Abstract
Objective
To determine whether Experience Corps (EC), a social service program, would improve age-vulnerable executive functions and increase activity in brain regions in a high-risk group through increased cognitive and physical activity.
Methods
Eight community-dwelling, older female volunteers and nine matched wait-list controls were recruited to serve in the ongoing EC: Baltimore program in three elementary schools. We employed functional magnetic resonance imaging (fMRI) preintervention and postintervention to examine whether EC volunteers improved executive function and showed increased activity in the prefrontal cortex relative to controls. fMRI volunteers were trained and placed with other volunteers 15 h/wk for 6 months during the academic year to assist teachers in kindergarten through third grade to promote children’s literacy and academic achievement.
Results
Participants were African American and had low education, low income, and low Mini-Mental State Examination scores (M = 24), indicative of elevated risk for cognitive impairment. Volunteers exhibited intervention-specific increases in brain activity in the left prefrontal cortex and anterior cingulate cortex over the 6-month interval relative to matched controls. Neural gains were matched by behavioral improvements in executive inhibitory ability.
Conclusions
Using fMRI, we demonstrated intervention-specific short-term gains in executive function and in the activity of prefrontal cortical regions in older adults at elevated risk for cognitive impairment. These pilot results provide proof of concept for use-dependent brain plasticity in later life, and, that interventions designed to promote health and function through everyday activity may enhance plasticity in key regions that support executive function.
Keywords: Prefrontal cortex, Executive function, fMRI, Aging, Social engagement
THE prevalence of one of the most costly and irreversible conditions, Alzheimer’s disease (AD), is expected to rise fourfold, to 8.6 million, over the next 50 years (1). In order to be responsive to this potential health crisis, Healthy People 2010 has emphasized efforts to increase the quality and years of healthy life and eliminate health disparities that magnify with age, particularly among those with low education and low income. Such efforts may include the design of activity programs that improve the health and well-being of our aging population and thus prevent or halt age-vulnerable cognitive and neurological declines.
Epidemiological observational studies have suggested that leisure-time cognitive, physical, and social activities help maintain cognitive and functional health (for reviews, see (2,3)). Executive planning and organizational skills appear to be important to maintaining functional independence (4–7) and appear to be particularly vulnerable to declines at later ages (8–10) along with the prefrontal cortical regions of the brain that support them (11–14). These findings suggest that executive functions may contribute to both memory and functional difficulties and serve as an important target for preventive interventions.
To date, little is known about the efficacy of community-based cognitive and physical exercise programs to improve a range of cognitive abilities (3). Engaging in complex work and leisure environments has been associated with improved mental flexibility over the long term, particularly among older adults (15). Complex environments impose cognitive challenges through the diversity of stimuli and the number of decisions required. As a result, they exercise organizational, inhibitory, and working memory skills, all components of executive function.
We now describe a new model designed to enhance physical, social, and cognitive activity simultaneously, and how cognitive activity, broadly, and exercise of executive function, in particular, were intentionally embedded within the design of program roles. The Experience Corps (EC) program was designed (16) to train and place volunteers in participating elementary schools for an academic year during which time they assisted teachers in grades kindergarten-third grade with literacy and library functions (17). A pilot randomized trial of this program in Baltimore demonstrated program-specific benefits in children’s academic achievement (18) and in the physical (17) and cognitive health (8) of senior volunteers. Specifically, we found that those at greatest risk for executive deficits showed substantial and clinically meaningful improvements in these and other functions because of participating in EC. Our promising short-term findings among individuals at risk for cognitive impairment suggest that they have sufficient neurocognitive reserves or plasticity to benefit immediately and substantially from this type of high-impact activity intervention.
We next sought to find preliminary evidence of brain plasticity in age-vulnerable executive functions among these cognitively at-risk older adults through a functional magnetic resonance imaging (fMRI) pilot study of EC in eight volunteers and nine matched controls (see Table 1). We describe results of this pilot study of EC, a program that provides an ideal environment in which to test the potential for a multimodal activity intervention to influence cognitive and brain health. Additionally, practical goals were to determine whether the use of fMRI would be feasible in participants who do not typically comprise volunteer samples for intervention.
Table 1.
