Abstract
Background
Balance during quiet stance involves the complex interactions of multiple postural control systems, which may degrade with frailty. The complexity of center of pressure (COP) dynamics, as quantified using multiscale entropy (MSE), during quiet standing is lower in older adults, especially those with falls. We hypothesized that COP dynamics from frail elderly individuals demonstrate less complexity than those from nonfrail elderly controls; complexity decreases when performing a dual task; and postural complexity during quiet standing is independent of other conventional correlates of balance control, such as age and vision.
Methods
We analyzed data from a population-based study of community-dwelling older adults. Frailty phenotype (nonfrail, prefrail, or frail) was determined for 550 participants (age 77.9 ± 5.5 years). COP excursions were quantified for 10 trials of 30 seconds each. Participants concurrently performed a serial subtraction task in half of the trials. Complexity of balance dynamics was quantified using MSE. Root-mean-square sway amplitude was also computed.
Results
Of the 550, 38% were prefrail and 9% were frail. Complexity of the COP dynamics in the anteroposterior direction was lower in prefrail (8.78 ± 1.91 [mean ± SD]) and frail (8.38 ± 2.13) versus nonfrail (9.20 ± 1.74) groups (p < .001). Complexity reduced by a comparable amount in all three groups while performing the subtraction task (p < .001). Quiet standing complexity was independently associated with frailty after adjusting for covariates related to balance while sway amplitude was not.
Conclusion
Cognitive distractions during standing may further compromise balance control in frail individuals, leading to an increased risk of falls.
Keywords: Aging, Frailty, Dual task, Posture control, Complexity
FRAILTY is a common geriatric syndrome, characterized by a constellation of systemic symptoms and a decreased resistance to stress. Fried and colleagues (1) defined a phenotype of frailty that predicts adverse outcomes, including falls, disability, and mortality. Previous work has examined individual components of the frailty syndrome, including sarcopenia (2) and cardiovascular abnormalities (3). However, the biological basis of frailty is not well understood. Beyond such individual components, the frailty syndrome may importantly involve impaired integration of regulatory mechanisms that control physiological function under both baseline (free-running) and stress conditions (4). An important challenge lies in quantifying the integrative dynamics that underlies adaptive systems such as those controlling human balance and its degradation with frailty, the subject of the present study.
Previously, we showed that the dynamics of center of pressure (COP) excursions on a balance platform exhibit complex fluctuations over a broad range of timescales, as quantified using the multiscale entropy (MSE) method (5). Furthermore, healthy individuals exhibited more complex postural sway fluctuations than elderly individuals with a history of falls. The results were consistent with the general conceptual framework that complexity decreases with aging and disease under free-running conditions. Although studies of other regulatory systems, such as those controlling blood pressure (6), hormone levels (7), and heart rate (8), were also consistent with this framework, the relationship between changes in dynamical complexity and aging has been difficult to probe. Apparently contradictory findings (9–11) may be due to (i) lack of an unifying definition of complexity; (ii) use of different methods to quantifying complexity, some of which are single scale based and fail to capture the multiscale properties inherent in physiological control systems; (iii) different preprocessing techniques; and (iv) different experimental protocols (e.g., free-running vs goal-directed challenge). Based on prior work (12,13), we relate complexity with the coexistence of the following properties in a given system: (i) variability over multiple timescales, (ii) long-range correlations, (iii) time irreversibility, and (iv) nonlinearity.
In this study of a diverse community-based population of elderly people, we sought to determine whether there is an association between frailty and a decrease in the complexity of COP dynamics during both free-running and stressed conditions associated with a cognitive task. We hypothesized that (i) COP time series from frail individuals are less complex than those from nonfrail during quiet standing; (ii) performance of a cognitive “dual” task reduces the complexity of balance dynamics in older adults, with greater reduction in frail individuals; and (iii) the loss of postural dynamical complexity associated with frailty is independent of other conventional correlates of balance control, such as age, vision, peripheral neuropathy, and executive function.
