Abstract
In the present study, we investigated the effects of Acori graminei rhizoma (AGR) on learning and memory for the Morris water maze task and on the central cholinergic system of the rats with excitotoxic medial septum (MS) lesion. On the water maze test, the rats were trained to find a platform that was in a fixed position during 6 days and then they received a 60 s probe trial in which the platform was removed from the pool on the 7th day. Ibotenic lesioning of the MS impaired the performance on the maze test and it caused degeneration of choline acetyltransferase and acetylcholine esterase in the hippocampus, which are markers of the central cholinergic system. Daily administrations of AGR (100 mg kg−1, i.p.) for 21 consecutive days produced reversals of the ibotenic acid-induced deficit in learning and memory. These treatments also reduced the loss of cholinergic immunoreactivity in the hippocampus that was induced by ibotenic acid. These results demonstrated that AGR ameliorated learning and memory deficits through their effects on the central nervous system, and neuroprotection was partly evaluated through the effect of AGR on the cholinergic system. Our studies suggest that AGR can possibly be used as treatment for Alzheimer's disease.
Keywords: Acori graminei rhizome, learning and memory, central cholinergic system, neuroprotection
Introduction
The neurotransmitter acetylcholine (Ach) has been demonstrated to modulate learning and memory processes (1). Thus, the cholinergic system has been of interest for studying cognitive disorders, including the neurodegenerative disorder known as Alzheimer's disease (AD). Cholinergic neurons originating in the nucleus basalis of Meynert (nbM) and the medial septum (MS) project to such brain areas as the cortex and hippocampus, and both these areas play roles in cognitive performance such as attention, learning and memory (2–4). The establishment and standardization of animal cognitive deficit models are required for testing of putative, cognition-enhancing agents. Cognitive dysfunctions after lesioning the MS are mainly considered to be due to hippocampal deafferentiation and MS lesioned animal has been widely accepted as an AD model (5,6). The damage in rats with injections of excitotoxic ibotenic acid into the MS leads to a profound Morris water maze deficit and regards as a model of AD at a more advanced stage of the neurodegeneration of the acetylcholinesterase (AchE) levels and the choline acetyltransferase (ChAT) activity (7). These observations indicate that the cholinergic system in compromise between AD and drugs, which stimulate cholinergic activity may provide treatment of AD.
Acori graminei rhizoma (AGR), the dry rhizomes of Acorus gramineus Solander (Araceae), have been used as a traditional Oriental medicine for more than hundreds of years. AGR is listed officially in the Chinese Pharmacopoeia and used as a digestant, an expectorant and a stimulant against digestive disorders and diarrhea (8,9). Clinically, AGR has been used as a traditional oriental medicine against stroke, AD and vascular dementia. In particular, AGR in combination with other herbal drugs, is one of the major components in oriental medical prescriptions for the treatment of stroke (10).
Several studies have demonstrated that the decoction and water extract from AGR induced sedation, decreased spontaneous activity, potentiated pentobarbital-induced sleeping time and antagonized pentylenetetrazole-induced convulsion in mice (11,12). Also α-asarone and β-asarone, the main volatile oils of AGR, produced sedation, reduced spontaneous activity and increased the hypnotic action of pentobarbital (9). It has also been reported that AGR and its active principles protect from the toxic effect of amyloid-β peptide in vitro and provide the therapeutic materials for the management of AD (13). AGR contains 0.11–0.42% of volatile oil that is mainly composed of β-asarone (63.2–81.2%) and α-asarone (8.8–13.7%) (9). Although AGR in combination with other herbal drugs is clinically used for improving learning and memory, and it has often been included in oriental medicine prescriptions for treating stroke (14–16), the effects of AGR on the ibotenic acid-induced amnesic rat model have not been evaluated. In the present study, we demonstrated that the methanol extract of AGR ameliorated learning and memory deficits on a Morris water maze test via neuroprotective effects on the Ach system in the central nervous system (CNS).