Baseline Demographic and Cognitive Characteristics of Women Participating in the fMRI Pilot Study, Stratified by Intervention and Control Groups
| Characteristic | EC, Mean | Control, Mean |
| N | 8 | 9 |
| Age, in y (SD) | 68 (5.0) | 67.78 (3.7) |
| African American, n (%) | 8 (100) | 9 (100) |
| Education, in y (SD) | 12.4 (1.3) | 11.6 (2.5) |
| Widowed, n (%) | 5 (62) | 1 (11.1) |
| MMSE (SD) | 24.5 (3.6) | 26.4 (1.7) |
Note: EC, Experience Corps; fMRI, functional magnetic resonance imaging; MMSE, Mini-Mental State Examination.
METHODS
Participants
All prospective volunteers attended information sessions to describe the EC program and participation requirements, if interested. Eligibility criteria included (a) being 60 years of age or older; (b) English speaking; (c) agreeing to commit to at least 1 year; (d) agreeing to participate at least 15 h/wk for the full school year; (e) meeting minimum criteria for cognitive functioning necessary to function successfully in a school setting via an education-sensitive, two-step process using the Mini-Mental State Examination (MMSE) (19) score = 24 or higher or if scoring 20–23, with a high school education or less, and successful completion of the Trail Making Test (TMT) (20) within the time allotted (420 seconds). The TMT served as a measure of mental flexibility, a skill presumed key to adaptability in the schools; (f) minimum fifth grade level reading literacy; (g) clearance on the Baltimore city public school’s criminal background check; and (h) completing a 2-week training to participate in EC. In addition, to participate in this fMRI pilot study, participants also had to (a) be free of a pacemaker or other ferrous metal objects in the body, (b) have no history of brain cancer or brain aneurism or stroke in the prior year, and (c) be right-hand dominant to avoid possible confounds due to laterality in left-handed individuals. They were then scheduled for a separate 1-hour fMRI visit at the FM Kirby Center at Johns Hopkins. This study was approved by the Johns Hopkins IRB, and each participant gave informed, written consent. All participants received a $50 honorarium for each fMRI visit.
Participants were African American, 80% of whom had a high school education and marginally normal global cognitive scores on the MMSE (average = 24.5). Half were trained and integrated with existing teams of experienced EC volunteers in two elementary schools, and half were wait-listed for enrollment the following academic year.
Intervention
This multimodal EC activity program is described in detail elsewhere (21). It was designed to bolster memory and executive functions by exercising working memory skills via reading comprehension activities with children, in literacy and library support, cooperative problem-solving skills with team members, students, and teachers, and through program activities that operated along multiple dimensions of cognitive ability and by exercising mental flexibility through the need to shift across EC roles.
All volunteers were trained on the following modules and provided with a corresponding tool book covering the following:
General Literacy Support: provides a literacy support guide to train adults who are reading with and to children. It aids adults in assessing children’s current reading levels to guide level-appropriate book selection, build vocabulary and comprehension, and ask questions about the book.
Library Support: supports library functions, including shelving or cataloguing books, reopening and helping staff school libraries, helping children pick books they will enjoy, and reading to/with children, all under the guidance of a librarian.
Conflict Resolution, entitled “Partners in Play” (18): teaches children conflict resolution through play in a supervised recess program. Volunteers are trained in how to lead, set goals, and play a variety of both quiet group activity games and board games.
Outcome Measure: Flanker Task
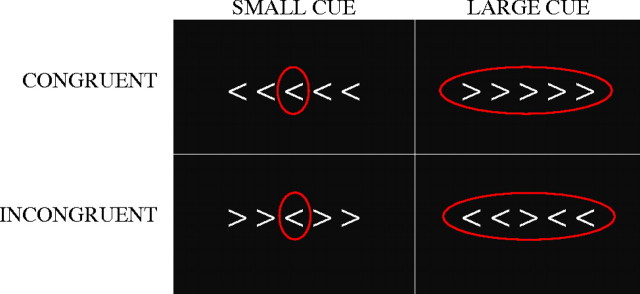
This selective attention task measures one’s ability to rapidly determine the direction of a central target (arrow) while effectively inhibiting distracting information that flanks the target and may conflict with the target response (e.g., central target points left, whereas flanking arrows point right). Each trial consisted of visual presentation of a central target arrow flanked on either side by two arrows using a magnetic resonance imaging (MRI)-safe back projection system. If the center arrow pointed right, participants were instructed to press the button in their right hand; if the center arrow pointed left, participants were instructed to press the button in their left hand. Speed and accuracy were emphasized. Task difficulty was manipulated across trials by varying the direction of the flankers, which were either incongruent (<<><< and >><>>) or congruent (<<<<< and >>>>>) with the central arrow. In the congruent condition, flanking arrows reinforced the target response. In the incongruent condition, flankers conflicted with the target response. The magnitude of interference from flankers was manipulated by cue size on each trial (small and large), which was composed of a red circle that was either drawn only around the central target (small) or around both target and flankers (large; see Figure 1). The small cue helped focus attention on the target and minimize the impact of distracting flankers, whereas the large cue provided no information.