METHODS
Participants
The MOBILIZE Boston Study (MBS), which stands for “Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston,” is a prospective study examining risk factors for falls, including pain, cerebral hypoperfusion, and foot disorders in the older population. The study includes a representative population sample of 765 elderly volunteers aged 70 years and older from the Boston area. After providing informed consent as approved by the Hebrew SeniorLife Institutional Review Board, all participants underwent a standardized evaluation. Study design details are presented elsewhere (14). Baseline data were available for the first 600 participants.
Frailty Definition
Frailty status was determined using an adaptation of the Fried and colleagues’ (1) definition. Frailty characteristics included weakness, unintentional loss of weight, slow gait, exhaustion from Center for Epidemiological Studies-Depression scale, and low daily activity (Appendix 1), with the following modifications. Weakness was defined using the time required to perform five repetitions of sit-to-stand (15). Low daily activity was determined using the Physical Activity Scale for the Elderly (16). Persons exhibiting three or more of these five characteristics were considered “frail,” one to two characteristics were considered “pre-frail,” and those exhibiting none were considered “nonfrail.”
Balance Assessment
Participants stood barefoot with the feet about 30 cm apart and eyes open on a force platform (Kistler 9286AA, Watertown, MA). No visual target was specified. The COP displacements under their feet in anteroposterior (AP) and mediolateral (ML) directions were sampled at 240 Hz. Participants performed two sets of five quiet standing trials, 30 seconds each. One set included a cognitive task (dual-task challenge). The order of the sets was randomized. Trials were grouped by sets of five to minimize carryover effects between conditions.
For the dual-task challenge, participants verbally counted backwards by 3 from 500 while standing. They continued the subtractions where they previously left off in subsequent trials. If five counting errors were made, the task was modified to: counting backward by 1 from 500, then counting backward by 1 from 100, then naming items found at a supermarket. Participants sat and rested for 1 minute between trials.
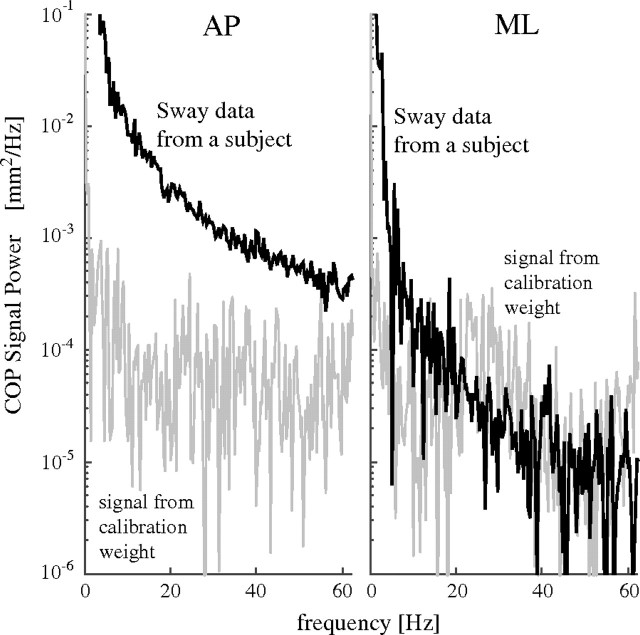
To assess the signal-to-noise ratio, we recorded the COP fluctuations for a 50 lb (22.7 kg), weight and compared its power with that of a COP time series from a nonfrail participant, across the bandwidth of interest (7.5–60 Hz). Because the signal-to-noise ratio in the ML direction was lower than 1 (Figure 1), we only analyzed the postural dynamics in the AP direction.
Figure 1.
Power spectral density of center of pressure (COP) time series from a nonfrail participant (black) and of the calibration signal (gray) in the anteroposterior (AP) and mediolateral (ML) directions. The signal-to-noise ratio between 7.5 and 60 Hz in the AP direction (top) is more than 10, but in ML direction, it is less than 1. Therefore, no further analyses of the COP time series in the ML direction were performed.
