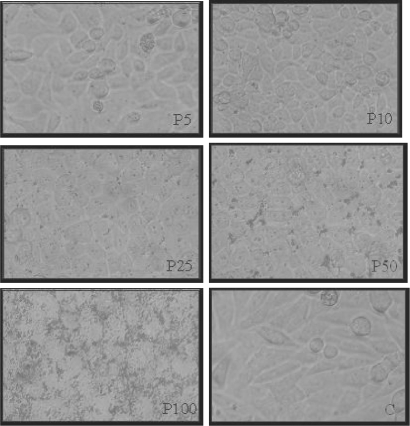
HEp-2 cells showed a typical morphology after 6, 24, 48 and 72 h of incubation with propolis, using 5 and 10 μg/well. In these concentrations, epithelioid-shaped cells were observed, with few rounded cells, similar to control. However, after 6 h of incubation with 25, 50 and 100 μg/well, changes in cell morphology were observed, such as cell lysis and disorganization of the monolayer, mainly using 100 μg/wells (Fig. 1).
Figure 1.
HEp-2 cell morphology: monolayers after 6 h of incubation with 5, 10, 25, 50 and 100 μg/well of propolis (P) in comparison to control (C) (20×). This figure represents five similar assays.
After 24 h of incubation, propolis cytotoxic activity was significantly detected using 100 μg/well (P < 0.05) (Fig. 2), affecting monolayers and numbers of viable cells (Fig. 4).
Figure 2.
HEp-2 cell morphology: monolayers after 24 h of incubation with 100 μg/well of propolis (P) in comparison to control (C) (20×). This figure represents five similar assays.
Figure 4.
Median of HEp-2 cells number after incubation with different propolis concentrations (5, 10, 25, 50 and 100 μg/well) for 6, 24, 48 and 72 h. This figure represents five similar assays.
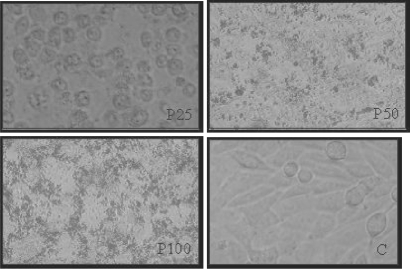
After 48 h, cell monolayers were affected significantly by propolis using 25 and 50 μg/well (P < 0.05), in comparison to control cells, showing a non-typical monolayer pattern (Fig. 3).
Figure 3.
HEp-2 cell morphology: monolayers after 48 h of incubation with 25, 50 and 100 μg/well of propolis (P) in comparison to control (C) (20×). This figure represents five similar assays.
Propolis Affected the Number of Viable HEp-2 Cells In Vitro
The number of viable cells diminished after incubation with propolis in different concentrations and time periods, in a dose- and time-dependent manner (Fig. 4).
Propolis Solvent (70% ethanol) Showed No Effects on Hep-2 Cells In Vitro
Seventy percent ethanol, in all dilutions (0.03, 0.06, 0.15, 0.29 and 0.59%–the respective concentrations of alcohol in propolis concentrations), had no effect either on cell morphology or on cell viability.
Discussion
Propolis's antitumor action has been widely investigated by several authors, and our group has also evaluated its effects both in vivo and in vitro (4,6–7).
Our propolis samples, collected in the Beekeeping Section of the University, UNESP, Campus of Botucatu, were analyzed by GC, GC-MS and TLC, revealing that its main components are phenolic compounds (flavonoids, aromatic acids, benzopyranes), di- and triterpenes, essential oils, among others.
The main constituents of our propolis sample were isolated and identified: flavonoids are present in small quantities in Brazilian propolis (kaempferid, 5,6,7-trihydroxy-3,4′-dimethoxyflavone, aromadendrine-4′-methyl ether); a prenylated p-coumaric acid and two benzopyranes: E and Z 2,2-dimethyl-6-carboxyethenyl-8-prenyl-2H-benzopyranes); essential oils (spathulenol, (2Z,6E)-farnesol, benzyl benzoate and prenylated acetophenones); aromatic acids (dihydrocinnamic acid, p-coumaric acid, ferulic acid, caffeic acid, which are common for poplar propolis, 3,5-diprenyl-p-coumaric acid, 2,2-dimethyl-6-carboxy-ethenyl-8-prenyl-2H-1-benzo-pyran); di- and triterpenes, among others (11–13).
The main vegetal sources of our propolis samples are Baccharis dracunculifolia DC, Eucalyptus citriodora Hook and Araucaria angustifolia (Bert.) O. Kuntze (15).
In this work, one may verify that propolis showed a cytotoxic effect in vitro against HEp-2 cells, in a dose- and time-dependent manner. Higher concentrations of propolis showed a short-term action, whereas its lower concentrations were effective with time. Propolis solvent had no effects on morphology and number of viable cells, suggesting that cytotoxic effects were exclusively due to propolis components. In our assays, the propolis samples collected in our university was employed. Samples from different geographic origins may show different chemical compositions, however, similarity in their activities may be found, due to different responsible compounds (16).