Figure 1.
Flanker task conditions in which participants were instructed to respond to the direction of the center arrow. The magnitude of interference from flankers was manipulated by cue size (small and large).
These task manipulations yielded four conditions (small circle incongruent, small circle congruent, large circle incongruent, and large circle congruent), which were each presented 40 times for a total of 160 trials in a rapid event-related paradigm. Each stimulus was displayed for 2 seconds on a black background in the middle of the screen. Baseline consisted of a 3-second presentation of a central fixation cross followed by a 40% interstimulus jitter optimized by optseq2 (http://surfer.nmr.mgh.harvard.edu/optseq/).
MRI Parameters and Preprocessing
All MRI data were collected on a 3.0T Phillips scanner (Best, the Netherlands). The functional MRI protocol employed a fast echo-planar imaging sequence with blood oxygenation level-dependent contrast acquiring 20 slices in sequence at a sampling rate of 1000 milliseconds. In addition, for each subject a high-resolution T1-weighted anatomical image was also collected, stripped of all nonbrain tissue (22), and subsequently used for image registration.
The functional MRI data were preprocessed using FSL version 4.0 (23). Images were slice-time and motion corrected using a rigid-body algorithm (24), temporally filtered with a bandpass filter cutoff at 30 seconds and 1 second, and spatially smoothed with a 7-mm FWHM 3D Gaussian kernel.
Residual noise from excessive head motion was isolated and corrected using MELODIC, an independent components analysis used in FSL. Residual motion artifacts for each participant were signal filtered from her respective time course before the first-level analysis (25).
Data Analysis
A repeated measures analysis of variance (ANOVA) was run on all behavioral data (response times [RTs] and accuracy) with time (baseline, postintervention) and condition (congruent large cue, congruent small cue, incongruent large cue, and incongruent small cue) as within-subjects factors and group (control and treatment) as a between-subjects factor.
The neuroimaging data were convolved using a double-gamma function with temporal derivatives in an event-related analysis. Each condition was added separately to the general linear model. For each participant, a parameter estimate was calculated at each voxel across each of the four conditions. Contrasts of the flanker task conditions were calculated at this level and then forwarded to a higher-level, group-wise analysis in which a mixed-effects ANOVA was carried out. All registration matrices to a standard-space template (Montreal Neurological Institute) were calculated on the individual level and then subsequently applied to the parameter estimates and variance estimates before forwarding to group level analyses. These analyses were conducted separately at each time point. To minimize statistical constraints associated with conducting multiple comparisons, we defined regions of interest (ROIs) based on main effects of congruency (incongruent > congruent collapsed across cue size) at baseline: the anterior cingulate cortex (ACC) and left and right dorsal and ventral prefrontal cortex (dPFC and vPFC, respectively). These ROIs were similar to activated regions found in previous studies of the flanker task (26). The main aim of this study was to examine the effects of the EC intervention; therefore, we used these regions to analyze the follow-up session so that our exploration of the data was restricted to well-defined and theoretically important regions. Data were extracted from these regions and analyzed via a repeated measures analysis in SPSS version 14.0 for Mac (Chicago, IL) to assess effects and interactions of group, time, and condition. We first assessed whether the intervention group exhibited a greater change in activation than the controls over the 6-month interval (Time × Group interaction). Second, we determined whether such intervention-specific change in activity would be selective to the most difficult flanker condition (incongruent) compared with the easier congruent conditions (Time × Group × Condition interaction).
Analysis of the neuroimaging data was carried out using FEAT (FMRI Expert Analysis Tool) version 5.1 part of FSL. Group level analyses were carried out using FLAME. All Z-statistic images were thresholded using clusters determined by Z > 3.1 and a corrected cluster significance threshold of p = .01.