COP Data Processing
The complexity of COP time series was quantified using the MSE method described elsewhere (17–19). Briefly, MSE quantifies the degree of irregularity of a time series over multiple timescales. Time series that are highly irregular, thus more entropic, over a broad range of timescales are considered more complex than those that show irregular behavior at only a single timescale. Single-scale methods, such as approximate entropy and sample entropy (SampEn), yield higher entropy values for uncorrelated random signals than for signals with long-range correlations, which have been interpreted as indicating that the former are more complex than the latter. In contrast, MSE that quantifies sample/approximate entropy over a range of scales resolves the apparent paradox between variability and complexity because it yields higher entropy values for correlated than for uncorrelated signals across a range of scales.
The MSE analysis consists of three steps: (i) coarse graining the original time series to derive multiple signals, each of which captures the system dynamics on a given scale; (ii) calculating a measure of entropy suitable for finite time series, SampEn in this case, for each coarse-grained time series; and (iii) integrating the entropy values over a predefined range of scales to obtain an index of complexity (CI).
The element j of the coarse-grained time series y for scale n is calculated according to the equation: (18), where xi, with 1 ≤ i ≤ N, are the data points of the original time series. MSE, as noted, uses SampEn to quantify the degree of irregularity of a time series. SampEn is a conditional probability measure that quantifies the likelihood that if a vector with m data points matches, within a tolerance r, a template of the same length, then the vector and the template will still match when their length increases from m to m + 1 data points. The MSE curve is obtained by plotting SampEn for each coarse-grained time series (ordinate) as a function of scale (Figure 2, right). The CI is the area under the MSE curve (17,18). The length of the original time series, N, determines the largest scale, n, analyzed (17). In this study, we used n = 8. Taking into consideration previous recommendations (17,20,21), we used m = 2 and r = 15% of the standard deviation of the original signal. Of note, SampEn is a self-consistent metric, that is, given two signals, A and B, if SampEnN1,m1,r1(A) > SampEnN1,m1,r1(B), then SampEnN2,m2,r2(A) > SampEnN2,m2,r2(B), for a wide range of parameter values. Consistently, high entropy values over a wide range of timescales, and thus high CI, indicate high complexity and vice versa. Scale 1 was excluded from the analysis because it is the most affected by superimposed uncorrelated noise generated by recording devices or rounding errors (13).
Figure 2.
Anteroposterior (AP) center of pressure time series from representative nonfrail and frail participants, root-mean-square (RMS) values, multiscale entropy (MSE) curves and CI values. Both the original and the high-pass filtered time series are presented during quiet stance and dual task. The RMS amplitudes were calculated for the original signals. The MSE plots and the CI values were derived from the high-pass filtered time series.
We preprocessed the data to remove low-frequency fluctuations and outliers that lead to spuriously low entropy values in short time series such as those analyzed here (17,22). We defined outliers as values outside the interval mean ±3 SDs. We used the empirical mode decomposition (EMD) method, described elsewhere (23), to high-pass filter the data. Briefly, the EMD method decomposes a signal into n intrinsic mode functions (IMFs), each of which has a dominant frequency given by: sampling frequency/2n Hz. We restricted our analysis to the first four IMFs (n = 1–4), thereby eliminating dynamical information on timescales larger than 133 milliseconds (25/240 seconds). This procedure is equivalent to applying a high-pass filter with a cutoff frequency at 7.5 Hz (Figure 2).
We next tested the hypothesis that the dynamical properties of the COP time series could not be generated by uncorrelated random processes. To this aim, we compared the complexity of the original COP time series with that of a randomized time series obtained by shuffling the order of the data points of the original time series. Because, by construction, both the physiological and the shuffled (surrogate) time series have the same mean, variance, and distribution, any differences in the complexity indexes are due to differences in the temporal order of the data points and their correlation properties.
In addition to CI, which is independent of the amplitude of the fluctuations, we calculated the following three traditional sway parameters: root-mean-square amplitude for each COP time series (RMS sway) before and after high-pass filtering, COP path length, and mean power frequency (MPF) of the COP data (24). Signal processing was performed using C and Matlab 7.04 (Mathworks, Natick, MA).
Measuring Factors Associated With Balance
Postural control involves the interaction of sensory systems, central processing, and the musculoskeletal system. We considered multiple covariates related to balance that might explain the association between the frailty and the dynamical measures described above. Vision was quantified using a Snellen eye chart. Tactile sensation in the feet was quantified using Semmes–Weinstein monofilaments of two sizes (4.1 and 5.6 g). Peripheral neuropathy was defined as sensing one of these of the filaments less than three times out of four in either foot. Executive function was determined using the Trail Making Test Part B (TMT-B) (25).