The antiproliferative action of propolis on tumor cells may be the result of the synergistic effect of propolis constituents, and some isolated compounds have been investigated (17). Caffeic acid phenethyl ester (CAPE) is one of propolis constituents most investigated with regards to antitumor action.
CAPE had a dose-dependent effect on cytotoxicity of C6 glioma cells, reducing viability to 42% in relation to control, and increasing the proportion of hypodiploid DNA, as indication of apoptosis (18). According to reports, antitumor activity of propolis also occurs through the induction of apoptosis via caspase pathways (19).
CAPE also interferes in cell cycle arrest. After incubation with CAPE for 24 h, cell number percentage of C6 glioma cells in G0/G1 phase increased to 85%, due to inhibition of retinoblastoma protein (pRB) phosphorylation. Phosphorylation of pRB by cyclin-dependent kinases (CDKs)/cyclins is believed to be a crucial event in regulation of S-phase entry, and appears to define the restriction point in the late G1 phase (20). CAPE derivatives were investigated on oral cancer using cultured cancer cell line (squamous cell carcinoma = SAS; oral epidermoid carcinoma-Meng 1 = OEC-M1) and normal human oral fibroblast (NHOF), examining their effects on cell growth pattern, their cytotoxicity and changes in the cell cycle. CAPEs showed cytotoxic effects on tumor cells but not on NHOF cell line. Flow cytometric analysis showed OEC-M1 cell arrest at G2/M phase. Such differential effects on cancer and normal cells suggested that these compounds might be useful in oral cancer chemotherapy (21).
CAPE prolonged the survival of mice implanted with colon adenocarcinoma cells (CT26 cells), reducing the pulmonary metastatic capacity of these cells (22). Recently, according to one suggestion the in vivo antitumor activity of propolis and some of its constituents is associated with their immunomodulatory action in mice, mainly due to augmentation of non-specific antitumor immunity, via macrophage activation, which in turn could produce soluble factors and interfere directly in tumor cells or in functions of other immune cells (23).
Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid), another propolis constituent with antitumor action and present in Brazilian propolis, was investigated for its effects on colon carcinogenesis, inducing G(0)/G(1) arrest via stimulation of Cip1/p21 expression in human colon cancer cells (24). Artepillin C induces apoptosis in human leukemia cell lines of different phenotypes (25).
Although the constituents of propolis responsible for its cytotoxic action were not investigated in this research, our work opens a new perspective for further investigation. The large amount of work dealing with antitumor action of propolis and its constituents indicates their promising usefulness, and claims for new investigations, in order to explore propolis's potential as a cancer chemopreventive and chemotherapeutic agent.
Since propolis shows several biological properties (26–29) and it is used as an alternative medicine for health amelioration and disease prevention, our laboratory is investigating propolis action in immunossuppressed mice, bearing melanoma cells.
Acknowledgement
Authors wish to thank FAPESP for financial support and Dr Lídia Raquel de Carvalho, for statistical analysis.
References
- 1.Bankova VS, Castro SL, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. [Google Scholar]
- 2.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–71. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 3.Huang MT, Ma W, Yen P, Xie JG, Han J, Frenkel K, et al. Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA and protein in HeLa cells. Carcinogenesis. 1996;17:761–5. doi: 10.1093/carcin/17.4.761. [DOI] [PubMed] [Google Scholar]
- 4.Sforcin JM, Kaneno R, Funari SRC. Absence of seasonal effect on the immunomodulatory action of Brazilian propolis on natural killer activity. J Venom Anim Toxins. 2002;8:19–29. [Google Scholar]
- 5.Scheller S, Gazda G, Pietsz G, Gabrys J, Szumlas J, Eckert L, et al. The ability of ethanol extract of propolis to stimulate plaque formation in immunized mouse spleen cells. Pharmacol Res Commun. 1988;20:323–8. doi: 10.1016/s0031-6989(88)80068-7. [DOI] [PubMed] [Google Scholar]
- 6.Bazo AP, Rodrigues MAM, Sforcin JM, Camargo JLV, Ribeiro LR, Salvadori DMF. Protective action of propolis on the rat colon carcinogenesis. Teratog Carcinog Mutagen. 2002;22:183–94. doi: 10.1002/tcm.10011. [DOI] [PubMed] [Google Scholar]
- 7.Alves De Lima RO, Bazo AP, Said RA, Sforcin JM, Bankova V, Darros BR, et al. Modifying effect of propolis on dimethylhydrazine-induced DNA damage but not colonic aberrant crypt foci in rats. Environ Mol Mutagen. 2005;45:8–16. doi: 10.1002/em.20082. [DOI] [PubMed] [Google Scholar]