RESULTS
As shown in Table 1, both groups were matched on all sociodemographic variables. Participants were African American, with low income, low education levels, and an average MMSE score of 25, a score lower than typically observed in volunteer samples. Only one participant, in the intervention group, dropped out prior to follow-up due to personal health reasons. No adverse events were reported in the intervention or control arms, and the fMRI protocol was well tolerated at baseline and follow-up.
Response Data
The control group and intervention group did not reliably differ for any condition at the baseline assessment (all p > .05). Furthermore, at baseline, RTs were slower for the incongruent condition compared with the congruent condition (F (1,15) = 21.27; p < .001) and for large cues compared with small cues (F (1,15) = 25.33; p < .001), as expected. At baseline, both groups showed improved performance on the incongruent condition when a small cue was available but showed no similar benefit of cue size on the congruent condition (F (1,15) = 19.52; p < .001).
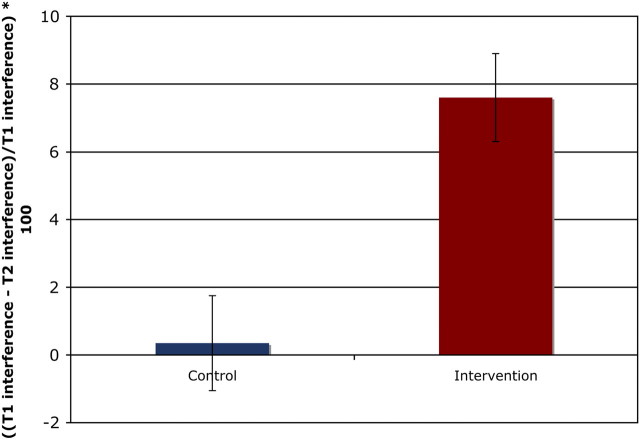
In pre–post comparisons, RTs were analyzed using repeated measures ANOVAs with intervention status as a between-subjects factor, time (baseline, postintervention) as a within-subjects factor, and cue size (small circle and large circle) as a within-subjects factor. Percent interference (((incongruent RT − congruent RT)/congruent RT) × 100) was calculated to adjust for general slowing effects related to aging (denominator) and served as the primary dependent variable. We observed a significant Time × Group interaction (F (1,13) = 5.28; p < .04) in interference scores such that the intervention group showed a greater reduction in interference over the 6-month interval compared with matched controls (see Figure 2). The percent reduction in interference was equivalent across large and small cue sizes. Similarly, the Time × Group × Cue-size interaction was not significant (F (1,13) = 1.80; p < .20).
Figure 2.
Change in percent interference from baseline to postintervention for both the intervention and the control groups collapsed across both small and large circle cues. *Group × Time interaction significant at p < .04.
Accuracy rates did not reliably differ between the control and the intervention groups at baseline (all p > .05). At baseline, accuracy was worse for the incongruent condition compared with the congruent condition (F (1,15) = 6.32; p < .02) and marginally worse for large cues compared with small cues (F (1,15) = 2.59; p < .06). In addition, the small cue improved accuracy more for the incongruent condition than the congruent condition (F (1,15) = 4.52; p < .05). No other main effects or interactions were significant (all p > .05).
Repeated measures ANOVAs were also conducted to examine accuracy rates by intervention status and task difficulty (congruent vs incongruent). Time (baseline and postintervention) and cue size (small circle and large circle) were also within-subjects factors. Compared with RT measures, there was no significant Time × Group interaction (F (1,13) = 1.28; p < .28). However, there was a significant Time × Group × Congruency interaction (F (1,13) = 5.77; p < .03). Post hoc comparisons revealed that this three-way interaction was due to a greater intervention-specific improvement in accuracy in the incongruent conditions (p < .05) that was independent of cue size (F (1,13) = 2.160; p < .16).
Neuroimaging
Results are first presented for baseline within- and between-group comparisons and then for intervention-related changes over time. Consistent with prior fMRI studies using the flanker task, we observed significant increases in activity in regions associated with the attentional network, including the left and right dorsal lateral prefrontal cortex, the ventral lateral prefrontal cortex, and the ACC (26). These regions showed elevated levels of activity for the incongruent condition compared with the congruent condition (collapsed across cue size) that met a voxel-wise threshold of p < .01 and a cluster-wise threshold of p < .05. Prior studies have extensively described these effects in relation to cognitive function, and age-related decline allowing this study to focus on how the EC intervention may impact on processing efficiency and plasticity in these regions. At baseline, both intervention and control groups showed comparable levels of activity across all three ROIs, the ACC, left ventral prefrontal cortex (vLPFC), and left dorsal prefrontal cortex (dLPFC). The effects of the intervention are highlighted subsequently.