Statistics
We compared the complexity indices among different groups, for quiet standing and dual-task conditions, and assessed their interactions using a mixed-model analysis of variance. To account for multiple observations per person, the analysis was done with empirical (“sandwich”) standard error estimation (26) and unstructured covariance. CI values were log-transformed to obtain a normal distribution. To determine if CI or other COP measures were independently associated with frailty, we employed a multivariate ordinal logistic regression model using the three frailty categories as the outcome and adjusting for the covariates that may affect standing balance: age, vision, peripheral neuropathy, and TMT-B as defined above. Analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Among 600 persons in the MBS data set, frailty status and postural sway could be determined for 550 participants (Table 1). Among these 550 participants, 38% were prefrail and 9% were frail. Frail individuals were older and exhibited more disability and depression, worse mini-mental state scores (p < .005), and reduced executive function (p < .001; Table 1). Of the 21 participants without balance data, 12 were prefrail and 6 were frail, most of who had difficulty standing. Of the 29 whose frailty status could not be determined due to missing data, 8 showed some frailty symptoms. CI of sway in these 29 participants was not different from the 550 considered in the final analysis (p = .88).
Table 1.
Participant Characteristics
| Not frail | Prefrail | Frail | p Value | |
| N (%) | 292 (53) | 209 (38) | 49 (9) | |
| Sex (% female) | 61 | 68 | 51 | .92* |
| Age (y ± SD) | 76.6 ± 5.1 | 78.8 ± 5.6 | 79.6 ± 5.6 | <.001 |
| 65–74 (n) | 122 | 55 | 11 | |
| 75–84 (n) | 147 | 117 | 28 | |
| 85+ (n) | 23 | 37 | 10 | |
| Race (% Caucasian) | 81 | 78 | 69 | .10* |
| Education (grades completed) | 14.7 ± 2.8 | 14.1 ± 3.0 | 12.2 ± 3.5 | <.001 |
| Cognitive function (MMSE) | 27.7 ± 2.3 | 27.0 ± 2.7 | 25.3 ± 3.5 | <.001 |
| Executive function (TMT-B(s)) | 127.9 ± 82.6 | 156.3 ± 104.8 | 208.7 ± 118.8 | <.001 |
| Depression (DSM-IV) | ||||
| % with minor symptoms | 2.7 | 9.6 | 16.3 | <.001* |
| % with major symptoms | 0 | 0.5 | 4.1 | |
| Height (m) | 1.63 ± 0.09 | 1.63 ± 0.10 | 1.65 ± 0.10 | .29 |
| Weight (kg) | 72.4 ± 14.4 | 73.2 ± 16.1 | 76.2 ± 19.6 | .28 |
| Body mass index (kg/m2) | 27.0 ± 4.5 | 27.3 ± 5.0 | 28.1 ± 5.9 | .27 |
| PASE | 128.2 ± 64.8 | 85.3 ± 63.5 | 38.7 ± 36.6 | <.001 |
| 4-m walk time (s) | 3.9 ± 0.7 | 4.7 ± 1.2 | 6.7 ± 1.6 | <.001 |
| 5× Sit-to-stand time (s) | 11.1 ± 2.2 | 14.3 ± 3.9 | 18.3 ±7 4.6 | <.001 |
| ADL | ||||
| % with little difficulty | 6.5 | 16.6 | 36.7 | <.001* |
| % with a lot of difficulty | 1.0 | 9.1 | 20.4 | |
| IADL | ||||
| % with little difficulty | 17.5 | 25.8 | 12.2 | <.001* |
| % with a lot of difficulty | 9.9 | 25.8 | 51.0 | |
| Hypertension | ||||
| % controlled | 48.8 | 55.8 | 59.6 | <.004* |
| % uncontrolled | 23.0 | 25.5 | 31.9 | |
| Falls Efficacy Scale | 98.0 ± 4.5 | 95.9 ± 8.0 | 88.0 ± 13.9 | <.001 |
| % with Neuropathy | 6.5 | 13.5 | 31.9 | <.001* |
| % Recurrent fallers | 17.8 | 14.4 | 25.0 | .60* |
| Falls over past year | 0.67 | 0.74 | 1.26 | .11 |
Notes: ADL: activities of daily living disability index; IADL: instrumental activities of daily living disability index; MMSE = Mini-Mental Status Examination; PASE: Physical Activity Scale for the Elderly; TMT-B: Trail Making Test Part B.