Methods
Subjects
Adult male Sprague-Dawley rats weighing 260–280 g were obtained from Samtaco Corp. (Kyungki-do, Korea). All the rats were housed in groups of five with continuous access to food and water ad libitum; they were maintained on a 12 h light/dark cycle regulated at 23°C room temperature. The experiments began at least 7 days after they had been acclimatized to their new environment. The experimental procedures were carried out according to the animal care guidelines of the NIH and the Catholic University Medical College Institutional Animal Care.
Methanol Extract of AGR
AGR was purchased from an oriental drug store (Jungdo Inc. Seoul, Korea). The voucher specimens (DG-AGR9801) have been deposited at the herbarium located at the College of Oriental Medicine, Kyung Hee University. AGR (100 g) was cut into small pieces and extracted three times in a reflux condenser for 24 h each with 85% methanol. The solution was combined, filtered through Whatman No.1 filter paper, and concentrated using a rotary vacuum evaporator followed by lyophilization. The yield of AGR was 10.1% (w/w).
Lesioning Procedure and Administration of AGR
The general procedures for surgery were the same for all groups, except that artificial cerebrospinal fluid (CSF) was microinjected into the sham group, whereas ibotenic acid (Sigma, St. Louis, MO, USA) was microinjected into the rats in the lesion group at a concentration of 4 μg μl−1 of artificial CSF. The anesthetized rat (50 mg kg−1, pentobarbital, i.p.) was placed in a stereotaxic apparatus. The skin over the rat's skull was shaved and cleaned with betadine, and then an incision was made through the skin and muscle to expose the skull; the skin was next retracted. Two holes were drilled in the skull at the location of the MS (AP: −0.2, L: ±0.3, DV: −6.2 referenced to the bregma) with using stereotaxic coordinates based on Paxinos and Watson brain atlas (17). A 22-gauge Hamilton syringe (Reno, NV, USA) filled with either artificial CSF or ibotenic acid was slowly infused at 0.2 μl min−1 using a microinjection pump (Pump 22, Harvard Apparatus, South Natick, MA) for 5 min. The syringe was left in place for 5 min following the 1 μl injection.
The AGR was dissolved in saline (100 mg ml−1). The day after surgery, the AGR suspension or saline was administered intraperitoneally for 3 weeks before testing of water maze. In the experiment, the sham and ibotenic lesioned + saline groups received saline (1 ml kg−1 per day), and the ibotenic lesioned + AGR group received AGR (100 mg kg−1 per day).
Morris Water Maze Test
A spatial memory test was performed by the method of Morris (18) with minor modification. The water maze was a circular pool (painted white, 2.0 m in diameter and 0.35 m in height) constructed from fiberglass. The pool contained water that was maintained at a temperature of 22 ± 2°C. The water was made opaque by the addition of 1 kg of powdered milk. During testing in the water maze, a platform, 15 cm in diameter, was located 1.5 cm below the water in one of four locations in the pool ∼50 cm from the side walls. The pool was surrounded by many cues external to the maze. A video camera was mounted to the ceiling above the pool and it was connected to a video recorder and tracking device (S-MART, Pan-Lab, Spain), which permitted on- and off-line automated tracking of the path taken by the rat. The rats were tested between 10:00 and 14:00. The rats received four trials per session. The rats were trained to locate the hidden escape platform, which remained in a fixed location throughout the testing procedures. The trials lasted a maximum of 180 s and the latency to find the submerged platform was recorded. The rats were tested in this way for 6 days, and then they received a probe trial on the 7th day. For the probe trial, the platform was removed from the pool and the rat was released from the quadrant opposite to where the platform would have been located. The length of the trial was 60 s, after which the rat was removed from the pool. The proportion of time the rat spent searching for the platform in the training quadrant, i.e. the previous location of the platform, was recorded, and this time was used as a measure of memory retention.
Neurobiological Analysis
At the end of the behavioral observation, the rats were deeply anesthetized with sodium pentobarbital (80 mg kg−1, i.p.) and then they were perfused through the ascending aorta with normal saline (0.9%) followed by 900 ml of 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS). The brains were removed, post-fixed overnight and cryoprotected in 20% sucrose with PBS. The brains were cut by a cryostat into 30 μm coronal sections, and these were processed immunohistochemically as free-floating sections.