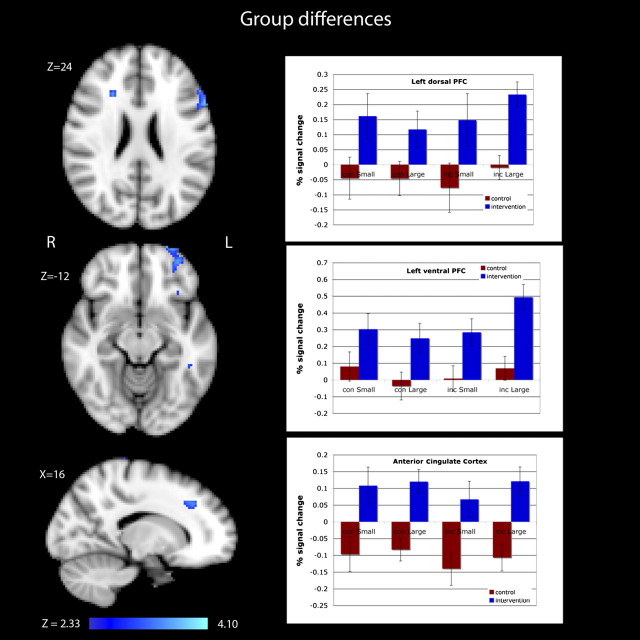
In a repeated measures analysis of the ACC (cluster size = 1704 mm3), we observed a significant Time × Group interaction (F (1,13) = 13.22; p < .003) that was due to a significant intervention-specific increase in activity over the 6-month follow-up (see Figure 3). There were no interactions with congruency or cue size, suggesting that intervention-specific gains in the ACC were independent of congruency condition or cue size or that there was insufficient power.
Figure 3.
Images and plots showing differences in activity between the Experience Corps (EC) group and the control group for the left dorsolateral prefrontal cortex, the left ventrolateral prefrontal cortex, and the anterior cingulate cortex. These regions were not reliably different between the groups at baseline but showed significant increases in activity at postintervention for the EC group (Time × Group, p < .05). PFC, prefrontal cortex.
Similar analyses of the left dLPFC (cluster size = 2704 mm3) also revealed a significant Time × Group interaction (F (1,13) = 5.16; p < .04) with the intervention group showing a significant increase in activity over time (Figure 2). As with the ACC, there were no significant interactions with congruency or cue size. The left vLPFC (cluster size = 1576 cubic mm) showed a similar Time × Group interaction (F (1,13) = 8.99; p < .01) with those in the intervention group showing a significant increase in activity at follow-up. Again, neither congruency nor cue size interacted with time or group, indicating that the intervention group showed increases in this region across all conditions (Figure 2).
No regions showed an intervention-related decrease in activity. The right prefrontal regions, although active at baseline for both groups, did not show significant changes in activation that met threshold. However, there was a nonsignificant increase in the right dorsal prefrontal activity for the incongruent condition for both the intervention and the control groups (p < .16).
Pre–post comparisons of each group’s ability to filter conflicting distracters (incongruent RT–congruent RT) for small and large cues are presented in Figure 3 and show EC-intervention–specific improvements in the ability to selectively attend during the most attention demanding task conditions (incongruent). Corresponding fMRI group comparisons similarly showed increased activity in attentional control regions of the PFC (middle and inferior frontal regions bilaterally) and the parietal regions, suggesting more efficient filtering or inhibiting of target information from distraction (Figure 3).
DISCUSSION
This pilot study provides proof of concept for the feasibility and utility of neuroimaging to begin to understand how a multimodal activity program in the community gets under the skin to improve executive functions and supporting brain regions. These at-risk individuals exhibited measurable brain plasticity in direct response to such environmental enrichment, providing initial evidence of this program’s potential to reverse cognitive and corresponding neural declines with age. Individuals exhibited use-dependent neural plasticity by exercising and reactivating skills that may have been relatively unused for years or even decades. This finding is best captured by a personal observation from one of the volunteers, who stated that “it [Experience Corps] removed the cobwebs from my brain.” Additionally, these previously sedentary at-risk participants were amenable to the fMRI environment on repeated exams, as demonstrated by 100% retention, and those enrolled in the program met the intensive service requirements, which led to unprecedented doses over a relatively short-exposure period.