Mantel–Hansel χ2 test.
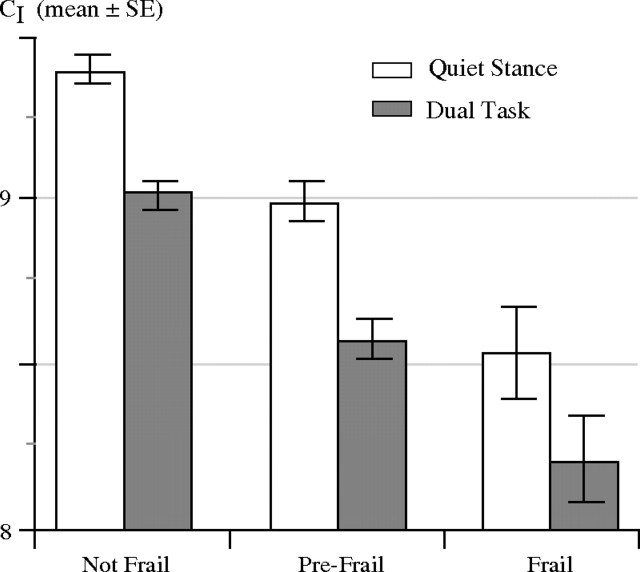
Figures 3 and 4 show that (i) for all three groups, CI values from quiet standing trials were higher than those from the dual-task trials (p < .001–0.04, Scales 2–7) and (ii) CI values were higher for the nonfrail than for the prefrail or frail groups (p < .001–.02, Scales 2–8). Differences in the CI between frailty groups remained significant (p = .006) when age (p < .001) was included as a covariate. The reduction of CI due to the dual task was not different among the three groups (Frailty × Dual-task interaction, p = .6).
Figure 3.
Multiscale entropy (MSE) analysis of COP sway dynamics for the nonfrail, prefrail, and frail groups during quiet standing and dual-task conditions. The MSE curves were obtained by connecting the group mean values of sample entropy for each scale. The error bars represent standard errors. The MSE curves for the surrogate shuffled time series (see text) are also presented.
Figure 4.
Complexity indices (CI) for nonfrail, prefrail, and frail groups during quiet standing and dual-task conditions. Solid bars and error bars represent group mean values and standard errors, respectively. The CI for the frail (p = .003; post hoc LSD) and prefrail (p < .001) groups were lower than for the nonfrail group and dropped further with dual task (p < .001). Prefrail and frail groups were not different (p = .2).
Of note, the MSE curves for the shuffled time series showed a monotonic decrease of SampEn values with scale factor, characteristic of uncorrelated noise (17) (Figure 3). In contrast, the MSE curves for the original COP time series show a “plateau” for large timescales. These results demonstrate that the COP sway dynamics over timescales ranging from 17 to 133 milliseconds (7.5–60 Hz) are not consistent with uncorrelated processes but represent complex behavior.
The CI values were reliable within a set (intraclass correlations [ICC](2,1) = 0.87 without dual task and 0.66 with dual task). No systematic order effects or fatigue effects were seen. Frail participants had greater difficulty in performing the subtractions by 3 and performed more of the easier tasks. However, the different dual tasks had similar effects on all three frailty groups (p ≥ .16, least-squared difference [LSD] post hoc comparisons).
Frailty and Traditional COP Measures
Root-mean-square (RMS) amplitude of sway both before and after high-pass filtering (p < .001), COP path length (p = .001), and MPF (p = .06) were higher in frail individuals and increased with the dual task (p < .001; Table 2). The CI and the RMS sway were not correlated (r = −.02, p = .6). Filtered RMS sway (r = −.56), MPF (r = −.58), and path length (r = −.57) were significantly correlated with CI (p < .001).