ChAT Immunohistochemistry
The sections were washed in PBS containing 0.3% Triton × -100 and 1% rabbit serum, and they were then incubated at 4°C for 72 h in the ChAT primary antibody (Cambridge Research Biochemicals, Wilmington, DE, USA) diluted 1:2000. After washing, the sections were incubated in a biotinylated anti-sheep serum and Avidin-Biotin-Complex (ABC) complex (Vectastain Elite Kit, Vector Lab., Burlingame, CA, USA) for 2 h. They were visualized with 0.05% diaminobenzidine with 0.02% H2O2. For measuring the cells, a grid was placed on the MS area and the hippocampal CA1 and CA3 areas according to the atlas of Paxinos and Watson (16). The number of cells was counted at ×100 magnification using a microscope rectangle grid that measured 100 × 100 microns. The cells were counted on each of three sections per rat (19).
AchE Histochemistry
The sections were washed in PBS and then incubated in a solution containing 25 mg acetylthiocholine iodine for 1 h. The solution was composed of 32.5 ml of 0.1 M sodium hydrogen phosphate buffer (NaH2PO4·H2O, pH 6.0), 2.5 ml of 0.1 M sodium citrate, 5 ml of 30 mM copper sulfate, 5 ml of 5 mM potassium ferricyanide and 5 ml of distilled water. The color of the mixing solution was a pretty green. The density of the stained nuclei of the hippocampal cells was measured using the Scion image program (Scion Corp., MD, USA) (20).
Statistical Analysis
The data were expressed as means ± SEM. Group differences in the escape latency in the Morris water maze training task were analyzed using one-way analysis of variance (ANOVA) with repeated measures. One-way ANOVA followed by Tukey's post hoc test for multiple group comparison was used to analyze group differences of the data collected during the successive training days, the probe trials, the immunohistochemical assay and the image analysis. Differences among groups were considered as statistically reliable if the associated probability (P-value) was <0.05.
Results
Morris Water Maze Test
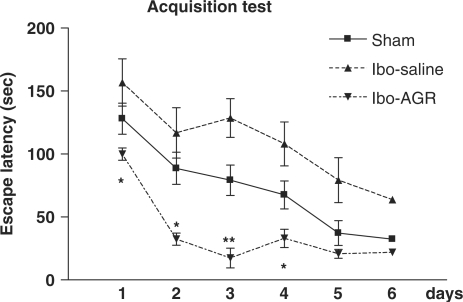
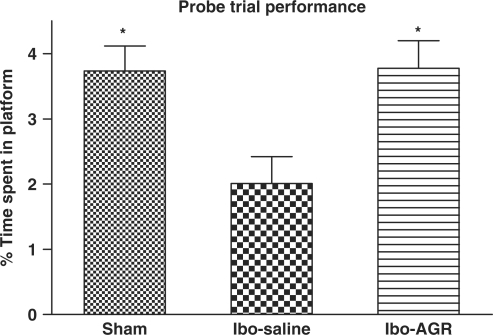
Results of the acquisition of the Morris water maze task are depicted in Fig. 1. The escape latency differed among the groups when this was averaged over all the session (F2,25 = 6.539, P < 0.01). Post hoc comparisons revealed that the Ibo lesion group needed more time to locate the platform than the Ibo-AGR group did. However, the sham group did not differ from the other two groups. During the experiment, the latency to escape diminished over time (F5,125 = 26.310, P < 0.001), but there was no interaction between the group and the day (F10.125 = 1.349, P > 0.212). Tukey's post hoc test revealed that the Ibo-AGR (P < 0.05 on day 1, 2 and 4, respectively, P < 0.01 on day 3) significantly reduced the latency of the swimming time compared with the Ibo-saline group. Analysis of the performance on the probe trial for comparing the percentage of time spent swimming above the platform is illustrated in Fig. 2. The time spent around the platform among the groups differed (F2,27 = 5.485, P < 0.05) and the sham and Ibo-AGR groups spent more time around the platform than the Ibo-saline group did (P < 0.05 for both groups). Ibotenic acid lesioning severely impaired the spatial cognition on the water maze task and AGR treatment attenuated ibotenic acid-induced learning and memory damage on the water maze.