The results replicate and build on the previous pilot trial of EC and cognition, and on an fMRI trial of physical activity in older adults. First, we replicated the impact of EC on executive functions using a sensitive measure that focuses on the age-vulnerable ability to inhibit distraction in one’s environment. Indeed, the present finding suggests generalization of EC improvements over different measures of executive function. These improvements extended to corresponding increases in the activity of supporting prefrontal cortical substrates, further replicate seminal findings on the neurocognitive benefit of physical activity (26–28). The patterns of increased functional activity here differed slightly from the exercise findings in two ways. First, we observed EC-related increases in the ACC during the executive function task, whereas the exercise intervention led to decreased activation in this region. The ACC has been implicated in the efficient filtering or inhibiting of conflicting information prior to generation of a motor response. Although both studies demonstrated improved inhibitory efficiency (speed), EC participants started with lower baseline inhibitory ability than those in the exercise intervention. Thus, as in a prior pilot trial of EC (21), these individuals were at elevated risk for executive dysfunction and likely exerted more effort to successfully develop inhibitory skills, which may be reflected by increased ACC activity. Second, we observed the lateralized increases in left prefrontal cortical activity in the EC sample while the exercise intervention observed the right prefrontal cortical increases. These laterality differences may be due to the nature of EC volunteer activities, which rely heavily on verbal communication and mediation strategies, and may thus elicit greater improvements in regions associated with communication, such as the left prefrontal and temporomedial cortices. These hypotheses require replication in a larger sample.
Although the functional significance of the laterality differences is unclear, greater unilateral activation of cortical regions following the EC intervention contributes to discussions in the functional neuroimaging literature (11,29) on the nature of brain plasticity, reserve, and compensatory function. We have yet to determine whether these changes were accompanied by structural changes and changes in supporting white matter tracts that facilitate rapid and efficient communication across regions.
The implications of these findings to the assessment of postretirement lifestyle activity are that a broader range of cognitive activities embedded within social settings may confer great cognitive and brain benefits for older adults. Recent epidemiological evidence in twin pairs suggests that socially engaging cognitive activities in midlife and early late-life may reduce risk for AD and dementia decades later (30) and may be indicative of an enriched environment, which enhances the proliferation of new brain cells and promotes brain repair in animal models (31–33). The implications of these findings to the assessment of postretirement lifestyle activity are that a broader range of cognitive activities may confer great cognitive benefits for older adults and may further confer neurocognitive protection.
Cognitive activity embedded within social settings may further increase task novelty, interactive problem-solving skills, and motivations to sustain these activities. In addition, these activities are generative in giving meaning and purpose to one’s life (volunteering, civic organizations, assisting others), which may make them more rewarding and personally enriching than highly stimulating activities performed alone (34). As a result, individuals may place more value on these activities beyond their immediate personal benefit and may sustain interest longer (35). This important developmental need to be generative could provide an important vehicle for enhancing and sustaining behaviors important to successful aging, namely remaining active—socially, physically, and cognitively (34).
Limitations of this pilot study include the small sample size necessarily restricted to women (due to gender differences in brain morphology), which limits generalizability but provides proof of concept for the potential of well-validated parametric fMRI tasks, such as this, to sensitively detect program-related functional brain changes in a larger randomized study of men and women. Second, although the sample represented an important and often under-studied segment of the aging adult population, we have yet to determine whether this program enhances or maintains cognition among more ethnically and socioeconomically diverse individuals. Finally, this study design does not allow us to definitively discern whether the effects of this intervention on cognition function were mediated primarily by cognitive and physical pathways, respectively, or whether benefits represent the synergy of increased activity in all domains. Understanding the mediating source may not be as critical as the observation that a multiple pathway approach set in the community was associated with high doses, good retention, and short-term effects spanning many abilities in the most at risk.
These findings offer next-level questions about the ability of this program, and others like it, to reset one’s trajectory of cognitive decline with age, particularly among those at elevated risk for dementia by virtue of impoverished environments over the life course, as marked by low or poor quality (36) education and low income. These individuals require further follow-up in order to determine the potential and boundaries of plasticity in a dose-dependent manner. Questions include whether a lower weekly exposure may confer equivalent benefits and whether continued exposure would lead to accruing benefits, perhaps in other brain regions interacting with these prefrontal circuits, such as parahippocampal and hippocampal regions that support some memory functions. Furthermore, it will be key to determine whether short-term benefits will be sustained after program exposure is discontinued. Overall, these pilot findings hold promise for enhancing functional reserve and neural plasticity among those at great risk.