Table 2.
Traditional COP Parameters
| Not Frail | Prefrail | Frail | p value | ||||||
| QS | DT | QS | DT | QS | DT | Frailty | Task | Interaction | |
| RMS sway (mm) | 4.45 (1.61) | 4.99 (2.18) | 4.70 (1.75) | 4.91 (2.04) | 5.52 (2.10) | 5.84 (2.66) | .005 | <.001 | .08 |
| RMS sway (high-pass filtered, mm) | 0.085 (0.074) | 0.124 (0.130) | 0.108 (0.097) | 0.145 (0.140) | 0.172 (0.224) | 0.213 (0.251) | .002 | <.001 | .99 |
| Mean power frequency (Hz) | 0.28 (0.13) | 0.33 (0.15) | 0.29 (0.14) | 0.34 (0.16) | 0.32 (0.17) | 0.38 (0.22) | .06 | <.001 | .53 |
| COP path length (mm) | 430.3 (95.9) | 487.9 (134.2) | 458.0 (119.9) | 512.8 (157.0) | 545.4 (225.9) | 595.7 (213.7) | .001 | <.001 | .86 |
Note: Mean (SD) are shown for each traditional COP measure (21). p Values are from the mixed-model analysis of variance as described in the text. All COP variables are calculated from anteroposterior sway. The RMS sway and COP path length of nonfrail and prefrail groups were not different (p = .41; post hoc least-squared difference [LSD]) from each other. The frail group exhibited larger sway amplitude than the other two (p ≤ .005). RMS for high-pass filtered time series could distinguish all three groups (p < .001; post hoc LSD). COP = center of pressure; DT= dual task; Interaction = interaction of frailty and dual task; QS= quiet stance; RMS = root-mean-square.
Determinants of Physiological Complexity and Frailty
In the adjusted logistic regression analysis with frailty status as outcome, CI during quiet stance (p = .017) was independently associated with frailty status after including covariates. CI was also associated with neuropathy (t = 3.35, p = .001), vision score (r = .12, p = .005) but not with TMT-B (r = −.01, p = .76). RMS sway was not associated with frailty when covariates were taken into account (Table 3).
Table 3.
Multivariable-Adjusted Associations of Balance Measures With Frailty
| Multivariate Models | OR | 95% CI* | p Value |
| CI | 0.303 | 0.114–0.805 | .017 |
| RMS sway | 1.039 | 0.943–1.144 | .44 |
| RMS sway (high-pass filtered) | 10.3 | 1.312–80.76 | .027 |
| Mean power frequency | 1.266 | 0.246–6.50 | .77 |
| COP path length | 1.002 | 1.000–1.004 | .017 |
Notes: Dependent variable: frailty status. Each line represents a different model that includes the listed COP-derived measure, with age, neuropathy, vision, and Trail Making Test Part B as covariates. All covariates were statistically significant in the each model. Including education in the model did not affect the final results. Neuropathy was defined dichotomously. CI, COP path length, and RMS of high-pass filtered sway signal were significantly associated with frailty even after adjusting for these covariates. CI = complexity index; COP = center of pressure; OR = odds ratio; RMS = root-mean-square.
95% CI = 95% confidence interval of the OR.
DISCUSSION
The results are consistent with the hypothesis that balance dynamics of frail individuals are less complex than those of nonfrail older adults during both quiet standing and dual-task challenges. Low dynamical complexity in frail older adults may be a marker of degraded postural control mechanisms similar to those with a history of falls. For all three groups, the superimposition of a concurrent mental task lowers the complexity of balance dynamics. The reduction of balance complexity during a dual task and the increase in RMS sway may be due to the diversion of attentional resources away from postural control. However, CI appears more sensitive to the onset of frailty than RMS sway (Table 2 and Figure 4) because it could distinguish prefrail from nonfrail individuals. Frail individuals did not exhibit a greater drop in CI with dual task than the others groups, possibly due to a relatively low number of frail individuals and the fact that their CI indexes may already be very close to a minimum value of postural complexity compatible with the ability to stand. The decrease of balance complexity in frail older adults may bring them closer to a threshold for falls. The contribution of cognitive distractions to fall risk needs further investigation.