Figure 1.
Comparisons of the acquisition performance on the Morris water maze task among the three groups of the rats. The results are the mean swimming time traveled per trial. The mean values of the four trials per day for 6 days for each group are shown. Repeated measures of ANOVA for the swimming time among the groups were followed by Tukey's test. *P < 0.05, **P < 0.01 as compared with the corresponding data of the Ibo-saline group.
Figure 2.
Comparisons of the retention performance on the Morris water maze task among the three groups of the rats. The results are the mean percentage of time spent on the platform swimming per trial. The mean values of the four trials for each group are shown. Separate measures of one-way ANOVA for the swimming time among the groups were followed by Tukey's test. *P < 0.05 as compared with the corresponding data of the Ibo-saline group.
ChAT Immunohistochemistry
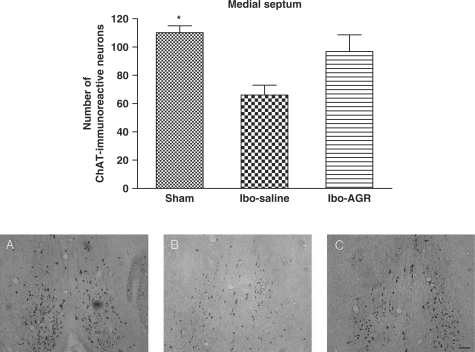
The results of the ChAT immunoreactive analysis of the MS are shown in Fig. 3. The number of ChAT positive neurons was 110.2 ± 4.9 (mean ± SEM) in the sham group and 66.0 ± 7.0 in the Ibo-saline group. This reduction exceeded 40% (F2,12 = 5.269, P < 0.05). However, the Ibo-ARG group showed no significant effect compared to the Ibo-saline group.
Figure 3.
The mean (±SEM) values of the quantities of ChAT immunoreactive nuclei in the MS of the experimental groups after the water maze learning task at 7 days post-operatively. Separate measures of one-way ANOVA for the number of neurons among the groups were followed by Tukey's test. *P < 0.05 as compared with the corresponding data of the Ibo-saline group. Scale bar represents 50 μm.
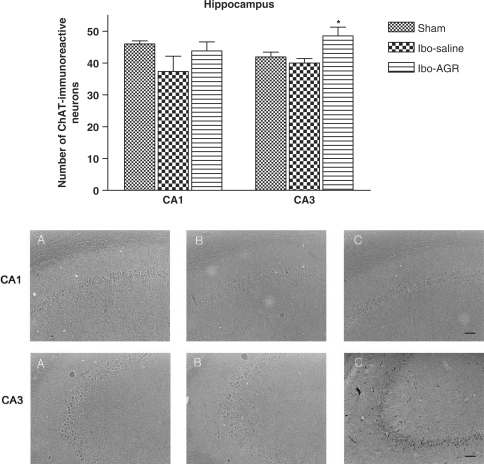
The results of ChAT immunoreactive cells per section from the different hippocampal formations are shown in Fig. 4. The number of ChAT positive neurons in the CA1 area was 46.0 ± 0.9 in the sham group, 37.3 ± 4.8 in the Ibo-saline group and 43.8 ± 2.8 in the Ibo-AGR group, but there was no significant difference among the groups (F2,12 = 2.286, P > 0.1). However, the number of immunoreactive cells in the CA3 area was 41.9 ± 1.5 in the sham group, 40.0 ± 1.4 in the Ibo-saline group and 48.5 ± 2.8 in the Ibo-AGR group (F2,12 = 96.591, P < 0.001).
Figure 4.