- 8.Bassani-Silva S, Sforcin JM, Amaral AS, Gaspar LFJ, Rocha NS. Propolis effect in vitro on venereal transmissible canine tumor. Rev Port Ciênc Vet. in press. [Google Scholar]
- 9.Lima ME, Rissino JD, Guimarães AC, Overal DJ, Khayat AS, Souza PC, et al. Drifter technique: a new method to obtain metaphases in HEp-2 cell line cultures. Braz Arch Biol Technol. 2005;48:537–40. [Google Scholar]
- 10.Orsi RO, Sforcin JM, Funari SRC, Bankova V. Effects of Brazilian and Bulgarian propolis on bactericidal activity of macrophages against Salmonella typhimurium. Int Immunopharmacol. 2005;5:359–68. doi: 10.1016/j.intimp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Boudourova-Krasteva G, Bankova V, Sforcin JM, Nikolova N, Popov S. Phenolics from Brazilian propolis. Z Naturforsch. 1997;52c:676–9. [Google Scholar]
- 12.Bankova V, Boudourova–Krasteva G, Popov S, Sforcin JM, Funari SRC. Seasonal variations in essential oil from Brazilian propolis. J Essent Oil Res. 1998a;10:693–6. [Google Scholar]
- 13.Bankova V, Boudourova–Krasteva G, Popov S, Sforcin JM, Funari SRC. Seasonal variations of the chemical composition of Brazilian propolis. Apidologie. 1998b;29:361–7. [Google Scholar]
- 14.ZarR JH. Biostatistical Analysis. 2nd. Englewood Cliffs: Prentice-Hall; 1996. p. 718. [Google Scholar]
- 15.Bankova V, Boudourova-Krasteva G, Sforcin JM, Frete X, Kujumgiev A, Maimoni-Rodella R, et al. Phytochemical evidence for the plant origin of Brazilian propolis from São Paulo state. Z Naturforsch. 1999;54c:401–5. doi: 10.1515/znc-1999-5-616. [DOI] [PubMed] [Google Scholar]
- 16.Bankova V. Recent trends and important developments in propolis research. Evid Based Complement Alternat Med. 2005;2:29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin UH, Chung TW, Kang SK, Suh SJ, Kim JK, Chung KH, et al. Caffeic acid phenyl ester in propolis is a strong inhibitor of matrix metalloproteinase-9 and invasion inhibitor: isolation and identification. Clin Chim Acta. 2005;362:57–64. doi: 10.1016/j.cccn.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Kuo HC, Chu CY, Wang CJ, Lin WC, Tseng TH. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol. 2003;66:2281–9. doi: 10.1016/j.bcp.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Aso K, Kanno S, Tadano T, Satoh S, Ishikawa M. Inhibitory effect of propolis of the growth of human leukemia U937. Biol Pharm Bull. 2004;27:727–30. doi: 10.1248/bpb.27.727. [DOI] [PubMed] [Google Scholar]
- 20.Kuo HC, Kuo WH, Lee YJ, Lin WL, Chou FP, Tseng TH. Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Lett. 2006;234:199–208. doi: 10.1016/j.canlet.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Lee YT, Don MJ, Hung PS, Shen YC, Lo YS, Chang KW, et al. Cytotoxicity of phenolic acid phenethyl esters on oral cancer cells. Cancer Lett. 2005;223:19–25. doi: 10.1016/j.canlet.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 22.Liao HF, Chen YY, Liu JJ, Hsu ML, Shieh HJ, Liao HJ, et al. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agr Food Chem. 2003;51:7907–12. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 23.Orsolic N, Saranovic AB, Basic I. Direct and indirect mechanism(s) of antitumour activity of propolis and its polyphenolic compounds. Planta Med. 2006;72:20–7. doi: 10.1055/s-2005-873167. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu K, Das SK, Hashimoto T, Sowa Y, Yoshida T, Sakai T, et al. Artepillin C in Brazilian propolis induces G(0)/G(1) arrest via stimulation of Cip1/p21 expression in human colon cancer cells. Mol Carcinog. 2005;44:293–9. doi: 10.1002/mc.20148. [DOI] [PubMed] [Google Scholar]
- 25.Kimoto T, Aga M, Hino K, Koya-Miyata S, Yamamoto Y, Micallef MJ, et al. Apoptosis of human leukemia cells induced by Artepillin C, an active ingredient of Brazilian propolis. Anticancer Res. 2001;21:221–8. [PubMed] [Google Scholar]
- 26.Inokuchi Y, Shimazawa M, Nakajima Y, Suemori S, Mishima S, Hara H. Brazilian green propolis protects against retinal damage in vitro and in vivo. Evid Based Complement Alternat Med. 2006;3:71–7. doi: 10.1093/ecam/nek005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trusheva B, Popova M, Bankova V, Simova S, Marcucci MC, Miorin PL, et al. Bioactive constituents of Brazilian red propolis. Evid Based Complement Alternat Med. 2006;3:249–54. doi: 10.1093/ecam/nel006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Missima F, Sforcin JM. Green Brazilian propolis action on macrophages and lymphoid organs of chronically stressed mice. Evid Based Complement Alternat Med. doi: 10.1093/ecam/nel112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol. 2007;113:1–14. doi: 10.1016/j.jep.2007.05.012. [DOI] [PubMed] [Google Scholar]