CI could independently predict frailty status after accounting for other physiological variables thought to affect balance control, thus suggesting that the MSE method may quantify an aspect of frailty not captured by functional tests of individual physiological systems. Our results support the notion that frailty is a global syndrome that degrades integrative physiological function beyond that of each subsystem.
We quantified the dynamical properties of sway on timescales shorter than 133 milliseconds (>7.5 Hz), not selectively analyzed in other COP studies. The small signal power in this bandwidth may affect the stability of these measures and comparability to previous work. However, we identified dynamics clearly distinct from random noise, and our complexity measures are stable as shown by the ICC values. Furthermore, both RMS amplitude and complexity of these high-frequency fluctuations that quantify complementary properties of the COP signal change with frailty status and dual task (27).
The high-frequency components of the dynamics that we quantified may arise from postural reflexes that operate over timescales as short as approximately 40 milliseconds related to tactile sensation from the feet, as CI was associated with neuropathy. In addition, leg muscle activity that produces force fluctuations around 8–12 Hz (28) may also contribute to the high-frequency fluctuations in COP sway. Force fluctuations become more variable with aging possibly due to loss and remodeling of muscle motor units (29) associated with sarcopenia (2). Additional roles of attention and verbal effects (30) in these high-frequency mechanisms need further examination.
To our knowledge, we present the first report on quantitative posturography measures in frail individuals during a dual-task paradigm, with a representative population sample. Apparent discrepancies between our results and other on the effects of dual-task challenges on postural sway amplitude may be due to different experimental paradigms (31–33). Recently, Duarte and Sternad (27) studied COP data from 30 minutes of standing using MSE. They did not find significant differences between young and old groups. However, comparison between their study and ours is difficult due to differences in participant groups (health status, age, and sample size) and the effects of marked low-frequency dynamics induced by prolonged standing.
There are several limitations to our study. To prevent participant fatigue, each trial lasted for only 30 seconds, thus limiting the timescales that could be analyzed. However, the effects of frailty and dual task still could be seen over the range of timescales considered. The observed association between frailty and complexity is cross-sectional. Future work is needed to assess whether the complexity of COP dynamics predicts the onset of frailty and related adverse outcomes.
FUNDING
This work was funded by grants R37AG025037, P01AG004390, T32AG023480, and U01EB008577 from the National Institutes of Health, the G. Harold and Leila Y. Mathers Charitable Foundation, the Ellison Medical Foundation Senior Scholars in Aging Program (A.L.G.), the James S. McDonnell Foundation, and the Defense Advanced Research Projects Agency.
CONFLICT OF INTEREST
The authors declare that no conflict of interest exists with regard to this work.
Acknowledgments
The authors acknowledge the MOBILIZE Boston research team and study participants for their time, effort, and dedication. We thank Dr. Jeffrey Hausdorff for his consultation.
Appendix 1
Criteria for Frailty Characteristics
Weight loss: Self-report of unintentionally losing 10 lb (4.53 kg) over the past year.
Exhaustion: Answering the Center for Epidemiological Studies-Depression scale question “I could not get going” as 3 or more days a week.
Physical activity: Lowest quintile of Physical Activity Scale for the Elderly score, stratified by sex: women <40; men <45.