The mean (±SEM) values of the quantities of ChAT immunoreactive nuclei in the different hippocampal areas of the experimental groups after the water maze learning task at 7 days post-operatively. Separate measures of one-way ANOVA for the number of neurons among the groups were followed by Tukey's test. *P < 0.05 as compared with the corresponding data of the Ibo-saline group. Scale bar represents 50 μm.
AchE Histochemistry
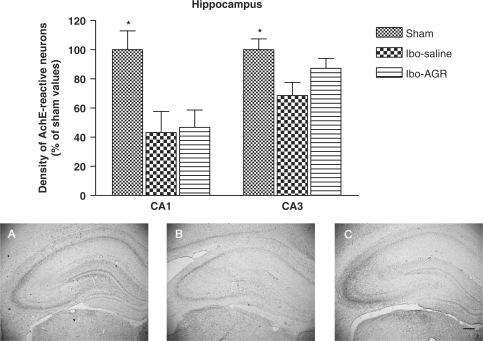
As shown in Fig. 5, the density of the AchE fibers in the hippocampal formation was decreased in the Ibo-saline group compared to the sham group. The density of the AchE neurons in the CA1 area was 24.0 ± 3.1 (100 ± 13.0%) in the sham group, 10.2 ± 3.1 (43.1 ± 14.5%) in the Ibo-saline group and 12.0 ± 2.6 (46.7 ± 11.9%) in the Ibo-AGR group (F2,14 = 6.484, P < 0.05). The density of the AchE neurons in the CA3 area was 48.6 ± 3.6 (100 ± 7.4%) in the sham group, 31.6 ± 3.5 (68.6 ± 9.0%) in the Ibo-saline group and 42.4 ± 3.3 (87.2 ± 6.8%) in the Ibo-AGR group (F2,14 = 6.154, P < 0.05). The AchE neurons in the CA3 area were more expressed than those in the CA1 area. Post hoc comparisons revealed that the Ibo lesion group showed differences in the CA1 and CA3 areas compared to the sham group (P < 0.05, both areas). The AchE-reactive neurons of the Ibo-AGR group had a significant effect in the CA3 area (P < 0.05), but not in the CA1 area (P = 0.9) compared to the Ibo-saline group in the hippocampus.
Figure 5.
The percentage (±SEM) of the sham values of the density of AchE stained nuclei in the different hippocampal formation areas of the experimental groups after the water maze learning task at 7 days post-operatively. Separate measures of one-way ANOVA for the neurons among the groups were followed by Tukey's test. *P < 0.05 as compared with the corresponding data of the Ibo-saline group. Scale bar represents 50 μm.
Discussion
The present study demonstrated that an infusion of ibotenic acid into the MS induced marked amnesic effects along with sign of neurodegeneration of AchE activity in the hippocampus of rats. In addition, AGR treatment significantly improved the performance on the Morris water maze and AGR attenuated the decrease of acetylcholinergic neurons by ibotecic acid lesioning.
AD is a neurodegenerative disease characterized by cognitive impairment and personality changes, and it affects from 5% to 10% of the adult population over 65 years of age. One of the consistent findings in the brains of AD patients is the loss the cholinergic markers, including levels of Ach and ChAT. The cholinergic approach to AD treatment involves counteracting this loss of cholinergic activity by pharmacological intervention to increase cholinergic transmission (21).