FUNDING
This work was supported in part by the Research Career Development Core of the Older Americans Independence Center (P30-AG021334) and a gift from S. B. Bechtel.
Acknowledgments
We would like to thank Mr. Vijay Varma for editing an earlier draft of this article.
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s and Dementia. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 3.Studenski S, Carlson MC, Fillit H, Greenough WT, Kramer A, Rebok GW. From bedside to bench: does mental and physical activity promote cognitive vitality in late life? Sci Aging Knowledge Environ. 2006;2006(10):pe21. doi: 10.1126/sageke.2006.10.pe21. [DOI] [PubMed] [Google Scholar]
- 4.Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol B Psychol Sci Soc Sci. 1999;54(5):S262–S270. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- 5.Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. J Am Geriatr Soc. 1998;46(5):590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62(10):1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royall DR, Palmer R, Chiodo LK, Polk MJ. Executive control mediates memory’s association with change in instrumental activities of daily living: the Freedom House Study. J Am Geriatr Soc. 2005;53(1):11–17. doi: 10.1111/j.1532-5415.2005.53004.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlson MC, Xue QL, Zhou J, Fried LP. Executive decline and dysfunction precedes declines in memory: the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2009;64(1):110–117. doi: 10.1093/gerona/gln008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempen GI, van Heuvelen MJ, van Sonderen E, van den Brink RH, Kooijman AC, Ormel J. The relationship of functional limitations to disability and the moderating effects of psychological attributes in community-dwelling older persons. Soc Sci Med. 1999;48(9):1161–1172. doi: 10.1016/s0277-9536(98)00427-4. [DOI] [PubMed] [Google Scholar]
- 10.Dulaney CL, Rogers WA. Mechanisms underlying reduction in Stroop interference with practice for young and old adults. J Exp Psychol Learn Mem Cogn. Mar 1994;20(2):470–484. doi: 10.1037//0278-7393.20.2.470. [DOI] [PubMed] [Google Scholar]
- 11.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Raz N. Aging of the brain and its impact on cogntive performance: Integration of structural and functional findings. In: Craik F, Salthouse T, editors. Handbook of Aging and Cognition. New Jersey: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- 13.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 15.Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol Aging. 1999;14(3):483–506. doi: 10.1037//0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Freedman M, Endres TE, Wasik B. Building communities that promote successful aging. West J Med. 1997;167(4):216–219. [PMC free article] [PubMed] [Google Scholar]
- 17.Fried LP, Carlson MC, Freedman M, et al. A social model for health promotion for an aging population: initial evidence on the Experience Corps model. J Urban Health. 2004;81(1):64–78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebok GW, Carlson MC, Glass TA, et al. Short-term impact of Experience Corps participation on children and schools: results from a pilot randomized trial. J Urban Health. 2004;81(1):79–93. doi: 10.1093/jurban/jth095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Motor Skills. 1958;8(4):271–276. [Google Scholar]
- 21.Carlson MC, Saczynski JS, Rebok GW, et al. Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps. Gerontologist. 2008;48(6):793–801. doi: 10.1093/geront/48.6.793. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 25.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 26.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 28.Kramer A, Erickson KI, Colcombe SJ. Exercise, cognition and the aging brain. J Appl Physiol. 2006;101(4):1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 29.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 30.Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement. 2008;4(5):324–331. doi: 10.1016/j.jalz.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 32.Greenough WT, Cohen NJ, Juraska JM. New neurons in old brains: learning to survive? Nat Neurosci. 1999;2(3):203–205. doi: 10.1038/6300. [DOI] [PubMed] [Google Scholar]
- 33.van Praag H, Kempermann G, Gage F. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 34.Carlson MC, Seeman T, Fried LP. Importance of generativity for healthy aging older women. Aging (Milano) 2000;12(2):132–140. doi: 10.1007/BF03339899. [DOI] [PubMed] [Google Scholar]
- 35.Erikson EH. Identity and Life Cycle: Selected Papers. New York: W. W. Norton; 1959. pp. 1–171. [Google Scholar]
- 36.Manly JJ, Schupf N, Tang MX, Stern Y. Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol. 2005;18(4):213–217. doi: 10.1177/0891988705281868. [DOI] [PubMed] [Google Scholar]