Slow gait: Highest quintile of 4-m walk time, stratified by sex and height (median split):
Women:
Height <1.583 m >5.9 s;
Height ≥1.583 m >5.0 s
Men:
Height <1.73 m >5.3 s;
Height ≥1.73 m >4.8 s
Weakness: Highest quintile of 5× sit-to-stand time: >15.2 s (did not differ by sex or body mass index) or inability to perform the task.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 4.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57(3):B115–B125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 5.Costa M, Priplata AA, Lipsitz LA, et al. Noise and poise: enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Europhys Lett. 2007;77:68008. doi: 10.1209/0295-5075/77/68008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler GC, Ando S, Floras JS. Fractal component of variability of heart rate and systolic blood pressure in congestive heart failure. Clin Sci (Lond) 1997;92(6):543–550. doi: 10.1042/cs0920543. [DOI] [PubMed] [Google Scholar]
- 7.Ilias I, Vgontzas AN, Provata A, Mastorakos G. Complexity and non-linear description of diurnal cortisol and growth hormone secretory patterns before and after sleep deprivation. Endocr Regul. 2002;36(2):63–72. [PubMed] [Google Scholar]
- 8.Peng CK, Havlin S, Hausdorff JM, Mietus JE, Stanley HE, Goldberger AL. Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdown with disease. J Electrocardiol. 1995;28(Suppl):59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 9.Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23(1):1–11. doi: 10.1016/s0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- 10.Donker SF, Roerdink M, Greven AJ, Beek PJ. Regularity of center-of-pressure trajectories depends on the amount of attention invested in postural control. Exp Brain Res. 2007;181(1):1–11. doi: 10.1007/s00221-007-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanaugh JT, Mercer VS, Stergiou N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: a methodological report. J Neuroeng Rehabil. 2007;4:42. doi: 10.1186/1743-0003-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberger AL. Giles F. Filley Lecture. Complex systems. Proc Am Thorac Soc. 2006;3:467–471. doi: 10.1513/pats.200603-028MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa MD, Peng CK, Goldberger AL. Multiscale analysis of heart rate dynamics: entropy and time irreversibility measures. Cardiovasc Eng. 2008;8(2):88–93. doi: 10.1007/s10558-007-9049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 17.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71(2 Pt 1):021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- 18.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89(6):068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- 19.Thuraisingham RA, Gottwald GA. On multiscale entropy analysis for physiological data. Physica A. 2006;366(1):323–332. [Google Scholar]
- 20.Pincus SM. Approximate entropy as a measure of system complexity. Proc Nat Acad Sci U S A. 1991;88(6):2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2002;283(3):R789–R797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- 22.Peng CK, Costa M, Goldberger AL. Adaptive data analysis of complex fluctuations in physiologic time series. Adv Adapt Data Anal. 2009;1(1):61–70. doi: 10.1142/S1793536909000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang NE, Shen Z, Long SR, et al. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc R Soc Lond A. 1998;454:903–995. [Google Scholar]
- 24.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng. 1996;43(9):956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 25.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2nd ed. Tuscon, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 26.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 27.Duarte M, Sternad D. Complexity of human postural control in young and older adults during prolonged standing. Exp Brain Res. 2008;191(3):265–276. doi: 10.1007/s00221-008-1521-7. [DOI] [PubMed] [Google Scholar]
- 28.Tracy BL, Dinenno DV, Jorgensen B, Welsh SJ. Aging, visuomotor correction, and force fluctuations in large muscles. Med Sci Sports Exerc. 2007;39(3):469–479. doi: 10.1249/mss.0b013e31802d3ad3. [DOI] [PubMed] [Google Scholar]
- 29.Roth SM, Ferrell RF, Hurley BF. Strength training for the prevention and treatment of sarcopenia. J Nutr Health Aging. 2000;4(3):143–155. [PubMed] [Google Scholar]
- 30.Dault MC, Yardley L, Frank JS. Does articulation contribute to modifications of postural control during dual-task paradigms? Brain Res Cogn Brain Res. 2003;16(3):434–440. doi: 10.1016/s0926-6410(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 31.Prado JM, Stoffregen TA, Duarte M. Postural sway during dual tasks in young and elderly adults. Gerontology. 2007;53(5):274–281. doi: 10.1159/000102938. [DOI] [PubMed] [Google Scholar]
- 32.Sturnieks DL, St George R, Fitzpatrick RC, Lord SR. Effects of spatial and nonspatial memory tasks on choice stepping reaction time in older people. J Gerontol A Biol Sci Med Sci. 2008;63(10):1063–1068. doi: 10.1093/gerona/63.10.1063. [DOI] [PubMed] [Google Scholar]
- 33.Melzer I, Benjuya N, Kaplanski J. Age-related changes of postural control: effect of cognitive tasks. Gerontology. 2001;47(4):189–194. doi: 10.1159/000052797. [DOI] [PubMed] [Google Scholar]