Animal models are playing a critical role in the ongoing attempts to understand the pathology and therapeutics of AD. Although no current model develops the full pathologic spectrum of this disease, injection of ibotenic acid into the MS has been shown to impair memory and elicit a degree of Alzheimer-type neurodegeneration (22). Ibotenic acid induces neuronal necrosis via hyperstimulation of the N-methyl-D-aspartate (NMDA) receptor leading to a calcium over-load. Its excitotoxic properties are confined to the somata of various neuron types; therefore, the axons and blood vessels that course through the target area remain intact (23). Ibotenic acid is also a pharmacologic tool used for studies of rat models involving lesion of cholinergic neurons by stereotaxic injections into the brain (24,25). After ibotenate lesions to the MS, at the source of the hippocampal branches of the forebrain cholinergic projection system, the rats displayed long-lasting stable impairment in their reference and working memory on both spatial (place) and associative (cue) radial maze tasks (22,26). The rats injected with ibotenic acid into the MS and nbM showed decreased ChAT activities in the hippocampus and frontal cortex, respectively, and this was followed by impairment of their memory acquisition compared with the sham-operated rats (27). In particular, the hippocampus is a vulnerable and sensitive region of the brain that is also very important for declarative and spatial learning and memory (28). The Morris water maze task, which is used to test the relatively pure spatial learning capability and reference memory might determine whether cholinergic depletion is sufficient to produce impairment (6,29,30). Learning and memory are essential requirements for every living organism, in order to cope with changing environmental demands, and the cholinergic systems are known to be involved in learning and memory.
In the current study, the performance of rats on the water maze task after AGR treatment showed significant differences between the escape latencies of the three groups for acquisition. The Ibo-ARG group revealed differences compared with the Ibo-saline group until the fourth day, and there were significant differences among the groups on the probe trial performance when compared the percentage of time spent swimming in the training quadrant. The AGR treatment group spent a greater proportion of the probe trial searching in the training quadrant. However, there were questionable results that the water maze acquisition time of the Ibo-AGR group was not longer than that of the sham group.
The ChAT activities of the groups were different. The Ibo-saline group showed a reduction in ChAT activity in the MS and the hippocampal formation. The Ibo-ARG group had a greater number of cholinergic neurons than did the Ibo-saline group in both the MS and the hippocampal area, and particularly the CA3 area. The AchE density of the groups was also significantly altered. However, the Ibo-ARG group had a slightly higher concentration of AchE than did the Ibo-saline group in the CA1 area. The Ibo-AGR group showed a significantly different from the Ibo-saline group in the CA3 area. It was likely that AGR had an ability to protect the brain neurons from ibotenic acid-induced damage. Such a protective effect might prove useful for slowing disease progression as opposed to mere symptomatic palliation.
Some of the oriental prescriptions, including AGR, have been shown to improve avoidance performance on learning and memory tasks (31,32), and also to ameliorate the impairment of learning and memory produced by thymectomy or lesioning of the amygdala (32,33). Further, the inhibitory effects of a methanol extract of AGR on excitotoxic neuronal death have been evaluated in cultured rat cortical neurons (34).
It has been reported that the central inhibitory effects of a water extract of AGR, which decreased locomotor activity, increased the pentobarbital-induced sleep, and exhibited a neuroprotective action of AGR essential oil against excitotoxicity through the blockade of NMDA receptor activity and antioxidative activities in vivo and in vitro assays (35,36). It has been demonstrated that an ethanol extract of AGR exhibited a wide range of CNS actions that were similar to those of asarone, but they differed in several other respects including the responses to electroshock, apomorphine-induced and isolation-induced aggressive behavior, amphetamine toxicity in aggressive mice, behavioral despair syndrome during forced swimming, etc. (11,12).
In conclusion, the present results demonstrated that the deficits after ibotenic acid lesions are pertinent to the degeneration of cholinergic neurons to memory impairment, and ameliorated learning and memory deficits through the effects on the Ach system. In addition, AGR improved performance on the spatial memory test and protected against the destruction of cholinergic cells in the hippocampus via ibotenic acid. Further, the anti-AD properties of AGR could also be due to cholinergic neurochemical abnormalities. It was likely that AGR had an inhibitory ability to protect brain neurons from excitotoxic ibotenic acid-induced damage. Such a protective effect might prove useful in slowing the progression of disease.
Acknowledgments
This research was supported by the Cognitive Neuro-science Program of the Korea Ministry of Science and Technology (M10644000017-06N4400-01710) and the Korea Research Foundation Grant (MOEHRD, Basic Research Promotion Fund, KRF-2005-050-E00005).
References
- 1.Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysgunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 2.Giacobini E. Cholinergic foundations of Alzheimer's disease therapy. J Physiol. 1988;92:283–7. doi: 10.1016/s0928-4257(98)80034-x. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- 4.McKinney M, Coyle JT, Hedreen JC. Topographic analysis of the innervation of the neocortex and hippocampus by the basal forebrain cholinergic system. J Comp Neurol. 1983;217:103–21. doi: 10.1002/cne.902170109. [DOI] [PubMed] [Google Scholar]
- 5.Hung MC, Shibasaki K, Nishizono S, Sato M, Ikeda I, Masuda Y, et al. Ibotenic acid-induced lesions of the medial septum increase hippocampal membrane associated protein kinase C activity and reduce acetylcholine systhesis: prevention by a phophatidylcholine/vitamin B12 diet. J Nurt Biochem. 2000;11:159–64. doi: 10.1016/s0955-2863(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 6.Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 7.Flicker C, Dean RL, Watkins DL, Fisher SK, Bartus RT. Behavioral and neurochemical effects following neurotoxin lesion of a major cholinergic input to the cerebral in the rat. Pharmacol Biochem Behav. 1983;18:973–81. doi: 10.1016/s0091-3057(83)80023-9. [DOI] [PubMed] [Google Scholar]
- 8.Tang W, Eisenbrand G. Chinese Drugs of Plant Origin. New York: Springer; 1992. [Google Scholar]
- 9.Chang HH, But PPH. Pharmacology and Application of Chinese Materia Medica. Vol. 1. Singapore: World Scientific Publishing; 1986. [Google Scholar]
- 10.Lee B, Choi Y, Kim H, Kim SY, Hahm DH, Lee HJ, et al. Protective effects of methanol extract of Acori graminei rhizoma and Uncariae Ramulus et Uncus on ischemia-induced neuronal death and cognitive impairments in the rat. Life Sci. 2003;74:435–50. doi: 10.1016/j.lfs.2003.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Vohora SB, Shah SA, Dandiya PC. Central nervous system studies on an ethanol extract of Acorus calamus rhizoma. J Ethnopharmacol. 1990;28:53–62. doi: 10.1016/0378-8741(90)90065-2. [DOI] [PubMed] [Google Scholar]
- 12.Liao J-F, Huang S-Y, Jan Y-M, Yu L-L, Chen C-F. Central inhibitory effects of water extract of Acori graminei rhizoma in mice. J Ethnopharmacol. 1998;61:185–93. doi: 10.1016/s0378-8741(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 13.Irie Y, Keung WM. Rhizoma acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid-β peptide. Brain Res. 2003;963:282–9. doi: 10.1016/s0006-8993(02)04050-7. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama N, Zhou Y, Takashina K, Saito H. Effects of DX-9386, a traditional Chinese preparation, on passive and active avoidance performance in mice. Biol Pharm Bull. 1994;17:1472–6. doi: 10.1248/bpb.17.1472. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama N, Zhou Y, Saito H. Ameliorative effects of chronic treatment using DX-9386, a traditional Chinese preparation, on learning performance and lipid peroxide content in senescence accelerated mouse. Biol Pharm Bull. 1994;17:1481–4. doi: 10.1248/bpb.17.1481. [DOI] [PubMed] [Google Scholar]
- 16.Cho J, Yang CH, Park CG, Lee H, Kim YH. Inhibition of excitotoxic neuronal cell death by total extracts from oriental medicines used for stroke treatment, Yakhak Hoeji. 2000;44:29–35. [Google Scholar]
- 17.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- 18.Morris RG. Development of a water maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 19.Cimino M, Cattabeni F, Di Luca M, Peruzzi G, Andena ML, Tirassa P, et al. Levels of NGF, p75NGFR and ChAT immunoreactivity in brain of adult and aged microencephalic rats. Neurobiol Aging. 1996;17:137–42. doi: 10.1016/0197-4580(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 20.Karnovsky MJ, Roots L. A “direct-coloring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–21. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- 21.Giacobini E. Cholinergic foundation of Alzheimer's disease therapy. J Physiol. 1998;92:283–7. doi: 10.1016/s0928-4257(98)80034-x. [DOI] [PubMed] [Google Scholar]
- 22.Hagan JJ, Salamone JD, Simpson J, Iversen SD, Morris RGM. Place navigation in rats is impaired by lesions of medial spetum and diagonal band but not nucleus basalis magnocellularis. Behav Brain Res. 1988;27:9–20. doi: 10.1016/0166-4328(88)90105-2. [DOI] [PubMed] [Google Scholar]
- 23.Choi DW, Koh J-H, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonist. J Neurosci. 1988;8:185–96. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baskys A. Metabotropic receptors and slow excitatory actions of glutamate agonists in the hippocampus. Trends Neurosci. 1992;15:92–6. doi: 10.1016/0166-2236(92)90018-4. [DOI] [PubMed] [Google Scholar]
- 25.Dunnet SB, Everitt BJ, Bobbins TW. The basal forebrain-cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- 26.Hodges H, Allen Y, Kershaw T, Lantos PL, Gray JA, Sinden J. Effects of cholinergic-rich neuronal grafts on radial maze performance of rats after excitotoxic lesions of the forebrain cholinergic projection system. I. Amelioration of cognitive deficits by transplants into cortex and hippocampus but not into basal forebrain. Neuroscience. 1991;45:587–607. doi: 10.1016/0306-4522(91)90273-q. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki R, Yamashita M, Taguch K, Okada M, Ikeda H. The role of two major cholinergic systems in memory acquisition and retention in eight-arm radial maze. Int J Geriatr Psychiatry. 1992;7:173–81. [Google Scholar]
- 28.Eichenbaum H. How does the brain organize memories? Science. 1997;277:330–2. doi: 10.1126/science.277.5324.330. [DOI] [PubMed] [Google Scholar]
- 29.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation is impaired in rats with hippocampus lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson RC, Shiffirm RM. The control of short-term memory. Sci Am. 1971;255:82–90. doi: 10.1038/scientificamerican0871-82. [DOI] [PubMed] [Google Scholar]
- 31.Nishiyama N, Zhou Y, Takashina K, Saito H. Effects of DX-9386, a traditional Chinese preparation, on passive and active avoidance performances in mice. Biol Pham Bull. 1994;17:1472–6. doi: 10.1248/bpb.17.1472. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Saito H, Nishiyama N. Improving effects of DX-9386, a traditional Chinese medicinal prescription on thymectomy-induced impairment of learning behaviors in mice. Biol Pharm Bull. 1994;17:1199–205. doi: 10.1248/bpb.17.1199. [DOI] [PubMed] [Google Scholar]
- 33.Nishiyama N, Zhou Y, Saito H. Beneficial effects of DX-9386, a traditional Chinese prescription, on memory disorder produced by lesioning the amygdala in mice. Biol Pharm Bull. 1994;17:1674–81. doi: 10.1248/bpb.17.1679. [DOI] [PubMed] [Google Scholar]
- 34.Cho J, Joo NE, Kong JY, Jeong DY, Lee KD, Kang BS. Inhibition of exitotoxic neuronal death by methanol extract of Acori graminei rhizoma in cultured rat cortical neusons. J Ethnopharmacol. 2000;73:31–7. doi: 10.1016/s0378-8741(00)00262-2. [DOI] [PubMed] [Google Scholar]
- 35.Cho J, Kong J, Yeong DY, Lee KD, Lee DU, Kang BS. Protection of cultured rat cortical neurons from excitotoxicity by asarone, a major essential oil component in the rhizomes of Acorus gramineus. Life Sci. 2002;71:591–9. doi: 10.1016/s0024-3205(02)01729-0. [DOI] [PubMed] [Google Scholar]
- 36.Koo BS, Park KS, Ha JH, Park JH, Lim HC, Lee DU. Inhibitory effects of the fragrance inhalation of essential oil from Acorus gramineus on central nervous system. Biol Pharm Bull. 2003;26:978–82. doi: 10.1248/bpb.26.978. [DOI] [PubMed] [Google Scholar]